Abstract
Heat shock protein 90 (HSP90) inhibitors are considered as new radiosensitizing agents. PU-H71, a novel HSP90 inhibitor, is under evaluation for the treatment of advanced cancer. It is however not known whether PU-H71 alters radiosensitivity of metastatic breast cancer. Hence, we here evaluated mechanisms of possible anti-tumoral and radiosensitizing effects of PU-H71 on breast carcinoma cells metastasized to vital organs such as the liver and brain. The effect of PU-H71 on proliferation of breast carcinoma cells was determined using 4T1 cells and its brain (4TBM), liver (4TLM), and heart (4THM) metastatic subsets as well as non-metastatic 67NR cells. Changes in radiation sensitivity were determined by clonogenic assays. Changes in client proteins and levels of angiogenic and inflammatory mediators from these cancer cell cultures and ex vivo cultures were detected. PU-H71 alone inhibited ERK1/2, p38, and Akt activation and reduced N-cadherin and HER2 which further documented the anti-tumoral effects of PU-H71. The combination of PU-H71 and radiotherapy induced cytotoxic effect than PU-H71 alone, and PU-H71 showed a radiosensitizing effect in vitro. On the other hand, PU-H71 and radiation co-treatment increased p38 phosphorylation which is one of the hallmarks of inflammatory response. Accordingly, IL-6 secretion was increased following PU-H71 and radiotherapy co-treatment ex vivo. Levels of angiogenic and inflammatory factors such as MIP-2, SDF-1, and VEGF were increased under in vitro conditions but not under ex vivo conditions. These results demonstrated for the first time that PU-H71 enhances therapeutic effects of radiotherapy especially in highly metastatic breast carcinoma but a possible increase in inflammatory response should also be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of deaths in breast cancer are due to metastasis to vital organs such as the brain and liver (Jemal et al. 2011; Weigelt et al. 2005). Small percentage of cells within the primary tumor can form metastasis, and these cells are resistant to conventional treatments (Valastyan and Weinberg 2011). Hence, new treatment modalities should have anti-tumoral effects on metastatic cell in order to achieve clinically promising results.
Heat shock protein 90 (HSP90) induced by cell stress stabilizes many of cellular proteins and protects them from degradation (Morimoto et al. 1997). These proteins are known as HSP90’s client proteins. Increased expression of HSP90 is closely associated with a poor prognosis and resistance to therapy in cancer. HSP90 has distinct expression profiles in normal and malignant cells (Guo et al. 2017; Neckers 2007). HSP90 derived from tumor cells has a higher binding affinity for HSP90 inhibitor 17-AAG compared to HSP90 from normal cells (Kamal et al. 2003). Hence, HSP90 inhibitors are promising drugs for cancer treatment.
HSP90 interacts with specific client proteins which have critical roles in signal transduction, cellular trafficking, cell growth, and differentiation (Butler et al. 2015). PU-H71 has a unique selectivity for binding the fraction of HSP90 that is associated with oncogenic client proteins that are enriched in tumor cells. For example, PU-H71 was shown to preferentially target HSP90-client protein complexes in tumor cells (Moulick et al. 2011). PU-H71, which is a purine scaffold HSP90 inhibitor, has a higher affinity for activated state of HSP90 (HSP90 complexes) and has anti-cancer activity on triple-negative breast cancer (Caldas-Lopes et al. 2009; Moulick et al. 2011). This property is thought to increase anti-tumoral effects of PU-H71 and decrease its possible toxicity in normal cells. Effectiveness of PU-H71 in combination with other anti-cancer agents was shown in myeloproliferative neoplasms, Ewing sarcoma, and lymphoma xenografts (Ambati et al. 2014; Bhagwat et al. 2014; Goldstein et al. 2015).
Several studies demonstrated that HSP90 inhibitors may enhance sensitivity of tumor cells to ionizing radiation (Gandhi et al. 2013; Ha et al. 2011; Lee et al. 2016; Li et al. 2016; Segawa et al. 2014; Yoshida et al. 2011). Up to our knowledge, the effect of HSP90 inhibitors on metastatic cells of breast carcinoma alone or in combination with radiotherapy (RT) was not studied before. Hence, one of the aims of this study was to examine possible anti-tumoral effects of HSP90 inhibitors alone and in combination with RT on breast cancer cells metastasized to vital organs such as the brain (4TBM), liver (4TLM), and heart (4THM).
Chemokines, such as stromal-derived factor-1 (SDF-1; also known as CXCL12) and macrophage inflammatory protein-2 (MIP-2; also known as CXCL2), play a critical role in tumor growth, angiogenesis, metastasis, and resistance to chemotherapy (Barbero et al. 2003; Lee et al. 1995; Shen et al. 2008; Singh et al. 2013; Xu et al. 2015). Vascular endothelial growth factor (VEGF) is another angiogenic factor involved in tumor progression and angiogenesis (McMahon 2000). Previous studies suggest that HSP90 inhibitors decrease secretion of angiogenic and inflammatory factors such as IL-8 (functional counterpart in mouse MIP-2), VEGF, and SDF-1 (Liu et al. 2017; Nagaraju et al. 2013; Seaton et al. 2009; Terwisscha van Scheltinga et al. 2014; Xiang et al. 2014; Xu et al. 2013). Interleukin-6 (IL-6), an inflammatory cytokine, plays an important role in cancer formation and metastasis (Knupfer and Preiss 2007). Previous studies suggest that inflammatory mediators may decrease radiosensitivity (Chin and Wang 2014). IL-6 also causes radioresistance which might be due to increased inflammation and angiogenesis (Wu et al. 2013). Up to our knowledge, the effects of HSP90 inhibitors on cancer-induced IL-6 secretion are not known. Hence, we here further examined changes in SDF-1, MIP-2, VEGF, and IL-6 secretion following treatment with PU-H71 alone or in combination with RT. Treatment-induced changes in HSP-client proteins were also determined.
Methods and materials
Cell culture, pre-irradiation treatments, and radiotherapy
4T1 (highly metastatic) and 67NR (non-metastatic) cells were previously derived from spontaneously formed breast tumors in Balb-c mice (Aslakson and Miller 1992). The 4THM cell line was derived from cardiac metastases of 4T1 cells (Erin et al. 2004; Erin et al. 2013; Erin et al. 2006). 4THM cells when implanted orthotopically into Balb/c mice generated macroscopic liver and brain metastases, which in turn were used to develop additional cell line, designated as 4TLM (liver metastatic) and 4TBM (brain metastatic) (Erin et al. 2009).
These cells were grown in DMEM-F12 (Gibco, 11320-074) supplemented with 5% FBS (fetal bovine serum), 2 mM l-glutamine, 1 mM sodium pyruvate, and 0.02 mM nonessential amino acids. PU-H71 (Adooq, A11130) and 17-AAG (LC laboratories, A-6880) were dissolved in dimethyl sulfoxide (DMSO).
Cells in culture dishes were irradiated with 2–10 Gy at room temperature using Cobalt 60 (Co60) irradiator at a dose rate of 0.4 Gy/min. Cells were irradiated for a field size of 40 × 40 cm2 at distance and SSD of 100 cm (skin-source distance).
Cell proliferation and viability assays
Cells, plated in 96-well plates (500 cells/well) in growth media, were treated with varying concentration of PU-H71 or 17-AAG for 72 h. After treatment, the number of viable cells was determined using the WST-1 (Roche) cell proliferation assay. The concentration of drugs at which cell growth was inhibited by 50% (IC50) was estimated using GraphPad Prism5 software.
Colony formation assay
Cells, plated in a 6-well plate at 300 cells/well density, were treated with various concentrations of PU-H71 (0.1 and 0.01 μM) 6 h after plating and were irradiated (2, 4, 6, or 8 Gy) 24 h after PU-H71 treatment. After RT, mediums were removed and fresh medium was added to remove PU-H71. The colonies were fixed and stained 12–14 days after RT. Colonies containing at least 50 cells were counted. The assay was repeated three times. Plating efficiencies and surviving fractions were calculated as described before (Franken et al. 2006). In addition, the effects of PU-H71 and RT co-treatment on cell proliferation were determined.
Measurement of VEGF, MIP-2, SDF-1, and IL-6
Cells, plated in 24-well plates (4 × 103 cells/well), were treated with 0.1 μM PU-H71 for 24 h followed by 10 Gy RT. Conditioned mediums (CM) were collected after 48 h of RT to determine VEGF (PEPROTECH 900-K99), CXCL12/SDF-1 (R&D Systems, DY460), MIP-2 (PEPROTECH 900-K152), and IL-6 (BioLegend, 78,417) levels using enzyme-linked immunosorbent assay. Protocols provided by the manufacturer were followed. All experiments were performed in triplicate.
Ex vivo culture
4TLM cells (105 cells /mouse) were injected orthotopically into the right upper mammary gland of 8–10-week-old female Balb-c mice (from Kobay, Ankara-Turkey). Necropsies were performed 15–20 days after injection of tumor cells. Primary cell cultures were prepared from primary tumors as described before (Erin et al. 2013). Primary tumor explants were exposed to varying dose of PU-H71 and RT when the cells reached 80% confluence. CM from primary tumor explants were collected 48 h after treatments to measure IL-6, VEGF, and MIP-2 levels.
Western blot analysis
Cells were treated with varying concentration of PU-H71 for 24 h followed by 5 Gy irradiation. Cell lysates were obtained 24 h after RT. Immunoblotting of target proteins was performed as described before (Erin et al. 2003). Dilutions of primary antibodies used were p-Akt (1:1000, Cell Signaling, 4060, Ser473), p-ERK1/2 (1:1500, p-p44/42, Cell Signaling, 9106, Thr202/Tyr204), p-p38 MAPK (1:1000, Cell Signaling, 4511, Thr180/Tyr182), N-Cadherin (1:1000, BD Biosciences, 610,921), HER-2 (1:1500, Millipore, 06-562), HSP90 (1:1000, Cell Signaling, 4875), and HSP70 (1:1000, Cell Signaling,4876). Proteins were visualized using enhanced chemiluminescence (ECL). GAPDH (1:100000, Meridian Life Science, H86504M) was used as a housekeeping protein to ensure equal loading and transfer of proteins. Experiments were repeated three times.
Statistical analysis
Data were presented as mean values ± standard error. Depending on the data, statistical comparisons among groups were performed using one of the following tests: ANOVA followed by Tukey post-test and Student’s t test with or without Welch correction. These analyses were performed using GraphPad Software.
Results
PU-H71 dose dependently inhibits cell proliferation and enhanced anti-proliferative effects of RT in metastatic breast cancer cells
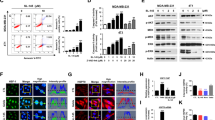
The effects of HSP90 inhibitor PU-H71 (0.01–10 μM) on cell viability and proliferation of metastatic breast cancer cells were determined, and its effects were compared with a classical HSP90 N-terminal inhibitor, 17-AAG (Supplementary Fig. 1). Although PU-H71 inhibited proliferation of cells in all metastatic cell lines, the sensitivity to PU-H71 differed. The IC50 of PU-H71 was 0.11 μM, 0.05 μM, 0.24 μM, and 0.04 μM for 4T1, 4TBM, 4THM, and 4TLM cells, respectively (Fig. 1a). Hence, 4TLM and 4TBM cells were more sensitive to PU-H71 than 4THM cells. Surprisingly non-metastatic breast carcinoma cells (67NR) were markedly less sensitive to PU-H71 (IC50: 0.23 μM) (Supplementary Fig. 1). In addition, PU-H71 was a more potent inhibitor of cell proliferation compared to 17-AAG, the effect observed mainly in 4TBM and 4TLM (Fig. 1a) cells as well as 67NR cells (Supplementary Fig. 1).
The effects of HSP90 inhibitors on cell proliferation alone or in combination with radiotherapy (RT). Cells, plated in 96-well plates (500 cells/well) in growth media, were treated with varying concentration of PU-H71 for 72 h. After treatment, the number of viable cells was determined using the WST-1 (Roche) cell proliferation assay. “Inhibitor concentration 50” values of PU-H71 were calculated and reported here as IC50. The IC50 values (in μM) for 4TBM, 4TLM, 4THM, and 4T1 cells are shown in a. RT and PU-H71 co-treatment-induced changes in cell proliferation for 4TLM and 4TBM cells are shown in b and c, respectively. T0 denotes number of cells just before treatment*,$ p<0,05.
The possible radiosensitizing effects of PU-H71 on the proliferation of metastatic cell lines were also determined. Initially, two different doses of RT were used to determine its effects on cell proliferation (Supplementary Fig. 2). PU-H71 at relatively low concentrations increased anti-proliferative effects of RT (10 Gy) on both 4TLM (Fig. 1b) and 4THM (Fig. 1c) cells. Ineffective concentration of PU-H71 (0.1 μM) markedly enhanced anti-proliferative effects of RT on 4TLM cells. Interestingly, 4THM cells were partly resistant to anti-proliferative effects of RT under this experimental setting, which was restored with the treatment of PU-H71, further demonstrating radiosensitizing effects of HSP90 inhibition (Fig. 1c).
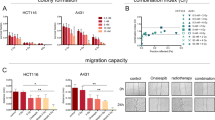
We used another approach to further examine possible radiosensitizing effects of PU-H71. Colony formation assay was used to determine the effect of PU-H71 administration (0.01 and 0.1 μM) on RT-induced (2, 4, 6, 8 Gy) inhibition of cell survival in 4THM (heart), 4TLM (liver), and 4TBM (brain) metastatic cells of 4T1 murine breast carcinoma cells. Results were given as survival fractions (Fig. 2). We also included actual changes in colony numbers in order to demonstrate the effects of PU-H71 treatment alone. PU-H71 at 0.1 μM concentration significantly inhibited colony formation in all cell lines. RT dose dependently inhibited colony formation in all cell lines. Brain metastatic cells (4TBM) were more sensitive to RT such that at 2 Gy RT inhibited colony formation in 4TBM cells but not in 4TLM and 4THM cells (Fig. 2a). 4TLM and 4THM cells became sensitive to 2 Gy RT in the presence of PU-H71 (0.1 μM) (Fig. 2a, c).
Clonogenic survival assay. Cells, plated in a 6-well plate at 300 cells/well density, were treated with various concentrations of PU-H71 (0.1 and 0.01 μM) 6 h after plating and were irradiated (2, 4, 6, or 8 Gy) 24 h after PU-H71 treatment. After RT, mediums were removed and a fresh medium was added to remove PU-H71. The colonies were fixed and stained 12–14 days after RT. Colonies containing at least 50 cells were counted. The assay was repeated three times. Number of colonies and surviving fractions were demonstrated for 4TBM (a), 4TLM (b) and 4THM (c). $p < 0.05 compared to the 0.1 μM PU + 0 GY group. *p < 0.05. T0 denotes number of cells just before treatments. $p < 0.05 compared to PU-H71-treated 4TLM and 4TBM cells. *p < 0.05
Radiosensitizing effects of PU-H71 at 0.01 μM concentration were also observed in all cell lines. Specifically, PU-H71 (0.01 μM) did not affect colony numbers in the absence of RT but markedly enhanced the inhibitor effects of 6 Gy irradiation in 4TBM cells (Fig. 2b). Similar effects of 0.01 μM PU-H71 were observed in 4TLM and 4THM cells exposed to 8 Gy RT (Fig. 2a, c).
PU-H71 inhibited ERK1/2, p38, and Akt activation and reduced HER2 and N-cadherin
PU-H71 inhibited activation (phosphorylation) of ERK1/2, p38, and Akt in metastatic cell lines especially at relatively high concentration (1 μM) 24 h after treatment (Fig. 3a, b). PU-H71 treatment did not alter the total level of ERK (Supplementary Fig. 3). Similarly, PU-H71 markedly decreased cellular levels of HER2, a well-known HSP90 client protein (Mimnaugh et al. 1996). The 4T1 model is considered as HER2 (−) based on the percent of cells expressing HER2 under in vivo conditions. Hence, it was shown that 4 T1 tumors do not overexpress HER2 (Bao et al. 2011). Our results are somewhat in agreement with these findings and 4T1 and 4T1-derived cells express detectable levels of HER-2. PU-H71 also increased HSP70, a marker for HSP90 inhibition, as demonstrated before in different models (Ambati et al. 2014; Caldas-Lopes et al. 2009). We here also examined changes in N-cadherin expression, which is not a classical client protein. PU-H71 decreased N-cadherin expression dose dependently. Surprisingly, we observed a hormetic effect (Lushchak 2014) such that PU-H71 at 1, 0.1, and 0.01 μM concentration markedly suppressed N-cadherin expression while the opposite effect was observed at 0.5 μM concentration in both 4THM and 4TLM.
PU-H71-induced changes in levels and/or phosphorylation (p) of intracellular proteins. Cells, plated in a 6-well plate at 300000 cells/well density, were treated with various concentrations of PU-H71 (1, 0.5, 0.1, and 0.01 μM). Cell lysates were prepared 24 h after treatment. Effects of PU-H71 on 4THM (a) and 4TLM (b) are shown. GAPDH was used as a loading control (housekeeping gene)
Low concentration of PU-H71 and radiation co-treatment inhibited ERK1/2 activation, but increased p38 phosphorylation
Low concentration of PU-H71 (0.1–0.001 μM) was used here because higher concentrations in combination with 5 Gy RT induced cell death in 48 h preventing evaluation of cellular changes. Co-treatment with PU-H71 and RT markedly suppressed ERK1/2 phosphorylation especially in 4TBM cells. Surprisingly PU-H71 alone or in combination with RT significantly increased p38 phosphorylation in 4TBM, 4THM, and 4TLM cells (Fig. 4a–c, respectively).
The effects of radiotherapy (RT) and/or PU-H71 treatment on the levels and/or phosphorylation (p) of intracellular molecules. Cells, plated in a 6-well plate at 300000 cells/well density, were treated with lower concentrations of PU-H71 (0.1, 0.05, 0.01, 0.001 μM) for 24 h followed by 5 Gy irradiation. After RT, the culture medium was changed to remove PU-H71. Cell lysates were obtained 24 h after RT. Effects of RT and or PU-H71 on 4TBM (a), 4THM (b), and 4TLM (c) are shown. GAPDH was used as a loading control (housekeeping gene)
Combination of PU-H71 and RT altered secretion of MIP-2, SDF-1, and VEGF from 4TLM and 4TBM cells under in vitro conditions
PU-H71 alone or in combination with RT significantly increased MIP-2 secretion correlating with enhanced p38 phosphorylation. RT alone also increased MIP-2 secretion in 4TLM cells (Fig. 5a). RT and PU-H71 co-treatment induced increases in MIP-2 secretion which were significantly higher compared to RT or PU-H71 treatment alone (Fig. 5a, d). Similar effects of RT and PU-H71 co-treatment were observed in SDF-1 secretion (Fig. 5b, e). VEGF secretion, detectable only in 4TLM cells, was also markedly increased after co-treatment (Fig. 5c). We also examined the changes in SDF-1, VEGF, and MIP-2 levels in 4THM cells. VEGF levels were low in 4THM cells. PU-H71 did not alter MIP-2 and SDF-1 levels in 4THM cells at concentrations that were effective in 4TLM and 4TBM cells. This is most likely due to the lower sensitivity of 4THM cells to PU-H71 (Supplementary Fig. 4).
The effects of radiotherapy (RT) and/or PU-H71 treatment on secretion of MIP-2, SDF-1, and VEGF in vitro. Cells, plated in 24-well plates (4 × 103 cells/well), were treated with 0.1 μM PU-H71 for 24 h followed by 10 Gy RT. Conditioned mediums (CM) were collected after 48 h of RT to determine secreted levels of VEGF, CXCL12/SDF-1, and MIP-2 using enzyme-linked immunosorbent assay. Changes in 4TLM cells (a–c) and 4TBM cells (d, e) are shown. *p < 0.05 compared to control, #p < 0.05 compared to PU-H71 or RT alone. SDF-1 stromal-derived factor-1, MIP-2 macrophage inflammatory protein-2, VEGF vascular endothelial growth factor
Effects of PU-H71 and RT on IL-6, VEGF, and MIP-2 under ex vivo conditions
The metastatic cell lines studied here do not secrete IL-6 under in vitro conditions. IL-6 secretion however was observed freshly prepared primary tumor explants (Erin et al. 2015b). Hence, ex vivo cultures from primary tumors of 4TLM-injected mice were prepared to determine possible treatment-induced changes in IL-6 secretion. We here found that ex vivo cultures are much more sensitive to cytotoxic effects of PU-H71 and combination treatment. Specifically, PU-H71 at 0.1 μM concentration induced excessive cell death such that WST-1 reading of the treated group was similar to the background reading. Hence, we decreased the drug concentration at 10 times magnitude in order to evaluate changes in IL-6, MIP-2, and VEGF levels. RT alone or in combination with PU-H71 treatment significantly increased IL-6 secretion (Fig. 6a). Surprisingly, PU-H71 alone decreased IL-6 secretion but markedly potentiated the effects of RT on IL-6 secretion. Because ex vivo cultures may reflect in vivo conditions better than in vitro cell culture, we also examined changes in VEGF and MIP-2 levels. RT alone increased MIP-2 levels similar to the results obtained under in vitro conditions. Differently, PU-H71 treatment alone did not increase VEGF and MIP-2 levels; on the contrary, PU-H71 treatment decreased VEGF levels and prevented RT-induced increases in VEGF and MIP-2 levels (Fig. 6b, c).
Ex vivo effects of radiotherapy (RT) and/or PU-H71 treatment on secretion of IL-6 (a), MIP-2 (b), and VEGF (c) in 4TLM tumor explants. 4TLM cells (105 cells /mouse) were injected orthotopically into the right upper mammary gland of 8–10-week-old female Balb-c mice. Necropsies were performed 15–20 days after injection of tumor cells. Primary cell cultures were prepared from primary tumors as described before (Erin et al. 2013). Primary tumor explants were exposed to varying dose of PU-H71 and RT when the cells reached 80% confluence. CM from primary tumor explants were collected 48 h after treatments to measure IL-6, VEGF, and MIP-2 levels.*p < 0.05 compared to control. MIP-2 macrophage inflammatory protein-2, VEGF vascular endothelial growth factor
Discussion
Cells metastasize to visceral organs comprise stem cell properties and are resistant to most conventional treatments (Charafe-Jauffret et al. 2009). We here demonstrated that HSP90 inhibitor PU-H71 inhibited the proliferation of brain (4TBM) and liver (4TLM) metastatic cells more efficiently than non-metastatic 67NR breast cancer cells. Furthermore, PU-H71 was a more potent inhibitor of cell proliferation compared to 17-AAG. 4TLM and 4TBM cells are more aggressive and induce more systemic metastasis compared to parental cell line (4T1) (Erin et al. 2013). The sensitivity of 4TLM cells, the most aggressive subset of breast carcinoma, to PU-H71 was approximately 5–6 times more than non-metastatic 67NR breast carcinoma cells. In addition, PU-H71 sensitized the metastatic breast cancer cells to growth inhibitory effects of irradiation. Similarly, radiosensitivity of PC3 and LNCaP metastatic prostate carcinoma increased with 17-DMAG, another HSP inhibitor, treatment (Rae and Mairs 2017). HSP90 inhibitors were shown to enhance the radiosensitivity of lung, cervical, breast, and bladder cancer cell lines in vitro (Ha et al. 2011; Hashida et al. 2015; Provencio and Sanchez 2014; Yoshida et al. 2011). PU-H71 enhanced the sensitivity of the SQ-5, A549 human lung cancer cells, and LM8 murine osteosarcoma cells but not fibroblasts to radiation (Lee et al. 2016; Li et al. 2016; Segawa et al. 2014). It is however not known whether PU-H71 alters radiosensitivity of breast cancer cells that metastasized to vital organs. Up to our knowledge, there are two studies that extensively examined the effects of PU-H71 in metastatic breast cancer. In the first study, PU-H71 inhibited tumor growth markedly without major toxicity demonstrating that PU-H71 could be considered for the treatment of metastatic breast cancer (Caldas-Lopes et al. 2009). In the second study, PU-H71 was found to induce death of MDA-MB-231 breast carcinoma cells in the presence of TNF-α (Qu et al. 2014). We here found that PU-H71 sensitized 4TLM, 4THM, and 4TBM cells to growth inhibitory effects of RT.
Previous studies suggest that HSP90 inhibitors decrease secretion of angiogenic and inflammatory factors such as IL-8 (functional counterpart in mouse MIP-2), VEGF, and SDF-1 (Liu et al. 2017; Nagaraju et al. 2013; Seaton et al. 2009; Terwisscha van Scheltinga et al. 2014; Xiang et al. 2014; Xu et al. 2013). We however found that PU-H71 alone increased MIP-2, SDF-1, and VEGF secretion correlating with enhanced p38 phosphorylation in 4TBM and 4TLM cells under in vitro conditions. Activation of p38 pathway is considered to be one of the hallmarks of inflammatory response (Gupta and Nebreda 2015). Inflammatory mediators may decrease radiosensitivity (Chin and Wang 2014). Hence, other inflammatory mediators such as MIP-2 and SDF-1 may also cause radioresistance. Specifically MIP-2 is known to be a chemoattractant for neutrophils and increases local inflammation as well as angiogenesis in tumor microenvironment (Erin et al. 2015a; Kollmar et al. 2006; Kwon et al. 2015; Wagner et al. 2012). Similarly, inhibition of SDF-1 activity decreases inflammation and suppresses cancer growth (Mao et al. 2015). Recently, it was shown that anti-VEGF treatment increases radiosensitivity of schwannoma demonstrating that increased local VEGF levels may cause radioresistance (Gao et al. 2015). Hence, increased MIP-2, SDF-1, and VEGF may counteract radiosensitizing effects of PU-H71.
IL-6, an inflammatory cytokine, enhances stemness of tumor cells and metastasis (Chin and Wang 2014). Previous studies documented that IL-6 causes radioresistance which might be due to increased inflammation and angiogenesis (Wu et al. 2013). Similarly, IL-6 was found to inhibit radiation-induced apoptosis of pancreatic cancer cells, suggesting a role for IL-6 in radioresistance (Miyamoto et al. 2001). RT was also found to elevate serum IL-6 levels in head and neck cancer patients (Akmansu et al. 2005). Similar IL-6 levels were increased after RT in colon cancer cell lines (Pathak et al. 2015). Accordingly, we here found that RT alone or in combination with PU-H71 treatment significantly increased IL-6 secretion under ex vivo conditions. Hence, increased IL-6 levels may limit the effectiveness of RT and PU-H71 co-treatment and inhibition of IL-6 activity in addition to PU-H71 and RT co-treatment should be considered clinically. Hence, caution should be taken during PU-H71 treatment of carcinomas that induce excessive inflammation.
The cell signaling molecules ERK1/2, p38, and Akt are client proteins of HSP90 and they play a crucial role in cell growth, survival, and apoptosis. Accordingly, we found that PU-H71 treatment inhibited phosphorylation of ERK1/2, p38, and Akt in breast cancer cells metastasized to visceral organs (Weigelt et al. 2005). Similarly, PU-H71 decreased phosphorylation of ERK1/2 and Akt in a dose-dependent manner in the triple-negative breast cancer cell lines, acute myeloid leukemia cells, and Burkitt lymphoma cells (Caldas-Lopes et al. 2009; Giulino-Roth et al. 2017; Zong et al. 2015). HER2 is a HSP90’s client protein that is amplified in approximately 25% of breast cancers. We found that PU-H71 decreased HER2 expression dose dependently in metastatic cells.
N-Cadherin, a mesenchymal marker, plays a critical role in breast cancer progression and in maintaining malignant phenotype (Rezaei et al. 2012). Hence, we also evaluated changes in N-cadherin levels. Although N-cadherin is not considered as a client protein, our results suggest that N-cadherin could be one of the HSP90’s client proteins because PU-H71 markedly decreased N-cadherin level which is highly expressed in liver metastatic breast carcinoma cells (Zuehlke and Johnson 2010). PU-H71 in combination with radiation also reduced activation of ERK1/2 in 4TBM and 4TLM cells. We however observed differential effects of PU-H71 and RT co-treatment in p38 activation. Specifically, co-treatment increased p38 phosphorylation in metastatic breast carcinoma cells. Similar effects were observed another HSP90 inhibitor, 17-AAG, which induced activation of p38 in EGFR overexpressed pancreatic cancer cells (Adachi et al. 2010).
Interestingly, we also found that experimental conditions affected the outcome of PU-H71 and RT co-treatment. Specifically, PU-H71 prevented RT-induced increases in VEGF and MIP-2 levels under ex vivo conditions in which freshly prepared tumor explants were used. This might be due to a stress response induce by RT that increases the secretion of chemokines and cytokines under ex vivo conditions since these cells are more vulnerable to cytotoxic effects of PU-H71. Prevention of these stress response with HSP90 inhibition was sufficient to reverse the increases in MIP-2 and VEGF. These results also demonstrate that different experimental conditions should be used to thoroughly evaluate the effects of possible therapeutic agents.
In conclusion, PU-H71 may enhance therapeutic effects of radiotherapy especially in highly metastatic breast carcinoma. Further clinical and basic studies however are required to evaluate the possible role of IL-6 in resistance to this treatment approach.
Abbreviations
- HSP90:
-
Heat shock protein 90
- SDF-1:
-
Stromal-derived factor-1
- MIP-2:
-
Macrophage inflammatory protein-2
- VEGF:
-
Vascular endothelial growth factor
- IL-6:
-
Interleukin 6
- RT:
-
Radiotherapy
- 4THM:
-
4T1 heart metastasis
- 4TLM:
-
4T1 liver metastasis
- 4TBM:
-
4T1 brain metastasis
References
Adachi S et al (2010) HSP90 inhibitors induce desensitization of EGF receptor via p38 MAPK-mediated phosphorylation at Ser1046/1047 in human pancreatic cancer cells. Oncol Rep 23:1709–1714
Akmansu M, Unsal D, Bora H, Elbeg S (2005) Influence of locoregional radiation treatment on tumor necrosis factor-alpha and interleukin-6 in the serum of patients with head and neck cancer. Cytokine 31:41–45. https://doi.org/10.1016/j.cyto.2005.02.009
Ambati SR et al (2014) Pre-clinical efficacy of PU-H71, a novel HSP90 inhibitor, alone and in combination with bortezomib in Ewing sarcoma. Mol Oncol 8:323–336. https://doi.org/10.1016/j.molonc.2013.12.005
Aslakson CJ, Miller FR (1992) Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer res 52:1399–1405
Bao L, Haque A, Jackson K, Hazari S, Moroz K, Jetly R, Dash S (2011) Increased expression of P-glycoprotein is associated with doxorubicin chemoresistance in the metastatic 4T1 breast cancer model. Am J Pathol 178:838–852. https://doi.org/10.1016/j.ajpath.2010.10.029
Barbero S et al (2003) Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res 63:1969–1974
Bhagwat N et al (2014) Improved targeting of JAK2 leads to increased therapeutic efficacy in myeloproliferative neoplasms. Blood, 123:2075–2083. https://doi.org/10.1182/blood-2014-01-547760
Butler LM, Ferraldeschi R, Armstrong HK, Centenera MM, Workman P (2015) Maximizing the therapeutic potential of HSP90 inhibitors. Mol cancer res 13:1445–1451. https://doi.org/10.1158/1541-7786.MCR-15-0234
Caldas-Lopes E et al (2009) Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci U S A 106:8368–8373. https://doi.org/10.1073/pnas.0903392106
Charafe-Jauffret E et al (2009) Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 69:1302–1313. https://doi.org/10.1158/0008-5472.CAN-08-2741
Chin AR, Wang SE (2014) Cytokines driving breast cancer stemness. Mol Cell Endocrinol 382:598–602. https://doi.org/10.1016/j.mce.2013.03.024
Erin N, Boyer PJ, Bonneau RH, Clawson GA, Welch DR (2004) Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart. Anticancer Res 24:1003–1009
Erin N, Kale S, Tanriover G, Koksoy S, Duymus O, Korcum AF (2013) Differential characteristics of heart, liver, and brain metastatic subsets of murine breast carcinoma. Breast Cancer Res Treat 139:677–689. https://doi.org/10.1007/s10549-013-2584-0
Erin N, Nizam E, Tanriover G, Koksoy S (2015a) Autocrine control of MIP-2 secretion from metastatic breast cancer cells is mediated by CXCR2: a mechanism for possible resistance to CXCR2 antagonists. Breast Cancer Res Treat 150:57–69. https://doi.org/10.1007/s10549-015-3297-3
Erin N, Podnos A, Tanriover G, Duymus O, Cote E, Khatri I, Gorczynski RM (2015b) Bidirectional effect of CD200 on breast cancer development and metastasis, with ultimate outcome determined by tumor aggressiveness and a cancer-induced inflammatory response. Oncogene 34:3860–3870. https://doi.org/10.1038/onc.2014.317
Erin N et al (2009) Altered gene expression in breast cancer liver metastases. Int J Cancer 124:1503–1516. https://doi.org/10.1002/ijc.24131
Erin N, Zhao W, Bylander J, Chase G, Clawson G (2006) Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res Treat 99:351–364. https://doi.org/10.1007/s10549-006-9219-7
Erin N, Bronson SK, Billingsley ML (2003) Calcium-dependent interaction of calcineurin with Bcl-2 in neuronal tissue. Neurosci. 117:541–555
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1:2315–2319. https://doi.org/10.1038/nprot.2006.339
Gandhi N et al (2013) Novel Hsp90 inhibitor NVP-AUY922 radiosensitizes prostate cancer cells. Cancer biol ther 14:347–356. https://doi.org/10.4161/cbt.23626
Gao X et al (2015) Anti-VEGF treatment improves neurological function and augments radiation response in NF2 schwannoma model. Proc Natl Acad Sci U S A 112:14676–14681. https://doi.org/10.1073/pnas.1512570112
Giulino-Roth L et al (2017) Inhibition of Hsp90 suppresses PI3K/AKT/mTOR signaling and has antitumor activity in Burkitt lymphoma. Mol Cancer Ther 16:1779–1790. https://doi.org/10.1158/1535-7163.MCT-16-0848
Goldstein RL et al (2015) Pharmacoproteomics identifies combinatorial therapy targets for diffuse large B cell lymphoma. J Clin Invest 125:4559–4571. https://doi.org/10.1172/JCI80714
Guo A, Lu P, Lee J, Zhen C, Chiosis G, Wang YL (2017) HSP90 stabilizes B-cell receptor kinases in a multi-client interactome: PU-H71 induces CLL apoptosis in a cytoprotective microenvironment. Oncogene 36:3441–3449. https://doi.org/10.1038/onc.2016.494
Gupta J, Nebreda AR (2015) Roles of p38alpha mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J 282:1841–1857. https://doi.org/10.1111/febs.13250
Ha K, Fiskus W, Rao R, Balusu R, Venkannagari S, Nalabothula NR, Bhalla KN (2011) Hsp90 inhibitor-mediated disruption of chaperone association of ATR with hsp90 sensitizes cancer cells to DNA damage. Mol Cancer Ther 10:1194–1206. https://doi.org/10.1158/1535-7163.MCT-11-0094
Hashida S et al (2015) Hsp90 inhibitor NVP-AUY922 enhances the radiation sensitivity of lung cancer cell lines with acquired resistance to EGFR-tyrosine kinase inhibitors. Oncol Rep 33:1499–1504. https://doi.org/10.3892/or.2015.3735
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90. https://doi.org/10.3322/caac.20107
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425:407–410. https://doi.org/10.1038/nature01913
Knupfer H, Preiss R (2007) Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat 102:129–135. https://doi.org/10.1007/s10549-006-9328-3
Kollmar O, Menger MD, Schilling MK (2006) Macrophage inflammatory protein-2 contributes to liver resection-induced acceleration of hepatic metastatic tumor growth. World J Gastroenterol 12:858–867
Kwon Y et al (2015) MicroRNA-26a/-26b-COX-2-MIP-2 loop regulates allergic inflammation and allergic inflammation-promoted enhanced tumorigenic and metastatic potential of cancer cells. J Biol Chem 290:14245–14266. https://doi.org/10.1074/jbc.M115.645580
Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI (1995) Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol 155:2158–2164
Lee Y et al (2016) The purine scaffold Hsp90 inhibitor PU-H71 sensitizes cancer cells to heavy ion radiation by inhibiting DNA repair by homologous recombination and non-homologous end joining. Radiother Oncol 121:162–168. https://doi.org/10.1016/j.radonc.2016.08.029
Li HK, Matsumoto Y, Furusawa Y, Kamada T (2016) PU-H71, a novel Hsp90 inhibitor, as a potential cancer-specific sensitizer to carbon-ion beam therapy. J Radiat Res 57:572–575. https://doi.org/10.1093/jrr/rrw054
Liu Y et al (2017) STK33 participates to HSP90-supported angiogenic program in hypoxic tumors by regulating HIF-1alpha/VEGF signaling pathway. Oncotarget 8:77474–77488. https://doi.org/10.18632/oncotarget.20535
Lushchak VI (2014) Dissection of the hormetic curve: analysis of components and mechanisms. Dose Response 12:466–479. https://doi.org/10.2203/dose-response.13-051.Lushchak
Mao AW, Jiang TH, Sun XJ, Peng J (2015) Application of chemokine receptor antagonist with stents reduces local inflammation and suppresses cancer growth. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 36:8637–8643. https://doi.org/10.1007/s13277-015-3557-1
McMahon G (2000) VEGF receptor signaling in tumor angiogenesis. The oncologist 5(Suppl 1):3–10
Mimnaugh EG, Chavany C, Neckers L (1996) Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. The Journal of biological chemistry 271:22796–22801
Miyamoto Y et al (2001) Interleukin-6 inhibits radiation induced apoptosis in pancreatic cancer cells. Anticancer research 21:2449–2456
Morimoto RI, Kline MP, Bimston DN, Cotto JJ (1997) The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem 32:17–29
Moulick K et al (2011) Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nature chemical biology 7:818–826. https://doi.org/10.1038/nchembio.670
Nagaraju GP et al (2013) Antiangiogenic effects of ganetespib in colorectal cancer mediated through inhibition of HIF-1alpha and STAT-3. Angiogenesis 16:903–917. https://doi.org/10.1007/s10456-013-9364-7
Neckers L (2007) Heat shock protein 90: the cancer chaperone. J Biosci 32:517–530
Pathak S et al (2015) Radiation and SN38 treatments modulate the expression of microRNAs, cytokines and chemokines in colon cancer cells in a p53-directed manner. Oncotarget. https://doi.org/10.18632/oncotarget.5815
Provencio M, Sanchez A (2014) Therapeutic integration of new molecule-targeted therapies with radiotherapy in lung cancer. Translational lung cancer research 3:89–94. https://doi.org/10.3978/j.issn.2218-6751.2014.03.06
Qu Z, Wang S, Teng R, Yi X (2014) PU-H71 effectively induces degradation of IkappaB kinase beta in the presence of TNF-alpha. Mol Cell Biochem 386:135–142. https://doi.org/10.1007/s11010-013-1852-y
Rae C, Mairs RJ (2017) Evaluation of the radiosensitizing potency of chemotherapeutic agents in prostate cancer cells. Int J Radiat Biol 93:194–203. https://doi.org/10.1080/09553002.2017.1231946
Rezaei M, Friedrich K, Wielockx B, Kuzmanov A, Kettelhake A, Labelle M, Schnittler H, Baretton G, Breier G (2012) Interplay between neural-cadherin and vascular endothelial-cadherin in breast cancer progression. Breast cancer res 14:R154. https://doi.org/10.1186/bcr3367
Seaton A, Maxwell PJ, Hill A, Gallagher R, Pettigrew J, Wilson RH, Waugh DJ (2009) Inhibition of constitutive and cxc-chemokine-induced NF-kappaB activity potentiates ansamycin-based HSP90-inhibitor cytotoxicity in castrate-resistant prostate cancer cells. Br j cancer 101:1620–1629. https://doi.org/10.1038/sj.bjc.6605356
Segawa T, Fujii Y, Tanaka A, Bando S, Okayasu R, Ohnishi K, Kubota N (2014) Radiosensitization of human lung cancer cells by the novel purine-scaffold Hsp90 inhibitor, PU-H71. Int j mol med 33:559–564. https://doi.org/10.3892/ijmm.2013.1594
Shen XY, Wang SH, Liang ML, Wang HB, Xiao L, Wang ZH (2008) The role and mechanism of CXCR4 and its ligand SDF-1 in the development of cervical cancer metastasis Ai zheng = Aizheng = Chinese journal of cancer 27:1044-1049
Singh JK, Simoes BM, Howell SJ, Farnie G, Clarke RB (2013) Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast cancer res 15:210. https://doi.org/10.1186/bcr3436
Terwisscha van Scheltinga AG et al (2014) Visualising dual downregulation of insulin-like growth factor receptor-1 and vascular endothelial growth factor-A by heat shock protein 90 inhibition effect in triple negative breast cancer. Eur j cancer 50:2508–2516. https://doi.org/10.1016/j.ejca.2014.06.008
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147:275–292. https://doi.org/10.1016/j.cell.2011.09.024
Wagner M, Bjerkvig R, Wiig H, Melero-Martin JM, Lin RZ, Klagsbrun M, Dudley AC (2012) Inflamed tumor-associated adipose tissue is a depot for macrophages that stimulate tumor growth and angiogenesis. Angiogenesis 15:481–495. https://doi.org/10.1007/s10456-012-9276-y
Weigelt B, Peterse JL, van’t Veer LJ (2005) Breast cancer metastasis: markers and models. Nat Rev Cancer 5:591–602. https://doi.org/10.1038/nrc1670
Wu CT, Chen MF, Chen WC, Hsieh CC (2013) The role of IL-6 in the radiation response of prostate cancer. Radiat oncol 8:159. https://doi.org/10.1186/1748-717X-8-159
Xiang L et al (2014) Ganetespib blocks HIF-1 activity and inhibits tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in orthotopic mouse models of triple-negative breast cancer. J mol med 92:151–164. https://doi.org/10.1007/s00109-013-1102-5
Xu C, Zhao H, Chen H, Yao Q (2015) CXCR4 in breast cancer: oncogenic role and therapeutic targeting. Drug des devel ther 9:4953–4964. https://doi.org/10.2147/DDDT.S84932
Xu Y, Zhang C, Chen D, Zhao J, Shen Z, Wu Y, Zhu Y (2013) Effect of HSP90 inhibitor in pheochromocytoma PC12 cells: an experimental investigation. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 34:4065–4071. https://doi.org/10.1007/s13277-013-0996-4
Yoshida S et al (2011) Low-dose Hsp90 inhibitors tumor-selectively sensitize bladder cancer cells to chemoradiotherapy. Cell cycle 10:4291–4299. https://doi.org/10.4161/cc.10.24.18616
Zong H et al (2015) A hyperactive signalosome in acute myeloid leukemia drives addiction to a tumor-specific Hsp90 species. Cell Rep 13:2159–2173. https://doi.org/10.1016/j.celrep.2015.10.073
Zuehlke A, Johnson JL (2010) Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers 93:211–217. https://doi.org/10.1002/bip.21292
Acknowledgments
This study was supported by Akdeniz University Research Unit, Grant No: 2014.03.0122.005.
Author information
Authors and Affiliations
Contributions
ŞK conducted the experiments; ŞK and NE were involved in planning and analyzing the experiments as well as writing the manuscript; AK and ED are involved in planning and conducting of the radiotherapy experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 541 kb)
Rights and permissions
About this article
Cite this article
Kale, Ş., Korcum, A.F., Dündar, E. et al. HSP90 inhibitor PU-H71 increases radiosensitivity of breast cancer cells metastasized to visceral organs and alters the levels of inflammatory mediators. Naunyn-Schmiedeberg's Arch Pharmacol 393, 253–262 (2020). https://doi.org/10.1007/s00210-019-01725-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01725-z