Abstract
This study aims to investigate whether the expression of heat shock protein 90 (HSP90) is associated with the malignant pheochromocytoma (PHEO) and the effects of 17-allylamino-17-demethoxygeldanamcyin (17-AAG) on the expression of vascular endothelial growth factor (VEGF) in PHEO cell line PC12. The expression of HSP90 was investigated in 38 paraffin-embedded samples of PHEO patients using immunohistochemistry. The time and concentration effects of 17-AAG were investigated in PHEO PC12 cells. Cell proliferation was measured by MTT assay and cell counting. Apoptosis was detected by flow cytometry. Positive staining for HSP90 was found in 14 of 17 malignant (82.35 %) and in 5 of 21 (23.81 %) benign PHEOs. There existed a significant statistical difference between the malignant group and the benign ones (P < 0.001). 17-AAG inhibited the proliferation of HCC cells in a time- and concentration-dependent manner. The apoptosis rates of PC12 cells after treatment with 0.1 μmol/L for 6, 12, 24, and 48 h were significantly higher than that in blank control group. 17-AAG significantly downregulated VEGF-165 protein level in PC12 cells. This study has confirmed that the specific HSP90 inhibitor 17-AAG can play a therapeutic role in malignant PHEO treatment, and HSP90 qualifies as a promising new target in malignant PHEO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pheochromocytomas (PHEOs) are rare neuroendocrine tumors that derive from chromaffin cells of the adrenal medulla [1]. Most of PHEOs are benign, and the rate of malignancy has long been cited as 10 %; however, recent study has estimated that it may be as high as 26 % [2]. There is currently no generally effective therapy for malignant PHEO. At the moment, chemotherapy and radionuclide therapy are the main treatment options of choice in the case of malignant PHEO. The 5-year overall survival of patients with unresectable metastatic disease is 40–72 % [3]. Therefore, this urges the need for innovative nonsurgical treatment options for patients with malignant PHEO.
Heat shock protein 90 (HSP90) is a molecular chaperone that comprises 1–2 % of total cellular protein content and regulates the correct conformation, activity, function, and stability of over 200 client proteins [4]. Several observations support the importance of the activation of HSP90 in a variety of human cancers, suggesting an increased reliance of cancer cells on HSP for maintenance of cellular viability [5, 6]. Enhanced or diminished expression of HSP90 promotes cell survival or growth inhibition, respectively [7, 8]. Targeting HSP90 may represent a therapeutic strategy that could potentially interfere with multiple oncogenic pathways.
Recently, HSP90 inhibitors have been considerably improved; 17-allylamino-17-demethoxygeldanamycin (17-AAG), as the first-in-class HSP90 inhibitor and the first to enter clinical trials, has shown significant antitumor activity in preclinical studies [9–11]. It can not only inhibit cell proliferation and induce apoptosis by mediating the HSP90, which results in inhibition of tumor growth, but also suppress angiogenesis. For many years, many investigators have tried to identify primary tumor phenotype of PHEO to tailor appropriate therapy and follow-up to the individual. However, expression of HSP90 in malignant PHEO and the effect of 17-AAG on malignant PHEO have not been clarified until recently. A recent study has suggested that HSP90 inhibitors may benefit patients with advanced PHEO [12], yet no large studies have been conducted on growth-inhibitory effect of HSP90 inhibitors on malignant PHEO through angiogenesis pathway. Therefore, the aim of our study is to investigate the expression of HSP90 in malignant PHEO tissue and evaluate the efficacy of 17-AAG in vitro through angiogenesis pathway.
Materials and methods
Patients and tissues
A total of 38 paraffin-embedded samples of PHEO were obtained from 38 patients undergoing surgery between 1990 and 2010. The preoperative diagnosis was confirmed by increased plasma and urinary catecholamine (adrenaline and norepinephrine) or their metabolites (metanephrine and occasionally vanillylmandelic acid) and by specific, unequivocal imaging findings (CT, MRI, or radiotracer-labeled metaiodobenzylguanidine scan). Syndromic or familial cases (multiple endocrine neoplasia type 2A or 2B, von Hippel–Lindau disease, or neurofibromatosis type 1) were not included in this study. For histological evaluation, sections were stained with hematoxylin and eosin. There were two clinically defined subject groups: 21 benign (male 10, female 11, aging from 19 to 77 years old, average 41.53) and 17 malignant intramedullar PHEOs (male 9, female 8, aging from 21 to 74 years old, average 47.05). The diagnosis of malignant was established according to the criteria: (1) presence of widespread local invasion at time of surgery, (2) history of recurrence, and (3) metastasis which occur to sites normally devoid of chromaffin tissue. Standard demographic data, information on presenting signs and symptoms, results of routine laboratory tests, and preoperative radiographic imaging studies were obtained by reviewing these records. Besides clinical diagnosis, histopathologic slides were classified by two pathologists independently and no discrepancy exists between them. However, when the tumor invades the vessel, massive necrotic area is found in the tumor tissue, or more than five out of ten HP mitosis cells ar found in the borderline tumor but no metastasis in any non-chromaffin issues, it is treated as benign. The tissues are from the patients who did not receive radiotherapy or chemotherapy. The follow-up data were obtained from patient's medical records. Each patient was evaluated at regular intervals postoperatively. Routine examination, including blood pressure measurement, plasma or urine catecholamine (MN/NMN), imaging examinations, was done every 3 to 6 months for 5 years and every 6 months thereafter to follow patients and possibly identify recurrence. The mean post-surgery follow-up of patients was 126.3 months (range, 97–264 months) for the benign group and 92.2 months (range, 11–220 months) for the malignant group, respectively. This study was approved by the Ethics Committee of Ruijin Hospital. All patients provided informed consent.
Cells culture and reagents
PC12, a rat PHEO cell line, was obtained from American Type Culture Collection (Manassas, VA) and cultured in Dulbecco's Modified Eagle Medium (Invitrogen Inc., Carlsbad, CA) supplemented with 10 % fetal bovine serum and antibiotic/antimycotic at 37 °C, 5 % CO2. The HSP90 inhibitor 17-AAG (Cayman, USA) was solubilized in dimethyl sulfoxide (DMSO) before use. Vascular endothelial growth factor (VEGF) protein (Roche, Switzerland), rabbit anti-rat VEGF antibody (PeproTech, USA), and mouse anti-human HSP90 antibody (Abcam, USA) were obtained.
Immunohistochemical analysis
To evaluate the HSP90 expression in malignant PHEO, the immunohistochemical analyses were performed using the EnVision method (DAKO, Glostrup, Denmark). Antigen retrieval was achieved by microwave at 750 W for 15 min, and the sections were incubated with 10 % normal goat serum at room temperature for 10 min to block nonspecific reactions. This was followed by a PBS wash and incubation with polyclonal mouse anti-human HSP90 antibody (Abcam, USA) diluted to 1:100 for 12 h at 4 °C. Localization of immunostaining was demonstrated by incubation with EnVision–peroxidase system. The staining results of pancreatic carcinoma tissue sections which HSP90 positive had already known were regarded as positive control, PBS instead of primary antibodies was as negative control.
Evaluation of immunohistochemical results
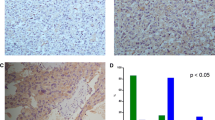
Positive HSP90 staining was characterized by purple–brown granules located diffusely in the cell cytoplasm. Lack of any obvious purple–brown or brown–red pigmentation in the cytoplasm of tumor cell was considered negative. For quantitative analyses of expression, five visual fields were randomized selected per section under high power microscope (×400), and 200 cells were counted in each high power field. Staining was scored according to the percent of positive staining tumor cells, including 0—less than 5 %, 1—5 to 29 %, 2—30 to 50 %, and 3—greater than 50 %, and as intensity, including 0—no, 1—weak, 2—moderate, and 3—strong staining. Positive or negative expression was determined according to the combinationof these 2 variables. A total score of greater than 3 was considered positive and a total score of 3 or less was considered negative, as previously reported [13]. The results were scored by two independent pathologists who were blinded to the diagnosis.
Cell viability measurement
Cell viability was analyzed by Thiazolyl Blue (MTT, Sigma-Aldrich, USA). The cells were divided into three groups: negative controls (the solvent DMSO treated cells served as control), blank controls, and experiment groups. The experiment group was divided into two groups. Cells of the first group that were grown to 70–80 % confluency in 96-well plates were treated with 17-AAG at a final concentration of 0.005, 0.025, 0.05, 0.1, 0.25, 0.5, 1.0, and 2.0 μmol/L for 12, 24, or 48 h, respectively. In addition, PC12 cells were treated with 150 μg/L VEGF (VEGF group), 0.1 μmol/L 17-AAG (17-AAG group), and 0.1 μmol 17-AAG + 150 μg/L VEGF (17-AAG + VEGF group), respectively. The blank group was only added culture fluid. Cells treated with 100 μL cell suspension and DMSO were used as negative control. After the reaction with the drugs for 12, 24, and 48 h, cells were then treated with MTT (10 mL/well) for 4 h at 37 °C. Cells were subjected to absorbance reading at 570 nm using a 96-well microplate reader. The OD values were normalized to cells treated with 0 nmol/L of 17-AAG. Percentage of residual cell viability was determined as [(OD of experiment group − OD of blank group) / (OD of negative group − OD of blank group)] × 100 %. Assays were performed three times.
Cell morphology and number counting
PC12 cells were seeded in 96-well plates at a density of 2 × 104 cells/ well. After continuous incubation for 24, 48, and 72 hours, cell suspensions were smeared on a slide and air-dried, fixed in methanol, and stained with Wright−Giemsa staining solution for 2 min. Cells were observed under inverted microscope. To count the increased cell number, PC12 cells were then trypsinized and cell number was counted using hemocytometer by two researchers blinded to the experimental design. The increased cell number was calculated as percentage of the cells treated with 0 nmol/L of 17-AAG.
Flow cytometry assay of cell apoptosis
After treating cells with 17-AAG, the PC12 cells were washed twice in cold PBS and labeled with Annexin V-FITC and propidium iodide (PI) and analyzed immediately after staining with a FACScan flow cytometer (BD Biosciences) and FlowJo software according to the manufacturer's recommendations. Quantification of Annexin V-FITC and PI binding was conducted with a FACScan flow cytometer. Cell flow cytometric analysis was used to differentiate between living, early apoptotic, late apoptotic/necrotic, and necrotic cells by staining with Annexin V-FITC and PI.
Cell extracts and immunoblotting
Cells were harvested and homogenized in cell lysis buffer containing the following: 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 % Triton X-100, 100 μg/mL phenylmethylsulfonyl fluoride, and 1 mM DTT. After centrifugation at 12,000 rev/min for 30 min at 4 °C, supernatants were used and separated by SDS-PAGE (using 10 % gels) and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA). The membranes were blocked with 5 % nonfat milk and then incubated with primary antibodies, including rabbit anti-rat VEGF-165 antibody (PeproTech, USA) and actin (1:10,000; MP Biomedicals, Germany). Membranes were washed three times for 10 min each with Tris-buffered saline (50 mM Tris, pH 7.4, 0.9 % NaCl) containing 0.05 % Tween 20 (TBS-T) and incubated with horseradish peroxidase-conjugated secondary antibodies. Membranes were then washed again three times for 10 min each with TBS-T. Target protein bands were visualized using the enhanced chemiluminescence method. Western blot experiments were repeated at least three times with independent cell preparations.
Statistical analysis
Data analyses were performed using SPSS statistical package 15.0. Patient characteristics are shown as the mean ± SD for continuous variables and as the count and percent for discrete variables. Phenotypic differences in quantitative traits were assessed by genotype using the t test or ANOVA. Differences in the distribution of qualitative traits by genotype were assessed by standard chi-square analysis and Fisher's exact test. A P value less than 0.05 was considered significant.
Results
HSP90 is expressed in overwhelming majority of malignant PHEOs
Positive staining for HSP90 was found in 14 of 17 malignant (82.35 %) and in 5 of 21 (23.81 %) benign PHEOs (Fig. 1). There existed a significant statistical difference between the malignant group and the benign ones (P < 0.001), which indicated the upregulation of HSP90 in malignant cells of the adrenal medulla.
17-AAG plays an inhibiting role in proliferation of PC12 cells
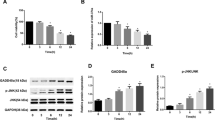
MTT assay shows that 17-AAG in experiment group 1 inhibit the proliferation of PC12 cells with time and dose dependence. As shown in Fig. 2, 17-AAG significantly reduced cell survival rate in PC12 cells in a time- and dose-dependent manner (P < 0.05). The half maximal growth inhibitory concentration after 24 h (IC50) was 0.05 μmol/L. In experiment group 2, PC12 cells were treated with 150 μg/L VEGF (VEGF group), 0.1 μmol/L 17-AAG (17-AAG group), and 0.1 μmol 17-AAG and 150 μg/L VEGF (17-AAG + VEGF group), respectively. In this group, PC12 cell survival rate in VEGF group and 17-AAG + VEGF group is much higher than 17-AAG group (P < 0.01), while the survival rate in 17-AAG + VEGF group is lower than VEGF group (P < 0.01) (Fig. 3).
Morphological observation of PC12 cells
Wright–Giemsa staining shows that the cell volume did not change apparently after intervention of 17-AAG in experiment group 1. There are bud hooks on the cell membrane and vacuole in the cytoplasm. The nucleus staining is not all-dyed. The nuclear deformation, karyopyknosis, and coenocytes can be seen. In the negative control group and blank control group, no karyopyknosis can be found and the nuclear remains normal, round, and all-dyed (Fig. 4).
HSP90 inhibition induces apoptosis in PC12 cells
In PC12 cells, apoptosis significantly increased after inhibition of HSP90. We test the survival rates after action of 17-AAG in 6, 12, 24, and 48 h in group 1. The results show that the cell survival rate in each point of time (Q1 + Q3) is much higher than the blank control (P < 0.01) (Fig. 5). The survival rate in 17-AAG + VEGF group is 12.3 %, much lower than the 17-AAG group (22.3 %), which has statistical significance (Fig. 6).
VEGF-165 expression tested in Western blotting
After action with 0.1 μmol/L 17-AAG in 6, 12, 24, and 48 h, the expression of VEGF-165 is gradually lower. The comparison between expression level of VEGF-165 and negative control groups is statistical significant (P < 0.01) (Figs. 7 and 8).
Discussion
Malignant PHEO has a poor prognosis and the 5-year survival rate is about 44 %, while the 5-year survival rate of surgically treated benign PHEOs is over 95 % [13]. The diagnosis of malignant PHEO can only be determined by the presence of recurrence or metastatic disease at a site where chromaffin cells do not normally exist [14]. However, the molecular mechanisms responsible for malignant PHEO development have not been elucidated. Therefore, novel approaches to diagnose malignant PHEO early to ensure effective therapy are drastically needed.
HSP90 regulates the fate of a variety of client proteins crucial for multiple cell signaling processes in eukaryotic cells, including AKT, IL-6R, BCR-ABL, and Apaf-1. Many client proteins of HSP90 are involved in signal transduction pathways whose deregulation may drive cancer [15]. A variety of receptor tyrosine kinases including VEGFR, IGFR, and EGFR are client proteins of HSP90, which depend on HSP90 to achieve active conformation or increase stability. These client proteins are available to promote growth factor independence, resistance to drugs, proliferation, tissue invasion, metastasis, and angiogenesis, all critical components for tumor progression and survival [16]. In this study, our findings on malignant PHEOs are in line with the previous reported overexpression of HSP90 in different tumor entities, including invasive breast tumors, gastrointestinal stromal tumors, and others [17, 18].
HSP90 inhibition has attracted considerable interest in the past two decades for the treatment of advanced cancers [19, 20]. In general, HSP90 inhibitors display remarkable selectivity for cancer cells as compared to normal cells [5, 6]. And due to specific conformations of the chaperone, it has been implicated in cancer versus normal cell sensitivity to HSP90 inhibitors: HSP90 was shown to display higher binding affinity for 17-AAG exclusively in cancer cells, leading to the formation of 17-AAG-sensitive HSP90-containing “superchaperone” complexes in malignant cells, whereas normal cells bearing a predominantly uncomplexed HSP90 are significantly less sensitive to these types of inhibitors. According to concerned research reports [21, 22], as the first-in-class HSP90 inhibitor, 17-AAG can inhibit various tumor cells from proliferation, induce the apoptosis of tumor cell, and show the time and dose dependence. Kaur et al. [23] found that 17-AAG can inhibit the endothelial cell from proliferation, motility, invasion, and inducement of apoptosis, all of which can be through angiogenesis pathway. However, whether 17-AAG possesses growth-inhibitory effect on PC12 cells through angiogenesis pathway has not yet been examined. In our study, we treated PC12 cells with different concentrations of 17-AAG. Cell survival rate was measured by MTT assay, cell morphology was observed by Wright–Giemsa staining, and cell apoptosis was detected by flow cytometry. All of this is to test the inhibiting effect of 17-AAG. The MTT assay showed that 17-AAG inhibited the growth of PC12 cell lines in a time- and dose-dependent manner. In this study, IC50s of 17-AAG for PC12 cells at 24 h was 0.05 μmol/L. These values were within the range of IC50 values, but at relatively low level, as previously reported in other cancer cells [24]. Our results validate HSP90 as an important target in malignant PHEO and provide rationale for the testing of HSP90 inhibitors as a promising therapeutic agent in clinical trials.
Angiogenesis is an essential process for growth of tumor and metastasis of solid malignancy. One of the most potent endothelial mitogens and mediators of angiogenesis is VEGF. The induction of VEGF in cancer cells can be mediated through activation of various signaling pathways such as PI3K/AKT. Nguyen et al. [25] reports that by observing H358 cell and rat tumor cell lines in vitro, 17-AAG can both obviously inhibit the creation of VEGF, which proves that 17-AAG is an effective medicine which can regulate the expression of genes of VEGF. In addition, Hur et al. [26] also affirms that 17-AAG can reduce the angiogenesis of tumor cells by inhibiting the genes of VEGF. In our previous study, we recognized that VEGF are highly expressed in the malignant PHEO [27]. To make sure whether the function of 17-AAG has an effect on VEGF expression, expression of VEGF-165 protein was determined by western blotting in PC12 cells treated with different concentrations of 17-AAG. Our study first demonstrated that 17-AAG significantly downregulated VEGF-165 protein level in PC12 cells.
In conclusion, our data show that HSP90 is overexpressed in malignant PHEOs. HSP90 inhibitor 17-AAG inhibited cell proliferation and induced apoptosis through the direct degradation of VEGF in a time- and dose-dependent manner. The 17-AAG treatment may provide a promising strategy for the antitumor therapy of PHEO, and HSP90 qualifies as a promising new target in malignant PHEO.
References
Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–75.
Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423–36.
Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96:717–25.
Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–49.
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on hsp90 inhibitors. Nature. 2003;425:407–10.
Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L. Gomes DaGama EM, Caldas-Lopes E, Beebe K, Perna F, Hatzi K, Vu LP, Zhao X, Zatorska D, Taldone T, Smith-Jones P, Alpaugh M, Gross SS, Pillarsetty N, Ku T, Lewis JS, Larson SM, Levine R, Erdjument-Bromage H, Guzman ML, Nimer SD, Melnick A, Neckers L, Chiosis G: Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7:818–26.
Zurawska A, Urbanski J, Matuliene J, Baraniak J, Klejman MP, Filipek S, et al. Mutations that increase both Hsp90 atpase activity in vitro and Hsp90 drug resistance in vivo. Biochim Biophys Acta. 1803;2010:575–83.
Schwock J, Pham NA, Cao MP, Hedley DW. Efficacy of Hsp90 inhibition for induction of apoptosis and inhibition of growth in cervical carcinoma cells in vitro and in vivo. Cancer Chemother Pharmacol. 2008;61:669–81.
Porter JR, Fritz CC, Depew KM. Discovery and development of Hsp90 inhibitors: a promising pathway for cancer therapy. Curr Opin Chem Biol. 2010;14:412–20.
Usmani SZ, Bona R, Li Z. 17 AAG for HSP90 inhibition in cancer—from bench to bedside. Curr Mol Med. 2009;9:654–64.
Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 1823;2012:742–55.
Giubellino A, Sourbier C, Lee MJ, Scroggins B, Bullova P, Landau M, et al. Targeting heat shock protein 90 for the treatment of malignant pheochromocytoma. PLoS One. 2013;8:e56083.
Zhu Y, He HC, Yuan F, Zhang J, Rui WB, Zhao JP, et al. Heparanase-1 and cyclooxygenase-2: prognostic indicators of malignancy in pheochromocytomas. Endocrine. 2010;38:93–9.
Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2007;14:569–85.
Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410:439–53.
Powers MV, Workman P. Targeting of multiple signalling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr Relat Cancer. 2006;13 Suppl 1:S125–35.
Kang GH, Lee EJ, Jang KT, Kim KM, Park CK, Lee CS, et al. Expression of HSP90 in gastrointestinal stromal tumours and mesenchymal tumours. Histopathology. 2010;56:694–701.
Diehl MC, Idowu MO, Kimmelshue K, York TP, Elmore LW, Holt SE. Elevated expression of nuclear Hsp90 in invasive breast tumors. Cancer Biol Ther. 2009;8:1952–61.
Gartner EM, Silverman P, Simon M, Flaherty L, Abrams J, Ivy P, et al. A phase II study of 17-allylamino-17-demethoxygeldanamycin in metastatic or locally advanced, unresectable breast cancer. Breast Cancer Res Treat. 2012;131:933–7.
Pacey S, Gore M, Chao D, Banerji U, Larkin J, Sarker S, et al. A phase II trial of 17-allylamino,17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Invest New Drugs. 2012;30:341–9.
Roforth MM, Tan C. Combination of rapamycin and 17-allylamino-17-demethoxygeldanamycin abrogates akt activation and potentiates mtor blockade in breast cancer cells. Anticancer Drugs. 2008;19:681–8.
Sauvageot CM, Weatherbee JL, Kesari S, Winters SE, Barnes J, Dellagatta J, et al. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro Oncol. 2009;11:109–21.
Kaur G, Belotti D, Burger AM, Fisher-Nielson K, Borsotti P, Riccardi E, et al. Antiangiogenic properties of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin: an orally bioavailable heat shock protein 90 modulator. Clin Cancer Res. 2004;10:4813–21.
Burger AM, Fiebig HH, Stinson SF, Sausville EA. 17-(Allylamino)-17-demethoxygeldanamycin activity in human melanoma models. Anticancer Drugs. 2004;15:377–87.
Nguyen DM, Lorang D, Chen GA. Stewart JHt, Tabibi E, Schrump DS: Enhancement of paclitaxel-mediated cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: in vitro and in vivo analysis. Ann Thorac Surg. 2001;72:371–8. discussion 378–379.
Hur E, Kim HH, Choi SM, Kim JH, Yim S, Kwon HJ, et al. Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1alpha/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Mol Pharmacol. 2002;62:975–82.
Feng F, Zhu Y, Wang X, Wu Y, Zhou W, Jin X, et al. Predictive factors for malignant pheochromocytoma: analysis of 136 patients. J Urol. 2011;185:1583–90.
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (no. 81272936) and Shanghai Municipal Natural Science Foundation (no. 134119a2700).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Xu, Y., Chen, D. et al. Effect of HSP90 inhibitor in pheochromocytoma PC12 cells: an experimental investigation. Tumor Biol. 34, 4065–4071 (2013). https://doi.org/10.1007/s13277-013-0996-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0996-4