Abstract
Retinoic acid (RA) plays an important role in the developing mammalian nervous system. Based on this concept, some studies have demonstrated the beneficial effects of RA administration on neurogenesis in neuropathological diseases. Some investigations have revealed the anti-inflammatory effects of RA treatment in multiple systems, in addition to its role in neurogenesis. To date, however, the neuroprotective efficacy of RA after cerebral ischemia, especially in the context of its anti-inflammatory effects, has been poorly demonstrated. Additionally, to the best of our knowledge, experiments of the therapeutic efficacy of RA treatment in a transient global ischemic model in the Mongolian gerbil have been lacking worldwide. Here, we studied the neuroprotective effects and neurobehavioral outcomes of intraperitoneally administered all-trans-RA (ATRA; a synthetic form of RA) on brains with transient global ischemia that was induced with the bilateral common carotid artery occlusion and reperfusion (BCCAO/R) model in the gerbil. In order to identify whether these neuroprotective mechanisms were due to the anti-inflammatory effects of ATRA, in vivo hippocampal expression of proinflammatory cytokines including tissue necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) after ATRA injection and in vitro levels of release of nitric oxide, TNF-α and IL-6 from lipopolysaccharide (LPS)-stimulated BV2 microglial cells after ATRA treatment were evaluated. The results showed that ATRA can protect pyramidal neurons in the hippocampal CA1 region against BCCAO-induced neuronal apoptosis and significantly reduce the extent of astrocytosis and microglial activation. In addition, the ischemia-induced neurobehavioral changes were normalized by ATRA injection. Consistent with these phenotypic data, we observed the diminishing effects of ATRA treatment on the production of proinflammatory mediators (e.g., TNF-α and IL-6) in hippocampal homogenates and LPS-stimulated BV2 cells, and these effects were dose-dependent. These results suggest a beneficial role of ATRA in the attenuation of global cerebral ischemia due to its anti-inflammatory properties, resulting in, at least partly, the inhibition of microglial secretion of variable proinflammatory cytokines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain lesions, such as those resulting from ischemia or neurodegenerative diseases, are accompanied by inflammatory reactions that involve the activation and proliferation of glial cells, such as astrocytes and microglial cells. In these inflammatory processes, inflammatory mediators play an important role [1]. Among these mediators, tissue necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which are quickly released in the course of brain injuries, are essential for the generation of immunological responses [2]. Anti-inflammatory agents that inhibit the release of these cytokines have been shown to be experimentally beneficial in various animal models of brain injuries [3–5]. Especially in cerebral ischemia, one process that may be involved in delayed neuronal death is post-ischemic neuroinflammation [6]. Therefore, the search for agents that diminish such inflammatory processes is important because these agents might suggest new therapeutic strategies that are targeted at the late phase of ischemic cerebral injury.
Retinoic acid (RA) plays an important role in the developing mammalian nervous system [7]. RA, which is a metabolite of vitamin A (retinol), mediates the functions of vitamin A that are required for growth and development. RA is essential for the initial anteroposterior neural patterning and the subsequent development of the spinal cord and hindbrain structures. Based on the concept that there is substantial overlap between the molecular and cellular events of neural development and adult neuroregeneration after nerve lesions, some studies have demonstrated the beneficial effects of RA administration in neuropathological diseases. In fact, in vivo experiments with rats and mice have demonstrated that RA treatment is beneficial in reducing ischemic volume and neurobehavioral performance [8, 9]. In these cases, of course, direct RA signaling within neurons through its nuclear receptors (e.g., RAR and RXR) may play a major role.
However, other than these direct effects on neurons, we cannot rule out the possibility that anti-inflammatory effects of RA might also be involved. It is likely that RA interferes in the later stages of inflammatory reactions because numerous reports have demonstrated negative effects of RA on the production of proinflammatory cytokines [10]. For example, in monocytes, RA has been shown to inhibit the synthesis of IL-1, IL-8, and IL-6 [11] and macrophage colony-stimulating factor [12]. After stimulation with lipopolysaccharide (LPS), RA reduces the levels of expression of cyclooxygenase-2 (COX-2), the associated release of prostaglandin E2 from mouse peritoneal macrophages, and TNF-α release from blood mononuclear cells [13]. Phospholipase-A2, which is involved in the synthesis of arachidonic acid, has also been shown to be inhibited [14], and there have been numerous reports about the beneficial effects of RA on pathophysiologic conditions that are not related to nerve injury [15–17]. However, as mentioned above, numerous investigations have confirmed the anti-inflammatory activities of RA in multiple systems, but the neuroprotective efficacy of RA after cerebral ischemia in the context of its anti-inflammatory effects have been poorly demonstrated. In addition, to the best of our knowledge, experiments on the efficacy of RA treatment in a transient global ischemic model in gerbils have not been conducted. Here, for the first time, we examined the neuroprotective effects of all-trans-RA (ATRA), which is a synthetic form of RA, in an experimental model of transient global cerebral ischemia that used the bilateral common carotid artery occlusion and reperfusion (BCCAO/R) method in Mongolian gerbils. In addition, we evaluated the effects of ATRA on the behavioral changes of ischemic gerbils in an open-field test. Furthermore, for demonstrating the involvement of its modulating effects on neuroinflammation for pursuing its neuroprotective role, we measured the expression of TNF-α and IL-6 in gerbils’ hippocampal homogenates and release of nitric oxide (NO), TNF-α and IL-6 from LPS-stimulated microglial cell line BV2, which is well established in vitro model of mimicking neuroinflammatory conditions accompanying cerebral ischemia [18–21], with or without ATRA treatment.
Materials and Methods
Experimental Animals
A total of 80 male Mongolian gerbils (Meriones unguiculatus) was obtained from the Experimental Animal Center, Konyang University, Daejeon, South Korea. The gerbils were used at 6 months (body weight, 65–75 g) of age. The animals were housed in a conventional state under adequate temperature (23 °C) and humidity (60 %) control with a 12-h light/12-h dark cycle, and they were provided free access to water and food. The procedures for handling the animals and their care conformed to the guidelines that are in compliance with current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996). All of the experiments were conducted in order to minimize the number of animals used and the suffering caused by such procedures. The gerbils were divided into the following 5 groups, each with 16 individuals: sham-operated (SHAM), operated (OP), 1-mg/kg-ATRA-injected, 5-mg/kg-ATRA-injected, and 10-mg/kg-ATRA-injected groups.
Surgeries (BCCAO/R) and Drug Administration
The animals were anesthetized with a mixture of 2.5 % isoflurane in 33 % oxygen and 67 % NO. Both of the common carotid arteries were isolated and occluded with nontraumatic aneurysm clips. Complete interruption of the blood flow was confirmed by observing the central artery in the retina with an ophthalmoscope. After 6 min of occlusion, the aneurysmal clips were removed from the common carotid arteries. Body (rectal) temperatures under free-regulating or normothermic (37 ± 0.5 °C) conditions were monitored with a rectal temperature probe (TR-100; Fine Science Tools, Inc., Foster City, CA, USA) and maintained with a thermometric blanket before, during, and after the surgery until the animals completely recovered from the anesthesia. Thereafter, the animals were maintained in a thermal incubator (Mirae Medical Industry, Seoul, South Korea) in order to maintain their body temperature until they were euthanized. The gerbils in the SHAM group were subjected to the same surgical procedures as the other groups except that the common carotid arteries were not occluded. The gerbils were treated with 1, 5, or 10 mg/kg of ATRA (Sigma-Aldrich Co. LLC, St. Louis, MO, USA) that was dissolved in a 1:1 mixture ratio of v/v dimethyl sulfoxide (DMSO): saline (0.9 % w/v sodium chloride) for 5 days before and over the variable days after the surgery until they were sacrificed for further studies. The gerbils in the OP group received saline with DMSO at a ratio of 1:1 v/v instead of the ATRA solution.
Tissue Processing and Hematoxylin-Eosin Staining
For the histological analyses i.e., hematoxylin-eosin (H-E) and Fluoro-Jade C (F-J C) histofluorescence staining, animals were sacrificed 3 days after the BCCAO/R surgery (n of each group subjected to different staining were indicated below). All the animals were anesthetized with sodium pentobarbital and perfused trans cordially with 0.1 M phosphate-buffered saline (PBS, pH 7.4), which was followed by 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed and postfixed in the same fixative for 6 h. For H-E staining (n of each group = 3), the brain tissues were dehydrated by placing the fixed brain tissues for 2 h in 50, 70, 80, 90, 95, and 100 % ethanol baths in a consecutive manner at room temperature. Then, the tissues were placed 2 times, each time for 1 h, in fresh pure xylene and then in molds containing melted paraffin (Sigma-Aldrich Co. LLC). Serial transverse sections, which were 5 μm in thickness, were made by a tissue microtome and mounted on microscopic slides. For measuring neuronal survival, the hippocampal CA1 region of the tissue sections of each group were stained with H-E. Observations of the slides were performed with the aid of a light microscope (Leica Microsystems CMS GmbH, Mannheim, Germany). The numbers of intact neurons in a 1-mm length of the middle portion of the hippocampal CA1 subfield from 5 randomly selected coronal sections (located between 0 and +2.00 mm from bregma in the stereotaxic frame) per animal were counted by 3 blinded observers with visualization with a microscope at 400× magnification. Only neurons with visible nuclei were counted.
Fluoro-Jade C Histofluorescence Staining
The tissue sections of each of the groups (n of each group = 3) were first immersed in a solution containing 1 % sodium hydroxide in 80 % alcohol, which was followed by immersion in 70 % alcohol. They were then transferred to a solution of 0.06 % potassium permanganate and then transferred to a 0.0004 % F-J C (Histo-Chem Inc., Jefferson, AR, USA) staining solution. After washing, the sections were placed in a slide warmer (approximately 50 °C) and then examined with the use of an epi fluorescent microscope (Olympus Corporation, Tokyo, Japan) and with blue (450–490 nm) excitation light and a barrier filter. With this method, neurons that undergo degeneration and that display pyknotic nuclei brightly fluoresce in comparison to the background. F-J C-positive cells were selected from 5 randomly selected coronal sections (located between 0 and +2.00 mm from bregma in the stereotaxic frame) and counted at the center of CA1 with the same methods described above.
Immunoblotting
Total protein extraction and western blot analyses were performed as described in a previous study [22]. For quantification of the poly (ADP-ribose) polymerase-1 (PARP-1) cleavage, TNF- α and IL-6 expression in the hippocampal homogenates of each group (n of each group for PARP-1 = 3; n for both TNF-α and IL-6 = 3), both hippocampi were gently isolated from the cerebral hemisphere in Hank’s Balanced Salt Solution (Lonza Ltd., Walkersville, MD, USA) on postoperative day (POD) 1, transferred to lysis buffer, and homogenized. For this study, the protein homogenates were incubated with rabbit anti-PARP-1 (1:1,000), anti-TNF-α (1:1,000), anti-IL-6 (1:500) and anti-β-actin (1:2,000) as primary antibodies. All antibodies were purchased by Santa Cruz Biotechnology (CA, USA). After subsequential incubation with biotinylated anti-rabbit IgG (1:1,000; Vector Laboratories, Inc., Burlingame, CA, USA) and 5 times washing in PBS, the proteins in PVDF membranes were detected by a chemiluminescence detection system according to the manufacturer’s instructions (ECL; GE Healthcare Life Sciences, Buckinghamshire, UK). The band intensity was quantified with a densitometric scanner (GE Healthcare Life Sciences).
Open-Field Test
Locomotor activity was video recorded and assessed in an open-field box that measured 72 × 72 cm with 36-cm walls and that was divided into 16 squares (18 × 18 cm each) that were drawn out with blue lines. One of the walls was clear Plexiglas so that the gerbils could be visible in the apparatus. The gerbils before being sacrificed for histological staining (i.e., H-E and F-J C histofluorescence staining; n of each group = 6) were placed at one corner of the box, and their activity was monitored by Noldus Ethovision XT software (Noldus Information Technology, Leesburg, VA, USA) installed in video-tracking system for 10 min at 5 time points (1 day before; on the same day of the operation; and 1–3 days after the operation). Three trials of spontaneous locomotor activities (line crossing: the number of squares that the gerbils crossed with all 4 paws) in each animal were measured by the modified method from Zhuang et al. [22] by 3 blinded observers.
Immunohistochemistry
In order to detect the extent of astrocytosis and microglial activation, immunohistochemical staining was performed with anti-glial fibrillary acidic protein (anti-GFAP; Santa Cruz Biotechnology, Inc.) and anti-ionized calcium binding adapter molecule 1 (anti-Iba1; Santa Cruz Biotechnology, Inc.). Briefly, for each gerbil (n of each group = 4), 5 randomly selected coronal paraffinized sections for both hippocampal CA1 regions (located between 0 and +2.00 mm from bregma in the stereotaxic frame) were deparaffinized in xylene, hydrated by immersion in baths featuring a decreasing ethanol gradient, and then washed in distilled water. Next, each section was incubated with primary antibodies that were diluted in blocking solution at a ratio of 1:200 for rabbit anti-GFAP and 1:100 for rabbit anti-Iba1 for 24 h at 4 °C. After 3 washes in PBS, the sections were sequentially incubated with secondary antibodies containing anti-rabbit IgG for 1 h at 36 °C and then avidin–biotin complex solution for 1 h at 36 °C. The immunoreactive regions turned dark brown in appearance after addition of the chromogen 3,3′-diaminobenzidine tetrachloride (DAB; Sigma-Aldrich Co. LLC). The densities of the GFAP-immunoreactive astrocytes and the numbers of Iba1-immunoreactive activated microglia in a 1-mm length of the hippocampal CA1 subfields in the 5 randomly selected coronal sections per n were evaluated by 3 blinded observers with an image analysis system (ImageJ software, National Institutes of Health, Bethesda, MD, USA).
BV2 Cell Culture and Cell Viability Assay
BV2 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) that was supplemented with 10 % fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 μg/mL). Cells were maintained in a humidified incubator with 5 % CO2. ATRA was dissolved in DMSO, and dilutions were made in DMEM. The final concentration of DMSO in the medium was less than 0.05 % (v/v), and this concentration showed no influence on cell growth (data not shown). In all of the experiments, the cells were pretreated with the indicated concentrations (0.78–50 μg/mL) of ATRA for 1 h with or without LPS (0.5 μg/mL) for 24 h. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay was used for the determination of cell viability. In brief, BV2 cells (4 × 105 cells/well) were seeded and treated with the indicated concentrations of ATRA 1 h before LPS (0.5 μg/mL) treatment for 24 h. After the treatments, the medium was removed, and the cells were incubated with 0.5 mg/mL of MTT solution. After incubation for 2 h at 37 °C and 5 % CO2, the supernatant was removed, and the formation of formazan was measured at 540 nm with a microplate reader (BioTek Instruments, Inc., Luton, UK).
Enzyme-Linked Immunosorbent Assay
In accordance with the manufacturer’s instructions, the levels of TNF-α (R&D Systems, Inc., Minneapolis, MN, USA) and IL-6 (BioLegend, Inc., San Diego, CA, USA) in the BV2 cell media that were pretreated with the indicated concentrations (0.78–50 μg/mL) of ATRA for 1 h with or without LPS (0.5 μg/mL) for 24 h were measured with enzyme-linked immunosorbent assay (ELISA) kits. Absorbance was determined at 540 nm with a microplate reader.
Statistical Analysis
All data are presented as mean ± SE of the means (SEMs). Comparisons of the data from the different groups were performed with Student’s t tests (IBM Corporation, Armonk, NY, USA). Differences with p values less than 0.05 were considered to be statistically significant. Each “n” value refers to the number of separate experiments conducted.
Results
Protective effects of ATRA on pyramidal neurons in the hippocampal CA1 region in a model of transient global cerebral ischemia.
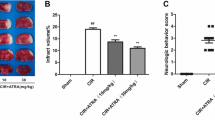
In the present study, the neuroprotective effects of ATRA against transient global cerebral ischemia were evaluated by histologic assessments of viable pyramidal neurons in the stratum pyramidale (SP) of the hippocampal CA1 region. 3 days after BCCAO/R, viable pyramidal neurons in the SP were rarely seen in the OP group (Fig. 1A-b, g). The cell numbers of the viable pyramidal neurons in the OP group were approximately estimated to be 5 % of that of the SHAM group (Fig. 1B). Importantly, intraperitoneal injections of 1, 5, or 10 mg/kg of ATRA significantly and dose-dependently increased the viable cell numbers in the SP of the hippocampal CA1 region. Especially in the 10-mg/kg-ATRA-injected group, viable neurons in the SP reached nearly 93 % of that in the SHAM group (Fig. 1A-e, j, B). Next, in order to rule out the possibility that morphologically viable neurons could be subjected to neurodegeneration and eventually to cell death, we examined the effects of ATRA treatment on neurodegeneration in the hippocampal CA1 region after ischemic insult with histofluorescence staining for F-J C, which is a high-affinity fluorescent marker that is used for the localization of neuronal degeneration, according to the methods described in a previous study [23]. Although some F-J C-positive glial cells were also seen in the stratum oriens (SO) and the stratum radiatum (SR), we demonstrated that the neuroprotective effects of ATRA were mainly due to an attenuation of neurodegeneration in the SP (Fig. 2A, B). More importantly, we confirmed that the morphologically viable neurons that we observed in the H-E-stained SP in the hippocampal CA1 of ATRA-treated groups were not subjected to ongoing death processes but were actually healthy and alive. Additionally, we confirmed that ATRA treatment inhibited neurodegeneration in a dose-dependent manner. In order to elucidate whether these neuroprotective effects of ATRA were due to the inhibition of apoptosis or the prevention of necrosis, the extent of cleaved PARP-1 fragments, which are produced by activated caspase-3, in the hippocampal homogenates of the OP group and the 10-mg/kg-ATRA-injected group was compared. As shown in Fig. 2C, ATRA treatment at this dose (10 mg/kg) clearly inhibited PARP-1 cleavage, which is a hallmark of apoptosis, compared to that in the OP group. These results demonstrated that neuroprotective effects of ATRA involves the mechanism of inhibition for neuronal apoptosis.
Effects of ATRA on neuronal survival in an in vivo gerbil model of global cerebral ischemia. A Histochemical examination with hematoxylin-eosin (H-E) staining of hippocampal areas 3 days after bilateral common carotid artery occlusion and reperfusion (BCCAO/R). Photomicrographs of low (×40, a–e) and high (×400, f–j) magnifications of representative H-E-stained slices of the hippocampi reveal dose-dependent neuroprotective effects of ATRA in the context of global cerebral ischemia. Three blind observers counted the pyramidal neurons with normal morphology (indicated by open arrows) and those with dark, shrunken, and damaged appearances (indicated by closed arrows) in a 1-mm length of the middle portion of the hippocampal CA1 subfield from 5 randomly selected coronal sections per animal. Scale bar = 200 μm. B The summed cell counts of viable neurons in the stratum pyramidale (SP) of the hippocampal CA1 region in 5 randomly selected slices. The graphs reveal that the total numbers of viable cells in the areas of interest in gerbils that were treated with 1, 5, and 10 mg/kg of ATRA were significantly higher compared to that of the operated (OP) group [78.2 ± 8.2, 96.3 ± 11.6, and 113.2 ± 9.8 vs. 8.9 ± 4.6, respectively; n of each group = 3 per group; ***p < 0.001 vs. OP group, ### p < 0.001 vs. sham-operated (SHAM) group]. The bars indicate SEs of the mean (SEMs)
Effects of ATRA on neurodegeneration in an in vivo gerbil model of global cerebral ischemia. A Histo fluorescent examination with Fluoro-Jade C (F-J C) staining of hippocampal areas 3 days after BCCAO/R. Photomicrographs of high magnifications (×400, a–e) of representative F-J C-stained slices of the hippocampi reveal a dose-dependent reduction effect of ATRA on neurodegeneration in the context of global cerebral ischemia. Three blind observers counted F-J C-positive neurons (indicated by closed arrows) in a 1-mm length of the middle portion of the hippocampal CA1 subfield from 5 randomly selected coronal sections per animal. Scale bar = 200 μm. B The summed cell counts of degenerated neurons in the SP of the hippocampal CA1 in 5 randomly selected slices. The graphs demonstrate that the numbers of degenerated neurons in the areas of interest in gerbils treated with 1, 5, or 10 mg/kg ATRA were significantly lower compared to that of the OP group (48.2 ± 6.7, 25.3 ± 10.6, and 14.2 ± 12.1 vs. 64.9 ± 4.1, respectively; n of each group = 3 per group; **p < 0.01, *p < 0.001 vs. OP group, ### p < 0.001 vs. SHAM group). The bars indicate SEMs. C Quantitative changes in poly (ADP-ribose) polymerase-1 (PARP-1) cleavage in the gerbil hippocampal tissue at postoperative day (POD) 1 with and without 10 mg/kg of ATRA. These results reveal that cleaved PARP-1 fragments (89 kDa) are significantly downregulated by 10-mg/kg-ATRA injections. The β-actin band is shown as a molecular reference
Effects of ATRA on Ischemia-Induced Hyperactivity in Gerbils
In order to determine the effects of ATRA treatment on the development of ischemia-induced neurobehavioral changes, the ischemia-induced hyperactivity levels in gerbils that had been treated with different doses of ATRA were compared to those of the OP group with an open-field test. Changes in locomotor activity that have been induced by cerebral ischemia in gerbils have been clearly documented, and this hyperactivity occurs immediately after occlusion in accordance with the progression of hippocampal CA1 neuronal death [24–27]. Wang and Corbett [27] have reported that the increased locomotor activity that results from ischemic damage is due to a reduction in the potential of the animal to form spatial maps. The OP group showed an abrupt increase in spontaneous locomotor activity to approximately 3 times that of the SHAM group on the day of the operation (POD 0) (Fig. 3). Interestingly, in the trials conducted on POD 0, a statistically significant attenuation of the ischemia-induced hyperactivity was evident in the 5- and 10-mg/kg-ATRA-injected groups compared to those in the OP group. These results suggested that, other than the biochemical evidence previously shown by morphologic and quantitative assessments, ATRA demonstrated neurobehavioral benefits on transient global cerebral ischemia.
Effects of ATRA on ischemia-induced locomotor activity. In an open-field test, the changes in locomotor activity were measured in the animals of each group before and after BCCAO/R surgery (n of each group = 6). Three trials of the locomotor activities (line crossing: number of squares that gerbils crossed with all 4 paws) in each animal were measured by 3 blind observers, and the averages were obtained for each trial. The overall activity levels of all of the experimental groups, including the OP group, were higher than that of the SHAM group at POD 0 (# p < 0.05, ## p < 0.01 vs. SHAM group). Interestingly, a statistically significant attenuation of ischemia-induced hyperactivity is evident in the 5- and 10-mg/kg-ATRA-injected groups compared to those of the OP group (**p < 0.01 vs. OP group). The bars indicate SEMs
Effects of ATRA on Neuroinflammation
By results from F-J C histofluorescence staining and quantitative observation of PARP-1 cleavage, now we could suggest the involvement of neuronal antiapoptotic mechanism for ATRA’s neuroprotection. On the other hand, as described in the introduction, ischemia is accompanied by inflammatory reactions that involve the activation and proliferation of astrocytes and microglia. For addressing possible involvement of ATRA’s modulating effects on neuroinflammation as a kind of the upstream events proceeded by these processes, we compared the extent of astrocytosis and the microglial activation in hippocampal CA1 regions in response to the different doses of ATRA in the ATRA-injected groups with the OP group with immunohistochemistry with antibodies to GFAP and Iba1, respectively (Fig. 4A). Although changes in the attenuation of the neuroinflammatory reactions were not observed in the 1- and 5-mg/kg-ATRA-injected group (data not shown), the 10-mg/kg injections of ATRA significantly reduced the extent of astrocytosis and microglial activation. A robust increase in GFAP-positive astrocytes with thick and short processes (the morphological characteristics of activated astrocytes) was observed not only in the SO and SR, but also in the SP in the OP group (Fig. 4A-c, f, B). However, these characteristics were obviously rarely seen in the SP of the 10-mg/kg-ATRA-injected group, although some activated astrocytes were observed in the SO and SR of that group. Increased Iba1-positive activated microglia were also evident in the OP group compared to the SHAM group, but 10 mg/kg of ATRA significantly attenuated these increases (Fig. 4A-i, l, B) in all 3 regions of the hippocampal CA1. Next, in order to examine whether ATRA acted as a neuroinflammatory modulator in ischemic processes in vivo and in vitro, we measured the level of the expression of TNF-α and IL-6 and the release of NO, TNF-α, and IL-6 with or without ATRA treatment in geril hippocampal homogenates and in LPS-stimulated BV2 cells, respectively. There has been abundant evidence showing that cerebral ischemia induces an acute neuroinflammatory reaction. To address and confirm this issue, we first determined the gerbils’ hippocampal expression of TNF-α and IL-6 as proinflammatory cytokines, 24 h after the BCCAO/R procedure. As it is shown in Fig. 5A, B, induction of global ischemia by BCCAO/R (i.e., OP group) intensly increased the hippocampal expression of TNF-α and IL-1β, compared with the SHAM group. However, those of 1- and 10-mg/kg-ATRA group were significantly lower than that of OP group, although these extents were not dose-dependent (n of each group = 4; **p < 0.01 vs. OP group; # p < 0.05, ## p < 0.01 vs. OP group). Next, for establishment of well-correlated in vitro data supporting in vivo, we first identified the applicable doses of ATRA that were not neurotoxic on BV2 cells with MTT assays after a 24-h incubation with different concentrations of ATRA. As shown in Fig. 5C, D, cell viability following treatment with 0.78–25 μg/mL of ATRA was not significantly different from that in the control in the absence or presence of LPS. However, the exposure of BV2 cells to 50 μg/mL of ATRA led to about a 50 % decrease in cell survival. After identifying the optimal dose of safety in this setting, BV2 cells were pretreated with ATRA (0.78–50 μg/mL) for 1 h and then stimulated with 0.5 μg/mL of LPS for a 24-h incubation period. As anticipated, LPS significantly enhanced NO release in the culture media, while pretreatment with a dose of 25 μg/mL of ATRA surprisingly reduced NO production by about 0 % (Fig. 5e). We further determined whether ATRA regulated the release of other proinflammatory cytokines that were secreted by microglia in the processes of neuroinflammation. As shown in Fig. 5F, G, LPS incubation caused a remarkable increase in the levels of TNF-α and IL-6, which were suppressed by ATRA in a dose-dependent manner.
Effects of ATRA on astrocytosis and microglial activation in gerbil hippocampal CA1 regions following BCCAO/R (n of each group = 4). A Immunohistochemical examinations of anti-GFAP (a–f) and anti-Iba1 (g–l) in the hippocampal CA1 regions 3 days after BCCAO/R reveal that 10-mg/kg-ATRA injections reduce the extent of astrogliosis and microglial activation, respectively. In photomicrographs of low (×200, a–c) and high (×400, d–f) magnifications, a robust increase of GFAP-positive astrocytes with thick and short processes is observed in hippocampal CA1 regions of the OP group compared to that of the SHAM group (b and e vs. a and d). However, these increases are obviously rarely seen in the regions of interest of the 10-mg/kg-ATRA-injected group (c, f). Similarly, low (×200, g–i) and high (×400, j–l) magnifications of images obtained of the immunohistochemistry with anti-Iba1 demonstrate that increases of activated microglia are also evident in the OP group compared to the SHAM group (h and k vs. g and j). However, 10 mg/kg of ATRA significantly attenuates these results in all 3 regions of the hippocampal CA1 (i, l). The arrows in e and f and k and l indicate the astrocytes and activated microglial cells, respectively. Scale bar = 200 μm. B Average relative optical intensity and cell counts of GFAP-positive astrocytes and Iba1-positive activated microglia in the hippocampal CA1 of 5 randomly selected slices. The graphs demonstrate that the density of astrocytes and the numbers of activated microglia in the areas of interest in the OP group are significantly increased compared to that of each SHAM group (9.52 ± 0.9 vs. 1 and 185.36 ± 21.72 vs. 47.30 ± 2.73, respectively; ## p < 0.05), while a 10-mg/kg-ATRA injection significantly reduces both of these values by approximately one-third for astrocytosis and half for microglial activation (**p < 0.01 vs. OP group). The bars indicate SEMs
Effects of ATRA on the expression and release of proinflammatory cytokines in gerbils’ hippocampal homogenates and lipopolysaccharide (LPS)-stimulated BV-2 cells, respectively. The representative photographs of immunoblots (a) and the bar graphs for its densitometric analysis (b) revealed that the BCCAO/R (i.e., OP) increased robustly the extent of hippocampal expression of tumor necrosis factor (TNF)-α and interleukin (IL)-6 (n of each group = 3; 3.89 ± 0.38 and 4.21 ± 0.22 for TNF-α and IL-6, respectively), however 1 (2.15 ± 0.21 and 2.66 ± 0.04 for TNF-α and IL-6, respectively; **p < 0.01 and # p < 0.05 vs. OP groups’ TNF-α and IL-6, respectively) and 10 mg/kg of ATRA treatment (2.31 ± 0.13 and 2.98 ± 0.20 for TNF-α and IL-6, respectively; **p < 0.01 and ## p < 0.01 vs. OP groups’ TNF-α and IL-6, respectively) significantly reduced these extent. After determining the maximal safe dose of ATRA in BV-2 cells with or without LPS (c and d, respectively), we evaluated the concentrations of nitric oxide (NO; e), TNF-α (f) and IL-6 (g) in culture media of LPS-stimulated BV-2 cells that were treated with different doses of ATRA for 24 h and that were demonstrated with enzyme-linked immunosorbent assays. A remarkable increase in the levels of NO, TNF-α and IL-6 secretion from BV-2 cells that were stimulated by LPS is significantly suppressed by ATRA in a dose-dependent manner (*p < 0.05, **p < 0.01, and ***p < 0.001 vs. control). The bars indicate SEMs. The numbers above the error bars indicate the actual measurements of the concentrations of each cytokine
Discussion
Ischemic stroke is caused by an interruption of cerebral blood flow, which can lead to a failure of energy production, the activation of proteases, impairments in metabolism, free radical production, excito toxicity, and altered calcium homeostasis [28–31]. Cerebral ischemia is associated with a severe deterioration in the ability of neuronal mitochondria to function effectively; this seriously compromises oxidative phosphorylation, which is a key mechanism by which adenosine triphosphate is produced. Furthermore, dysfunctional mitochondria may increase the production of reactive oxygen species (ROS), and they are then unable to maintain a normal [Ca2+]i. This causes depolarization of the cytoplasmic membrane potential. Such changes can contribute to both Ca2+-induced membrane damage and increases in the levels of Ca2+-induced proteases, the extent of ROS-mediated cell damage (to membranes and DNA), and lipid peroxidation [32]. These changes can induce a failure of cytoskeletal organization, the activation of apoptotic signals that lead to cell death, and mitochondrial dysfunction [33]. In turn, the downstream cytotoxic cascades that are thus activated render Ca2+ homeostasis dysfunctional, and the positive feedback loop that is thus created eventually kills the cell.
Apart from these so-called intrinsic mechanisms of neuronal apoptosis, the extrinsic mechanisms of neuronal apoptosis in the pathogenesis of cerebral ischemia involve the engagement of interactions between death receptors that are located on the plasma membrane and various cytokines that are secreted by activated glia, and this is referred to as the “death receptor pathway.” Neuronal surface death receptors belong to the tumor necrosis factor receptor superfamily, which is activated by its ligand, TNF-α. Together with IL-6 receptors (IL-6R) that are also expressed on the neuronal plasma membrane, these receptors trigger the recruitment of the Fas-associated death domain protein (FADD). FADD then activates caspase-8 and mediates the cleavage of Bid and caspase-3. Caspase-3 cleaves PARP-1 and inactivates it, resulting in the breakdown of DNA structures.
The results of these studies demonstrated that ATRA treatment maintains the neuronal population in the SP of the hippocampal CA1 region in gerbils that are exposed to global cerebral ischemia by antiapoptotic activity. Additionally, ATRA showed beneficial effects on the behavioral outcomes, as demonstrated by it altering the ischemia-induced hyperactivity of gerbils. These effects were due, at least in part, to inhibition of the release of NO, TNF-α, and IL-6 by activated microglia. These results demonstrated that ATRA treatment protects neurons by attenuating neuroinflammatory processes in the hippocampal CA1 region from ischemia-induced neurodegeneration in a transient global cerebral ischemia model.
However, we cannot exclude the possibility that direct effects on the neurons were also involved in the protective effects of ATRA, as RARs are also expressed in neurons. In fact, previous research on brain slice cultures has shown that RA protects neurons from inflammatory degeneration, presumably through an upregulation of brain-derived neurotrophic factor [34]. Because RA has a role in patterning in mammalian developmental stages and neurogenesis in rodent adult brain, we cannot rule out the possibility that neuroregeneration processes participated in these process [35–37]. However, the chances of the latter possibility occurring are relatively low, as we focused on screening for the short-term efficacy of ATRA on cerebral ischemia with the experimental models at POD 3. Nevertheless, whether neurotrophin expression or neuroregeneration processes contributed to the protective effects of ATRA in cerebral ischemia is an interesting point to be addressed in the future.
In this study, we focused on examining the synthetic form of retinoids as an immune modulator. Retinoids refer to natural or synthetic compounds that are related to RA and that act on RARs and RXRs. Both RARs and RXRs include 3 subtypes that are designated as α, β, and γ, and the expression of these subtypes has been demonstrated in the adult central nervous system [38]. The detailed roles of retinoids in physiological conditions remain to be investigated, but some evidence has indicated that retinoids may protect neurons from neuroinflammatory processes. A synthetic RAR agonist, Am80 (tamibarotene), has been shown to ameliorate the symptoms of experimental autoimmune encephalomyelitis [39, 40]. Here, the neuroprotective effects of ATRA likely involved the suppression of inflammatory reactions in the transient global cerebral ischemia model. This assumption was supported by previous findings that RAs inhibit the expression of inducible nitric oxide synthase and proinflammatory cytokines in LPS-stimulated primary isolated microglial cells [41, 42].
Some fundamental questions about the relationships of neuroinflammatory processes that originate from the surrounding glial population with RA signaling remain. What might be the underlying mechanisms of the attenuation of microglial activation after ATRA treatment? One possibility is that microglial activation is associated with the initial glutamate-induced excitotoxic events that occur due to cerebral ischemia [43], and ATRA might inhibit these steps. A more intriguing possibility is that microglial activation might be due to an innate immune response to CNS injury and that RA signaling is engaged in these kinds of immune response. In fact, much of the neuronal damage that is seen in LPS-induced CNS injury models appears to be mediated through Toll-like receptors (TLR) that are present on the microglial plasma membrane [44]. Actually, cerebral ischemia induces one of the endogenous ligands of microglial TLRs [45]. In a study by Liu et al., the effects of ATRA on TLR expression in primary human monocytes have been studied by measuring the levels of mRNA and protein expression of TLRs 1–10 and the TLR-2 co receptor, CD14, in cells after ATRA exposure [46, 47]. TLR-2 and CD14 mRNA encoding was decreased by 34 and 65 %, respectively, while TLR-4 mRNA levels were maintained. Furthermore, ATRA decreased TLR-2 and CD14 protein expression by 41 and 42 %, respectively. These findings suggest that ATRA directly suppresses TLR-mediated inflammation, most likely by effecting TLR signaling or mRNA stability. Thus, identifying the direct path of ATRA action in signaling cascades in microglial cells (e.g., cascades of glutamate excito toxicity and/or TLRs signaling) during cerebral ischemia remains for further investigation.
References
Benveniste EN (1998) Cytokine actions in the central nervous system. Cytokine Growth Factor Rev 9:259–275
Donnelly DJ, Popovich PG (2008) Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 209:378–388
Knoblach SM, Fan L, Faden AI (1999) Early neuronal expression of tumor necrosis factor alpha after experimental brain injury contributes to neurological impairment. J Neuroimmunol 95:115–125
Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA (2003) Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol 140:471–476
Hailer NP (2008) Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol 84:211–233
Barone FC, Feuerstein GZ (1999) Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab 19(8):819–834
Maden M (2001) Role and distribution of retinoic acid during CNS development. Int Rev Cytol 209:1–77
Harvey BK, Shen H, Chen GJ, Yoshida Y, Wang Y (2004) Midkine and retinoic acid reduce cerebral infarction induced by middle cerebral artery ligation in rats. Neurosci Lett 369:138–141
Sato Y, Meller R, Yang T, Taki W, Simon RP (2008) Stereo-selective neuroprotection against stroke with vitamin A derivatives. Brain Res 19(1241):188–192
Choi WH, Ji KA, Jeon SB, Yang, Kim H, Min KJ, Shong M, Jou I, Joe EH (2005) Anti-inflammatory roles of retinoic acid in rat brain astrocytes: suppression of interferon-gamma-induced JAK/STAT phosphorylation. Biochem Biophys Res Commun 329(1):125–131
Gross V, Villiger PM, Zhang B, Lotz M (1993) Retinoic acid inhibits interleukin-1-induced cytokine synthesis in human monocytes. J Leukoc Biol 54(2):125–132
Kreutz M, Fritsche J, Ackermann U, Krause SW, Andreesen R (1998) Retinoic acid inhibits monocyte to macrophage survival and differentiation. Blood 91(12):4796–4802
Kim BH, Kang KS, Lee YS (2004) Effect of retinoids on LPS-induced COX-2 expression and COX-2 associated PGE (2) release from mouse peritoneal macrophages and TNF-alpha release from rat peripheral blood mononuclear cells. Toxicol Lett 21 150(2):191–201
Fawzy AA, Vishwanath BS, Franson RC (1988) Inhibition of human non-pancreatic phospholipases A2 by retinoids and flavonoids. Mechanism of action. Agents Act 25(3–4):394–400
Motomura K, Sakai H, Isobe H, Nawata H (1997) All-trans retinoic acid suppresses liver injury induced by propionibacterium acnes and lipopolysaccharide in rats. J Gastroenterol Hepatol 12(12):887–892
Motomura K, Ohata M, Satre M, Tsukamoto H (2001) Destabilization of TNF-alpha mRNA by retinoic acid in hepatic macrophages: implications for alcoholic liver disease. Am J Physiol Endocrinol Metab 281(3):420–429
Sirsjö A, Gidlöf AC, Olsson A, Törmä H, Ares M, Kleinert H, Förstermann U, Hansson GK (2000) Retinoic acid inhibits nitric oxide synthase-2 expression through the retinoic acid receptor-alpha. Biochem Biophys Res Commun 270(3):846–851
Lee DH, Ha N, Bu YM, Choi HI, Park YG, Kim YB, Kim MY, Kim H (2006) Neuroprotective effect of Buddleja officinalis extract on transient middle cerebral artery occlusion in rats. Biol Pharm Bull 29(8):1608–1612
Ha SK, Moon E, Lee P, Ryu JH, Oh MS, Kim SY (2012) Acacetin attenuates neuro inflammation via regulation the response to LPS stimuli in vitro and in vivo. Neurochem Res 37(7):1560–1567
Ha SK, Moon E, Ju MS, Kim DH, Ryu JH, Oh MS, Kim SY (2012) 6-Shogaol, a ginger product, modulates neuro inflammation: a new approach to neuroprotection. Neuropharmacology 63(2):211–223
Wu KJ, Hsieh MT, Wu CR, Wood WG, Chen YF (2012) Green tea extract ameliorates learning and memory deficits in ischemic rats via its active component polyphenol epigallocatechin-3-gallate by modulation of oxidative stress and neuro inflammation. Evid Based Complement Alternat Med 2012:1–11
Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R (2001) Hyperactivity and impaired response habituation in hyper dopaminergic mice. Proc Natl Acad Sci USA 98(4):1982–1987
Yoo DY, Kim WS, Nam SM, Chung JY, Choi JH, Yoon YS, Won MH, Hwang IK (2012) Chronic effects of pyridoxine in the gerbil hippocampal CA1 region after transient forebrain ischemia. Neurochem Res 37:1011–1018
Chandler MJ, Deleo J, Carney JM (1985) An unanesthetized-gerbil model of cerebral ischemia-induced behavioral changes. J Pharmacol Methods 14:137–146
Kuroiwa T, Bonnekoh P, Hossman KA (1991) Therapeutic window of halothane for reversal of delayed neuronal injury in gerbils: relationship to postischemic motor hyperactivity. Brain Res 563:33–38
Mileson BE, Schwartz RD (1991) The use of locomotor activity as a behavioral screen for neuronal damage following transient forebrain ischemia in gerbils. Neurosci Lett 128:71–76
Wang D, Corbett D (1990) Cerebral ischemia, locomotor activity and spatial mapping. Brain Res 533:78–82
Wexler BC (1970) Metabolic changes in response to acute cerebral ischemia following bilateral carotid artery ligation in arteriosclerotic versus non arteriosclerotic rats. Stroke 1:112–121
Martins E, Inamura K, Themner K, Malmqvist KG, Siesjö BK (1988) Accumulation of calcium and loss of potassium in the hippocampus following transient cerebral ischemia: a proton microprobe study. J Cereb Blood Flow Metab 8:531–538
Choi DW (1988) Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11:465–469
Panickar KS, Norenberg MD (2005) Astrocytes in cerebral ischemic injury: morphological and general considerations. Glia 50:287–298
Sims NR, Muyderman H (2010) Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta 1802:80–91
Szydlowska K, Tymianski M (2010) Calcium, ischemia and excitotoxicity. Cell Calcium 47:122–129
Hideaki M, Masanori H, Akinori H, Yoichiro I, Koichi S, Hiroshi K (2011) A retinoic acid receptor agonist Am 80 rescues neurons, attenuates inflammatory reactions, and improves behavioral recovery after intracerebral hemorrhage in mice. J Cereb Blood Flow Metab 31(1):222–234
Anastassia V, Anna F, Tammy R, Ashraf AM, Ilona SS (2011) Ascl1/Mash1 is a novel target of gli2 during gli2-induced neurogenesis in P19 EC cells. PLoS One 6(4):19174
Maureen AK, Alexandra EF, Chao W, Joseph LN (2010) Ethanol elevates physiological all-trans-retinoic acid levels in select loci through altering retinoid metabolism in multiple loci: a potential mechanism of ethanol toxicity. FASEB J 24(3):823–832
Timothy G, James EC, Sonia EN, Loredana Q, Kirsty S, Alexander R, Peter MC (2012) Patterning of retinoic acid signaling and cell proliferation in the hippocampus. Hippocampus 22(11):2171–2183
Malcolm M, Matthew H (2004) Retinoic acid in alveolar development, maintenance and regeneration. Philos Trans R Soc Lond B Biol Sci 359(1445):799–808
Shinya Y, Xiao-Ying Z, Mitsuyo M, Katsuyuki M, Shelley W, Robert VF, Hiroshi I, Thomas LI (2000) Essential role of NAT1/p97/DAP5 in embryonic differentiation and the retinoic acid pathway. EMBO J 19(20):5533–5541
Christian K, Benjamin JE, Anna KK, Tomoko O, Stephan VH, Koichi S, Shinji O, Takashi Y (2009) Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol 174(6):2234–2245
Jihong X, Paul DD (2006) 9-Cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. J Neuroimmunol 171(1–2):135–144
Cindy X, Paul DD (2007) Liver X receptor and retinoid X receptor agonists inhibit inflammatory responses of microglia and astrocytes. J Neuroimmunol 183(1–2):50–59
Casha S, Yu WR, Fehlings MG (2001) Oligodendoglial apoptosis occursa along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in rats. Neuroscience 103:203–218
Matute C, Alberdi E, Domercq M, Pérez-Cerdá F, Pérez-Samartín A, Sánchez-Gómez MV (2001) The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci 24:224–230
Lehnardt S, Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T (2003) Activation of innate immunity in the CNS triggers neurodegeneration through a toll-like receptor 4 pathway. Proc Natl Acad Sci USA 100:8514–8519
Tsan MF, Gao B (2004) Endogenous ligands of toll-like receptors. J Leukoc Biol 76:514–519
Liu PT, Krutzik SR, Kim J, Modlin RL (2005) Cutting edge: all-trans retinoic acid down regulates TLR2 expression and function. J Immunol 174(5):2467–2470
Acknowledgments
The authors would like to thank Miss Yeji Lee for technical assistance. The work was evenly supported by a researching fund by Korea Research Foundation in Korea (No. KRF-2011-0012388) and Myunggok Research Institute, College of Medicine, Konyang University (No. 2010-17).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.H., Yu, K.S., Jeong, J.H. et al. All-Trans-Retinoic Acid Rescues Neurons After Global Ischemia by Attenuating Neuroinflammatory Reactions. Neurochem Res 38, 2604–2615 (2013). https://doi.org/10.1007/s11064-013-1178-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1178-x