Abstract
The problem of new psychoactive substances (NPS) is emerging globally. However, the immunotoxicity of synthetic cannabinoids is not evaluated extensively yet. The purpose of the present study was to investigate whether synthetic cannabinoids (JWH-210 and JWH-030) induce adverse effects on lymphoid organs, viability of splenocytes and thymocytes, and immune cell activator and cytokines in mice. JWH-210 (10 mg/kg, 3 days, i.p.) is more likely to have cytotoxicity and reduce lymphoid organ weight than JWH-030 of ICR mice in vivo. We also demonstrated that JWH-210 administration resulted in the decrease of expression levels of T-cell activator including Cd3e, Cd3g, Cd74p31, and Cd74p41, while JWH-030 increased Cd3g levels. In addition, JWH-210 reduced expression levels of cytokines, such as interleukin-3, interleukin-5, and interleukin-6. Furthermore, we demonstrated that a CB2 receptor antagonist, AM630 inhibited JWH-210-induced cytotoxicity, whereas a CB1 receptor antagonist, rimonabant did not in primary cultured splenocytes. These results suggest that JWH-210 has a cytotoxicity via CB2 receptor action and results in decrement of lymphoid organ weights, T-cell activator, and cytokine mRNA expression levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
New psychoactive substances (NPS) have diverse adverse effects including cardiovascular, neurological, gastrointestinal, and pulmonary. Currently, there is a dearth of knowledge on many types of NPS. Most of the available data on NPS-induced toxicity are derived from retro- or prospectively analyzed cases of intoxication as well as interviews with drug users and, therefore, are of limited scientific value (Hohmann et al. 2014). Therefore, to evaluate the adverse effect of NPS, preclinical studies are required to study their toxicity. However, most preclinical research studies have investigated the dependence potential and neuropsychiatric effects of NPS. Synthetic cannabinoids are one of most abused NPS and have dependence liability potential that is similar to that of natural and botanical components. Research on the cannabinoids has revealed several hundred agonists that might be abused, with varying affinity for the CB1 and CB2 receptors (Fattore and Fratta 2011). The endocannabinoid system participated in the regulation of physiological process such as caloric balance and the control of arterial smooth muscle tone (Hohmann et al. 2014). CB1 receptors are found mainly in the nervous system, as well as on particular types of neurons (Seely et al. 2011). Synthetic cannabinoids are potent CB1 agonist and exert a delta-9-tetrahydrocannabinol (THC)-like effect, with alteration of mood, perception, sleep and wakefulness, body temperature, and cardiovascular function (Hermanns-Clausen et al. 2013). Their side effects are more varied and more severe than those of THC, with the more common ones being tachycardia, arterial hypertension, hyperglycemia, hypokalemia, hallucinations, and agitation (Hohmann et al. 2014). Because of expression patterns of CB receptors in the immune system, it can be assumed that cannabinoids have significant effects on immune response. It has been reported that cannabinoids significantly hampered the resistance to infection in human as well as several animal species (Friedman et al. 2003). However, the exact harmful effects of synthetic cannabinoids on the immune system are not fully understood yet. In the present study, we investigated the effects of the synthetic cannabinoids, which have differences in CB receptor binding affinity on the immune system in vivo and ex vivo. JWH-030 is regarded as agonists at both the CB1 and CB2 with Ki values of 87 and 320 nM, respectively. JWH-210 has a higher binding affinity with Ki values of 0.46 nM at CB1 and 0.69 nM at CB2 (Showalter et al. 1996; Griffin et al. 1998; Huffman et al. 2005; Rieder et al. 2010). The cytotoxicity of the synthetic cannabinoids was evaluated in thymocytes and splenocytes. We also measured lymphoid organ weight and T-cell activator and cytokine levels of lymphoid organs in mice after administration of JWH-210 and JWH-030. Furthermore, to clarify a mechanism underlying synthetic cannabinoid-induced toxicity, a pharmacological rescue study of the cytotoxicity of the agents was performed using CB1 and CB2 receptor antagonists.
Materials and methods
Animals
All experimental procedures were approved by the Animal Ethics Committee, National Institute of Food and Drug Safety Evaluation and complied with the Guide for the Care and Use of Laboratory Animals (Institue of Laboratory Animal Resources (U.S.) 1996).
All efforts were made to minimize animal distress and prevent suffering. Male and female ICR mice (4 weeks old) were obtained from the Ministry of Food and Drug Safety (Association for Assessment and Accreditation of Laboratory Animal Care, AAALAC member, Osong, Republic of Korea). The mice were maintained in an animal facility where they were transferred to cages on arrival and were housed in adequate group sizes. They were allowed to acclimatize for 1 week before being used in the experiments. The animal holding rooms were maintained at a temperature of 21–24 °C and 40–60% relative humidity with a 12-h light/dark cycle (lights on at 08:00 to 20:00). The animals received a solid diet, tap water ad libitum.
Materials
All the synthetic cannabinoids tested (JWH-210 and JWH-030 illustrated in Fig. 1) were purchased from Cayman Chemical (Ann Arbor, MI, USA). Rimonabant hydrochloride and AM630 were purchased from Sigma-Aldrich (St. Louis, MO, USA). The other routine chemicals were purchased from Sigma-Aldrich unless otherwise specified.
Lymphoid organ weight
ICR mice were weighted every day during injection period and just before being euthanized. JWH-210 or JWH-030 was administered (10 mg/kg, i.p.) for 3 days. Vehicle is 0.5% DMSO and 0.5% Tween80 in saline. Mice were sacrificed by cervical dislocation, and spleen and thymus were removed 24 h after last administration. Body weight and selected absolute organ weights were scaled, and relative organ to body weight ratios were calculated for spleen and thymus.
Splenocyte and thymocyte culture
Spleen and thymus were isolated from mice. A 100-μm-pore cell strainer (Falcon, USA) was used for dividing single cells. Single cell suspensions of spleen and thymus were prepared by gently pressing the tissues with sterilized slide glass. Splenocytes and thymocytes were pooled from isolated organs and seeded in plates with the RPMI (Gibco, Waltham, MA, USA) supplemented with β-mercaptoethanol (50 μM), HEPES (10 mM), FBS (5%), l-glutamine (1 mM), and antibiotics/antimycotics (Invitrogen, Waltham, MA, USA).
Cell viability (in vivo administration)
Splenocytes were cultured from JWH-210- (10 mg/kg, 3 days, i.p.) or JWH-030- (10 mg/kg, 3 days, i.p.) treated mice as mentioned previously. The cells were seeded in 96-well plates (5 × 105 cells/well) with 100 μL media. After seeding single cell suspensions, the plates were incubated for 6 h in 95% air:5% CO2 using standard culture methods. Cell viability was measured using LIVE/DEAD viability/cytotoxicity kit (Thermo Fisher, Waltham, MA, USA) according to the manufacturer’s manual. In brief, prior to the assay, the cells were washed twice with phosphate-buffered saline (PBS) and added 100 μL PBS to each well with supplied EthD-1 and calcein AM. The cells were incubated for 30 min at room temperature in the dark, and cell viability was measured by microplate-based cell cytometer (Celigo, Lawrence, MA, USA). The data of Live (% corrected) was used to analyze cell viability.
Cell viability (ex vivo treatment)
Splenocytes were cultured from naïve mice as mentioned previously. The cells were seeded in 96-well plates (5 × 105 cells/well) with 100 μL media. After seeding single cell suspensions, the plates were incubated for 6 h in 95% air:5% CO2 using standard culture methods. To confirm cell viability by synthetic cannabinoids, the splenocytes were treated with synthetic cannabinoids (0, 1, 10, and 100 μM) for 16 h. To observe relationship between JWH-210 and CB receptors, the splenocytes were treated with media or JWH-210 or JWH-210/CB receptor antagonist (rimonabant or AM630) for 4 h. Cell viability was measured as mentioned previously.
T-cell and B-cell activation-related gene profiling and quantitative PCR
Spleen were isolated from mice that were injected with JWH-210 (10 mg/kg, i.p., 3 days). Total RNA was extracted by easy-spin DNA free total RNA extraction kit (Intron, Seoul, Republic of Korea). Complementary DNA (cDNA) was synthesized from total isolated RNA using a SuperScript III first-strand synthesis system (Invitrogen) for quantitative PCR (qPCR). RT2 Profiler™ PCR Array Mouse T-Cell & B-Cell Activation (PAMM-053Z, QIAGEN, Valencia, CA, USA) was used for pathway-focused T-cell and B-cell activation-related gene expression analysis. Subsequent qPCR was performed with an iCycler iQ5 real-time detection system (Bio-Rad, Hercules, CA, USA) using the SYBR Green PCR master mix (Thermo Fisher Scientific, Waltham, MA, USA). For the polymerase activation, the initial incubation was conducted at 50 °C for 2 min followed by 95 °C for 10 min. After that, 40 cycles were performed at 95 °C for 15 s and then at 60 °C for 1 min. cDNA was included in a 25-μL-volume PCR reaction with the following components: 0.125 μL each of forward and reverse primer, 12.5 μL SYBR green, and 0.5 μg of cDNA with sterilized water. The primers used were as follows: mouse Cd3ε, forward 5′-CGTCCGCCATCTTGGTAGAG-3′, reverse 5′-ATTCAATGTTCTCGGCATCGT-3′; mouse Cd3γ, forward 5′-TGGAGAAGCAAAGAGACTGACA-3′, reverse 5′-GCCATCCACTTGTACCAAATTC-3′; mouse Cd74p41, forward 5′-TTCCTCACACCAAGAGCCG-3′, reverse 5′-TGTCCAGTGGCTCACTGCAG-3′; mouse Cd74p31, forward 5′-ACCGAGGCTCCACCTAAAGAG-3′, reverse 5′-TTGACCCAGTTCCTGCCTG-3′; mouse IL-3 (N-4011, Bioneer, Daejeon, Republic of Korea); mouse IL-5 (N-4012, Bioneer, Daejeon, Republic of Korea); mouse IL-6 (N-4013, Bioneer, Daejeon, Republic of Korea); mouse TNF-α (N-4015, Bioneer, Daejeon, Republic of Korea); mouse TNF-β (N-4018, Bioneer, Daejeon, Republic of Korea); mouse IL-1β (N-4009, Bioneer, Daejeon, Republic of Korea); mouse IL-10 (N-4014, Bioneer, Daejeon, Republic of Korea); mouse GAPDH (used as an internal control), forward 5′-TGTCAAGCTCATTTCCTGGT-3′, reverse 5′-CTTACTCCTTGGAGGCCATG-3′.
Immunofluorescence
The ICR mice were treated with JWH-210 (10 mg/kg, 3 days, i.p.), and then, the mice were perfused with phosphate-buffered saline (PBS, pH 7.4) with heparin under inhaled CO2 anesthetization. The mice spleen were immediately fixed with 4% paraformaldehyde for 3 days at 4 °C and transferred to 30% sucrose solutions at 4 °C. After being transferred to 30% sucrose solutions, the mice spleen were cut into 16 μm sections by using a cryostat microtome (Leica CM 1850; Leica Microsystems, Seoul, Republic of Korea) and mounted on MAS-GP type A-coated slides. After two 10-min washes in PBS (pH 7.4), endogenous peroxidase activity was quenched by incubating prepared spinal cord sections in 3% hydrogen peroxide in PBS for 20 min, followed by an additional two 10-min washes in PBS. Sections were blocked for 1 h in 5% bovine serum albumin (BSA) and incubated overnight at 4 °C with a mouse polyclonal antibody against CD3 (1:200; Santa Cruz Biotechnology, CA, USA) and a rat polyclonal antibody against CD8 (1:200; eBioscience, USA). Sections were then washed three times (10 min each) in PBS and incubated for 2 h at room temperature with a secondary antibody conjugated to Alexa Fluor 488 and 594 (Invitrogen Molecular Probes, Carlsbad, CA, USA). Sections were then washed three times (10 min each) in PBS, incubated for 30 s at room temperature in the dark for DAPI staining, and mounted with VectaMount™ AQ (Vecta Laboratories, Burlingame, CA, USA). IF images were acquired using an inverted Zeiss Axiovert 200 M fluorescent microscope (Microscope Axio Imager.A2, Carl Zeiss, Oberkochen, Germany) (×200).
Measurement of CD3/CD4- and CD3/CD8-positive cell
Splenocytes and thymocytes were cultured from mice that were injected with JWH-210 (10 mg/kg, i.p., 3 days) as mentioned previously. After seeding single cell suspensions, the plates were incubated for 6 h in 95% air:5% CO2 using standard culture methods. The cultured cells were fixed with 4% formaldehyde for 15 min. After two washes in PBS, endogenous peroxidase activity was quenched by incubating prepared cells in 0.3% hydrogen peroxide and 0.2% Triton X-100 in PBS for 15 min, followed by an additional two washes in PBS. The cells were blocked for 1 h in 5% bovine serum albumin (BSA) and incubated overnight at 4 °C with the following primary antibodies: CD3 (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), CD4 (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and CD8 (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The cells were washed three times (10 min each) in PBS and incubated for 2 h at room temperature in the dark with the following secondary antibodies conjugated to Alexa Fluor 488 and 594 (1:500, Invitrogen Molecular Probes, Carlsbad, CA, USA). The cells were washed three times (10 min each) in PBS and incubated for 5 min at room temperature in the dark with DAPI (2 mg/mL stock, 1:1000, Sigma-Aldrich, St. Louis, MO). Fluorescent intensity was measured by microplate-based cell cytometer (Celigo, Lawrence, MA, USA), and cell populations were identified using color-coding overlay methods according to the manufacturer’s instructions.
Data analysis
The data represent the mean ± S.E. Statistically significant differences from the values in vehicle-treated group were analyzed by Student’s t test, one-way or two-way ANOVA followed by Bonferroni’s test for equal variance data, and followed by Dunnett’s rank test for non-equal variance data using SigmaPlot 13 software (SigmaPlot, Chicago, IL, USA). Probabilities less than 5% (p < 0.05) were considered to be statistically significant.
Results
JWH-210 reduced the weight of spleen and thymus
To investigate the effect of synthetic cannabinoids on lymphoid organ, ICR mice were injected with JWH-210 or JWH-030 (10 mg/kg, i.p. 3 days). We observed that JWH-210 decreased the weight of spleen and thymus, whereas JWH-030 had no effects in organ weight (Fig. 2).
Effects of synthetic cannabinoids on spleen and thymus. The organ weight was scaled and presented relatively to body weight. (a) JWH-210 administration reduced the organ weight of spleen, while (b) JWH-030 had no effects. (c) JWH-210 administration reduced the organ weight of thymus, while (d) JWH-030 had no effects. Data are shown as the means ± S.E. (n = 8). **p < 0.01 vs each vehicle-treated control group (Student’s t test)
JWH-210 reduced cell viability of splenocytes and thymocytes
We aimed to determine whether JWH-210, which has reduced organ weight, induced cytotoxicity. JWH-210 (10 mg/kg, i.p. 3 days) reduced cell viability in splenocytes (Fig. 3a, b), while JWH-030 (10 mg/kg, i.p. 3 days) had no effects on cell viability (Supplementary Fig. 1). In addition, JWH-210 (1, 10, and 100 μM) or JWH-030 (10 and 100 μM) treatments induced cytotoxicity in splenocytes and thymocytes (Fig. 3c, d). These results suggest that JWH-210 induced cytotoxicity of lymphoid organ.
Effects of JWH-210 on cell viability of splenocytes and thymocytes. (a) Representative photographs show live (green) and dead (cell) cell images of splenocytes isolated from JWH-210 treated mice (10 mg/kg, 3 days, i.p.) and control mice. (b) JWH-210 administration reduced the cell viability of splenocytes. Data are shown as the means ± S.E. (n = 4). **p < 0.01 vs each vehicle-treated control group (Student’s t test). Cell viability was measured after treatment of JWH-210 (c) in splenocytes and JWH-030 (d) in thymocytes isolated from naïve mice at indicated concentrations. JWH-210 and JWH-030 reduced cell viability of splenocytes and thymocytes. Data are shown as the means ± S.E. (n = 8). *p < 0.05, **p < 0.01 vs each vehicle-treated control group (one-way ANOVA, Bonferroni’s)
JWH-210 reduced the expression of T-cell activators in mice spleen
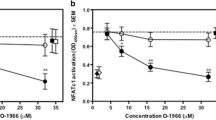
To investigate the effects of JWH-210 on immune system activator levels, mouse T-cell and B-cell activation PCR array was performed. The gene profiling results showed that 25 genes out of 85 genes were downregulated, and no gene was upregulated in the splenocytes of JWH-210-treated mice (Table 1 and Supplementary Fig. 2). Most of the downregulated genes were related with T-cell activation. Among those genes, qPCR assay demonstrated that CD3 antigen epsilon polypeptide (Cd3ε), CD3 antigen gamma polypeptide (Cd3γ), Cd74 antigen (invariant polypeptide of major histocompatibility complex, class II antigen-associated) p31, and Cd74 p41 genes were downregulated significantly in the splenocyte of JWH-210-treated mice (Fig. 4). These results suggest that JWH-210 may affect the immune system via the regulation of T-cell activator levels.
JWH-210 reduced the expression of T-cell marker in spleen
To confirm the expression of T-cell marker (CD8 and CD3), we used immunohistochemistry. The results showed that JWH-210-treated mice decreased the expression of these markers in comparison with the control (Fig. 5a). We also measured CD3/CD4- and CD3/CD8-positive cells in mice splenocytes. Both cells were decreased by treatment of JWH-210 in mice splenocytes compared with the control (Fig. 5b–e). These results suggest that JWH-210 may have an effect on the immune system via the regulation of T-cell in spleen.
JWH-210 reduced the mRNA level of cytokines in spleen
To clarify the effects of JWH-210 on the cytokine levels and T-cell marker levels in spleen, qPCR assay was performed. As Fig. 6a–c showed, JWH-210 significantly reduced IL-3, IL-5, and IL-6 mRNA levels. Otherwise, decreasing trends in TNF-α, TNF-β, IL-1α, and IL-10 mRNA levels were observed but not significantly (Fig. 6d–g). These results suggest that JWH-210 may have an effect on the immune system via the regulation of levels of cytokine associated with T-cell.
Effects of JWH-210 on mRNA levels of cytokines. JWH-210 reduced mRNA levels of (a) IL-3, (b) IL-5, and (c) IL-6 in splenocytes. On the other hand, mRNA levels of (d) TNFα, (e) TNFβ, (f) IL-1α, and (g) IL-10 were not changed significantly. Data are shown as the means ± S.E. (n = 6). *p < 0.05, **p < 0.01 vs vehicle-treated control group (Student’s t test)
AM630 rescued JWH-210-induced decrement in cell viability of splenocytes
In order to examine the role of CB1 and CB2 receptors in the decrement of cell viability, JWH-210 (10 μM) was treated in the presence of various concentrations of CB1 and CB2 receptor antagonists (0.1, 1, and 10 μM) for 4 h simultaneously. As Fig. 7 showed, the JWH-210-induced decrement of cell viability was rescued by AM630 (CB2 antagonist) co-treatment at 10 μM, while rimonabant (CB1 receptor antagonist) had no effects. These results suggested that CB2 receptors may play a role in the decrement of cell viability induced by JWH-210.
Effects of cannabinoid receptor antagonists on the JWH-210-induced decrement of cell viability in splenocytes. Cell viability was measured after treatment of JWH-210 (10 μM) and antagonists in splenocytes isolated from naïve mice at indicated concentrations. Data are shown as the means ± S.E. (n = 12). **p < 0.01 and #p < 0.05 vs. vehicle-treated control and JWH-210-treated groups, respectively (one-way ANOVA, Bonferroni’s)
Discussion
Synthetic cannabinoids are one of most abused novel psychoactive substances. A little information of toxicity and pharmacological activity of synthetic cannabinoids may mislead people to abuse substances without concerning health risks. We have previously reported that JWH series induced conditioned place preferences in parallel with CB1 receptor binding affinity (Cha et al. 2014). However, cannabinoids increase susceptibility to various infectious agents in human and animal (Friedman et al. 2003), while the effects of synthetic cannabinoids on the immune system are not fully elucidated yet. In the present study, we demonstrated that JWH-210 has severe harmful effects on the immune system. JWH-210 administration (10 mg/kg, 3 days, i.p) resulted in decrease of weight of spleen and thymus, whereas a single acute injection (1 and 10 mg/kg, i.p.) and chronic injection (0.1 mg/kg, 5 days, i.p.) did not (Supplementary Fig. 3). JWH-030 administration had no effects on lymphoid organ weights. In addition, JWH-210 reduced cell viability in vivo and ex vivo. Cell viability of primary cultured splenocytes from JWH-210-treated mice was reduced compared to the control group, and JWH-210 treatment also decreased cell viability of primary cultured splenocytes and thymocytes from naive mice. However, the cytotoxicity was not observed in primary cultured splenocytes from JWH-030-treated mice. These differences may be associated with the binding affinity of JWH-210 (Ki; 0.69 nM) and JWH-030 (Ki; 320 nM) to CB2 receptors, which mediate immunotoxicity (Showalter et al. 1996; Huffman et al. 2005; Rieder et al. 2010). JWH-210 has effects on cell viability at 1 μM in the present study, and JWH-210 could be detected in the abusers’ serum/plasma sample up to the concentration of about 0.5 μM (Karinen et al. 2015); therefore, the toxicity of JWH-210 on the immune system was needed to be further evaluated. Immune cells express high levels of CB2 receptors mediating cannabinoid anti-inflammatory effects, immunomodulation, and immunosuppression (McKallip et al. 2002a, b; Yao and Mackie 2009; Rieder et al. 2010). We further studied the role of the cannabinoid receptors in JHW-210-induced cytotoxicity. AM630, a CB2 receptor antagonist rescued JWH-210-induced decrement in cell viability of splenocytes; however, rimonabant, a CB1 antagonist, did not. These results suggest that CB2 receptor may play a role in the synthetic cannabinoid-induced harmful effects on the immune system. In addition, we demonstrated that JWH-210 reduced the mRNA expression levels of T-cell activators, such as, Cd3ε, Cd3γ, Cd74 p41, and Cd74 p31. Cd3ε and Cd3γ form the T-cell receptor-CD3 complex and play an essential role in T-cell development as well as immune response (Gagnon et al. 2012; Brazin et al. 2014). Cd74 is a nonpolymorphic type II integral membrane protein and is thought to function mainly as an MHC class II chaperone and generates two different isoforms, p31 and p41 (Starlets et al. 2006). Furthermore, the mRNA expression levels of cytokines including IL-3, IL-5, and IL-6 were reduced in the splenocytes of JWH-210 treated mice. However, there were no effects on the expression levels of TNFα, TNFβ, IL-1α, and IL-10. The qRT-PCR can determine accurately immune cell density and cytokine gene profile (Vremec et al. 2000; Mocellin et al. 2003; Tanaka et al. 2004). It has been reported that cannabinoids suppress T-cell proliferation and cytokine production in mice spleen cells (Robinson et al. 2013, 2015). These results suggest that JWH-210 may suppress T-cell density and cytokine production through the regulation of expression levels of T-cell activators.
In conclusion, our present study indicates that synthetic cannabinoids have harmful effects on the immune system. JWH-210 induced significant reductions in lymphoid organ weights, cell viability, and T-cell activator levels. Furthermore, JWH-210 inhibited the production of cytokines, which may be associated with immunosuppressive effects in drug abusers.
References
Brazin KN, Mallis RJ, Li C, Keskin DB, Arthanari H, Gao Y, Wu SL, Karger BL, Wagner G, Reinherz EL (2014) Constitutively oxidized CXXC motifs within the CD3 heterodimeric ectodomains of the T cell receptor complex enforce the conformation of juxtaposed segments. J Biol Chem 289:18880–18892
Cha HJ, Lee KW, Song MJ, Hyeon YJ, Hwang JY, Jang CG, Ahn JI, Jeon SH, Kim HU, Kim YH, Seong WK, Kang H, Yoo HS, Jeong HS (2014) Dependence potential of the synthetic cannabinoids JWH-073, JWH-081, and JWH-210: in vivo and in vitro approaches. Biomol Ther (Seoul) 22:363–369
Fattore L, Fratta W (2011) Beyond THC: the new generation of cannabinoid designer drugs. Front Behav Neurosci 5:60
Friedman H, Newton C, Klein TW (2003) Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev 16:209–219
Gagnon E, Schubert DA, Gordo S, Chu HH, Wucherpfennig KW (2012) Local changes in lipid environment of TCR microclusters regulate membrane binding by the CD3epsilon cytoplasmic domain. J Exp Med 209:2423–2439
Griffin G, Atkinson PJ, Showalter VM, Martin BR, Abood ME (1998) Evaluation of cannabinoid receptor agonists and antagonists using the guanosine-5′-O-(3-[35S]thio)-triphosphate binding assay in rat cerebellar membranes. J Pharmacol Exp Ther 285:553–560
Hermanns-Clausen M, Kneisel S, Szabo B, Auwarter V (2013) Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction 108:534–544
Hohmann N, Mikus G, Czock D (2014) Effects and risks associated with novel psychoactive substances: mislabeling and sale as bath salts, spice, and research chemicals. Dtsch Arztebl Int 111:139–147
Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, Cassidy MP, Wiley JL, Martin BR (2005) 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett 15:4110–4113
Institute of Laboratory Animal Resources (U.S.) (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C., pp xii–125
Karinen R, Tuv SS, Oiestad EL, Vindenes V (2015) Concentrations of APINACA, 5F-APINACA, UR-144 and its degradant product in blood samples from six impaired drivers compared to previous reported concentrations of other synthetic cannabinoids. Forensic Sci Int 246:98–103
McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu S, Grant S, Nagarkatti PS, Nagarkatti M (2002a) Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood 100:627–634
McKallip RJ, Lombard C, Martin BR, Nagarkatti M, Nagarkatti PS (2002b) Delta(9)-tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J Pharmacol Exp Ther 302:451–465
Mocellin S, Provenzano M, Rossi CR, Pilati P, Nitti D, Lise M (2003) Use of quantitative real-time PCR to determine immune cell density and cytokine gene profile in the tumor microenvironment. J Immunol Methods 280:1–11
Rieder SA, Chauhan A, Singh U, Nagarkatti M, Nagarkatti P (2010) Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 215:598–605
Robinson RH, Meissler JJ, Breslow-Deckman JM, Gaughan J, Adler MW, Eisenstein TK (2013) Cannabinoids inhibit T-cells via cannabinoid receptor 2 in an in vitro assay for graft rejection, the mixed lymphocyte reaction. J Neuroimmune Pharmacol 8:1239–1250
Robinson RH, Meissler JJ, Fan X, Yu D, Adler MW, Eisenstein TK (2015) A CB2-selective cannabinoid suppresses T-cell activities and increases Tregs and IL-10. J Neuroimmune Pharmacol 10:318–332
Seely KA, Prather PL, James LP, Moran JH (2011) Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol Interv 11:36–51
Showalter VM, Compton DR, Martin BR, Abood ME (1996) Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther 278:989–999
Starlets D, Gore Y, Binsky I, Haran M, Harpaz N, Shvidel L, Becker-Herman S, Berrebi A, Shachar I (2006) Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood 107:4807–4816
Tanaka Y, Koido S, Xia J, Ohana M, Liu C, Cote GM, Sawyer DB, Calderwood S, Gong J (2004) Development of antigen-specific CD8+ CTL in MHC class I-deficient mice through CD4 to CD8 conversion. J Immunol 172:7848–7858
Vremec D, Pooley J, Hochrein H, Wu L, Shortman K (2000) CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol 164:2978–2986
Yao B, Mackie K (2009) Endocannabinoid receptor pharmacology. Curr Top Behav Neurosci 1:37–63
Acknowledgments
This study was funded by the Ministry of Food and Drug Safety, Republic of Korea (grant number 15181MFDS482).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All experimental procedures were approved by the Animal Ethics Committee, National Institute of Food and Drug Safety Evaluation and complied with the Guide for the Care and Use of Laboratory Animals (National Research Council (NRC) 1996).
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gu, S.M., Lee, H.J., Lee, Th. et al. A synthetic cannabinoid JWH-210 reduces lymphoid organ weights and T-cell activator levels in mice via CB2 receptors. Naunyn-Schmiedeberg's Arch Pharmacol 390, 1201–1209 (2017). https://doi.org/10.1007/s00210-017-1418-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-017-1418-8