Abstract
Despite extensive studies, there is no effective treatment currently available other than pirfenidone for idiopathic pulmonary fibrosis. A protective effect of pantothenic acid and its derivatives on cell damage produced by oxygen radicals has been reported, but it has not been tested in bleomycin (BLM)--induced pulmonary fibrosis in rats. Therefore, we aimed to investigate the preventive effect of dexpanthenol (Dxp) on pulmonary fibrosis. Thirty-two rats were assigned to four groups as follows: (1) control group, (2) dexpanthenol (Dxp) group; 500 mg/kg Dxp continued intraperitoneally for 14 days, (3) bleomycin (BLM) group; a single intratracheal injection of BLM (2.5 mg/kg body weight in 0.25-ml phosphate buffered saline), and (4) BLM + Dxp-treated group; 500 mg/kg Dxp was administered 1 h before the intratracheal BLM injection and continued for 14 days i.p. The histopathological grades of lung inflammation and collagen deposition, tissue levels of malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and myeloperoxidase (MPO) were measured. BLM provoked inflammation and collagen deposition (p < 0.0001), with a marked increase in myeloperoxidase (MPO) activity resembling increased inflammatory activity (p < 0.0001), which was prevented by Dxp (p < 0.0001, p = 0.02). BLM reduced tissue activities of SOD, GPx, and CAT compared to controls (p = 0.01, 0.03, 0.009). MDA was increased with BLM (p = 0.003). SOD (p = 0.001) and MDA (p = 0.016) levels were improved in group 4. The CAT levels in the BLM + Dxp group were close to those in the control group (p > 0.05). We showed that Dxp significantly prevents BLM-induced lung fibrosis in rats. Further studies are required to evaluate the role of Dxp in the treatment of lung fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic pulmonary fibrosis (IPF) is defined as a specific form of chronic progressive lung disease of unknown cause associated with inflammation, oxidative stress, and accumulation of fibroblasts/myofibroblasts, leading to abnormal deposition of extracellular collagen, particularly in the early stage of the disease (Raghu et al. 2011; Gross and Hunninghake 2001). Unfortunately, IPF exhibits a poor prognosis and despite intensive investigations, there is no effective treatment other than pirfenidone, which is available in Europe, Japan, and Canada, for this disorder. In the absence of lung transplantation, the 5-year mortality rate is 80 %. Therefore, novel therapeutic agents with improved efficacy are needed (Cottin 2013).
Dexpanthenol (d-panthenol; (+)-2,4-dihydroxy-N-(3-hydroxypropyl)-3,3 dimethylbutyramide; Dxp) is an alcoholic analog of pantothenic acid (PA), and is oxidized to PA inside the peripheral tissues (Ebner et al. 2002). It is well established that PA and its derivatives increase the levels of reduced glutathione (GSH), co-enzyme A (Co A) (especially mitochondrial Co A), and adenosine-5′-triphosphate (ATP) synthesis within the cell (Slyshenkov et al. 2001, 2004). All of these play a major role in cellular defense and in the repair systems against oxidative stress and inflammatory response (Slyshenkov et al. 1995).
Bleomycin (BLM)-induced pulmonary fibrosis is widely used as an experimental model of human IPF, as intratracheal BLM administration causes alveolar cell damage, an inflammatory response, fibroblast proliferation, and collagen content deposition in rats (Ozyurt et al. 2004; Yildirim et al. 2004; Sogut et al. 2004).
Activated inflammatory cells accumulating in the lungs are known to release reactive oxygen species (ROS), leading to parenchymal damage, as well as proteolytic enzymes, further increasing the burden of injury (Ozyurt et al. 2004). Studies that tested several antioxidant substances, such as N-acetylcysteine (Mata et al. 2003), erdosteine (Sogut et al. 2004), caffeic acid phenethyl ester (Ozyurt et al. 2004), melatonin (Yildirim et al. 2006), ginkgo biloba (Iraz et al. 2006), cordyceps (Mengli et al. 2012), and resveratrol (Akgedik et al. 2012) as a prophylactic approach in BLM-induced lung fibrosis in rats, found that these substances usually reduced or prevented lung fibrosis according to Ashcroft's criteria and lung hydroxyproline content.
Therefore, antioxidant strategies may be a logical approach to the prevention or treatment of BLM-induced pulmonary fibrosis. Using this model, we investigated the preventive effect of Dxp on IPF. We evaluated histological and biochemical findings of lung fibrosis induced by BLM exposure, and measured oxidative stress and inflammatory markers in a rat model with damaged lungs.
Materials and methods
Study design
This study was approved by the Animal ethics Committee (Reference No.: 2012/A-59) and conducted in accordance with the “Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (NIH publication No. 5377–3, 1996), Animal ethics Committee.” This animal experimental study was design to ARRIVE guidelines (Colak and Parlakpinar 2012).
Animals and groups
For this study, a total of 32 female Wistar Albino rats of 10–12 weeks of age and weighing 250–300 g were obtained from Inonu University Laboratory Animals Research Center and placed in a room with controlled temperature (21 ± 2 °C) and humidity (60 ± 5 %), where a 12:12h light/dark cycle was maintained. The rats were equally and randomly assigned to four groups and treated as follows: (1) control group, (2) dexpanthenol (Dxp) group; 500 mg/kg Dxp (bepanthene ampule®, 500 mg, Bayer Corp., Istanbul, Turkey) was continued intraperitoneally (i.p.) for 14 days, (3) bleomycin (BLM) group, and (4) BLM + Dxp-treated group; 500 mg/kg Dxp was administered 1 h before the intratracheal BLM injection and continued for 14 days i.p.
BLM-induced lung fibrosis
The rats were weighed and then anesthetized with ketamine (75 mg/kg) and xylazine (5 mg/kg) i.p., followed by a single intratracheal injection of BLM hydrochloride (2.5 mg/kg body weight in 0.25-ml phosphate buffered saline, Nippon Kayaku, Japan). The control group received the same amount of intratracheal saline by the same route. All rats were sacrificed after 14 days of BLM injection. After sacrificing the animals by an overdose of the anesthesia, the lung tissue specimens were rapidly and meticulously harvested for biochemical and histopathological analysis. The right section of the lung was placed in liquid nitrogen and stored at −70 °C until the assay for thiobarbituric acid-reactive substances (TBARS)—a lipid peroxidation product, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and myeloperoxidase (MPO) contents. The left part of the lung was placed in formaldehyde solution for routine histopathological examination by light microscopy.
The dosages of BLM and Dxp were chosen according to previous dose–response studies, which reported the doses causing lung fibrosis and marked antioxidative effects in rats, respectively (Aytemur et al. 2012; Ceylan et al. 2011; Altintas et al. 2012).
Biochemical analyzes
Two hundred milligrams of frozen lung tissue specimen was dissected into pieces on dry ice, homogenized in 1.15 % KCl buffer (1:9, w/v) using a manual glass homogenizer for approximately 5 min and flushed with centrifugation for approximately 10 s to remove large debris. The supernatant was used for analysis.
Determination of tissue TBARS representing MDA content
The TBARS contents of homogenates were determined spectrophotometrically using thiobarbituric acid reaction (Uchiyama and Mihara 1978). Three milliliters of 1 % phosphoric acid and 1 ml 0.6 % thiobarbituric acid solution were added to 0.5 ml of plasma pipetted into a tube. The mixture was heated in boiling water for 45 min. After the mixture had cooled, the color was extracted into 4 ml of n-butanol. The absorbance was measured by a spectrophotometer (UV-1601; Shimadzu, Kyoto, Japan) at 532 nm. The amount of lipid peroxides was calculated as per TBARS of lipid peroxidation. The results were expressed in nanomoles per gram tissue according to a standard graph, which was prepared using the measurements of standard solutions (1,1,3,3-tetramethoxypropane).
Determination of superoxide dismutase
Total (Cu–Zn and Mn) SOD (EC 1.15.1.1) activity was determined based on the method of Sun et al. (Sun et al. 1988). The principle of this method is the inhibition of nitroblue tetrazolium (NBT) reduction by the xanthine–xanthine oxidase system as a superoxide (O2•−) generator. One unit of SOD was defined as the enzyme amount causing 50 % inhibition in the NBT reduction rate. SOD activity was expressed as units per milligram protein.
Determination of glutathione peroxidase
Determination of GPx activity (EC 1.6.4.2) was measured by the method of Paglia and Valentine (Paglia and Valentine 1967). An enzymatic reaction was initiated in a tube containing NADPH, reduced GSH, sodium azide, and glutathione reductase by adding H2O2, and the change in absorbance at 340 nm was monitored by a spectrophotometer. Activity was given in units per gram protein.
Determination of MPO activity
MPO (EC 1.11.1.7) activity was determined by using a 4-aminoantipyrine/phenol solution as the substrate for MPO-mediated oxidation by H2O2, and the change in absorbance at 510 nm was recorded (Wei and Frenkel 1993). One unit of MPO activity was defined as the amount causing degradation of 1 μmol H2O2/min at 25 °C. The results were given in micro units per gram protein.
Determination of catalase
Catalase (CAT, EC 1.11.1.6) activity was determined according to Aebi's method (Aebi 1974). The principle of the assay is based on the determination of the rate constant (k, s−1) or the H2O2 decomposition rate at 240 nm. Results are expressed in kilos per gram protein.
Histological evaluation of lung injury
Formalin-fixed (10 % formalin) lung tissues were embedded in paraffin. Sections 5 μm in thickness were dissected and stained with hematoxylin and eosin (H-E) for inflammation, with Van Gieson (V-G) to identify collagen deposition and with periodic acid Schiff (PAS) to identify alveolar macrophages. The histological grades of lung inflammation and collagen deposition were assessed with ×20 magnification in 10 microscopic fields for each specimen. The histological score was determined by pathologists unaware of the status of animals, according to the following criteria: 0, no lung abnormalities; 1, presence of inflammation and collagen deposition involving <25 % of the lung parenchyma; 2, lesions involving 25–50 % of the lung; and 3, lesions involving >50 % of the lung (Fukumoto et al. 2010). Quantification of the number of alveolar macrophages in tissue samples was performed by the point-counting technique with a high-power objective (×40) using Leica QWin Image Analysis System (Leica Micro Imaging Solutions, Cambridge, UK).
Statistics
In order to detect all effects including minor ones, the required sample sizes used in this experiment were identified using a statistical power analysis. The sample sizes required for a power of 0.80 were estimated using NCSS software. Data were analyzed using the SPSS software program for Windows, version 18.0 (SPSS, Inc., Chicago, IL). The normality of the distribution was confirmed using the Kolmogorov–Smirnov test. According to the results obtained from the normality test, one-way analysis of variance (ANOVA), and the Kruskal–Wallis H test were used for the statistical analysis, as appropriate. Multiple comparisons were carried out by Tamhane's test (for non-homogeneous variances) after the ANOVA test. The results are expressed as mean ± standard deviation (SD) for TBARS, SOD, GPx, and MPO. After a significant Kruskal–Wallis H test, a Conover test was also performed for CAT and histopathological results. P values less than 0.05 were regarded as statistically significant. The values are given as median (min–max).
Results
Changes in histology
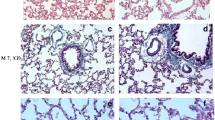
The control group showed normal histological lung structure except slight inflammation, likely due to local trauma. No sign of collagen deposition was observed and the appearance of macrophages was normal in the lung of sham rats (Fig. 1a, c, and e). The findings from the Dxp group were similar to those from the control group (Fig. 1b, d, and f). However, the lung sections of the BLM group exhibited major histological alterations such as dense inflammation (Fig. 2a) and collagen deposition (Fig. 2c). In addition, the PAS staining method demonstrated an increased number of alveolar macrophages in the intra-alveolar space in this group (Fig. 2e). BLM administration caused a markedly intense leukocyte migration, not only in peribronchial and perivascular areas, but also in the interstitial and intrabronchial spaces. Migrated leukocytes consisted of neutrophils, eosinophils and lymphocytes (Fig. 3a, b, and c). BLM-induced fibrosis was characterized by a dramatic increase in collagen fibers. The bundles of collagen were distinguished by their red color with Van Gieson staining methods. On the other hand, the severity of lung inflammation and collagen deposition was reduced in the rats treated with both BLM and Dxp compared to the rats treated with BLM alone (Fig. 2b and d). The degree of inflammation and collagen deposition was significantly decreased in the BLM + Dxp group compared to the BLM group (p < 0.0001). Inflammatory cells were observed only in the interalveolar septum and the peribronchial area. Moreover, there was a decreased accumulation of macrophages in the intra-alveolar space (Fig. 2f). The difference in the number of macrophages in the BLM and BLM + Dxp groups was statistically significant (p < 0.0001).
Histopathological findings in the control and Dxp groups. The normal histological lung, (a and b) (×10). The appearance of collagen fibers around the arteriole (major arrow) and capillary (small arrows) (red color), (c and d) (×20). Note the alveolar macrophages stained positive (pink) in the intraalveolar space (arrows), (e and f) (×40). H-E hematoxylin and eosin, V-G Van Gieson to identify collagen deposition, PAS: periodic acid-Schiff
Histopathological findings in the BLM and BLM + Dxp groups. a Intense leukocyte accumulation is evident in the bronchial lumens (stars), and the peribronchial and perivascular areas. b Leukocyte accumulation is observed only in the interalveolar septum (×10). c Dense bundles of collagen are recognized as red color (arrows). d The clear reduction of dense collagen fibers (×20). e Note the increased number of PAS positive alveolar macrophages observed in the intraalveolar area. f The number of alveolar macrophages is significantly decreased compared to the BLM group (×40). H-E hematoxylin and eosin, V-G Van Gieson to identify collagen deposition, PAS periodic acid-Schiff
The results of the severity of inflammation, collagen deposition, and the number of alveolar macrophages are shown Tables 1, 2, and 3, respectively.
Changes in oxidative stress parameters
SOD
The lung tissue SOD levels were decreased in the rats treated with BLM compared to the controls (0.15 ± 0.02 vs. 0.24 ± 0.07 nmol/g, p = 0.01), and there was a greater decrease in tissue SOD levels of the Dxp + BLM group (0.27 ± 0.07 nmol/g, p = 0.001) (Table 4).
GPx
The lung tissue GPx levels were decreased in rats treated with BLM compared to the controls (6.6 ± 1.8 vs. 9.6 ± 2.3 U/mg, p = 0.03). There was a slight but insignificant change after 14 days of Dxp treatment (7.0 ± 1.6, p > 0.05).
Catalase
The level of CAT activity in lung tissue was reduced by the exposure to BLM, and the reduction was significant compared to the controls (1.12 k/g [0.47–2.04] vs. 2.1 k/g [1.68–3.27], p = 0.009). Although not significant, there was an increase in the reduced CAT activity in the Dxp + BLM group compared to the group treated with BLM alone (1.75 k/g [1.05–2.33], p > 0.05).
Malondialdehyde (MDA)content
The MDA levels reflecting lipid peroxidation end-products were increased by the exposure to BLM (60.1 ± 12 vs. 37.8 ± 11 nmol/g, p = 0.003). Increased tissue MDA levels were significantly reduced in the Dxp + BLM group compared to the group treated with BLM alone (41.4 ± 14.5 nmol/g, p = 0.016).
Changes in MPO activity
MPO indicating the polymorphonuclear leucocyte (PMN) activity was increased by BLM exposure compared to the controls (66.9 ± 21.3 vs. 27.6 ± 10.1 mU/g, p < 0.0001). MPO activity was reduced in the Dxp + BLM group compared to BLM group (44.3 ± 15.6 mU/g, p = 0.02).
Discussion
Currently, pulmonary fibrosis is considered to result from repeated epithelial injury and apoptosis followed by inadequate re-epithelialization and aberrant wound healing (Coward et al. 2010; Harari and Caminati 2010). There are certain difficulties in disease modeling of IPF, including the difficulty of reproducing the chronic nature of the disease in animal models and no clear understanding of the injury that triggers fibrosis. BLM is the most commonly used agent and is often regarded as the standard in modeling pulmonary fibrosis. It is thought to induce lung damage by (1) causing direct DNA strand breakage, and by (2) generating free radicals and therefore inducing oxidative stress (Iraz et al. 2006; Inghilleri et al. 2006; Demerdash 2011). The most commonly employed reference model uses a single intratracheal dose of the drug during the first 7 days to induce an inflammatory response (Chaudhary et al. 2006) and increase the epithelial apoptosis (Mungunsukh et al. 2010), closely mimicking acute lung injury. Subsequently, the inflammation resolves and fibrosis is detected during the following transitional period of 3 days. The fibrotic stage persists for 3–4 weeks following BLM administration and is characterized by excessive deposition of extracellular matrix, resulting in fibrotic tissue (Mouratis and Aidinis 2011). In the present study, BLM caused significant lung injury demonstrated with histopathological findings such as dense inflammation characterized by alveolar macrophage accumulation, intense leukocyte migration, not only in peribronchial and perivascular areas but also in the interstitial and intrabronchial spaces, and remarkable collagen fiber deposition. Additionally, we showed prominent MPO activity in lung tissues of BLM-exposed rats, indicating increased PMN activity. These findings confirm the induction of lung injury, consistent with other studies that utilized BLM-induced lung fibrosis models.
One of the widely accepted mechanisms for BLM-induced lung destruction and tissue remodeling is its ability to generate ROS. BLM is known to form a complex by binding to DNA/Fe2+ (Caspary et al. 1982; El-Khouly et al. 2012). The DNA/Fe2+/BLM complex undergoes redox cycling and generates ROS such as superoxide and hydroxyl radicals. In the present study, we evaluated the levels of oxidative stress markers. In addition to the BLM-induced inflammation and collagen deposition, we found that BLM also induced a significant increase in lipid peroxide levels, accompanied by significantly decreased levels of the antioxidant enzymes GPx, CAT, and SOD compared to the control group. These results supported the previous studies evaluating the role of oxidative stress in BLM-induced pulmonary fibrosis (Coward et al. 2010; Harari and Caminati 2010; Demerdash 2011; Mouratis and Aidinis 2011).
Dxp is oxidized to PA within the tissues. A protective effect of PA and its derivatives against cell damage produced by oxygen free radicals has been reported for over a decade (Ebner et al. 2002; Slyshenkov et al. 1999, 2001; Wojtczak and Slyshenkov 2003). It is well established that PA and its derivatives increase the levels of reduced GSH and Co A, as well as ATP synthesis within the cell (Slyshenkov et al. 2001, 2004). All of these play a major role in cellular defense and in the repair systems against oxidative stress and inflammatory response (Slyshenkov et al. 1995, 1996).
In the present study, the histopathological degree of inflammation and collagen deposition induced by a single intratracheal bleomycin administration was significantly decreased by day 14 of the continued Dxp treatment. Moreover, we also observed the improving effect of Dxp on oxidative stress parameters such as CAT, MDA, and SOD. Although the tissue GPx activity was close to the control values, the increased GPx activity was not statistically significant in the Dxp + BLM group compared to the BLM group. The MDA content of rat lung tissues in the Dxp + BLM group was very close to that of the control group. Concurrent treatment with Dxp significantly counteracted the inflammatory effects of BLM treatment in these animals, or the antioxidant capabilities of Dxp resulted in an at least partial prophylactic effect. Most of the antioxidant agents tested for the treatment of BLM-induced lung fibrosis models have shown anti-inflammatory effects related to decreased oxidative stress (Sogut et al. 2004; Mata et al. 2003; Yildirim et al. 2006; Iraz et al. 2006; Mengli et al. 2012; Akgedik et al. 2012).
Previously, Altintas et al. studied Dxp on ischemia-reperfusion induced renal injury in a rat model (Altintas et al. 2012) and reported beneficial effects related to the reduction in oxidative stress. In another study, it was tested in testicular an ischemia-reperfusion model and was found to be helpful in reducing lipid peroxidation, which translates into protection against the sequences of oxidative stress that may amplify the inflammatory response (Etensel et al. 2007). Slyshenkov et al. tested pantothenol on the deleterious effects of gamma radiation and apoptosis, and reported positive results with reduced oxidative stress (Slyshenkov et al. 1998, 2001). To date, there has been no study performed to test Dxp in lung fibrosis.
Limitations
The BLM-induced lung fibrosis model fails to recapitulate several important characteristics of usual interstitial pneumonia, namely the lack of fibroblastic foci, hyperplastic epithelium, and temporal heterogeneity. In addition, an inflammatory, neutrophil-rich process is observed following the injury caused by a single dose of BLM, and this finding is more indicative of an acute lung injury model than a fibrosis model. However, despite these limitations, the intratracheal BLM model has undoubtedly been extremely important in clarifying critical aspects of pulmonary fibrosis, and remains an important tool for ongoing and future use in relevant studies (Mouratis and Aidinis 2011). Additionally, collagen deposition is typically measured by the hydroxyproline or sircol assays in most pulmonary fibrosis models. However, because of our limited facilities, the simplest collagen-identifying methods of Van Gieson's staining technique was used for displaying collagen fibers. It is well known that the bundles of collagen are distinguished by their red color with Van Gieson staining methods, as in our study. Another limitation is initiation of Dxp concomitantly with BLM, which indicates a protective study design rather than therapeutic effect.
In conclusion, the readily available and safe agent Dxp significantly prevents BLM-induced lung fibrosis in rats. Further studies are required to evaluate the role of Dxp in the treatment of lung fibrosis, which has no definitive treatment.
References
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic, New York, pp 673–677
Akgedik R, Akgedik S, Karamanli H, Uysal S, Bozkurt B, Ozol D, Armutcu F, Yildirim Z (2012) Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats. Inflammation 35(5):1732–1741
Altintas R, Parlakpinar H, Beytur A, Vardi N, Polat A, Sagir M, Odabas GP (2012) Protective effect of dexpanthenol on ischemia-reperfusion-induced renal injury in rats. Kidney Blood Press Res 36:220–230
Aytemur ZA, Hacievliyagil SS, Iraz M, Samdanci E, Ozerol E, Kuku I, Nurkabulov Z, Yildiz K (2012) Effects of iloprost on bleomycin-induced pulmonary fibrosis in rats compared with methyl-prednisolone. Rev Port Pneumol 18(6):272–277
Caspary WJ, Lanzo DA, Niziak C (1982) Effect of deoxyribonucleic acid on the production of reduced oxygen by bleomycin and iron. Biochemistry 21:334–338
Ceylan H, Yapici S, Tutar E, Ceylan NO, Tarakcioglu M, Demiryurek AT (2011) Protective effects of dexpanthenol and y-27632 on stricture formation in a rat model of caustic esophageal injury. J Surg Res 171:517–523
Chaudhary NI, Schnapp A, Park JE (2006) Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med 173:769–776
Colak C, Parlakpinar H (2012) Hayvan Deneyleri: In Vivo Denemelerin Bildirimi: ARRIVE Kılavuzu-Derleme. J Turgut Ozal Med Cent 19:128–131
Cottin V (2013) The role of pirfenidone in the treatment of idiopathic pulmonary fibrosis. Respir Res 14(1):5. doi:10.1186/1465-9921-14-S1-S5
Coward WR, Saini G, Jenkins G (2010) The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis 4:367–388
Demerdash EE (2011) Anti-inflammatory and antifibrotic effects of methyl palmitate. Toxicol Appl Pharmacol 254:238–244
Ebner F, Heller A, Rippke F, Tausch I (2002) Topical use of dexpanthenol in skin disorders. Am J Clin Dermatol 3:427–433
El-Khouly D, El-Bakly W, Awad AS, El-Mesallamy HO, El-Demerdash E (2012) Thymoquinone blocks lung injury and fibrosis by attenuating bleomycin-induced oxidative stress and activation of nuclear factor Kappa-B in rats. Toxicology 302:106–113
Etensel B, Ozkisacik S, Ozkara E, Karul A, Oztan O, Yazici M, Gursoy H (2007) Dexpanthenol attenuates lipid peroxidation and testicular damage at experimental ischemia and reperfusion injury. Pediatr Surg Int 23:177–181
Fukumoto J, Harada C, Kawaguchi T, Suetsugu S et al (2010) Amphiregulin attenuates bleomycin-induced pneumopathy in mice. Am J Physiol Lung Cell Mol Physiol 298(2):L131–L138
Gross TJ, Hunninghake GW (2001) Idiopathic pulmonary fibrosis. N Engl J Med 345:517–525
Harari S, Caminati A (2010) IPF: new insight on pathogenesis and treatment. Allergy 65:537–553
Inghilleri S, Morbini P, Oggionni T, Barni S, Fenoglio C (2006) In situ assessment of oxidant and nitrogenic stress in bleomycin pulmonary fibrosis. Histochem Cell Biol 125:661–669
Iraz M, Erdogan H, Kotuk M, Yagmurca M, Kilic T, Ermis H, Fadillioglu E, Yildirim Z (2006) Ginkgo biloba inhibits bleomycin-induced lung fibrosis in rats. Pharmacol Res 53:310–316
Mata M, Ruíz A, Cerdá M, Martinez-Losa M, Cortijo J, Santangelo F, Serrano-Mollar A, Llombart-Bosch A, Morcillo EJ (2003) Oral N-acetylcysteine reduces bleomycin-induced lung damage and mucin Muc5ac expression in rats. Eur Respir J 22(6):900–905
Mengli C, Cheung FWK, Ming Hung C, Pak Kwan H, Siu-Po I, Yick Hin L, Chun-Tao C, Wing Keung L (2012) Protective roles of Cordyceps on lung fibrosis in cellular and rat models. J ethnopharmacol 143(2):448–54
Mouratis MA, Aidinis V (2011) Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med 17:355–361
Mungunsukh O, Griffin AJ, Lee YH, Day RM (2010) Bleomycin induces the extrinsic apoptotic pathway in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 298:696–703
Ozyurt H, Sogut S, Yildirim Z et al (2004) Inhibitory effect of caffeic acid phenethyl ester (cape) on bleomycine-induced lung fibrosis in rats. Clin Chim Acta 339:65–75
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–170
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA et al (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183:788–824
Slyshenkov VS, Rakowska M, Moiseenok AG, Wojtczak L (1995) Pantothenic acid and its derivatives protect Ehrlich ascites tumor cells against lipid peroxidation. Free Radic Biol Med 19:767–772
Slyshenkov VS, Rakowska M, Wojtczak L (1996) Protective effect of pantothenic acid and related compounds against permeabilization of Ehrlich ascites tumour cells by digitonin. Acta Biochim Pol 43:407–410
Slyshenkov VS, Omelyanchik SN, Moiseenok AG, Trebukhina RV, Wojtczak L (1998) Pantothenol protects rats against some deleterious effects of gamma radiation. Free Radic Biol Med 24(6):894–899
Slyshenkov VS, Omelyanchik SN, Moiseenok AG, Petushok NE, Wojtczak L (1999) Protection by pantothenol and beta-carotene against liver damage produced by low-dose gamma radiation. Acta Biochim Pol 46:239–248
Slyshenkov VS, Piwocka K, Sikora E, Wojtczak L (2001) Pantothenic acid protects jurkat cells against ultraviolet light-induced apoptosis. Free Radic Biol Med 30:1303–1310
Slyshenkov VS, Dymkowska D, Wojtczak L (2004) Pantothenic acid and pantothenol increase biosynthesis of glutathione by boosting cell energetics. FEBS Lett 569:169–172
Sogut S, Ozyurt H, Armutcu F et al (2004) Erdosteine prevents bleomycin-induced pulmonary fibrosis in rats. Eur J Pharmacol 494:213–220
Sun Y, Oberley L, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by tiobarbituric acid test. Anal Biochem 34:271–278
Wei H, Frenkel K (1993) Relationship of oxidative events and DNA oxidation in Sencar mice to in vivo promoting activity of phorbol ester-type tumor promoters. Carcinogenesis 14:1195–1201
Wojtczak L, Slyshenkov VS (2003) Protection by pantothenic acid againts apoptosis and cell damage by oxygen free radicals-the role of glutathion. Biofactors 17:61–73
Yildirim Z, Turkoz Y, Kotuk M et al (2004) Effects of aminoguanidine and antioxidant erdosteine on bleomycin-induced lung fibrosis in rats. Nitric Oxide 11:156–165
Yildirim Z, Kotuk M, Erdogan H, Iraz M, Yagmurca M, Kuku I, Fadillioglu E (2006) Preventive effect of melatonin on bleomycin-induced lung fibrosis in rats. J Pineal Res 40:27–33
Conflict of interest
The authors have no conflict of interest to declare. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ermis, H., Parlakpinar, H., Gulbas, G. et al. Protective effect of dexpanthenol on bleomycin-induced pulmonary fibrosis in rats. Naunyn-Schmiedeberg's Arch Pharmacol 386, 1103–1110 (2013). https://doi.org/10.1007/s00210-013-0908-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-013-0908-6