Abstract
Recent studies have emphasized the contribution of neuroinflammation and oxido-nitrosative stress to neuropathic pain. Both, heme oxygenase (HO)-1 and carbon monoxide (CO) play an important role in regulating free radical generation and inflammation. Herein, we investigated the role of HO-1/CO pathway, by using hemin, a selective HO activator, and CO-releasing molecule (CORM)-2, a CO-releasing agent, in rat sciatic nerve chronic constriction injury (CCI)-induced neuropathic pain. CCI rats exhibited full development of behavioral hypersensitivity symptoms, including cold allodynia, mechanical and thermal hyperalgesia and also exhibit of a significant increase in spinal cord pro-inflammatory cytokines (TNF-α and IL-1β) and oxido-nitrosative stress markers, both in spinal cord and ipsilateral sciatic nerve homogenate. Spinal (10 and 30 μg/rat, intrathecal (i.t.)), but not systemic (5 and 10 mg/kg, subcutaneous (s.c.)), administration of hemin for 14 days significantly prevented the development of behavioral hypersensitivity. Further, simultaneous administration of hemin via spinal (10 μg/rat, i.t.) and systemic (5 mg/kg, s.c.) routes led to a more pronounced inhibition of the development of behavioral hypersensitivity. Further, administration of CORM-2 (1 and 5 mg/kg, s.c.), dose-dependently and most effectively, prevented the development of behavioral hypersensitivity. Both hemin and CORM-2 produced ameliorative beneficial effects that paralleled with the extent of reduction of oxido-nitrosative stress and pro-inflammatory cytokines. Also, hemin and CORM-2 significantly improved the levels of HO-1 and activity of anti-oxidant enzymes such as superoxide dismutase and catalase. Thus, it may be concluded that chronic pharmacological activation of HO-1/CO pathway may prevent the development of behavioral symptoms of neuropathic pain, through an activation of anti-inflammatory and anti-oxidant mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropathic pain (NP) is a chronic debilitating condition, initiated or caused by a primary lesion or dysfunction in the nervous system that is primarily characterized by stimulus-independent persistent pain or abnormal sensory perception of pain such as allodynia and hyperalgesia (Merskey and Bogduk 1994; Sandkuhler 2009). It is generally acknowledged that NP is extremely difficult to treat, and a major factor affecting outcomes is the presence of comorbidities such as poor sleep, depressed mood, and anxiety (McDermott et al. 2006; Agarwal et al. 2008). Despite many pharmacotherapeutic options, the treatment of neuropathic pain is often not fully effective and is unsatisfactory due to dose-limiting side effects (Davis 2007; Dray 2008). Thus, there is an unmet need to understand disease pathogenesis, identify and characterize novel targets, and develop newer agents which act at one or more sites in the pathogenesis of neuropathic pain.
Now, it has become clear that inflammatory and immune mechanisms in the peripheral and central nervous systems play an important role in the development and maintenance of peripheral neuropathic pain (Perkins and Tracey 2000; Moalem and Tracey 2006; Hu et al. 2007; Milligan and Watkins 2009; Vallejo et al. 2010). Also, accumulating evidence indicates that oxido-nitrosative stress plays an important role in the peripheral and central sensitization of NP (Guedes et al. 2006; Naik et al. 2006; Park et al. 2006; Gao et al. 2007). Furthermore, pharmacological inhibition of recruitment and/or activation of inflammatory cells and subsequent release of pro-inflammatory cytokines have been shown to ameliorate various behavioral hypersensitivity symptoms following nerve injury (Raghavendra et al. 2003; Mika et al. 2007; Padi and Kulkarni 2008). Heme oxygenase (HO)-1 is a rate-limiting enzyme that catalyzes the oxidative degradation of heme into biliverdin, carbon monoxide (CO), and iron (Maines 1997; Abraham and Kappas 2008). Wide variety of in vitro and in vivo studies has demonstrated that pharmacological upregulation of HO-1 provides a robust anti-inflammatory and anti-oxidant response (Benallaoua et al. 2007; Innamorato et al. 2009; Paine et al. 2010). Further, an upregulation of HO-1/CO pathway has been shown to mediate the anti-inflammatory effect of IL-10, an endogenous anti-inflammatory and anti-nociceptive cytokine (Lee and Chau 2002; Milligan et al. 2006). Importantly, nuclear factor-erythroid 2-related factor 2 (Nrf2), a Cap'n'Collar transcription factor, is primarily involved in the regulation of neuroinflammation via expression of HO-1 (Alam et al. 1999; Syapin 2008; Innamorato et al. 2009). Hemin has been reported to selectively induce the expression and activity of HO via increasing nuclear export Nrf2, which is secondary to increased degradation of endogenous transcription repressor of Nrf2, i.e., Bach1 (Zenke-Kawasaki et al. 2007). Systemic administration of hemin (1 and 3 mg/kg, i.p.) has been shown to inhibit the acetic acid-induced writhing response. Also, HO-1 has been shown to mediate the anti-nociceptive effect of etoricoxib, a selective COX-2 inhibitor (Grangeiro et al. 2011). Recently, we have reported that prophylactic administration of hemin (5 mg/kg) prevents the development of thermal and mechanical hyperalgesia associated with Fruend’s adjuvant-induced rheumatoid arthritis in rats (Kaur et al. 2011).
The peripheral upregulation of HO-1 via Nrf has been shown to mediate the analgesic effect of epibatidine in formalin test (Rosa et al. 2008; Egea et al. 2009). Moreover, pharmacological activation of peripheral HO-1/CO pathway has been implicated in the anti-hyperalgesic effect in carrageenan-induced inflammatory pain in rats (Steiner et al. 2001). Also, a synergistic interaction between the peripheral and spinal HO/CO pathways has been observed for providing anti-nociceptive effects in formalin-induced flinching responses in rats (Nascimento and Branco 2009). The endogenous CO is mostly generated by the action of HO-1 in the human body (Maines 1997). The potential beneficial effects of exogenous application of CO may be limited due to its high affinity for hemoglobin and subsequent formation of harmful levels of toxic carboxyhemoglobin (Motterlini and Otterbein 2010). CO-releasing molecule (CORM)-2 (tricarbonyldichlororuthenium II dimer) is reported to release carbon monoxide in a controlled manner (Motterlini et al. 2002). Hence, the present study was designed to elucidate the role of HO-1/CO pathway, by using hemin, a selective HO activator, and CORM-2, a CO-releasing agent, in rat chronic constriction sciatic nerve injury (CCI)-induced neuropathic pain.
Materials and methods
Animals
Wistar rats (180–250 g) of either sex were used. Animals were housed under standard conditions of light and dark cycle in the central animal house of I.S.F College of Pharmacy, Moga, India, with food and water ad libitum. Animals were acclimatized to laboratory conditions before the behavioral tests. All experiments were carried between 0900 and 1400 hours. The experimental protocol was approved by the Institutional Animal Ethics Committee (approval no. ISF/IAEC/CPCSEA/33/2011; dated 05.02.2011) and were carried out in accordance with the guidelines for Control and Supervision of Experimentation on Animals, Government of India.
Development of chronic constriction injury of sciatic nerve
The unilateral mononeuropathy was produced according to the method described by Bennett and Xie (1988). Briefly, the Wistar rats were anesthetized with thiopental sodium (30 mg/k i.p.) and the common sciatic nerve of the left hind paw was exposed at the level of the middle of the thigh by blunt dissection through the biceps femoris muscle. Proximal to the sciatic trifurcation, approximately 7-mm of nerve was freed and four ligatures of 3–0 silk thread were tied loosely around the sciatic nerve at 1-mm intervals.
Intrathecal catheterization
Intrathecal catheters were implanted as described by Yaksh and Rudy (1976). In brief, rats were anesthetized with ketamine and xylazine, and polyethylene catheters (PE-10) were inserted via an incision in the atlantooccipital membrane and advanced 7.5 cm caudally to the level of the lumbar enlargement and secured to the musculature at the incision site. Only rats with no evidence of neurological deficit after catheter insertion were used in the study.
Measurement of thermal hyperalgesia
The response to noxious thermal stimulus was determined using a plantar test Apparatus (Model 37370; Ugo Basile, Comerio, Italy) (Hargreaves et al. 1988; Kaur et al. 2011). Briefly, Wistar rats were accustomed to the device; a movable infrared radiant heat source was placed directly under the plantar surface on the hind paw, and the time taken for hind-paw withdrawal latency was noted. A cutoff time of 20 s was used in all experiments.
Assessment of mechanical allodynia (Automatic von Frey test)
For each animal, threshold for touch sensitivity was measured in both hind paws using an automated apparatus for applying reproducible light touch using a dynamic plantar esthesiometer, which is an automated von Frey-type system (Model 37450; Ugo Basile, Comerio, Italy) as described earlier (Meregalli et al. 2010; Kaur et al. 2011). In this, Wistar rats were placed individually on an elevated wire mesh floor in a Plexiglas chamber and a metal filament was applied to the central region on the plantar surface until the animal moves its paw or until the point at which greatest present force is met. The maximum value of force in grams (50 g) was previously fixed.
Assessment of cold allodynia
Cold allodynia was evaluated as the paw withdrawal latency (PWL) to thermal, non-noxious stimulus of hind paws by dipping in water maintained at 10 ± 0.5 °C (Padi and Kulkarni 2004). The PWLs were taken twice, 5 min apart, and averaged. A cutoff time of 15 s was imposed.
Measurement of motor behavior (Locomotor activity)
The locomotor activity was monitored by using photoactometer (IMCORP, India) and was expressed in terms of total photobeam interruption counts for 5 min (Padi and Kulkarni 2008).
Collection of sciatic nerve and spinal cord in Wistar rats
The animals were euthanized by overdose of thiopental sodium (200 mg/kg, i.p.) and then subjected to cervical dislocation, immediately after behavioral assays. After which, the ipsilateral sciatic nerve of each rat were removed by giving a blunt cut in the thigh and collection of ipsilateral lumbar region of spinal cord with L4–L6 segments as the epicenter. The isolated tissues were separately weighed and homogenized in ice cold phosphate buffer pH 7.0 and divided into two portions. One part of the homogenate was centrifuged for 15 min at 2,000 × g to obtain the clear supernatant for the estimation of oxidative stress markers, and another part of homogenate was mixed with 4 μL mL−1 protease inhibitor cocktail. These samples were cold centrifuged at 14,000 × g at 4 °C for 15 min and the supernatant was stored at −70 °C (New Brunswick Ultra Deep Freezer, U410 premium, Eppenddorf India Ltd), until assay using biochemical and enzyme-linked immunosorbent assay (ELISA) methods.
Measurement of pro-inflammatory cytokines
The supernatant of spinal cord homogenate was used for the estimation of levels of IL-1β and TNF-α using the quantitative ELISA according to manufacturer’s instructions. The cytokine level was determined by comparing samples to the standard curve generated from the respective kits by determining the optical density (OD) at 450 nm using iMARK Microplate Absorbance Reader (Bio-Rad Lab 10699, India) and values were expressed as picograms per milligram wet weight of sciatic nerve.
Estimation of lipid peroxidation
Concentration of thiobarbituric acid reactive substances (TBARS) was determined as an index of lipid peroxidation, as described by Niehius and Samuelson (1968). Absorbance was measured spectrophotometrically at 532 nm; the values calculated using molar extinction coefficient of chromophore (1.56 × 105 M−1 cm−1) and expressed as nanomoles per gram tissue.
Estimation of catalase
Catalase was assayed as described by Sinha (1972). The rate of decomposition of H2O2 was measured spectrophotometrically at 620 nm. Activity of catalase was calculated and expressed as % activity of sham control.
Estimation of superoxide dismutase
Superoxide dismutase (SOD) activity was measured according to the method described by Misra and Fridovich (1972). Absorbance was measured spectrophotometrically at 480 nm. The activity of SOD was calculated, and expressed as % activity of sham control.
Measurement of nitrite
The nitrite concentration in the sciatic nerve and spinal cord was measured by the Griess reaction (Sastry et al. 2002). In this method, 0.1 mL of supernatant of the tissue homogenate was mixed with 0.25 mL of 1 % sulfanilamide (prepared in 3 N HCl) and 0.25 mL of 0.1 % N-(1-naphthyl) ethylene diamine dihydrochloride with shaking. After 10 min, optical density at 550 nm was measured using iMARK Microplate Absorbance Reader (Bio-Rad Lab 10699, India). Values of nitrite concentration were obtained from sodium nitrite standard curve, and expressed as in nanomoles per gram tissue.
Heme oxygenase (HO)-1 estimation
HO-1 concentration was estimated by quantitative ELISA (CUSABIO BIOTECH Co., Ltd) according to the vendor’s instructions. The HO-1 quantity was determined by comparing samples to the standard curve generated from the standard kit by determining the OD at 450 nm using iMARK Microplate Absorbance Reader (Bio-Rad Lab 10699, India), and values were expressed as picograms per milligram wet weight of spinal cord tissues.
Drugs and reagents
Hemin (Sanjay biological, Amritsar, India), tricarbonyldichlororuthenium (II) dimer (CORM-2) (Sigma Aldrich Ltd, St. Louis, USA), were purchased. Rat IL-1β and TNF-α ELISA kits (RayBiotech, Inc, Norcross, CA) were used to quantify cytokines. HO-1 activity was quantified by HO-1 ELISA kit (CUSABIO BIOTECH Co., Ltd). Unless stated, all other chemicals and biochemical reagents of highest analar quality were used.
Preparation and administration of drugs
Hemin was dissolved in 0.5 mol/L NaOH and then reconstituted to pH 7.4 with HCl in normal saline. The solution was prepared in darkness just before use and protected from light. CORM-2 was prepared by dissolving in 5 % DMSO solution after reconstituting with saline and was administered to rats by subcutaneous route. The treatment of hemin and CORM-2 were initiated at 2 and 24 h, respectively, before sciatic nerve ligation and was continued once daily for next 14 days. The daily dose of hemin for intrathecal route was injected in a total volume of 10 μL followed by 10 μL saline to flush the catheter.
Experimental protocol
All animals were acclimatized to laboratory environment for at least 3 days before testing. The behavioral tests were started before and post-sciatic nerve ligation at various pre-sleeted time schedule (Fig. 1). All the post-treated animals were humanely killed to measure the markers of oxido-nitrosative stress and pro-inflammatory cytokines. The total number of eleven groups, consisting of six animals each, was employed.
Group I: | Sciatic surgery subjected, sciatic nerve not ligated, intrathecal catheterized and vehicle (s) treated sham control rats. |
Group II: | Sciatic nerve ligated (CCI), intrathecal catheterized and vehicle(s) treated control rats. |
Group III: | Hemin (30 μg/rat/day, intrathecal (i.t.)), for 2 weeks treated normal per se rats |
Group IV: | CORM-2 (5 mg/kg/day, s.c), for 2 weeks treated normal per se rats |
Group V: | CCI + Systemic hemin (5 mg/kg/day, subcutaneous (s.c.)), for 2 weeks |
Group VI: | CCI + Systemic hemin (5 mg/kg/day, s.c.), for 2 weeks |
Group VII: | CCI + Spinal hemin (10 μg/rat/day, i.t.), for 2 weeks |
Group VIII: | CCI + Spinal hemin (30 μg/rat/day, i.t.), for 2 weeks |
Group IX: | CCI + hemin both spinal (10 μg/rat/day, i.t.) and systemic (5 mg/kg/day, s.c.), for 2 weeks |
Group X: | CCI + CORM-2 (1 mg/ kg/day, s.c), for 2 weeks |
Group XI: | CCI + CORM-2 (5 mg/kg/day, s.c), for 2 weeks |
Statistical analysis
All the results obtained are expressed as mean ± S.E.M. The data obtained for all behavioral parameters were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. The area under the curve (AUC) for the repeated measurements of behavioral hypersensitivity symptoms over entire observation period was calculated by the trapezoidal method (Pruessener et al. 2003). AUC was analyzed using one-way ANOVA followed by Dunnett post hoc test. Whereas, data obtained for all biochemical and ELISA test were analyzed using one-way ANOVA followed by Tukey’s multiple comparisons post hoc test. P < 0.05 was considered statistically significant.
Results
The ipsilateral PWLs to thermal and cold stimuli and mechanical threshold (in grams) in sham-operated animals remained unchanged from baseline values throughout the observation period. The ipsilateral PWLs of a vehicle-treated CCI rats were significantly less than that of sham-operated rats from day 3 onwards and reached steady state between days 7 and 14 post-sciatic nerve ligation, indicating the development and maintenance of allodynia and hyperalgesia.
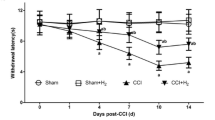
Effect of hemin and CORM-2 treatment on development of nerve injury-induced mechanical hyperalgesia and thermal hyperalgesia
The repeated administration of hemin via i.t. administration (10 and 30 μg/rat, daily, for 2 weeks), but not systemic (5 and 10 mg/kg, s.c. daily, for 2 weeks), significantly inhibited the development of both mechanical hyperalgesia (MH) and thermal hyperalgesia (TH), as compared to vehicle-treated CCI rats. Further, simultaneous administration of hemin via spinal (10 μg/rat, i.t.) and systemic (5 mg/kg, s.c.) routes, led to a more pronounced inhibition in the development of both MH and TH, as compared with either treatment alone. Similarly, repeated administration of CORM-2 (1 and 5 mg/kg, s.c. daily, for 2 weeks) significantly and dose-dependently inhibited the development of both MH and TH, as compared to vehicle-treated CCI rats (Figs. 2a, b, c, d and 3a, b).
Effect repeated administration of hemin and CORM-2 on mechanical threshold (a and b), paw withdrawal latency (PWL) to thermal stimuli (c and d), and PWL to cold stimuli (e and f) in CCI rats. All the values are expressed as Mean ± S.E.M. The single asterisk indicates P < 0.05 vs CCI; double asterisks indicate P < 0.01 vs CCI; triple asterisks indicate P < 0.001 vs CCI; CCI chronic nerve constriction injury; H Hemin; i.t. intrathecal; i.p. intra peritoneal; s.c. subcutaneous
Effect repeated administration of CORM-2 alone and hemin either alone or in combination via systemic and spinal routes, on the area under the curve (AUC) obtained from the repeated measurements of mechanical threshold (a), paw withdrawal latency (PWL) to thermal stimuli (b), and PWL to cold stimuli (c). Bars are the Mean ± S.E.M. (N = 6/group). The single asterisk indicates P < 0.05 vs CCI; double asterisks indicate P < 0.01 vs CCI; triple asterisks indicate P < 0.001 vs CCI; Abbreviation: CCI chronic nerve constriction injury; i.t. intrathecal; s.c. subcutaneous
Effect of hemin and CORM-2 treatment on development of nerve injury-induced cold allodynia
The repeated i.t. administration of hemin only at a dose of 30 μg/rat, daily, for 2 weeks significantly increased the PWLs, as compared to vehicle-treated CCI rats. However, repeated i.t. administration of hemin at a dose of 10 μg/rat, daily, for 2 weeks as well as systemic administration of hemin (5 and 10 mg/kg, s.c, daily, for 2 weeks) did not significantly increase the PWLs. On the other hand, simultaneous administration of hemin via spinal (30 μg, i.t.) and systemic (5 mg/kg, s.c.) route significantly increased PWLs, as compared to vehicle-treated CCI rats. Further, administration of CORM-2 (1 and 5 mg/kg s.c. daily, for 2 weeks), significantly increased the PWLs, as compared to vehicle-treated CCI rats (Figs. 2e, f and 3c).
Effect of hemin and CORM-2 treatment on spinal cord IL-1β and TNF-α level
The concentrations of spinal cord IL-1β and TNF-α were significantly elevated in vehicle-treated nerve-injured control rats, as compared to that of vehicle-treated sham-operated rats. However, repeated i.t. administration (10 and 30 μg/rat, daily, for 2 weeks), but not systemic administration of hemin (5 and 10 mg/kg, s.c. daily, for 2 weeks) significantly attenuated spinal cord IL-1β and TNF-α levels, as compared to CCI control group. Similarly, simultaneous administration of hemin via spinal (10 μg/rat, i.t.) and systemic (5 mg/kg, s.c.) routes as well as CORM-2 treatment (1 and 5 mg/kg s.c. daily, for 2 weeks) significantly lowered the concentrations of these cytokines in spinal cord of CCI rats, as compared with vehicle-treated CCI control group (Fig. 4).
Effect repeated administration of CORM-2 alone and hemin either alone or in combination via systemic and spinal routes, on interleukin (IL)-1β and tumor necrosis factor (TNF)-α levels, in whole dorsolumbar region of spinal cord homogenate. All the values are expressed as Mean ± S.E.M. The single asterisk indicates P < 0.05 vs CCI; double asterisks indicate P < 0.01 vs CCI; triple asterisks indicate P < 0.001 vs CCI; CCI chronic nerve constriction injury; i.t. intrathecal; s.c. subcutaneous
Effect of hemin and CORM-2 on spinal cord heme oxygenase (HO)-1
The levels of spinal cord HO-1 was slightly, but not significantly, elevated in vehicle-treated nerve-injured control rats, as compared to that of vehicle-treated sham-operated rats. However, repeated i.t. administration (10 and 30 μg/rat, daily, for 2 weeks), but not systemic administration, of hemin (5 and 10 mg/kg, s.c. daily, for 2 weeks) significantly increased the amount of HO-1, as compared to CCI control group. Similarly, simultaneous administration of hemin via spinal (10 μg/rat, i.t.) and systemic (5 mg/kg, s.c.) routes as well as CORM-2 treatment (1 and 5 mg/kg s.c. daily, for 2 weeks) also significantly increased the concentrations of HO-1 in spinal cord of CCI rats, as compared with vehicle-treated CCI control group (Fig. 5).
Effect repeated administration of CORM-2 alone and hemin either alone or in combination via systemic and spinal routes, on the ipsilateral lumbar region (L4–L6) of spinal cord heme oxygenase (HO)-1 level. All the values are expressed as Mean ± S.E.M. The single asterisk indicates P < 0.05 vs CCI; triple asterisks indicate P < 0.001 vs CCI; CCI chronic nerve constriction injury; i.t. intrathecal; s.c. subcutaneous
Effect of hemin and CORM-2 on altered sciatic nerve and spinal cord SOD, catalase, lipid peroxidation, and nitrite levels in CCI rats
Vehicle-treated CCI control rats showed a significant increase in the levels of TBARS and nitrite, while significant decrease in levels of SOD and catalase activity in both ipsilateral sciatic nerve and spinal cord homogenate were observed, as compared to sham-operated rats (Tables 1 and 2). However, repeated systemic administration of CORM-2 (1 and 5 mg/kg s.c., for 2 weeks) as well as simultaneous administration of hemin via spinal (10 μg/rat, i.t.) and systemic (5 mg/kg, s.c.) routes significantly attenuated the sciatic oxido-nitrosative stress, along with significant improvement in anti-oxidant levels, as compared to vehicle-treated CCI rats. On the other hand, spinal administration of hemin (10 and 30 μg/rat, i.t., for 2 weeks) did selectively improve the altered sciatic oxido-nitrosative stress parameters in spinal cord, but not in sciatic nerve homogenate, as compared to vehicle-treated CCI rats. However, systemic administration of hemin (5 and 10 mg/kg, s.c, daily, for 2 weeks) significantly altered the sciatic nerve, but not the spinal cord oxido-nitrosative stress parameters, as compared to vehicle-treated CCI rats (Tables 1 and 2).
Effect of hemin and CORM-2 on locomotor activity in CCI rats
The motor activity scores were normal in both sham-operated and CCI rats on days 3, 7, and14 following surgery, as compared to their respective scores observed on day 0 (basal). Also, chronic systemic and spinal (5 and 10 mg/kg, s.c.; 10 and 30 μg, i.t., respectively) administration of hemin and CORM-2 (1 and 5 mg/kg, s.c., for 2 weeks) did not produce any significant effect on locomotor activity, as compared to vehicle-treated CCI group (Table 3).
Discussion
The results obtained in the present study demonstrate that chronic pharmacological activation of HO-1/CO pathway prevented the development of behavioral symptoms of allodynia and hyperalgesia in sciatic nerve CCI-induced peripheral neuropathy in Wistar rats. The observed beneficial effects of hemin and CORM-2 on behavioral symptoms of NP were associated with decreased levels of pro-inflammatory cytokines, oxido-nitrosative stress, along with improved anti-oxidant enzymes levels.
The peripheral upregulation of HO-1 has been shown to mediate the analgesic effect of epibatidine in formalin test (Rosa et al. 2008; Egea et al. 2009). Further, pharmacological activation of peripheral HO-1/CO pathway has been implicated in the anti-hyperalgesic effect of drugs in carrageenan-induced mechanical hypersensitivity in rats (Steiner et al. 2001). However, in the present study, systemic administration of hemin (5 and 10 mg/kg, s.c, daily, for 2 weeks) failed to produce significant effect on the development of nerve injury-induced allodynia and hyperalgesia. It is well kwon that hemin is blood–brain barrier-impermeable in nature (Mancuso 2004) and, therefore, may not activate the central HO-1 pathway after s.c. administration. Hence, it is unlikely that selective activation of peripheral HO-1/CO pathway may not be sufficient to elicit an anti-hyperalgesic and anti-allodynic effect in experimental paradigm of neuropathic pain. This is supported by the finding that at the employed doses, intrathecal repeated administration of hemin significantly inhibit the development of allodynia and hyperalgesia. Furthermore, concurrent administration of hemin via spinal (10 μg/rat, i.t.) and systemic (5 mg/kg, s.c.) routes produced a more pronounced inhibitory effect on the development of behavioral symptoms of NP, as compared with i.t. dose of hemin (30 μg/rat) alone-treated group. Consistent with this, a previous study demonstrated that a low-dose injection of heme-lysinate, a substrate of HO pathway, either into paw or spinal site alone, did not modulate the nociceptive behavior induced by formalin flinching test. Also, spinal HO-1 has been shown to mediate the amitriptyline-induced reversal of anti-nociceptive tolerance observed in chronic morphine-infused rats (Tai et al. 2009). In addition, simultaneous pharmacological activation of HO/CO pathway both at peripheral and spinal sites has been shown to provide a more pronounced anti-nociceptive effect in formalin-induced flinching responses in rats (Nascimento and Branco 2009). Similarly, the data obtained in the present study revealed, for the first time, the existence of an anti-nociceptive synergy between peripheral and spinal HO pathways in the chronic pharmacological activation of HO-1 pathway CCI-induced NP. In contrast to this, single dose systemic administration of HO-1 inhibitors, tin protoporphyrin, dose-dependently reversed the mechanical allodynia and thermal hyperalgesia induced by the L5 and L6 nerve roots ligation in rats (Li and Clark 2000). These discrepancies may be due to use of difference in nature, dosing schedule (preventive vs post-emptive) of pharmacological agents and animal models.
Recently, it has been shown that an activation of central HO-CO-cGMP pathway produced anti-nociceptive effect in the tail flick test, a model of non-inflammatory acute pain (Carvalho et al. 2011). These previous observations and the present study data clearly suggest that simultaneous activation of peripheral, spinal, and perhaps, supraspinal HO-1/CO pathway may be necessary for obtaining maximal anti-nociceptive activity in NP. This notion is supported further by the results obtained in this study that even combined administration of hemin via spinal and systemic route was unable to produce equivalent beneficial effects, as compared to CORM-2-treated group. The data obtained from in vitro studies using whole-cell patch recordings have demonstrated that CORM-2 act as an effective antagonist at human P2X4 receptors (Wilkinson and Kemp 2011), and an increased expression of P2X4 receptors in microglia of the dorsal horn has been reported to play an important role in peripheral nerve injury-induced pain behaviors in rats (Tsuda et al. 2003, 2008). Therefore, the possible involvement of P2X4 receptors in CORM-2-induced beneficial effects cannot be excluded, and further studies are needed in this regard.
Now, it has become clear that inflammatory and immune mechanisms in the peripheral and central nervous systems play an important role in the development and maintenance of peripheral NP (Moalem and Tracey 2006; Vallejo et al. 2010). Particularly, microglial activation and subsequent release of pro-inflammatory cytokines in spinal cord has been documented in the development of nerve injury-induced peripheral NP in rats (Raghavendra et al. 2003; Ledeboer et al. 2005; Watkins et al. 2007). Once released, these pro-inflammatory cytokines may increase expression of enzymes like COX-2 and iNOS, which lead to enhanced synthesis of prostaglandins and NO, well known mediators involved in spinal hypersensitization (Thacker et al. 2007). In addition, oxido-nitrosative stress has also been shown to play an important pathogenic role in the peripheral and central sensitization and subsequent development of peripheral NP (Levy and Zochodne 1998; Kim et al. 2004; Naik et al. 2006). Further, it is also well demonstrated that exogenous administration of anti-oxidant, including SOD, SOD mimetic, nitric oxide synthase inhibitors and microglial inhibitors are effective in preventing the development and maintenance of NP (Kim et al. 2004; Park et al. 2006; Padi and Kulkarni 2008). Consistent with these reports, in the present study, vehicle-treated CCI control rats developed a significant increase in inflammatory and oxido-nitrosative stress, along with significant decrease in anti-oxidant levels. This indicates that these pathogenic mechanisms play an important role in the development and maintenance NP. Although NF-κB has not been estimated in the present study, accumulating evidences from in vitro and in vivo studies, however, suggest that hemin and CORM-2 did suppress the activation of NF-κB activation and subsequent inhibition of the expression of pro-inflammatory cytokines, particularly TNF-α and IL-1β and development of oxido-nitrosative stress (Otterbein et al. 2000; Zuckerbraun et al. 2007; Jadhav et al. 2008; Chen et al. 2010; Ahanger et al. 2011). Pharmacological upregulation of HO-1 has also been shown to downregulate the release of pro-inflammatory cytokines and oxido-nitrosative stress markers, in activated microglia, macrophages, and hippocampal HT22 cells, in vitro (Li et al. 2012). In tune with this, in this study, treatments with both CORM-2 and hemin have significantly and dose-dependently attenuated the elevated oxido-nitrosative stress and pro-inflammatory cytokines in the sciatic nerve and spinal cord. In addition, both CORM-2 and hemin treatment have also significantly improved the levels of anti-oxidant enzymes. Therefore, these data along with the results of the present study reveal that pharmacological activation of HO-1/CO pathway induced inhibition of development of NP, which may be mediated through anti-inflammatory and anti-oxidant mechanisms.
Conclusion
On the basis of the discussion above, it may be concluded that the spinal administration, but not systemic administration, of hemin significantly attenuated the development of nerve injury-induced NP. On the other hand, simultaneous administration of hemin via spinal and systemic routes, as well as CORM-2 alone, produced a more pronounced inhibition of the development of NP, through activation of anti-inflammatory and anti-oxidant mechanisms. It suggests that concurrent pharmacological activation of HO-1/CO pathway both at spinal and peripheral sites, but not peripheral site alone, is needed to prevent the development of sciatic nerve CCI-induced neuropathic pain in Wistar rats.
References
Abraham NG, Kappas A (2008) Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60:79–127
Agarwal CS, Anand M, Arjundas D, Baheti DK, Balaji V, Banerjee T, Joshi M, Prasannakumar K, Mehrotra R, Mohan V, Rais N, Roy T, Sharda A, Sinha B, Sridharan R, Srikanta, Srinivasa R, Srinivasan AV, Sule N, Talwalkar P, Viswanathan V, Yograj S (2008) Burden of neuropathic pain in Indian patients attending urban, specialty clinics: results from a cross sectional study. Pain Pract 8(5):362–378
Ahanger AA, Prawez S, Kumar D, Prasad R, Amarpal, Tandan SK, Kumar D (2011) Wound healing activity of carbon monoxide liberated from CO-releasing molecule (CO-RM). Naunyn Schmiedebergs Arch Pharmacol 384(1):93–102
Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL (1999) Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274(37):26071–26078
Benallaoua M, François M, Batteux F, Thelier N, Shyy JY, Fitting C (2007) Pharmacologic induction of heme oxygenase 1 reduces acute inflammatory arthritis in mice. Arthritis Rheum 56:2585–2594
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Carvalho PG, Branco LG, Panissi CR (2011) Involvement of the heme oxygenase-carbon monoxide-cGMP pathway in the nociception induced by acute painful stimulus in rats. Brain Res 1385:107–113
Chen P, Sun B, Chen H, Wang G, Pan S, Kong R, Bai X, Wang S (2010) Effects of carbon monoxide releasing molecule-liberated CO on severe acute pancreatitis in rats. Cytokine 49(1):15–23
Davis M (2007) What is new in neuropathic pain? Support Care Cancer 15:363–372
Dray A (2008) Neuropathic pain: emerging treatments. Bri J Anaesth 101:48–58
Egea J, Rosa AO, Lorrio S, del Barrio L, Cuadrado A, López MG (2009) Haeme oxygenase-1 overexpression via nAChRs and the transcription factor Nrf2 has antinociceptive effects in the formalin test. Pain 146(1–2):75–83
Gao X, Kim HK, Chung JM, Chung K (2007) Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 131:262–271
Grangeiro NM, Aguiar JA, Chaves HV, Silva AA, Lima V, Benevides NM, Brito GA, da Graça JR, Bezerra MM (2011) Heme oxygenase/carbon monoxide-biliverdin pathway may be involved in the antinociceptive activity of etoricoxib, a selective COX-2 inhibitor. Pharmacol Rep 63(1):112–119
Guedes RP, Bosco LD, Teixeira CM, Araújo AS, Llesuy S, Belló-Klein A, Ribeiro MF, Partata WA (2006) Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res 31:603–609
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Hu P, Bembrick AL, Keay KA, McLachlan EM (2007) Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun 21:599–616
Innamorato NG, Lastres-Becker I, Cuadrado A (2009) Role of microglial redox balance in modulation of neuroinflammation. Curr Opin Neurol 22(3):308–314
Jadhav A, Torlakovic E, Ndisang JF (2008) Interaction among heme oxygenase, nuclear factor-kappaB, and transcription activating factors in cardiac hypertrophy in hypertension. Hypertension 52(5):910–917
Kaur S, Bijjem KR, Sharma PL (2011) Anti-inflammatory and antihyperalgesic effects of the combination of ibuprofen and hemin in adjuvant-induced arthritis in the Wistar rat. Inflammopharmacology 19(5):265–272
Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM (2004) Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 111(1–2):116–124
Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR (2005) Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115:71–83
Lee TS, Chau LY (2002) Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature Medicine 8:240
Levy D, Zochodne DW (1998) Local nitric oxide synthase activity in a model of neuropathic pain. Eur J Neurosci 10:1846–1855
Li B, Lee D-S, Jeong G-S, Kim Y-C (2012) Involvement of heme oxygenase-1 induction in the cytoprotective and immunomodulatory activities of 6,4′-dihydroxy-7-methoxyflavanone in murine hippocampal and microglia cells. Eur J Pharmacol 674:153–162
Li X, Clark JD (2000) The role of heme oxygenase in neuropathic and incisional pain. Anesth Analg 90(3):677–682
Maines MD (1997) The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharm Toxicol 37:517–554
Mancuso C (2004) Heme oxygenase and its products in the nervous system. Antioxid Redox Signal 6(5):878–887
McDermott AM, Toelle TR, Rowbotham DJ, Schaefer CP, Dukes EM (2006) The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain 10(2):127–135
Meregalli C, Canta A, Carozzi VA, Chiorazzi A, Oggioni N, Gilardini A, Ceresa C, Avezza F, Crippa L, Marmiroli P, Cavaletti G (2010) Bortezomib-induced painful neuropathy in rats: a behavioral, neurophysiological and pathological study in rats. Eur J Pain 14:343–350
Merskey H, Bogduk N (eds) (1994) Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms, 2nd edn. IASP, Seattle
Mika J, Osikowicz M, Makuch W, Przewlocka B (2007) Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol 560(2–3):142–149
Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, Flotte TR, Johnson KW, Mahoney MM, Chavez RA, Leinwand LA, Watkins LR (2006) Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain 126(1–3):294–308
Milligan ED, Watkins LR (2009) Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10:23–36
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Moalem G, Tracey DJ (2006) Immune and inflammatory mechanisms in neuropathic pain. Brain Res Brain Res Rev 51:240–264
Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ (2002) Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res 90:E17–E24
Motterlini R, Otterbein LE (2010) The therapeutic potential of carbon monoxide. Nat Rev Drug Discov 9:728–743
Naik AK, Tandan SK, Kumar D, Dudhgaonkar SP (2006) Nitric oxide and its modulators in chronic constriction injury-induced neuropathic pain in rats. Eur J Pharmacol 530:59–69
Nascimento CG, Branco LG (2009) Antinociception synergy between the peripheral and spinal sites of the heme oxygenase-carbon monoxide pathway. Braz J Med Biol Res 42(1):141–147
Niehius WG Jr, Samuelsson B (1968) Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130
Otterbein LE, Bach FH, Alam J, Soares M, Tao LH, Wysk M, Davis RJ, Flavell RA, Choi AM (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6:422–428
Padi SSV, Kulkarni SK (2004) Differential effects of naproxen and rofecoxib on the development of hypersensitivity following nerve injury in rats. Pharmacol Biochem Behav 79:349–358
Padi SSV, Kulkarni SK (2008) Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. Eur J Pharmacol 601:79–87
Paine A, Eiz-Vesper B, Blasczyk R et al (2010) Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol 80:1895–1903
Park ES, Gao X, Chung JM, Chung K (2006) Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett 391:108–111
Perkins NM, Tracey DJ (2000) Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience 101(3):745–757
Pruessener JC, Kirschbaum C, Meinlschmid G, Hellhammer DH (2003) Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinol 28:916–931
Raghavendra V, Tanga F, DeLeo JA (2003) Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 306:624–630
Rosa AO, Egea J, Lorrio S, Rojo AI, Cuadrado A, López MG (2008) Nrf2-mediated haeme oxygenase-1 up-regulation induced by cobalt protoporphyrin has antinociceptive effects against inflammatory pain in the formalin test in mice. Pain 137(2):332–339
Sandkuhler J (2009) Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 89:707–758
Sastry KV, Moudgal RP, Mohan J, Tyagi JS, Rao GS (2002) Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem 306(1):79–82
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Steiner AA, Branco LGS, Cunha FQ, Ferreira SH (2001) Role of the haeme oxygenase/carbon monoxide pathway in mechanical nociceptor hypersensitivity. Br J Pharmacol 132:1673–1682
Syapin PJ (2008) Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol 155:623–640
Tai YH, Tsai RY, Lin SL, Yeh CC, Wang JJ, Tao PL (2009) Amitriptyline suppresses neuroinflammation-dependent interleukin-10-p38 mitogen-activated protein kinase-heme oxygenase-1 signaling pathway in chronic morphine-infused rats. Anesthesiology 110(6):1379–1389
Thacker MA, Clark AK, Marchand F, McMahon SB (2007) Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg 105:838–847
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424(6950):778–783
Tsuda M, Tozaki-Saitoh H, Masuda T, Toyomitsu E, Tezuka T, Yamamoto T, Inoue K (2008) Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheralnerve injury. Glia 56:50–58
Vallejo R, Tilley DM, Vogel L, Benyamin R (2010) The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract 10(3):167–184
Watkins LR, Hutchinson MR, Milligan ED, Maier SF (2007) “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev 56:148–169
Wilkinson WJ, Kemp PJ (2011) The carbon monoxide donor, CORM-2, is an antagonist of ATP-gated, human P2X4 receptors. Purinergic Signal 7(1):57–64
Yaksh TL, Rudy TA (1976) Chronic catheterization of the spinal subarachnoid space. Physiol Behav 17:1031–1036
Zenke-Kawasaki Y, Dohi Y, Katoh Y, Ikura T, Ikura M, Asahara T, Tokunaga F, Iwai K, Igarashi K (2007) Heme induces ubiquitination and degradation of the transcription factor Bach1. Mol Cell Biol 27(19):6962–6971
Zuckerbraun BS, Chin BY, Bilban M, d’Avila JC, Rao J, Billiar TR, Otterbein LE (2007) Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J 21:1099–1106
Acknowledgments
We express our sincere thanks to the management, ISF College of Pharmacy, Moga, Punjab, India for providing necessary facilities. The authors are also thankful to AICTE New Delhi, India (RPS grant: File.No. 8023/BOR/RID/RPS-193/2008-09) and School of Biomedical Sciences, The University of Edinburgh, Edinburgh (CAEN fund), for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bijjem, K.R.V., Padi, S.S.V. & lal Sharma, P. Pharmacological activation of heme oxygenase (HO)-1/carbon monoxide pathway prevents the development of peripheral neuropathic pain in Wistar rats. Naunyn-Schmiedeberg's Arch Pharmacol 386, 79–90 (2013). https://doi.org/10.1007/s00210-012-0816-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-012-0816-1