Abstract
Fludarabine, clofarabine, and cladribine are anticancer agents which are analogues of the purine nucleoside adenosine. These agents have been associated with cardiac and neurological toxicities. Because these agents are analogues of adenosine, they may act through adenosine receptors to elicit their toxic effects. The objective of this study was to evaluate the ability of cytotoxic nucleoside analogues to bind and activate adenosine receptor subtypes (A1, A2A, A2B, and A3). Radioligand binding studies utilizing Chinese hamster ovary cells, stably transfected with adenosine A1, A2A, or A3 receptor subtype, were used to assess the binding affinities of these compounds, whereas adenylyl cyclase activity was used to assess the binding to A2B receptors. Clofarabine and cladribine both bound to the A2A receptor with a K i of 17 and 15 μM, respectively. Clofarabine was the only adenosine analogue to bind to the A3 receptor with a K i of 10 μM, and none of these compounds bound to the A2B receptor. Results show that clofarabine, cladribine, and fludarabine bind to the A1 receptor. In addition, clofarabine, cladribine, and fludarabine were A1 agonists (IC50 3.1, 30, and 30 μM, respectively). Neither pyrimidine nucleoside analogues gemcitabine nor cytarabine associated with any of the adenosine receptor subtypes (K i > 100μM). This is the first report of an interaction between all adenosine receptor subtypes and chemotherapeutic nucleoside analogues commonly used in the treatment of cancer. Therefore, activation of these receptors may be at least one mechanism through which fludarabine-associated toxicity occurs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenosine is a ubiquitous purine nucleoside found in the extracellular space and generally exerts cytoprotective effects by accumulating in response to tissue damage and stress (Sitkovsky et al. 2004). Elevated adenosine levels promote tissue protection and repair by increasing available oxygen, protecting against ischemic damage, initiating anti-inflammatory responses, and stimulating angiogenesis (Linden 2005). However, there are reports of adenosine receptor stimulation also linked to heightened tissue damage (Cunha 2005; Picano and Abbracchio 2000). These biologic processes are primarily mediated through the seven-transmembrane G-protein-coupled adenosine receptors which include A1, A2A, A2B, and A3 receptor subtypes occurring in a tissue-specific manner (Fredholm et al. 2005; Jacobson and Gao 2006; Ralevic and Burnstock 1998). Signaling events upon receptor binding modulate the production of intracellular cyclic adenosine monophosphate (cAMP) through G-protein effects on adenylyl cyclase. Agonists binding to A1 and A3 receptors inhibit adenylyl cyclase activity thereby decreasing cAMP while both A2 receptor subtypes stimulate adenylyl cyclase promoting cAMP formation (Fredholm et al. 2005; Gessi et al. 2008).
Considered an inhibitory neuromodulator, extracellular adenosine has an important role in the central nervous system where it modulates cognition, sleep, locomotion, anxiety, and memory (Ribeiro et al. 2002). Due to a wide range of effects, adenosine receptors have become a focal point for targeted therapies in numerous CNS diseases including Parkinson’s disease, epilepsy, and ischemic brain damage (Cunha 2005; de Mendonca et al. 2000; Fredholm et al. 2005; Picano and Abbracchio 2000; Ribeiro et al. 2002).
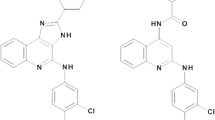
The anti-metabolite class of cytotoxic agents includes the adenosine-based purine and pyrimidine nucleoside analogues, which are widely used in the treatment of hematological malignancies (Robak et al. 2005) and as part of preparative regimens for hematopoietic cell transplantation (Barker et al. 2003). These agents, cladribine, clofarabine, and fludarabine, are deoxyadenosine nucleoside analogues (Fig. 1) and require intracellular phosphorylation to their triphosphate moieties for activity. These compounds have similar mechanisms of action whereby the triphosphate moieties are incorporated into DNA and/or RNA, thus, inhibiting ribonucleotide reductase, DNA polymerases, and promoting apoptosis (Hentosh and Peffley 2010, Vasova et al. 1997; Zhenchuk et al. 2009).
One of the complications associated with fludarabine is a progressive and severe neurotoxicity (Merkel et al. 1986; Spriggs et al. 1986; Von Hoff 1990; Warrell and Berman 1986). Usually noted with high-dose therapy (fludarabine >40 mg/m2/day), the onset of neurological symptoms typically begins weeks after the final dose and is often irreversible and fatal (Cheson et al. 1994). Protection from fludarabine neurotoxicity in mice has been associated with reduced uptake of fludarabine across the luminal membrane of the blood–brain barrier (BBB) with the addition of 5′-phosphate of nitrobenzylthioinosine (NBMPR-P), an inhibitor of the equilibrative nucleoside transporter 1 (ENT1) (Adjei et al. 1992). Currently, the mechanisms precipitating these neurotoxic events are unknown. With evidence that fludarabine is able to cross the BBB (Lindemalm et al. 1999), the present study evaluated the ability of fludarabine and other cytotoxic nucleoside analogues to interact with the adenosine receptors, possibly identifying a mechanism of neurotoxicity.
Material and methods
Chemicals
Fludarabine (2-fluoroadenine-9-β-d-arabinofuranoside, the unphosphorylated form), clofarabine (2-chloro-2′-arabino-fluoro-2′-deoxyadenosine), cladribine (2-chloro-2′-deoxyadenosine), cytarabine (cytosine β-d-arabinofuranoside), and gemcitabine (2′,2′-difluoro-2′-deoxycytidine) were obtained from Sigma-Aldrich (St. Louis, MO). Fludarabine monophosphate (2-fluoroadenine-9-β-d-arabinofuranoside-5′phosphate) was obtained from Fischer Scientific (Pittsburgh, PA). [3H]CCPA (2-chloro-N6-cyclopentyladenosine) and [3H]NECA (N-ethylcarboxamidoadenosine) were obtained from GE Healthcare, Freiburg, Germany, [3H]HEMADO (2-(1-Hexynyl)-N-methyladenosine) was from Tocris, Bristol, UK, and [α-32P]ATP was from Hartmann Analytic, Braunschweig, Germany.
Cell culture
Chinese hamster ovary (CHO) cells were grown adherently and maintained in Dulbecco’s Modified Eagles Medium with nutrient mixture F12 without nucleosides, containing 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 mg/ml), l-glutamine (2 mM), and geneticin (G418, 0.2 mg/ml; A2B, 0.5 mg/ml) at 37°C in 5% CO2/95% air. For binding assays, the culture medium was removed, cells washed with PBS and frozen in dishes until preparation of membranes.
Membrane preparation
Membranes from CHO cells, stably transfected with one human adenosine receptor subtype, were removed from petri dishes via scraping in the presence of ice-cold hypotonic buffer (5 mM Tris/HCl, 2 mM EDTA, pH 7.4). The cell suspension was homogenized on ice (Ultra-Turrax, 2 × 15 s at full speed) followed by low-speed centrifugation (×1,000g). A second, high-speed centrifugation (100,000×g) separated a crude membrane fraction from supernatant. The crude membrane was resuspended in buffers specific for each receptor subtype, frozen in liquid nitrogen, and immediately stored at −80 C. For measurement of adenylyl cyclase activity, a slightly modified protocol with only one centrifugation step was used. Fresh cells were homogenized and the homogenate centrifuged for 30 min at 54,000×g. The resulting pellet was resuspended in 50 mM Tris/HCl pH 7.4 and used for the adenylyl cyclase assay immediately.
Adenosine receptor radioligand binding assay
Competition binding experiments were employed to determine K i values of the purine analogues (fludarabine, fludarabine monophosphate, clofarabine, and cladribine) and the pyrimidine analogues (gemcitabine and cytarabine) for the adenosine A1, A2A, and A3 receptor subtypes. Briefly, competitors for radioligand binding experiments were 1 nM [3H]CCPA for A1 receptors, 30 nM [3H]NECA for A2A receptors, and 1 nM [3H]HEMADO for A3 receptors. Crude membranes expressing A1, A2A, or A3 receptor subtypes were incubated with the specified radioligand and 0–100 μM fludarabine, 0–30 μM clofarabine, 0–100 μM cladribine, 0–100 μM cytarabine, or 0–100 μM gemcitabine in 96-well microplates with filter bottoms for 3 h at 25°C. After that, filtration membranes were washed with cold-binding buffer to remove unbound ligand. Following the addition of scintillation fluid, samples were counted using a Wallac Micro-Beta counter. With no high-affinity ligands available for A2B receptor, potency and efficacy of compounds were assessed through their effect on adenylyl cyclase activity (Klotz et al. 1998).
Adenylyl cyclase activity
To determine the agonist or antagonist properties of fludarabine, clofarabine, and cladribine, CHO membranes transfected with one receptor subtype (A1, A2A, or A2B) were incubated with approximately 150,000 cpm of [α-32P]ATP and either 0–100 μM fludarabine, 0–30 μM clofarabine, or 0–100 μM cladribine for 20 min in the incubation mixture as previously described (Klotz et al. 1985) without EGTA and NaCl (only stimulatory experiments). As agonist controls, 1 μM CCPA (A1) or 1 μM NECA (A2A) was used.
Data analysis
K i values from competition experiments were determined utilizing the program SCTFIT. For agonists, the EC50 values for the stimulation of adenylyl cyclase were calculated with the Hill equation. The IC50 values for concentration-dependent inhibition of forskolin-stimulated adenylyl cyclase via A1 receptors were calculated accordingly. All mean values are from at least three experiments and given with 95% confidence intervals.
Results
Adenosine receptor radioligand binding affinity of nucleoside analogues
The binding affinities of cladribine, clofarabine, and fludarabine to the A1, A2A, and A3 adenosine receptors were assessed by radioligand competition binding. All three compounds showed the highest affinity for the A1 receptor (Table 1). The rank order of affinity at A1 receptors was clofarabine > cladribine > fludarabine (Fig. 2a). Clofarabine and cladribine bound with low affinity to the A2A subtype while only clofarabine showed detectable binding to the A3 receptor (Table 1). Figure 2b illustrates the affinity of clofarabine as determined in competition binding assays at A1, A2A, and A3 receptors. Due to the lack of a useful radioligand, the interaction with the A2B subtype was only tested in adenylyl cyclase experiments (see below). Fludarabine monophosphate and the pyrimidine nucleoside analogues gemcitabine and cytarabine showed no detectable affinity for any adenosine receptor subtype (data not shown).
Radioligand competition experiments with clofarabine, cladribine, and fludarabine. The curves in A represent competition for [3H]CCPA binding to A1 adenosine receptors with clofarabine showing the highest affinity (K i 248 nM), followed by cladribine (K i 1,643 nM) and fludarabine (K i 2,238 nM). In B, competition binding of clofarabine to A1, A2A, and A3 receptors using [3H]CCPA, [3H]NECA, and [3H]HEMADO as radioligands, respectively (corresponding K i values are 248 nM, 17,500 nM, and 8,280 nM). The shown curves are from single experiments, for data in detail see Table 1
Adenylyl cyclase activity
Modulation of adenylyl cyclase activity, as measured by [³²P]cAMP formation from [α-32P]ATP, was determined for the inhibitory A1 receptors and both stimulatory A2 subtypes. All interacting compounds were determined to be full agonists at the A1 receptor as they inhibited cAMP production in a concentration-dependent manner to the same extent as the prototypical A1 agonist CCPA (Fig. 3). The IC50 value for clofarabine was 3.1 μM and above 10 μM for both cladribine and fludarabine (Table 2). As commonly observed for the inhibitory adenosine receptor subtypes, IC50 values for cyclase inhibition are lower than binding K D values by at least one order of magnitude.
Inhibition of adenylyl cyclase activity via stimulation of A1 receptors. Adenylyl cyclase was stimulated with forskolin (F) and concentration-dependent inhibition by fludarabine, clofarabine, and cladribine is shown. The maximal inhibition is comparable to the full A1 receptor agonist CCPA. Data represent percent of forskolin-stimulated cyclase activity over basal (n = 3, error bars show SEM)
The concentration–response curves in Fig. 4a show A2A receptor-mediated stimulation of adenylyl cyclase by clofarabine and cladribine. Figure 4b illustrates that both compounds are partial agonists at the A2A adenosine receptor with about 50% and 40% efficacy, respectively, compared to the full agonist NECA. As no measurable binding affinity from our previous experiments was detected at the A2Areceptor subtype, fludarabine was not tested in cyclase experiments. As expected, the EC50 values were in the same order of magnitude as the K D values from binding experiments (Tables 1 and 2).
A2A receptor-mediated stimulation of adenylyl cyclase with clofarabine and cladribine. A Curves are from a representative experiment resulting in EC50 values of 10,400 and 15,400 nm for clofarabine and cladribine, respectively. The maximal [32P]cAMP production with the prototypical full agonist NECA (1 μM) amounts to 1,660 cpm. B Both compounds are partial agonists with about 50% and 40% efficacy, respectively, compared to the full agonist NECA. Error bars represent SEM, n = 3
There was no A2B-mediated activation of adenylyl cyclase found by any purine analogue. EC50 values for all compounds were >100 μM (Table 2). No inhibition of NECA-stimulated cyclase activity was detected by concentrations of 100 μM, demonstrating that these compounds are not antagonists at the A2B receptor (not shown).
Discussion
Fludarabine, cladribine, and clofarabine are adenosine analogues that bind to various degrees to all adenosine receptor subtypes except the A2B receptor. Previous studies in murine models led to the conclusion that the nucleoside analogue, cladribine, is a partial agonist of the adenosine receptor subtype A1 (Lorenzen et al. 1998). Additionally, other murine models have demonstrated that fludarabine can alter natural killer cell activity through adenosine receptors (Priebe et al. 1990). This study demonstrates an interaction between human adenosine receptor subtypes and adenosine analogues which are readily used in the treatment of cancer. Structure–activity considerations suggest such an interaction to be possible due to their close structural similarity to adenosine. The main requirements for a ligand to act as adenosine receptor agonist are more or less intact purine and ribose structures with modifications allowed in 2-N 6- and 5′-positions of adenosine (Klotz 2000). Modifications in other positions may affect different subtypes to various degrees ranging from complete loss of binding affinity to reduced affinity and/or efficacy only. One such case is the substitution of the 2′-position of the ribose with a methyl group which yields high-affinity agonists with high selectivity for A1 adenosine receptors (Franchetti et al. 2009). The modifications of adenosine leading to clofarabine, cladribine, and fludarabine are also introduced in 2-position of the purine ring and in 2′-position of the ribose, both modifications being compatible with the preconditions for adenosine receptor binding. The absence of measurable interaction of pyrimidine derivatives is in keeping with established requirements for adenosine receptor binding. The lack of affinity of phosphorylated fludarabine is also consistent with previous observations that phosphorylation of nucleosides abolishes receptor binding (Schwabe and Trost 1980).
Although we found clofarabine and cladribine to be partial agonists at A2A receptors, we detected full agonistic activity at A1 receptors for these compounds as well as for fludarabine. This contrasts a previous study by Lorenzen et al. (1998) who suggested that cladribine is a partial agonist at the A1 receptor. These contrasting findings may be explained by fundamental differences in the experimental approach. Our result is based on direct measurement of A1 receptor-mediated inhibition of adenylyl cyclase activity, whereas Lorenzen et al. measured agonist-stimulated binding of [35S]GTPγS in rat brain membranes after deletion of endogenous Gi-like G proteins and reconstitution of various Gα subtypes. The observation that stimulation of [35S]GTPγS binding with the prototypical full agonist CCPA in these reconstituted membranes reaches only about 20% of untreated membranes may well explain why a lower affinity agonist presents as a partial agonist.
The overall role of adenosine in the central nervous system is very complex. It plays an important role in physiological as well as pathophysiological situations (Dunwiddie and Masino 2001). Stimulation of A1 adenosine receptors is considered to be neuroprotective, most importantly through the presynaptic inhibition of excitatory neurotransmitters (Dunwiddie and Masino 2001; Fredholm et al. 2005). Neuroprotection is also achieved with A2A receptor antagonists, probably by blockade of presynaptic receptors that enhance the release of excitatory neurotransmitters (Fredholm et al. 2005). The situation is further complicated as data show that A2A agonists also offer neuroprotection in some experimental models. These effects might be mediated indirectly via non-neuronal targets (Fredholm et al. 2005).
Because fludarabine, cladribine, and clofarabine exhibit close similarity to adenosine, they might elicit unwanted side effects through interaction with adenosine receptors. For example, it is well documented in hematopoietic cell transplant that 2–10% of patients experience mild to severe neurological toxicities associated with fludarabine (Beitinjaneh et al. 2011; Chun et al. 1986; Ding et al. 2008). However, patients receiving either clofarabine- or cladribine-based regimens do not experience these neurological side effects. One plausible mechanism for fludarabine-associated neurotoxicity is through interaction with the adenosine A1 receptor. This receptor is primarily located in the brain and is highly expressed in neurons. Over activation of the A1 receptor induces sleep and coma, whereas inhibition may increase the occurrence of seizures; thus, the downstream effect of activation or inhibition results in neurological responses such as epilepsy, sleep, and neurological ischemia (Sebastiao and Ribeiro 2009). In the case of fludarabine, we have determined that fludarabine binds with agonistic properties to the A1 receptor at plasma concentrations previously determined to be clinically relevant (Long-Boyle et al. 2011b). We also determined that clofarabine and cladribine are also agonists of the A1 receptor at concentrations previously determined to be clinically relevant (Long-Boyle et al. 2011a). The binding affinities for the A1 receptor were clofarabine > cladribine > fludarabine. Clofarabine (10 μM) was observed to be more efficacious in decreasing adenylyl cyclase activity than equivalent concentrations of fludarabine or cladribine. Our data demonstrate that clofarabine has a higher affinity than cladribine and fludarabine at the A1 receptor site. However, this finding is contradictory to the fact that clofarabine and cladribine have a safer neurological toxicity profile when compared to fludarabine (Faderl et al. 2005). Both clofarabine and cladribine exhibit some affinity for A2A receptors that involves partial agonistic activity. It is possible that A1 stimulation together with a partial A2A effect may afford neuroprotection which is absent in the case of fludarabine with lower A1 affinity and no interaction with A2A receptors. The combined A1- and A2A-mediated effects on cAMP levels are hard to predict as they depend on the endogenous tonic stimulation of all subtypes. In particular, in a situation with higher adenosine levels, a partial blockade of A2A receptors by clofarabine and cladribine might potentiate the A1-mediated decrease of cAMP and thereby provide neuroprotection that is absent with fludarabine. In addition, alternative mechanisms for fludarabine-induced neurotoxicity may exist. One possibility is that these compounds may mediate their neurotoxic effects through direct activation of intracellular signaling pathways, bypassing the adenosine receptors.
Ultimately, the ability for either of these agents to cause neurological effects is dependent on their ability to penetrate the blood–brain barrier. Autoradiography studies in mice have shown that tritiated adenosine-like compounds (such as clofarabine, fludarabine, and cladribine) distribute into the brain; however, fludarabine uptake into the brain is ~2.5 times higher than both cladribine and clofarabine (Lindemalm et al. 1999). Furthermore, animal studies have demonstrated that fludarabine neurotoxicity can be decreased by reducing its uptake across the BBB by NBMPR-P, an ENT1 uptake transporter inhibitor (Adjei et al. 1992). Despite cladribine and clofarabine’s higher binding affinities to the A1 receptor relative to fludarabine, poor penetration across the BBB would likely limit their ability to accumulate brain concentrations approximate to their K i values. Hence, poor brain penetration may be one explanation for the lack of neurotoxicity associated with these two compounds.
There is also an abundance of A1 receptors in the heart (Fredholm et al. 2005). However rare, fludarabine-induced grade IV/V cardiotoxicity has been observed in four separate studies in patients who were undergoing nonmyeloablative hematopoietic cell transplant and received fludarabine (Martino et al. 2001; Ritchie et al. 2001; Van Besien et al. 2003). Alternatively, interaction of clofarabine and cladribine with the A2A receptor may be protective towards the neurological effects associated with the activation of A1 receptor, thus making clofarabine and cladribine more suitable when attempting to avoid the neurological complications which are associated with fludarabine.
In conclusion, these results suggest that clofarabine, cladribine, and fludarabine bind as agonists to adenosine receptors with various affinities. Even though these compounds bind A1, one must account for their limited CNS penetration, which could ultimately restrict their ability to cause neurotoxicity, especially cladribine and clofarabine. Further studies are needed to fully determine the molecular pathways involved in the cytotoxic nature of these agents in order to develop compounds with reduced toxicities.
References
Adjei AA, Dagnino L, Wong MM, Paterson AR (1992) Protection against fludarabine neurotoxicity in leukemic mice by the nucleoside transport inhibitor nitrobenzylthioinosine. Cancer Chemother Pharmacol 31:71–75
Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE (2003) Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood 102:1915–1919
Beitinjaneh AM, McKinney AM, Cao Q, Weisdorf DJ (2011) Toxic leukoencephalopathy following fludarabine-associated hematopoietic cell transplantation. Biol Blood Marrow Transplant 17:300–308
Cheson BD, Vena DA, Foss FM, Sorensen JM (1994) Neurotoxicity of purine analogs: a review. J Clin Oncol 12:2216–2228
Chun HG, Leyland-Jones BR, Caryk SM, Hoth DF (1986) Central nervous system toxicity of fludarabine phosphate. Cancer Treat Rep 70:1225–1228
Cunha RA (2005) Neuroprotection by adenosine in the brain: from A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal 1:111–134
de Mendonca A, Sebastiao AM, Ribeiro JA (2000) Adenosine: does it have a neuroprotective role after all? Brain Res Brain Res Rev 33:258–274
Ding X, Herzlich AA, Bishop R, Tuo J, Chan CC (2008) Ocular toxicity of fludarabine: a purine analog. Expert Rev Ophthalmol 3:97–109
Dunwiddie TV, Masino SA (2001) The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24:31–55
Faderl S, Gandhi V, Keating MJ, Jeha S, Plunkett W, Kantarjian HM (2005) The role of clofarabine in hematologic and solid malignancies—development of a next-generation nucleoside analog. Cancer 103:1985–1995
Franchetti P, Cappelacci L, Vita P, Petrelli R, Lavecchia A, Kachler S, Klotz KN, Marabese I, Luongo L, Maione S, Grifantini M (2009) N 6-Cycloalkyl- and N 6-bicycloalkyl-C5′(C2′)-modified adenosine derivatives as high-affinity and selective agonists at the human A1 adenosine receptor with antinociceptive effects in mice. J Med Chem 52:2393–2406
Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM (2005) Adenosine and brain function. Int Rev Neurobiol 63:191–270
Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA (2008) The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther 117:123–140
Hentosh P, Peffley DM (2010) The cladribine conundrum: deciphering the drug's mechanism of action. Expert Opin Drug Metab Toxicol 6:75–81
Jacobson KA, Gao ZG (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5:247–264
Klotz KN (2000) Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch Pharmacol 362:382–391
Klotz KN, Cristalli G, Grifantini M, Vittori S, Lohse MJ (1985) Photoaffinity labeling of A1-adenosine receptors. J Biol Chem 260:14659–14664
Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, Lohse MJ (1998) Comparative pharmacology of human adenosine receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol 357:1–9
Lindemalm S, Liliemark J, Larsson BS, Albertioni F (1999) Distribution of 2-chloro-2′-deoxyadenosine, 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine, fludarabine and cytarabine in mice: a whole-body autoradiography study. Med Oncol 16:239–244
Linden J (2005) Adenosine in tissue protection and tissue regeneration. Mol Pharmacol 67:1385–1387
Long-Boyle J, Huang J, Rydholm N, Smith A, Orchard P, Tolar J, Jacobson P (2011a) Pharmacokinetics of clofarabine in patients with high-risk inherited metabolic disorders undergoing brain-sparing hematopoietic cell transplantation. J Clin Pharmacol 51:679–686
Long-Boyle JR, Green KG, Brunstein CG, Cao Q, Rogosheske J, Weisdorf DJ, Miller JS, Wagner JE, McGlave PB, Jacobson PA (2011b) High fludarabine exposure and relationship with treatment-related mortality after nonmyeloablative hematopoietic cell transplantation. Bone Marrow Transplant 46:20–26
Lorenzen A, Lang H, Schwabe U (1998) Activation of various subtypes of G-protein alpha subunits by partial agonists of the adenosine A1 receptor. Biochem Pharmacol 56:1287–1293
Martino R, Caballero MD, Canals C, Simon JA, Solano C, Urbano-Ispizua A, Bargay J, Rayon C, Leon A, Sarra J, Odriozola J, Conde JG, Sierra J, San Miguel J, ALLOPBSCT Subcommittee of the Spanish Group for Haematopoietic Transplantation (GETH), Group GEL-TAMO (2001) Allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning: results of a prospective multicentre study. Br J Haematol 115:653–659
Merkel DE, Griffin NL, Kagan-Hallet K, Von Hoff DD (1986) Central nervous system toxicity with fludarabine. Cancer Treat Rep 70:1449–1450
Picano E, Abbracchio MP (2000) Adenosine, the imperfect endogenous anti-ischemic cardio-neuroprotector. Brain Res Bull 52:75–82
Priebe T, Platsoucas CD, Nelson JA (1990) Adenosine receptors and modulation of natural killer cell activity by purine nucleosides. Cancer Res 50:4328–4331
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Ribeiro JA, Sebastiao AM, de Mendonca A (2002) Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol 68:377–392
Ritchie DS, Seymour JF, Roberts AW, Szer J, Grigg AP (2001) Acute left ventricular failure following melphalan and fludarabine conditioning. Bone Marrow Transplant 28:101–103
Robak T, Korycka A, Kasznicki M, Wrzesien-Kus A, Smolewski P (2005) Purine nucleoside analogues for the treatment of hematological malignancies: pharmacology and clinical applications. Curr Cancer Drug Targets 5:421–444
Schwabe U, Trost T (1980) Characterization of adenosine receptors in rat brain by (-)[3H]N6-phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol 313:179–187
Sebastiao AM, Ribeiro JA (2009) Adenosine receptors and the central nervous system. Handb Exp Pharmacol 193:471–534
Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M (2004) Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol 22:657–682
Spriggs DR, Stopa E, Mayer RJ, Schoene W, Kufe DW (1986) Fludarabine phosphate (NSC 312878) infusions for the treatment of acute leukemia: phase I and neuropathological study. Cancer Res 46:5953–5958
Van Besien K, Devine S, Wickrema A, Jessop E, Amin K, Yassine M, Maynard V, Stock W, Peace D, Ravandi F, Chen YH, Hoffman R, Sossman J (2003) Regimen-related toxicity after fludarabine-melphalan conditioning: a prospective study of 31 patients with hematologic malignancies. Bone Marrow Transplant 32:471–476
Vasova I, Penka M, Hajek R, Mayer J, Krahulcova E (1997) A new purine analog in the treatment of hematologic malignancy I. Fludarabine. Vnitr Lek 43:45–50
Von Hoff DD (1990) Phase I clinical trials with fludarabine phosphate. Semin Oncol 17:33–38
Warrell RP Jr, Berman E (1986) Phase I and II study of fludarabine phosphate in leukemia: therapeutic efficacy with delayed central nervous system toxicity. J Clin Oncol 4:74–79
Zhenchuk A, Lotfi K, Juliusson G, Albertioni F (2009) Mechanisms of anti-cancer action and pharmacology of clofarabine. Biochem Pharmacol 78:1351–1359
Acknowledgments
This research was supported by NIH T32 CA099936 (K.J. and L.J.) and Children’s Cancer Research Fund, Minneapolis, MN (K.J. and L.J.), and the Cancer Center Support Grant CA077598 (P.J. and M.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jensen, K., Johnson, L.A., Jacobson, P.A. et al. Cytotoxic purine nucleoside analogues bind to A1, A2A, and A3 adenosine receptors. Naunyn-Schmiedeberg's Arch Pharmacol 385, 519–525 (2012). https://doi.org/10.1007/s00210-011-0719-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-011-0719-6