Abstract
Quercetin has antioxidants properties which may increase nitric oxide (NO) bioavailability. However, the effects of quercetin on NO status have been poorly studied. We evaluated whether quercetin improves the plasma levels of NO metabolites in two-kidney one-clip (2K1C) hypertensive rats and assessed its effect on endothelial function. Sham-operated and 2K1C rats were treated with quercetin (10 mg−1 kg−1 day−1 by gavage) or vehicle for 3 weeks. Systolic blood pressure (SBP) was monitored weekly. Vascular responses to acetylcholine (Ach) and sodium nitroprusside (SNP) were assessed in hindquarter vascular bed. Plasma nitrate levels were assessed by Griess reagent and plasma nitrite and nitroso species (S, N-nitroso species) were assessed by ozone- based chemiluminescence. Aortic NADPH oxidase activity and superoxide production were evaluated. While quercetin had no effects in control normotensive rats (P > 0.05), it significantly reduced SBP in 2K1C rats (P < 0.05). At the end of treatment, plasma nitrate levels were similar in all experimental groups (P > 0.05). However, plasma nitrite and the nitroso species levels were significantly lower in 2K1C rats when compared with controls (P < 0.05). Quercetin treatment restored plasma nitrite and nitroso species levels to those found in the sham-vehicle group (P < 0.05). While quercetin treatment induced no significant changes in responses to SNP (P > 0.05), it restored the vascular responses to Ach. Quercetin significantly attenuated 2K1C-hypertension-induced increases in NADPH oxidase activity and vascular superoxide production (P < 0.05). These results suggest that the antihypertensive effects of quercetin were associated with increased NO formation and improved endothelial function, which probably result from its antioxidant effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several clinical and experimental studies have shown that flavonoid quercetin exerts several biological effects including improved vascular reactivity, antioxidant, and antihypertensive effects (Duarte et al. 2001, 2002; Galisteo et al. 2004a; Garcia-Saura et al. 2005; Mackraj et al. 2008; Edwards et al. 2007; Yamamoto and Oue 2006). The mechanisms explaining the beneficial effects produced by quercetin are attributed mainly to its antioxidants properties, which might include increases in nitric oxide (NO) bioavailability. However, there is controversy with regards to the role induced by quercetin on NO status. While some studies have associated the antihypertensive effects of quercetin with increases in plasma NOx (nitrate + nitrite) levels (Garcia-Saura et al. 2005; Yamamoto and Oue 2006), another study showed that quercetin paradoxically reverted the reduced NOx plasma levels in an experimental model of hypertension induced by L-NAME (Duarte et al. 2002), without altering nitric oxide synthase (NOS) activity (Duarte et al. 2002). Moreover, Loke et al. showed that while the ingestion of pure quercetin by normotensive volunteers induced no changes in plasma nitrate levels (which represent most of NOx content (Lauer et al. 2001)), nitrite and S-nitrosothiols levels increased significantly (Loke et al. 2008). Finally, an in vitro study suggested that quercetin is a NO scavenger (Lopez-Lopez et al. 2004).
In order to clarify the effects of quercetin on NO status, the present study was designed to examine the effects induced by treatment with quercetin on markers of NO production, such as NOx, nitrite, and nitroso species (S, N-nitroso species) in an animal model of renovascular hypertension. In addition, we have also evaluated the impact of quercetin treatment on the vascular function, which was assessed in hindquarters of rats, a relevant vascular resistance bed. We demonstrated that quercetin induces no changes on plasma NOx, but significantly increases both plasma nitrite and nitroso species in renovascular hypertension, thus improving the vascular function.
Methods
Animals and treatments
This study complied with guidelines of the Faculty of Medicine of Ribeirão Preto, University of São Paulo, and the animals were handled according to the guiding principles published by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Wistar rats (180–200 g) obtained from the colony at University of São Paulo (Ribeirão Preto Campus, Brazil) were maintained on a 12-h light/dark cycle at a room temperature (22–25°C) with free access to standard rat chow and water.
2K1C hypertension was induced by clipping the left renal artery with a silver clip (0.2 mm)(Castro et al. 2008; Martinez et al. 2008). Sham-operated rats underwent the same surgical procedure except for the clip placement. ketamine 100 mg.kg−1 and xylazine 10 mg.kg−1 i.p. were used to anesthetize the animals. Treatment with quercetin (or vehicle) was started 2 weeks after surgery and maintained for 3 weeks. The rats were randomly divided into FOUR groups (n = 12/group) and were treated for 3 weeks with daily gavage of vehicle (sham and 2K1C groups; 100 μl of dimethyl sulfoxide to both) or quercetin (sham-quercetin and 2K1C-quercetin groups; 10 mg−1 kg−1 day−1, both groups). Quercetin was dissolved in dimethyl sulfoxide. The doses of quercetin were chosen with basis on previous works showing significant antihypertensive effects in different experimental models of hypertension (Duarte et al. 2001, 2002; Galisteo et al. 2004b; Garcia-Saura et al. 2005; Perez-Vizcaino et al. 2009).
Body weight and tail systolic blood pressure (SBP) were assessed weekly by tail-cuff plethysmography. To minimize the effects of stress induced by this method on blood pressure measurement, the animals were trained for a week before surgery.
Measurement of cardiovascular responses
At the end of treatment, rats (500–550 g) were anesthetized with urethane (1.25 g/kg, i.p.) and a tracheotomy was performed using polyethylene tubing (PE200). Another polyethylene catheter (PE50) was inserted into the left carotid and the mean arterial pressure was recorded using a data acquisition system (MP150CE; Biopac Systems Inc. CA, USA) connected to a computer (Acknowledge 3.2, for windows). Heart rate values were derived from the blood pressure recordings and processed online. The absence of somatic motor reflexes in response to tail pitching or blinking in response to a low-pressure corneal stimulation indicated deep anesthesia and analgesia. The data acquisitions were initiated 30 min after anesthesia onset.
Preparation of rat hindquarters vascular bed
After baseline assessment of mean arterial pressure and heart rate, a midline abdominal incision was performed and the aorta was cannulated above the iliac bifurcation. The hindquarters were then perfused in situ with Krebs' bicarbonate solution bubbled with 95% O2, 5% CO2, at 37°C, pH 7.4, containing (in mmol/L): NaCl 118; NaHCO3 25.0; KCl 4.7; KH2PO4 1.2; MgSO4 1.2; glucose 11.0; CaCl2.2.H2O 2.5, and using a peristaltic pump (Carter-Manostat, Barnant Com, IL, USA) at a constant rate of 16 ml−1 min−1 kg−1. The vena cava was sectioned to allow the perfusate to escape. The basal perfusion pressure was similar in all experimental groups (approximately 25–30 mmHg). Resting perfusion pressure was adjusted to approximately 100 mmHg by adding methoxamine (50–100 μM) to the perfusate and remained stable throughout the experiment. There were no significant differences between groups in relation to the concentration of methoxamine used in the study groups (data not shown). Because the flow was constant, changes in perfusion pressure reflected changes in vascular tone. After hindquarters perfusion was initiated, the rat was killed by an intracardiac injection of KCl 3 M, and 30 min of perfusion were then allowed for the preparation to stabilize before responses to vasodilators were measured. A side-arm of the perfusion circuit, between the peristaltic pump and the aortic catheter, was connected to a pressure transducer, and perfusion pressure was monitored on real-time using a data acquisition system (MP150CE; Biopac Systems Inc. CA, USA). Dose–response curves to acetylcholine and sodium nitroprusside were examined. Acetylcholine (0.1, 1, 10 and 100 ng) or sodium nitroprusside (0.01, 0.1, 1, and 10 μg) were injected in small volumes (10–100 μl) directly into the perfusion circuit, as we described previously (Montenegro et al. 2009).
Measurement of plasma NOx concentrations
Arterial blood samples were collected in tubes containing heparin and immediately centrifuged at 1,000×g for 3 min. Plasma aliquots were stored at −70°C until analyzed. The plasma NOx concentrations were determined in duplicate by using the Griess reaction as we described previously (Montenegro et al. 2009; Sandrim et al. 2010). Briefly, 40 μl of plasma was incubated with the same volume of nitrate reductase buffer (0.1 M potassium phosphate, pH 7.5, containing 1 mM beta nicotinamide adenine dinucleotide phosphate (NADPH), and 2 units of nitrate reductase/mL) in individual wells of a 96-well plate. Samples were allowed to incubate overnight at 37°C in the dark. Eighty microliters of freshly prepared Griess reagent (1% sulphanilamide, 0.1% naphthylethylenediamine dihydrochloride in 5% phosphoric acid) were added to each well and the plate was incubated for an additional 15 min at room temperature. A standard nitrate curve was obtained by incubating sodium nitrate (0.2–200 μM) with the same reductase buffer. The total amount of nitrite recovery was >98% and absorbances were measured at 540 nm using a microplate reader. The values of nitrite were subtracted of total NOx of each sample, thus the results are reported as micromoles (μM) per liter of nitrate. The assay results were not significantly affected by the presence of plasma proteins.
Measurement of plasma nitrite concentrations
Plasma aliquots were analyzed in duplicate for their nitrite content using an ozone-based reductive chemiluminescence assay as previously described (Feelisch et al. 2002; Dias-Junior et al. 2006; Sandrim et al. 2010). Briefly, to measure nitrite concentrations in plasma, 300 μl of plasma samples were injected into a solution of acidified tri-iodide, purging with nitrogen in-line with a gas-phase chemiluminescence NO analyzer (Sievers Model 280 NO analyzer, Boulder, CO, USA). Approximately 8 ml of tri-iodide solution (2 g potassium iodide and 1.3 g iodine dissolved in 40 ml water with 140 ml acetic acid) was placed in the purge vessel into which plasma samples were injected. The tri-iodide solution reduces nitrites to NO gas, which is detected by the NO analyzer.
Measurement of plasma nitroso species concentrations
Plasma aliquots were analyzed in duplicate for their nitroso species using an ozone-based reductive chemiluminescence assay with tri-iodide solution described above. Briefly, plasma samples (500 μL) were previously treated with acidified sulfanilamide (5 wt.%; 9:1, v/v) for 3 min to eliminate nitrite signal and injected in tri-iodine solution. After pretreatment with acidified sulfanilamide, the resulting signal is corresponding to the sum of the nitroso species (i.e., RSNO and others minor nitroso species, such as Fe-nitrosyl, N-nitrosamine and nitrated lipids) remaining in the sample. A standard curve was constructed by injecting known amounts of S-nitrosoglutathione (GSNO; 0–50 nmol) in duplicate, and GSNO (25 nmol) previously treated with HgCl2 (5 mM final, which degrade nitrosothiols) were used as control. The standard curve was constructed by plotting the area under curve and raw data were export from the Sievers program into a second program, origin 6.1 (Microcal Software, Inc., Northampton, MA). Using Origin, we can denoise the data and the peaks can be measured with greater precision and increased accuracy (MacArthur et al. 2007).
Assessment of vascular ROS
Dihydroethidium (DHE) was used to evaluate in situ reactive oxygen species (ROS) production by fluorescence microscopy as described previously (Ceron et al. 2010). The aortic cryosections (5-μm thick) were incubated at 37°C with DHE (1 μmol/l) or vehicle for 30 min. After triple washing with phosphate buffer, images were obtained with a microscope (Carl Zeiss, Axioskop, Germany) with ×40 objective lens, equipped for fluorescence. The intensity of the fluorescent signal was measured as arbitrary units and compared with that found in vessels from controls rats within 30 min of staining to avoid nonspecific signals.
Measurement of NADPH oxidase activity
NADPH-dependent superoxide production was measured in aortic rings from all experimental groups. Aortic rings were transferred to luminescence vials containing 1 mL of Krebs-HEPES buffer, pH 7.4. After equilibration and background counts, a nonredox cycling concentration of lucigenin (5 μmol/L) and NADPH (12 μmol) were automatically added and the luminescence counts measured continuously for 15 min in a Berthold FB12 single tube luminometer at 37°C. Background signals from aortic rings were subtracted from the NADPH-driven signals and the results were normalized for the dry weight and reported as lucigenin chemiluminescence/mg of dry tissue, as described previously (Ceron et al. 2010).
Drugs and solutions
All drugs and reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA). Acetylcholine and sodium nitroprusside were dissolved in saline solution immediately before use.
Statistical analysis
The results are expressed as means ± SEM. The comparisons between groups were assessed by one- or two way analysis of variance followed by Dunnett's multiple comparison tests (GraphPad Prism 3 Software, San Diego CA). A probability value <0.05 was considered significant.
Results
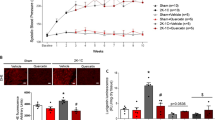
Treatment with quercetin ameliorates 2K1C hypertension
Baseline SBP and body weight were similar in the four experimental groups (Fig. 1a, b). While no significant changes in SBP were seen in sham and sham-quercetin groups, SBP increased in 2K1C and 2K1C-quercetin groups after the first week of the renal artery surgery, and additional increases in SBP were found in the 2K1C group throughout the study period (Fig. 1a). However, quercetin treatment induced a significant reduction in SBP in the 2K1C-quercetin group (P < 0.05; Fig. 1a). We found no significant differences of body weight among all experimental groups (P > 0.05; Fig. 1b).
Quercetin treatment improves vascular dysfunction associated with 2K1C hypertension
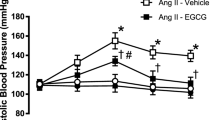
To evaluate the vascular effects of quercetin, rat hindquarters vascular beds were isolated and their functional responses to acetylcholine and sodium nitroprusside (n = 6–8, per group) were assessed. Fig. 1a, b shows endothelial-dependent and endothelial-independent vasorelaxation, respectively, induced by acetylcholine (0.1, 1, 10, and 100 ng) and sodium nitroprusside (0.01, 0.1, 1, and 10 μg), respectively, in the hindquarter vascular bed. The 2K1C group showed reduced responses to acetylcholine when compared with the sham group (P < 0.01; Fig. 2a). Conversely, the treatment of 2K1C animals with quercetin produced significant increases in acetylcholine-induced relaxation (P < 0.001; Fig. 2a). Finally, while quercetin improved the endothelium-dependent responses of 2K1C hypertensive rats, no significant differences were observed in endothelial-independent relaxation responses to increasing doses of sodium nitroprusside among the experimental groups (P > 0.05; Fig. 2b).
Endothelial-dependent (a) and endothelial-independent (b) vasorelaxation induced by acetylcholine and nitroprusside, respectively, in rat hindquarter vascular bed from animals treated with vehicle or Quercetin. Vascular responses were studied after preconstriction with methoxamine to a perfusion pressure of ∼100 mmHg. Data are shown as mean ± SEM (n = 6–8 per group). #P < 0.01 versus sham group; *P < 0.01; ***P < 0.001 versus 2K1C group
Quercetin treatment restores plasma nitrite and nitroso species levels in 2K1C hypertension
We evaluated if quercetin could alter the impaired NO formation associated with 2K1C hypertension. While no significant differences were found in plasma NOx (the sum of nitrate + nitrite) levels measured by Griess reagent (P > 0.05; Fig. 3), the plasma levels of both nitrite and nitroso species were significantly reduced in the 2K1C group (P < 0.05; Figs. 4 and 5, respectively). Conversely, the treatment of 2K1C hypertensive rats with quercetin completely restored the plasma levels of both nitrite and nitroso species to those found in the sham group (P < 0.05 to both; Figs. 4 and 5, respectively). Fig. 4b, c) shows representative tracings obtained during nitrite analysis. Likewise, Fig. 5b shows a representative tracing obtained by injecting samples from animals to study nitroso species concentrations and the standard curves constructed with GSNO, which was used to calculate the total nitroso species concentrations (Fig. 5c). After the pretreatment of GSNO standards with HgCl2, which degrades nitrosothiols, the signal produced by the NO analyzer was totally abolished (Fig. 5c).
Plasma nitrite levels at end of treatment. a Plasma nitrite concentrations (μmol/l). b Depicts a representative tracing showing signal changes produced by injection of 300 μL of plasma from animals from each experimental group into the tri-iodine solution. c The peaks corresponding to injections of known amounts of nitrite (0–1,000 pmol) injected in triplicate. The insert shows a standard curve constructed by plotting the area under each peak shown in c against the corresponding amount of nitrite. Data are shown as mean ± SEM (n = 6–8 per group). *P < 0.05 versus the sham group; #P < 0.05 versus the 2K1C group
Nitroso species levels in plasma at end of treatment. a The concentrations of nitroso species (nmol/l), which represent the circulating pool of bioactive nitric oxide, in plasma from animals from all experimental groups. b Depicts a representative tracing showing signal changes produced by injection of plasma samples into the tri-iodine solution. The tracings were denoised to facilitate the interpretation. Plasma samples (500 μL) were previously treated with acidified sulfanilamide (5 wt.%; 9:1, v/v) for 3 min to eliminate nitrite signal. c The peaks corresponding to injections of known amounts of S-nitrosoglutathione (GSNO; 0–50 nmol) injected in duplicate as well as GSNO previously treated with HgCl2, which degrades nitrosothiols. The insert shows a standard curve constructed by plotting the area under each peak shown in c against the corresponding amount of GSNO. Data are shown as mean ± SEM (n = 6–8 per group). *P < 0.05 versus the sham group; #P < 0.05 versus the 2K1C group
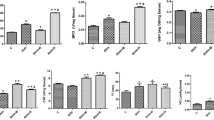
Quercetin treatment reduces the NADPH oxidase activity and decreases the production of vascular ROS in 2K1C hypertension
To assess if the increased nitric oxide levels induced by quercetin involves a reduction in ROS production, we evaluated ROS production by using the sensitive probe DHE and we also measured NADPH oxidase activity in aortic tissues from animals. We found that DHE oxidation was significantly increased in 2K1C groups when compared with the sham and sham + quercetin groups (p < 0.05, Fig. 6a). Treatment with quercetin significantly attenuated this increase (p < 0.05, Fig. 6a). Interestingly, in support of these findings, we found that the NADPH oxidase activity in the 2K1C group was significantly increased when compared with the sham and sham + quercetin groups (p < 0.05, Fig. 6b, and that treatment with quercetin attenuated this increase (p < 0.05, Fig. 6b).
Effects of quercetin treatment on vascular \( {{\hbox{O}}_2}^{ \bullet - } \) production measured by dihydroethidium (DHE) fluorescence and NADPH-dependent \( {{\hbox{O}}_2}^{ \bullet - } \) production measured in the aortic rings. a The fluorescence intensity in each experimental group and a representative picture (to the right) of arteries incubated in the presence of DHE, which produces a red fluorescence when oxidized to hydroxyethidium by \( {{\hbox{O}}_2}^{ \bullet - } \). b Left, shows the NADPH oxidase activity and representative tracing (to the right) showing signal changes produced by adding β-NADPH to the aortic ring in the presence of lucigenin (5 μmol/l). The tracings were denoised to facilitate the interpretation. Data are shown as mean ± SEM (n = 6–8 per group). *P < 0.05 versus the sham group; #P < 0.05 versus the 2K1C group
Discussion
The main findings of this study were: (1) quercetin treatment restored the plasma nitrite and nitroso species levels in 2K1C hypertensive rats, while induces no significant changes on plasma NOx; (2) these effects produced by quercetin on NO formation was associated with an antihypertensive effect and improved endothelial function; (3) quercetin treatment reduces vascular NADPH oxidase activity and produces remarkable antioxidant effects. These data suggest that the overall beneficial cardiovascular effects ascribed to quercetin are likely associated with its antioxidants properties, which improves NO bioavailability.
Several clinical and experimental reports have ascribed antihypertensive effects to quercetin (Duarte et al. 2001, 2002; Galisteo et al. 2004a; Garcia-Saura et al. 2005; Mackraj et al. 2008; Edwards et al. 2007; Yamamoto and Oue 2006). In this regard, the antioxidant properties produced by quercetin could increase NO bioavailability and contribute to the beneficial cardiovascular effects produced by this drug. However, the effects of quercetin on NO status have been poorly studied. Previous reports have associated the antihypertensive effects of quercetin with increases in plasma NOx levels (Garcia-Saura et al. 2005; Yamamoto and Oue 2006). Conversely, here we found that treatment with quercetin induced no significant changes in plasma NOx (nitrate + nitrite), either in normotensive or in hypertensive rats. In support of our findings, Loke et al. showed that the ingestion of pure quercetin by normotensive volunteers induced no changes in plasma nitrate levels, which reflect most of NOx content (Lauer et al. 2001). However, the use of NOx levels as a maker of NO production has been criticized (Kleinbongard et al. 2003, 2006). This because plasma NOx (total of nitrate + nitrate) reflects mostly the nitrate contents in the plasma, which are usually found at micromolar range, whereas nitrite levels are usually at nanomolar range, therefore at least two orders of magnitude below that of nitrate levels. In other words, the high levels of nitrate found in the plasma makes it very difficult to detect minor changes in plasma nitrate concentrations, thereby limiting the sensitivity of NOx levels as a marker of NO formation and NOS activity (Lauer et al. 2001). For this reason, we decided to assess the plasma levels of nitrite and nitroso compounds to evaluate the effects of quercetin on NO formation.
The plasma levels of nitrite and nitroso compounds (the sum of S-, N-nitroso species) have been recently recognized, rather than plasma NOx, as an important marker of nitric oxide formation (Metzger et al. 2006, 2007; Heiss et al. 2006; Kleinbongard et al. 2006). In the present study, in contrast to plasma NOx levels, we found lower nitrite and lower nitroso species concentrations in 2K1C hypertensive rats when compared with the sham group. In this regard, we evaluated if the antioxidant properties of quercetin were involved in the increased nitric oxide bioavailability reported in the present study. The vascular reactive oxygen species (ROS) production in aortic slices was significantly reduced in 2K1C hypertensive rats treated with quercetin, thus suggesting that quercetin attenuates the oxidative stress. A key enzyme promoting oxidative stress during hypertension is vascular NADPH oxidase, which produces superoxide anion and contributes to hypertension (Sedeek et al. 2009). Importantly, previous studies showed that reduced NADPH oxidase activity downregulates superoxide anion production and contributes to increase nitric oxide bioavailability (Schulz et al. 2008; Sedeek et al. 2009; Harrison and Gongora 2009). In the present study, we show higher NADPH oxidase activity in the 2K1C group compared with the sham and sham + quercetin groups. Treatment with quercetin attenuated 2K1C-induced increases in NADPH activity, thus suggesting that the antioxidant properties of quercetin involve reduction in NADPH oxidase activity. These findings confirm previous results by Sanchez et al., which support the idea that quercetin downregulates NADPH oxidase activity (Sanchez et al. 2006, 2007). Our data expand these previous findings and show, for the first time, that the antioxidants propeties of quercetin may improve NO biovaiability.
The lower nitrite and nitroso species concentrations reported in the present study were associated with impaired vasorelaxation in response to acetylcholine. This functional test using an endothelium-dependent agonist in a relevant vascular resistance bed (the hindquarter vascular bed) clearly shows endothelial-dependent dysfunction, which was confirmed by the reduced nitrite and nitroso species concentrations (Heiss et al. 2006; Kleinbongard et al. 2006). Treatment with quercetin improved both endothelial-dependent responses to acetylcholine and the concentrations of relevant markers of NO formation (nitrite and nitroso species), thus clearly indicating that quercetin produces beneficial effects on the endothelial dysfunction associated with 2K1C hypertension. In fact, quercetin is a well-known antioxidant, and several studies have shown that antioxidants can improve endothelium-dependent relaxation (Cai and Harrison 2000; Montenegro et al. 2009). Therefore, it is highly probable that antioxidants properties of quercetin may have improved NO bioavailability, thus producing antihypertensive effects with improved endothelial function.
Some limitations of this study should be taken into consideration. Firstly, we have not examined the activity and expression of endothelial nitric oxide synthase (eNOS), an approach that could help to explain the increases of nitrite and nitroso compounds described here. In this regard, previous reports have shown that treatment with quercetin at the same dose used in the present study increases eNOS expression and NOS activity in hypertensive rats (Sanchez et al. 2006; Duarte et al. 2002). In addition, in vitro studies showed that quercetin induces increases cGMP levels (Zhao and Gu 1996; Kuhlmann et al. 2005), consistent with the idea that activation of NO-cGMP pathway by nitric oxide is probably the main factor responsible for antihypertensive effects produced by quercetin. It is possible that increased eNOS expression and NOS activity are responsible for enhancing nitrite and nitroso species as was reported here because nitrite concentrations in plasma reflect NO synthase activity, and approximately 70% of plasma nitrite is derive from the NO synthase activity in the endothelium (Kleinbongard et al. 2003, 2006; Lauer et al. 2001). Secondly, we examined the effects produced by quercetin in only one experimental model of hypertension. It would be interesting to examine the effects produced by quercetin on nitrite and nitroso species status in other experimental models of hypertension and in human hypertension.
In conclusion, our results provide data suggesting that quercetin treatment induces no changes in plasma NOx but restores plasma nitrite and nitroso species in renovascular hypertension. In addition, quercetin significantly improved the endothelial dysfunction and attenuated 2K1C hypertension. Improved NO formation, probably resulting from antioxidant effects of quercetin may explain, at least in part, the beneficial cardiovascular effects ascribed to this drug.
References
Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844
Castro MM, Rizzi E, Figueiredo-Lopes L, Fernandes K, Bendhack LM, Pitol DL, Gerlach RF, Tanus-Santos JE (2008) Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis 198:320–331
Ceron CS, Castro MM, Rizzi E, Montenegro MF, Fontana V, Salgado MC, Gerlach RF, Tanus-Santos JE (2010) Spironolactone and hydrochlorothiazide exert antioxidant effects and reduce vascular matrix metalloproteinase-2 activity and expression in a model of renovascular hypertension. Br J Pharmacol 160:77–87
Dias-Junior CA, Gladwin MT, Tanus-Santos JE (2006) Low-dose intravenous nitrite improves hemodynamics in a canine model of acute pulmonary thromboembolism. Free Radic Biol Med 41:1764–1770
Duarte J, Perez-Palencia R, Vargas F, Ocete MA, Perez-Vizcaino F, Zarzuelo A, Tamargo J (2001) Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br J Pharmacol 133:117–124
Duarte J, Jimenez R, O’Valle F, Galisteo M, Perez-Palencia R, Vargas F, Perez-Vizcaino F, Zarzuelo A, Tamargo J (2002) Protective effects of the flavonoid quercetin in chronic nitric oxide deficient rats. J Hypertens 20:1843–1854
Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T (2007) Quercetin reduces blood pressure in hypertensive subjects. J Nutr 137:2405–2411
Feelisch M, Rassaf T, Mnaimneh S, Singh N, Bryan NS, Jourd’Heuil D, Kelm M (2002) Concomitant s-, n-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of no in vivo. FASEB J 16:1775–1785
Galisteo M, Garcia-Saura MF, Jimenez R, Villar IC, Wangensteen R, Zarzuelo A, Vargas F, Duarte J (2004a) Effects of quercetin treatment on vascular function in deoxycorticosterone acetate-salt hypertensive rats. Comparative study with verapamil. Planta Med 70:334–341
Galisteo M, Garcia-Saura MF, Jimenez R, Villar IC, Zarzuelo A, Vargas F, Duarte J (2004b) Effects of chronic quercetin treatment on antioxidant defence system and oxidative status of deoxycorticosterone acetate-salt-hypertensive rats. Mol Cell Biochem 259:91–99
Garcia-Saura MF, Galisteo M, Villar IC, Bermejo A, Zarzuelo A, Vargas F, Duarte J (2005) Effects of chronic quercetin treatment in experimental renovascular hypertension. Mol Cell Biochem 270:147–155
Harrison DG, Gongora MC (2009) Oxidative stress and hypertension. Med Clin North Am 93:621–635
Heiss C, Lauer T, Dejam A, Kleinbongard P, Hamada S, Rassaf T, Matern S, Feelisch M, Kelm M (2006) Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J Am Coll Cardiol 47:573–579
Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M (2003) Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 35:790–796
Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M (2006) Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med 40:295–302
Kuhlmann CR, Schaefer CA, Kosok C, Abdallah Y, Walther S, Ludders DW, Neumann T, Tillmanns H, Schafer C, Piper HM, Erdogan A (2005) Quercetin-induced induction of the no/cgmp pathway depends on ca2+-activated k+ channel-induced hyperpolarization-mediated ca2+-entry into cultured human endothelial cells. Planta Med 71:520–524
Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M (2001) Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 98:12814–12819
Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD (2008) Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am J Clin Nutr 88:1018–1025
Lopez-Lopez G, Moreno L, Cogolludo A, Galisteo M, Ibarra M, Duarte J, Lodi F, Tamargo J, Perez-Vizcaino F (2004) Nitric oxide (no) scavenging and no protecting effects of quercetin and their biological significance in vascular smooth muscle. Mol Pharmacol 65:851–859
MacArthur PH, Shiva S, Gladwin MT (2007) Measurement of circulating nitrite and s-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci 851:93–105
Mackraj I, Govender T, Ramesar S (2008) The antihypertensive effects of quercetin in a salt-sensitive model of hypertension. J Cardiovasc Pharmacol 51:239–245
Martinez ML, Castro MM, Rizzi E, Fernandes K, Demacq C, Bendhack LM, Gerlach RF, Tanus-Santos JE (2008) Lercanidipine reduces matrix metalloproteinase-2 activity and reverses vascular dysfunction in renovascular hypertensive rats. Eur J Pharmacol 591:224–230
Metzger IF, Sertorio JT, Tanus-Santos JE (2006) Relationship between systemic nitric oxide metabolites and cyclic gmp in healthy male volunteers. Acta Physiol (Oxf) 188:123–127
Metzger IF, Sertorio JT, Tanus-Santos JE (2007) Modulation of nitric oxide formation by endothelial nitric oxide synthase gene haplotypes. Free Radic Biol Med 43:987–992
Montenegro MF, Pessa LR, Gomes VA, Desta Z, Flockhart DA, Tanus-Santos JE (2009) Assessment of vascular effects of tamoxifen and its metabolites on the rat perfused hindquarter vascular bed. Basic Clin Pharmacol Toxicol 104:400–407
Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A (2009) Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep 61:67–75
Sanchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, Tamargo J, Perez-Vizcaino F, Duarte J (2006) Quercetin downregulates nadph oxidase, increases enos activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens 24:75–84
Sanchez M, Lodi F, Vera R, Villar IC, Cogolludo A, Jimenez R, Moreno L, Romero M, Tamargo J, Perez-Vizcaino F, Duarte J (2007) Quercetin and isorhamnetin prevent endothelial dysfunction, superoxide production, and overexpression of p47phox induced by angiotensin ii in rat aorta. J Nutr 137:910–915
Sandrim VC, Montenegro MF, Palei AC, Metzger IF, Sertorio JT, Cavalli RC, Tanus-Santos JE (2010) Increased circulating cell-free hemoglobin levels reduce nitric oxide bioavailability in preeclampsia. Free Radic Biol Med 49:493–500
Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T (2008) Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 10:1115–1126
Sedeek M, Hebert RL, Kennedy CR, Burns KD, Touyz RM (2009) Molecular mechanisms of hypertension: role of nox family nadph oxidases. Curr Opin Nephrol Hypertens 18:122–127
Yamamoto Y, Oue E (2006) Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosci Biotechnol Biochem 70:933–939
Zhao XY, Gu ZL (1996) Effects of quercetin on production and release of endothelin and cgmp from cultured endothelial cells. Zhongguo Yao Li Xue Bao 17:442–444
Acknowledgements
This study was funded by the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP-Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil). The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montenegro, M.F., Neto-Neves, E.M., Dias-Junior, C.A. et al. Quercetin restores plasma nitrite and nitroso species levels in renovascular hypertension. Naunyn-Schmied Arch Pharmacol 382, 293–301 (2010). https://doi.org/10.1007/s00210-010-0546-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-010-0546-1