Abstract
Microtubules are crucial targets for cancer chemotherapeutic drugs, and new microtubule-directed agents are of continued interest in drug development. A novel microtubule-directed agent, ethyl-2-[N-ρ-chlorobenzyl-(2′-methoxy)]-anilino-4-oxo -4, 5-dihydro-furan-3-carboxylate, was identified. The compound, designated K2154, inhibited cell proliferation, with IC50 values of 10.3, 15.3, 9.6, 11.2, 12.8 and 12.1 μM in prostate cancer PC-3, hepatocellular carcinoma Hep3B, non-small cell lung cancer A549, colorectal cancer HT29 and HCT116, and P-glycoprotein-rich breast cancer NCI/ADR-RES cells, respectively. Because NCI/ADR-RES cells were susceptible to inhibition by K2154, it indicated that this compound is a poor substrate for P-glycoprotein. In this study, PC-3 cells were used to identify the anticancer mechanisms of K2154. K2154 induced an arrest of the cell cycle at G2/M phase and a subsequent increase of hypodiploid phase in PC-3 cells, whereas it only induced a moderate level of G2/M arrest with little increase of hypodiploid phase in normal prostate cells. K2154 inhibited microtubule assembly in both in vitro turbidity assay and in vivo microtubule spin-down experiment. Immunochemical examination showed that K2154 caused formation of abnormal mitotic characteristics with bipolar spindles, particularly, in βII- and βIII-tubulin staining. It also induced several pathways, including cyclin B1 up-regulation, dephosphorylation on Tyr15 and phosphorylation on Thr161 of Cdk1 and Cdc25C phosphorylation, and roscovitine (a Cdk1 inhibitor) significantly inhibited K2154-induced apoptosis, suggesting a pro-apoptotic role of Cdk1. Phosphorylation of Bcl-2 and Bcl-xL and cleavage of Mcl-1, together with activation of caspase-9 and -3, indicated that mitochondrial pathway played a central role in K2154-mediated apoptotic cell death. Additionally, AIF contributed to a late phase of K2154-induced apoptotic pathway. In conclusion, it is suggested that K2154 displays an anticancer activity through a target on microtubules and a subsequent signaling cascade on cell cycle regulation and apoptotic machinery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microtubules are structural proteins and composed of α- and β-tubulin heterodimers. There are at least six α-tubulin and seven β-tubulin isotypes that have been discovered in vertebrates (Luduena 1998). The seven β-tubulin isotypes are encoded by a multigene family with highly homologous gene products, including βI, βII, βIII, βIVa, βIVb, βV and βVI, but differ in the last 15 carboxyl amino acid residues (Luduena 1998; Cleveland and Sullivan 1986). Since the tissue distributions and cellular localizations of β-tubulin isotypes are diverse, their functions may be different, although the details have not been fully elucidated (Walss-Bass et al. 2002; Aria and Matsumoto 1988; Burgoyne et al. 1988). It has been suggested that βIII-tubulin is a central target for taxanes. Both basic researches and clinical studies reveal that βIII-tubulin overexpression has been associated with microtubule destabilization and resistance to taxanes, and constitutes a prognostic factor in several solid tumors (Seve et al. 2005; Derry et al. 1997). Furthermore, there are several lines of evidence that βIII-tubulin is present in neoplastic but not in normal differentiated glial cells (Katsetos et al. 2003). Therefore, the investigation of molecular mechanisms responsible for the targeting on this isotype may provide significant insights into the regulation of the growth and progression of cancer cells. Importantly, microtubules are highly dynamic and play a crucial role during mitosis, such as the support for kinetochore attachment to chromosomes at the prometaphase, the proper position of chromosomes aligned at the metaphase plate and the segregation of the chromosomes at the anaphase and telophase (Jordan and Wilson 2004). The interruption of microtubule dynamics can result in mitotic arrest and inhibition of cell proliferation and, eventually, the induction of cell death. Accordingly, microtubules have become a potential target for the development of cancer chemotherapeutic agents (Sengupta et al. 2005; Orosz et al. 1997).
The activation of the Cdk1/cyclin B1 complex in the nucleus drives the progression from G2- to M-phase of the cell cycle. It has been suggested that Cdk1 could be a target for the induction of apoptosis. For example, the inactivation of Cdk1 increases apoptotic cell death induced by DNA damage (Ongkeko et al. 1995). Nevertheless, there are several lines of evidence that Cdk1 plays a pro-apoptosis other than an anti-apoptosis role in numerous tumor cell types. The tubulin-binding agents, such as taxol and vinca alkaloids, are able to increase the Cdk1 activity and induce apoptosis in tumor cells (Yu et al. 1998). However, the concern is how tubulin-binding agents drive the perturbation of cell cycle progression toward apoptotic cell death. It has been suggested that mitochondria may play a central role in the signaling pathway and that Cdk1 can trigger mitochondrial membrane permeabilization by targeting on Bcl-2 family proteins and subsequently induce cell apoptosis (Debatin et al. 2002).
Based on the basic researches and clinical studies, the target on tubulins can be an effective mechanism for cancer chemotherapy. Nonetheless, most of the tubulin-binding agents are derived from natural products and have complex chemical structures that limit chemical modification. Therefore, simple chemical structures with the aforementioned mechanisms could be valuable as lead structures for future therapeutic agents. K2154 (ethyl-2-[N-ρ-chlorobenzyl-(2′-methoxy)]anilino-4-oxo- 4,5-dihydro-furan-3-carboxylate) is a new synthetic compound with a key structure of 3-furancarboxylate (a furancarboxylate derivative). It has been suggested that several furancarboxylate derivatives display the pharmacological effects. One is 2-furancarboxylate derivative, which exhibits the binding activity with protein kinase C (Lee et al. 2001). The other two are derivatives of 3-furancarboxylate and 4-furancarboxylate. These two compounds have been reported to display the inhibitory effect on platelet-activating factor (PAF) receptor (Peterson et al. 1989a,b). The structure of K2154 fits the criteria as a pharmacological probe. In this study, the action mechanisms of K2154 have been identified from the characterization of tubulin isotypes and cell cycle regulators to mitochondrial proteins and related apoptotic cascades.
Methods
Tissue explants and cell culture
Human hyperplastic prostates were obtained from three males by transurethral resection of the prostate in the National Taiwan University Hospital. All patients had histories of prostatism and were diagnosed to have benign prostate hyperplasia by rectal digital examination, transrectal sonography of the prostate and urodynamic studies. Isolation of human prostatic cells from prostatic tissue explants was described in the previous study (Guh et al. 1998). NCI/ADR-RES cell line was from DTP Human Tumor Cell Line Screen (Developmental Therapeutics Program, NCI). The other cancer cell lines were from American Type Culture Collection (Rockville, Md.). Human cancer cells were cultured in RPMI1640 medium with 10% FBS (v/v) and penicillin (100 units/ml)/streptomycin (100 μg/ml). Cultures were maintained in a humidified incubator at 37°C in 5% CO2:95% air.
Sulforhodamine B (SRB) Assays
Cells were seeded in 96-well plates in medium with 5% FBS. After 24 h, cells were fixed with 10% trichloroacetic acid (TCA) to represent cell population at the time of K2154 addition (T0). After additional incubation of vehicle (0.1% DMSO) or K2154 for 48 h, cells were fixed with 10% TCA. SRB at 0.4% (w/v) in 1% acetic acid was added to stain cells. Unbound SRB was washed out by 1% acetic acid and SRB bound cells were solubilized with 10 mM Trizma base. The absorbance was read at a wavelength of 515 nm. Using the following absorbance measurements, such as time zero (T0), control growth (C), and cell growth in the presence of K2154 (Tx), the percentage growth was calculated at each of the compound concentrations. Percentage growth inhibition was calculated as [(Tx−T0)/(C−T0)]×100 for concentrations for which Tx≥T0. Growth inhibition of 50% (IC50) is determined at the drug concentration which results in 50% reduction of total protein increase in control cells during the compound incubation.
FACScan flow cytometric analysis
After the treatment of cells with vehicle (0.1% DMSO) or K2154 for the indicated time courses, the cells were harvested by trypsinization, fixed with 70% (v/v) alcohol at 4°C for 30 min and washed with phosphate-buffered saline (PBS). After centrifugation, cells were incubated in 0.1 ml of phosphate-citric acid buffer (0.2 M NaHPO4, 0.1 M citric acid, pH 7.8) for 30 min at room temperature. The cells were centrifuged and resuspended with 0.5 ml propidium iodide solution containing Triton X-100 (0.1%, v/v), RNase (100 μ/ml) and propidium iodide (80 μg/ml). DNA content was analyzed using the FACScan and CellQuest software (Becton Dickinson, Mountain View, Calif.).
Immunofluorescence microscopic examination
Cells were seeded in 8-well chamber slides. After the compound treatment, the cells were fixed with 100% methanol at −20°C for 5 min and incubated in 1% bovine serum albumin (BSA) containing 0.1% Triton X-100 at 37°C for 30 min. The cells were washed twice with PBS for 5 min and incubated with primary antibodies at 37°C for 1 h. The cells were washed twice with PBS and incubated with the fluorescein isothiocyanate (FITC, 1:100) or tetramethylrhodamine isocyanate (TRITC, 1:100)-conjugated secondary antibody at 37°C for 40 min. The nuclei were recognized by the staining with DAPI (1 mg/ml). The labeled targets in cells were detected by a confocal laser microscopic system (Leica TCS SP2).
In situ labeling of apoptotic cells
Cells were seeded in 4-well chamber slides. After the treatment, cells were stained with Heochest 33342 (0.1 μg/ml) at 37°C for 15 min and fixed with 4% paraformaldehyde for another 15 min at room temperature. The labeled targets in cells were detected by microscopic examination.
Western blotting
Cells were seeded in 100-mm Petri dishes. After the treatment, cells were harvested with trypsinization, centrifuged and lysed in 0.1 ml of lysis buffer containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EGTA, 1% Triton X-100, 1 mM phenylmethylsulfonnylfluoride (PMSF), 10 μg/ml leupeptinin, 10 μg/ml aprotinin, 50 mM NaF and 100 μM sodium orthovanadate. In some experiments, the mitochondrial/cytosol fractionation kit (Biovision, Mountain View, Calif.) was used to separate mitochondrial and cytosolic fraction. Total protein was quantified, mixed with sample buffer and boiled at 90°C for 5 min. Equal amount of protein (30 μg) was separated by electrophoresis in either 10 or 12% SDS-PAGE, transferred to PVDF membranes and detected with specific antibodies. The immunoreactive proteins after incubation with appropriately labeled secondary antibody were detected with an enhanced chemiluminescence detection kit (Amersham, Buckinghamshire, UK).
In vitro tubulin turbidity assay
The tubulin polymerization was detected by the use of CytoDYNAMIX Screen 03 kit (Cytoskeleton, Denver, Colo.). Tubulin proteins (>99% purity) were suspended in G-PEM buffer containing 80 mM PIPES, 2 mM MgCl2, 0.5 mM EDTA, and 1.0 mM GTP (pH 6.9) and 5% glycerol with or without the compound. Then, the mixture was transferred to a 96-well plate and the absorbance was measured at 340 nm (37°C) for 60 min (SpectraMAX Plus, Molecular Devices, Sunnyvale, Calif.).
In vivo microtubule assembly assay
After the treatment with the indicated compound, the cells were harvested by trypsinization and collected by centrifugation. The cells were lysed with 0.1 ml of hypotonic buffer (1 mM MgCl2, 2 mM EGTA, 0.5% NP-40, 2 mM PMSF, 200 U/ml aprotinin, 100 μg/ml soybean trypsin inhibitor, 5.0 mM ɛ-amino caproic acid, 1 mM bezamidine and 20 mM Tris-HCl, pH 6.8). The cytosolic and cytoskeletal fraction of cell lysate were separated by centrifugation at 16,000 × g for 15 min. The supernatant contained cytosolic tubulin. The pellet representing the particulate fraction of polymerized tubulin was resuspended in 0.1 ml hypotonic buffer. Tubulin contents in both fractions were detected by western blotting.
Regents
RPMI 1640 medium, FBS, penicillin, streptomycin, and all other tissue culture regents were obtained from GIBCO/BRL Life Technologies (Grand Island, N.Y.). All of the chemical reagents and antibodies to βI, βII, βIII, and βIV tubulin isotypes and FITC- or TRITC-conjugated secondary antibodies were obtained from Sigma (St Louis, Miss.). Antibodies to Bcl-2, Bcl-xL, Mcl-1, Survivin, Cyclin B1, Cdk1, Cdc25C, poly-(ADP-ribose) polymerase (PARP), cytochrome c, Smac, apoptosis inducing factor (AIF), and anti-mouse and anti-rabbit IgGs were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). Antibodies to caspase-9, caspase-8, phospho-cdc2Tyr15, phospho-cdc2Thr161, phospho-Bcl-2Ser70 and Bid were from Cell Signaling Technologies (Boston, Mass.). Antibody to OxPhos Complex IV subunit 2 was from Molecular Probes (Leiden, The Netherlands). Antibody to caspase-3 was from Imgenex (San Diego, Calif.). Antibodies to α- and β-tubulins were from Serotec Products (Beverly, MA) and BD Biosciences PharMingen (San Diego, Calif.), respectively. Antibody to MPM-2 was from Upstate Biotechnology (Lake Placid, N.Y.). K2154 (ethyl-2-[N-ρ-chlorobenzyl- (2′-methoxy)]anilino-4-oxo-4,5-dihydro- furan-3-carboxylate) was synthesized and provided by our colleagues (Dr. Sheng-Chu Kuo).
Results
Effect of K2154 on mammary cancer cell proliferation and cell cycle progression
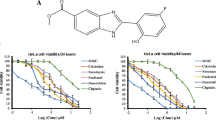
Treatment of human hormone-resistant prostate cancer PC-3 cells with K2154 inhibited cell proliferation in a dose-dependent manner with an IC50 of 10.3 μM (Fig. 1c). K2154 also displayed anti-proliferative effect in several types of cancer cells, including hepatocellular carcinoma Hep3B, non-small cell lung cancer A549, colorectal cancer HT29 and HCT116, and P-glycoprotein (P-gp)-rich breast cancer NCI/ADR-RES cells, with IC50 values of 15.3, 9.6, 11.2, 12.8 and 12.1 μM, respectively. Furthermore, K2154 was able to induce apoptotic cell death by detection of condensed DNA with Hoechst33342, a sign of apoptosis (Fig. 1b). Next, the effect of K2154 on cell cycle progression was determined by FACScan flow cytometric analysis of PI staining in PC-3 cells. The data demonstrated that K2154 induced a time-dependent increase of G2/M phase and a subsequent increase of hypodiploid (sub-G1) phase of the cell cycle, indicating the occurrence of mitotic arrest and apoptotic cell death (Fig. 1c). Furthermore, the selectivity of K2154 between tumor and normal prostatic cells was also examined. The data showed that K2154 only exhibited a modest effect on the induction of mitotic arrest of the cell cycle and apoptotic cell death in normal prostatic cells (Fig. 1c). The doubling time of PC-3 and normal prostatic cells was 26.1 and 31.6 h, respectively, in this study.
Effect of K2154 on cell proliferation and cell cycle progression. a The graded concentrations of K2154 were added to PC-3 cells for 48 h. Then, the growth inhibitory effect of K2154 was examined by SRB assays. b Cells were cultured in chamber slides for 24 h and then treated with graded concentrations of K2154 for another 24 h. After the incubation period, cells were washed and stained with Hoechst 33342, and examined under a fluorescence microscope. c PC-3 or primary prostatic cells were incubated in the control vehicle (0.1% DMSO), K2154 (30 μM) or taxol (0.1 μM) for the indicated time course. Then, cells were fixed and stained with propidium iodide to analyze DNA content by FACScan flow cytometry
Effect of K2154 on in vitro and in vivo microtubule assembly and characterization of β-tubulin isotypes
To determine whether tubulins were targets of K2154-induced anti-tumor effect, the in vitro tubulin polymerization of turbidity assay was carried out. As demonstrated in Fig. 2a, the tubulins polymerized in a time-dependent manner in the presence of GTP at 37°C. As expected, taxol promoted, while vincristine inhibited, the tubulin polymerization. K2154 displayed an inhibitory effect on tubulin polymerization in a concentration-dependent manner (Fig. 2a). We also performed an in vivo microtubule assembly assay, which separated assembly and disassembly microtubules in particulate and soluble fractions, respectively. The data showed that K2154 markedly reduced the particulate fraction of β-tubulins by 58%, confirming the inhibitory effect on microtubule assembly (Fig. 2b). In addition, based on the target of β-tubulins, four different β-tubulin isotypes were further examined. The immunochemical examination showed that taxol promoted, whereas vincristine inhibited, the formation of multipolar spindles in all types of β-tubulin staining. In contrast, K2154 induced the formation of dipolar spindle with a characteristic of pro-metaphase arrest, in particular, by staining with βII- and βIII-tubulin (Fig. 2c).
Effect of K2154 on in vitro and in vivo tubulin assembly assays. a Tubulins were incubated in 37°C with GTP in the presence of 0.1% DMSO (●), K2154 (◯, 10 μM; ▼, 30 μM; ∇, 100 μM; ■, 300 μM), taxol (□, 10 μM) or vincristine (□, 10 μM). Tubulin assembly was examined turbidimetrically. Change in absorbance at 340 nm is plotted as a function of time in minutes. b Cells were incubated in the presence of the indicated agent (K2154, 30 μM; taxol 0.01 μM; vincristine 0.1 μM) for 9 h. Then, the cells were harvested and separated into soluble (S, microtubule disassembly) and particulate form (P, microtubule assembly), and proteins were detected by western blot analysis. The relative expression of particulate fraction is also shown. c Cells were incubated in the vehicle or the indicated agent (K2154, 30 μM; taxol 0.01 μM; vincristine 0.1 μM) for 15 h. Then, the cells were fixed for the detection of microtubule organization by immunochemical staining with antibodies of β-tubulin isotypes and DAPI for nuclear examination. Scale bar, 20 μm
Regulation of the cyclin B1 and Cdk1 by K2154
Progression from G2- to M-phase of the cell cycle is driven by activation of the Cdk1/cyclin B1 complex. The perfect regulation of the Cdk1/cyclin B activity is crucial for the accurate progression of the cell cycle. After the exposure to K2154, the cyclin B1 expression was soon elevated. Furthermore, the dephosphorylation on inhibitory Tyr15 and phosphorylation on activating Thr161 of Cdk1 were induced. These data, together with the phosphorylation of Cdc25C, indicated that Cdk1 was activated in PC-3 cells when treated with K2154 (Fig. 3a). Additionally, the levels of the mitotic markers MPM2 were also dramatically increased, revealing the arrest of the cells at mitosis to K2154 action. Notably, the sudden decline of the MPM2 expression at a 36-h treatment of K2154 might result from the occurrence of apoptotic cell death (Fig. 3a).
Function examination of the involvement of Cdk1. a Cells were incubated in 0.1% DMSO or K2154 (30 μM) for the indicated time courses. Then, the cells were harvested and lysed for the detection of protein expressions with antibodies of mitotic regulators by western blot analysis. b, c Cells were pre-incubated in 0.1% DMSO (i and ii) or roscovitine (iii and iv, 10 μM) for 30 min and then K2154 (ii and iv, 30 μM) was added for another 24 h. The cells were observed by microscope or stained with propidium iodide to analyze DNA content by FACScan flow cytometry
An increase in Cdk1 activity has been found in cells during several apoptotic insults. In some cases the Cdk1 activation is suggested to be a consequence rather than a cause of apoptotic cell death (Zhou et al. 1998). Nonetheless, several studies have suggested that Cdk1 activation can be a mechanism of cell killing and lead to apoptosis (Konishi et al. 2002). To test the role of Cdk1, roscovitine (a pharmacological Cdk1 inhibitor) was used to suppress the Cdk1 activity. The data showed that K2154-induced apoptosis was significantly inhibited by roscovitine (10 μM, Fig. 3b,c) suggesting that Cdk1 acted as a pro-apoptotic mediator in this study.
Effect of K2154 on numerous anti- and pro-apoptotic proteins
Recently, a lot of attention has been directed at investigating the role of apoptosis regulators in controlling survival decisions in tumor cells. Currently, two broad categories have been focused on. Within the first category, several pro-apoptotic proteins (e.g., Bax, Bak and Bim) promote release of cytochrome c, whereas other anti-apoptotic proteins (e.g., Bcl-2, Bcl-xL and Mcl-1) are able to antagonize the pro-apoptotic member proteins and/or preserve mitochondrial integrity by preventing loss of mitochondrial membrane potential (Abuharbeid et al. 2005). Within the second category, proteins such as the inhibitors of apoptosis proteins (e.g., XIAP, CIAP-1, CIAP-2 and survivin) inhibit the activation of caspases, whereas Smac/DIABLO binds to several IAP proteins, thwarting their caspase-inhibitory functions (Holcik et al. 2001). In this study, K2154 had little effect on the pro-apoptotic members of Bcl-2 family proteins (data not shown). However, it induced the phosphorylation of Bcl-2 and Bcl-xL and the cleavage of Mcl-1 after 6- and 18-h treatments, respectively (Fig. 4). Furthermore, survivin was down-regulated while Smac/DIABLO was dramatically released from the mitochondria in a long-term treatment (48 h) with K2154 (Fig. 4). In addition to Smac/DIABLO, we also examined several apoptogenic factors including cytochrome c and AIF, which were also released from the mitochondrial intermembrane space. By using western blotting examination, there was no Complex IV expression indicating the cytosolic fraction without the existence of mitochondria (Fig. 5). The data also demonstrated that the release of cytochrome c and AIF from mitochondria was induced by K2154 after treatments for 18 and 48 h, respectively. (Fig. 5).
Effect of K2154 on the expression of family proteins of Bcl-2 and IAP. Cells were incubated in 0.1% DMSO or K2154 (30 μM) for the indicated time courses. Then, the cells were harvested and lysed for the detection of protein expressions with antibodies of family proteins of Bcl-2 and IAP by western blot analysis
Effect of K2154 on the expression of mitochondrial apoptogenic factors. Cells were incubated in 0.1% DMSO or K2154 (30 μM) for the indicated time courses. After the treatment, the cells were harvested and separated into mitochondrial and cytosolic fractions as described in Methods. The cytosolic protein expression was detected with antibodies of mitochondrial apoptogenic factors by western blot analysis. Complex IV is used to detect the presence of mitochondria
Effect of K2154 on the activation of caspases
Activation of caspases during apoptosis results in the cleavage and activation/inactivation of a range of critical cellular substrates, including the DNA repair enzyme PARP. In order to determine which caspases were involved in K2154-induced apoptosis, the expressions of activated caspases were detected by western blotting. In control cells, PARP and caspase-8, -9 and -3 were all present as un-cleaved forms. After the treatment with K2154, the cleavage of PARP and caspases to catalytically active fragments was clearly detected in a time-dependent manner, suggesting the activation of these caspases. The data also showed that the time of caspase-9 activation, which preceded that of caspase-8, corresponded with the cytochrome c release (Fig. 6).
Effect of K2154 on the cleavage of procaspases and related substrates. Cells were incubated in 0.1% DMSO or K2154 (30 μM) for the indicated time courses. Then, the cells were harvested and lysed for the detection of protein expressions with antibodies of several caspases and related substrates by western blot analysis
Discussion
Microtubules, composed of α- and β-tubulin heterodimers, are important cytoskeletal components involved in the regulation of cell proliferation, differentiation, and apoptosis. The microtubular dynamics are delicately regulated by polymerization and depolymerization of tubulin. Numerous natural products inhibit cell proliferation by acting on tubulin, affecting its assembly properties. Accordingly, tubulin-directed agents are potential ligands as chemotherapeutic drugs for the treatment of a variety of tumor types (Ehrlichova et al. 2005; Sengupta et al. 2005; Jordan and Wilson 2004). K2154 is a chemically synthetic compound with a simple chemical structure. It displayed anti-proliferative activity in numerous types of human cancer cells. In particular, K2154 showed anticancer effect in P-gp-rich NCI/ADR-RES cells with similar potency to that in other cancer types. The data indicated that K2154 was not a P-gp substrate. It is meaningful for the development of cancer chemotherapeutic agents since P-gp confers multiple drug resistance by effluxing a diverse array of anticancer drugs, such as vincristine, vinblastine, docetaxel and taxol (Silverman 1999; Wils et al. 1994). Furthermore, our data showed that K2154 displayed more potency in inducing mitotic arrest and apoptosis in PC-3 cells than those in normal prostatic cells. The shorter doubling time of PC-3 than normal prostatic cells may partly explain the selectivity.
To further characterize the anti-tumor effect, K2154 was found to inhibit the polymerization of tubulin (>99% purity) in an in vitro turbidity assay and reduce the particulate fraction of β-tubulin in an in vivo assay. These data suggested that the direct target on tubulin, especially on β-tubulin, leading to the inhibition of tubulin polymerization might primarily contribute to K2154-induced effect. It has been suggested that the different affinity of the agents on tubulin isotype as well as various isotype compositions in different cancer types determine the efficacy and toxicity of the agents (Pellegrini and Budman 2005; Lobert et al. 1998; Correia 1991). After further investigation, our data demonstrated that K2154 exhibited more selectivity toward βII- and βIII-tubulin, although it inhibited tubulin polymerization, an action similar to vinca alkaloids. Numerous studies suggest that vincristine triggers dissolution of microtubules, inducing formation of tubulin paracrystals and causing neurotoxicity (Muller et al. 1988). In contrast, although K2154 inhibited tubulin polymerization (Fig. 2a), the cells with a moderate degree of microtubule association were apparent (Fig. 2c). This discrepancy may, possibly, explain the lesser cytotoxic effect of K2154 on human normal prostatic cells and rat primary neuronal and microglial cells (data not shown). Furthermore, βI- and βIV-tubulin were found in most of the mammalian axonemal structures, including the olfactory sensory neurons and ciliated epithelial cells of the nose, trachea, ependyma, fallopian tube and testis, suggesting their functional involvement (Vent et al. 2005; Jensen-Smith et al. 2003). Our data showed that K2154 exhibited minor effect on βI and βIV-isotypes, suggesting a limited toxicity on cilia function.
It has been widely accepted that the mitotic arrest of the cell cycle or an aberrant mitotic exit into a G1-like multinucleate condition is a crucial causative factor for apoptosis induced by tubulin-binding agents (Chen and Horwitz 2002; Wang et al. 1999; Lin et al. 1998). Furthermore, an increase in Cdk1 activity has been found in cells during several apoptotic insults. Several studies have suggested that Cdk1 activation can be a mechanism of cell killing and lead to apoptosis (Konishi et al. 2002). In this study, the mitotic arrest induced by K2154 was found to precede the cell death. Accordingly, the activation of Cdk1 was identified to contribute to K2154-induced cell death by several observations, namely, (1) the elevation of cyclin B1 expression, (2) the phosphorylation of Cdc25C, (3) the dephosphorylation on inhibitory Tyr15 and phosphorylation on activating Thr161 of Cdk1, and (4) the suppression of cell death by roscovitine (a Cdk1 inhibitor). Mitotic catastrophe, a type of cell death thought to share some apoptotic pathways, is extensively characterized in the effect induced by tubulin-binding agents (Hyzy et al. 2005; Castedo et al. 2004). Mitotic catastrophe is controlled by numerous molecular regulators, such as cyclin B1-dependent Cdk1, aurora kinases, cell-cycle checkpoint proteins, survivin, p53, caspases and the Bcl-2 family of proteins (Hyzy et al. 2005; Castedo et al. 2004). In this study, K2154-treated cells appeared to stay arrested in the mitotic phase from which they consequently entered a cell death pathway or exited mitosis, but the nuclei were deformed (Fig. 2c). Beside the morphological observations, K2154-treated cells showed various biochemical criteria, including the activation of Cdk1/cyclin B1, the dramatic appearance of mitotic epitope MPM-2, the activation of caspases and alteration of theBcl-2 family of proteins (see below). It is likely that the cells treated with K2154 at least share some characteristics with those of undergoing mitotic catastrophe.
Next to the function in respiration, mitochondria play a crucial role in the control of apoptosis. Numerous stimuli and stresses are able to stimulate the release of apoptogenic factors from the mitochondrial intermembrane space, leading to the programmed demise of the cell. There is increasing evidence that the regulation of the Bcl-2 family of proteins shares the signaling pathways induced by tubulin-binding agents (Wang et al. 1999). Our data demonstrated that K2154 induced the phosphorylation of Bcl-2 at ser70, which was widely reported to be associated with loss of its anti-apoptotic function (Wang et al. 1999; Haldar et al. 1995). Additionally, the Bcl-xL phosphorylation was also stimulated by K2154 and other tubulin-binding agents (Poruchynsky et al. 1998). It is believed that Bcl-xL phosphorylation may play a crucial role in regulating its function and may participate in the determination of tubulin/microtubule-related chemotherapeutic efficacy in human tumors (Poruchynsky et al. 1998). Interestingly, K2154 also stimulated the cleavage of Mcl-1 protein. It has been suggested that Mcl-1 is a substrate for caspases, in particular caspase-3, during induction of apoptosis. The Mcl-1 cleavage occurs after Asp127 and Asp157 and generates four products of 24, 19, 17 and 12 kDa (Michels et al. 2005; Herrant et al. 2004). Our data showed that the onset of Mcl-1 cleavage correlated with the activation of caspase-3, resulting in a predominant fragment of 24 kDa, suggesting the involvement of caspase-3 in Mcl-1 cleavage.
Caspases are intracellular cysteine proteases responsible for and associated with apoptosis. In this study, the western blot analysis revealed that the cytochrome c release and activation of caspase-9 and -3, which preceded caspase-8, were time-correlated, indicating that K2154-induced apoptosis might be primarily mediated by the mitochondrial (intrinsic) apoptosis pathway. Furthermore, the release of AIF was also induced by K2154. Recent studies have identified the relationship between PARP-1 activation and nuclear translocation of AIF, suggesting that AIF is a crucial downstream effector of PARP-1-mediated cell death (Hong et al. 2004). Although the link between PARP-1 activation and AIF release reaction could not be made in the present work, the AIF-mediated caspase-independent pathway might contribute to K2154-induced anti-tumor effect in this study. Taken together, our data suggest that the mechanism of action of K2154 involves an interaction with tubulin resulting in disturbance of regular function of mitotic spindles, leading to mitotic arrest and activation of apoptotic signaling cascades. K2154 is an effective anti-tubulin agent with simple chemical structure, which allows further design and synthesis of derivatives to minimize toxic effect and maximize aqueous solubility for the development of cancer chemotherapeutic drugs.
References
Abuharbeid S, Apel J, Zugmaier G, Knabbe C, Sander M, Gilbert S, Czubayko F, Aigner A (2005) Inhibition of HER-2 by three independent targeting strategies increases paclitaxel resistance of SKOV-3 ovarian carcinoma cells. Naunyn Schmiedebergs Arch Pharmacol 371:141–151
Aria T, Matsumoto G (1988) Subcellular localization of functionally differentiated microtubules in squid neurons: regional distribution of microtubule-associated proteins and β-tubulin isotypes. J Neurochem 51:1825–1838
Burgoyne RD, Cambray-Deakin MA, Lewis SA, Sarkar S, Cowan NJ (1988) Differential distribution of β-tubulin isotypes in cerebellum. EMBO J 7:2311–2319
Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G (2004) Cell death by mitotic catastrophe: a molecular definition. Oncogene 23:2825–2837
Chen JG, Horwitz SB (2002) Differential mitotic responses to microtubule-stabilizing and -destabilizing drugs. Cancer Res 62:1935–1938
Cleveland DW, Sullivan KF (1986) Identification of conserved isotype-defining variable region sequences for four vertebrate β tubulin polypeptide classes. Proc Natl Acad Sci USA 83:4327–4331
Correia JJ (1991) Effects of antimitotic agents on tubulin-nucleotide interactions. Pharmacol Ther 52:127–147
Debatin KM, Poncet D, Kroemer G (2002) Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene 21:8786–8803
Derry WB, Wilson L, Khan IA, Luduena RF, Jordan MA (1997) Taxol differentially modulates the dynamics of microtubules assembled from unfractionated and purified β-tubulin isotypes. Biochemistry 36:3554–3562
Ehrlichova M, Vaclavikova R, Ojima I, Pepe A, Kuznetsova LV, Chen J, Truksa J, Kovar J, Gut I (2005) Transport and cytotoxicity of paclitaxel, docetaxel, and novel taxanes in human breast cancer cells. Naunyn Schmiedebergs Arch Pharmacol 372:95–105
Guh JH, Hwang TL, Ko FN, Chueh SC, Lai MK, Teng CM (1998) Antiproliferative effect in human prostatic smooth muscle cells by nitric oxide donor. Mol Pharmacol 53:467–474
Haldar S, Jena N, Croce CM (1995) Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci USA 92:4507–4511
Herrant M, Jacquel A, Marchetti S, Belhacene N, Colosetti P, Luciano F, Auberger P (2004) Cleavage of Mcl-1 by caspases impaired its ability to counteract Bim-induced apoptosis. Oncogene 23:7863–7873
Holcik M, Gibson H, Korneluk RG (2001) XIAP: apoptotic brake and promising therapeutic target. Apoptosis 6:253–261
Hong SJ, Dawson TM, Dawson VL (2004) Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol Sci 25:259–264
Hyzy M, Bozko P, Konopa J, Skladanowski A (2005) Antitumour imidazoacridone c-1311 induces cell death by mitotic catastrophe in human colon carcinoma cells. Biochem Pharmacol 69:801–809
Jensen-Smith HC, Ludueña RF, Hallworth R (2003) Requirement for the βI and βIV tubulin isotypes in mammalian cilia. Cell Motil Cytoskeleton 55:213–220
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nature 4:253–265
Katsetos CD, Legido A, Perentes E, Mork SJ (2003) Class III β-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J Child Neurol 18:851–866
Konishi Y, Lehtinen M, Donovan N, Bonni A (2002) Cdc2 phosphorylation of Bad links the cell cycle to the cell death machinery. Mol Cell 9:1005–1016
Lee J, Han KC, Lee SY, Kim SY, Kang JH, Lewin NE, Best LS, Blumberg PM, Marquez VE (2001) 5-acyloxy-5-hydroxymethyltetrahydro-2-furancarboxylate as a novel template for protein kinase C (PKC) binding. Farmaco 56:203–210
Lin HL, Chang YF, Liu TY, Wu CW, Chi CW (1998) Submicromolar paclitaxel induces apoptosis in human gastric cancer cells at early G1 phase. Anticancer Res 18:3443–3449
Lobert S, Frankfurter A, Correia JJ (1998) Energetics of vinca alkaloid interactions with tubulin isotypes: implications for drug efficacy and toxicity. Cell Motil Cytoskeleton 39:107–121
Luduena RF (1998) Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol 178:207–275
Michels J, Johnson PW, Packham G (2005) Mcl-1. Int J Biochem Cell Biol 37:267–271
Muller LJ, Moorer-Van Delft CM, Roubos EW (1988) Snail neurons as a possible model for testing neurotoxic side effects of antitumor agents: paracrystal formation by vinca alkaloids. Cancer Res 48(24 pt 1):7184–7188
Ongkeko W, Ferguson DJ, Harris AL, Norbury C (1995) Inactivation of Cdc2 increases the level of apoptosis induced by DNA damage. J Cell Sci 108:2897–2904
Orosz F, Vértessy BG, Salerno C, Capuozzo E, Ovádi J (1997) The interaction of a new anti-tumour drug, KAR-2 with calmodulin. Br J Pharmacol 121:955-962
Pellegrini F, Budman DR (2005) Review: tubulin function, action of antitubulin drugs, and new drug development. Cancer Invest 23:264–273
Peterson JR, Smillie TJ, Rogers RD (1989a) Platelet activating factor antagonist design: structure of methyl trans-5-(3,4-dimethoxyphenyl)- 2,3,4,5-tetrahydro-2-oxo-4- furancarboxylate. Acta Crystallogr C 45(Pt 2):297–300
Peterson JR, Horsley DB, Brozik JA, Rogers RD (1989b) Platelet activating factor antagonist design. 3. X-ray crystal structure and intermolecular crystal lattice interactions of methyl trans-4-acetoxymethyl-4,5-dihydro- 2,5-bis(3,4-methylenedioxyphenyl)- 3-furancarboxylate. Acta Crystallogr C 45 (Pt 8):1164–1167
Poruchynsky MS, Wang EE, Rudin CM, Blagosklonny MV, Fojo T (1998) Bcl-xL is phosphorylated in malignant cells following microtubule disruption. Cancer Res 58:3331–3338
Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R (2005) Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 436:568-572
Seve P, Mackey J, Isaac S, Tredan O, Souquet PJ, Perol M, Lai R, Voloch A, Dumontet C (2005) Class III β-tubulin expression in tumor cells predicts response and outcome in patients with non-small cell lung cancer receiving paclitaxel. Mol Cancer Ther 4:2001–2007
Silverman JA (1999) Multidrug-resistance transporters. Pharm Biotechnol 12:353–386
Vent J, Wyatt TA, Smith DD, Banerjee A, Luduena RF, Sisson JH, Hallworth R (2005) Direct involvement of the isotype-specific c-terminus of β tubulin in ciliary beating. J Cell Sci 118:4333–4341
Walss-Bass C, Xu K, David S, Fellous A, Luduena RF (2002) Occurrence of nuclear βII-tubulin in cultured cells. Cell Tissue Res 308:215–223
Wang LG, Liu XM, Kreis W, Budman DR (1999) The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review. Cancer Chemother Pharmacol 44:355–361
Wils P, Phung-Ba V, Warnery A, Lechardeur D, Raeissi S, Hidalgo IJ, Scherman D (1994) Polarized transport of docetaxel and vinblastine mediated by P-glycoprotein in human intestinal epithelial cell monolayers. Biochem Pharmacol 48:1528–1530
Yu D, Liu B, Yao J, Tan M, Mcdonnell TJ, Hung MC (1998) Overexpression of Erbb2 blocks taxol-induced apoptosis by upregulation of p21cip1, which inhibits p34Cdc2 kinase. Mol Cell 2:581–591
Zhou BB, Li H, Yuan J, Kirschner MW (1998) Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc Natl Acad Sci USA 95:6785–6790
Acknowledgement
This work was supported by a research grant of the National Science Council of the Republic of China (NSC 94-2323-B-002 -006 and NSC 93-2323-B-002-003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, PH., Kung, FL., Kuo, SC. et al. Investigation of anti-tumor mechanisms of K2154: characterization of tubulin isotypes, mitotic arrest and apoptotic machinery. Naunyn-Schmied Arch Pharmacol 374, 223–233 (2006). https://doi.org/10.1007/s00210-006-0114-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-006-0114-x