Abstract
Propiverine is a commonly used antimuscarinic drug used as therapy for symptoms of an overactive bladder. Propiverine is extensively biotransformed into several metabolites that could contribute to its spasmolytic action. In fact, three propiverine metabolites (M-5, M-6 and M-14) have been shown to affect various detrusor functions, including contractile responses and L-type calcium-currents, in humans, pigs and mice, albeit with different potency. The aim of our study was to provide experimental evidence for the relationship between the binding of propiverine and its metabolites to human muscarinic receptor subtypes (hM1–hM5) expressed in chinese hamster ovary cells, and to examine the effects of these compounds on muscarinic receptor-mediated detrusor function. Propiverine, M-5, M-6 and M-14 bound to hM1–hM5 receptors with the same order of affinity for all five subtypes: M-6 > propiverine > M-14 > M-5. In HEK-293 cells expressing hM3, carbachol-induced release of intracellular Ca2+ ([Ca2+]i) was suppressed by propiverine and its metabolites; the respective concentration-response curves for carbachol-induced Ca2+-responses were shifted to the right. At higher concentrations, propiverine and M-14, but not M-5 and M-6, directly elevated [Ca2+]i. These results were confirmed for propiverine in human detrusor smooth muscle cells (hDSMC). Propiverine and the three metabolites decreased detrusor contractions evoked by electric field stimulation in a concentration-dependent manner, the order of potency being the same as the order of binding affinity. We conclude that, in comparison with the parent compound, loss of the aliphatic side chain in propiverine metabolites is associated with higher binding affinity to hM1–hM5 receptors and higher functional potency. Change from a tertiary to a secondary amine (M-14) results in lower binding affinity and reduced potency. Oxidation of the nitrogen (M-5) further lowers binding affinity as well as functional potency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscarinic receptor antagonists are the most widely used drugs for the therapy of overactive bladder (OAB) symptoms (Sellers et al. 2001; Andersson et al. 2002; Andersson and Yoshida 2003; Hedge 2006). Spasmolytic agents with various properties appear to be effective, i.e. nonselective antimuscarinics, muscarinic receptor subtype 3 (M3)-selective compounds, and drugs with additional pharmacological activity (Chapple 2004; Ouslander 2004; Oki et al. 2005).
Besides its antimuscarinic properties, propiverine possesses other mechanisms of action, including direct spasmolytic effects (Andersson et al. 1999; Wuest et al. 2002, 2005b). In human detrusor strips, propiverine inhibits contractile responses elicited by electric field stimulation (EFS), stimulation with acetylcholine (Wada et al. 1995) and with carbachol (Yono et al. 1999; Wuest et al. 2005a). Previous studies have revealed that propiverine reduces KCl-induced contractions in guinea pig (Haruno 1992; Tokuno et al. 1993), and KCl- as well as CaCl2-induced contractions in human bladder strips (Wada et al. 1995). In rabbit detrusor, propiverine also appears to impair intracellular Ca2+ homeostasis (Madersbacher and Mürtz 2001).
Propiverine is extensively biotransformed in the liver to several active metabolites. After oral application of a single dose and after 5 days of treatment, the propiverine N-oxide M-5 was found to be the main metabolite in serum, while the N-oxide lacking the aliphatic side chain (M-6) and the secondary amine (M-14; for chemical structures see Fig. 1) were detectable only in urine. The pharmacological properties of propiverine metabolites have not been characterised in detail (Andersson et al. 1999). We recently reported that, in isolated detrusor strips of humans, pigs and mice, metabolites with an aliphatic side chain like the parent compound, i.e. M-5 and M-14, suppressed the effect maximum in the concentration-response curve for carbachol, whereas the compound lacking the aliphatic side chain (M-6) shifted the concentration-response curve to higher concentrations without any reduction of the maximum effect (Wuest et al. 2005b). Furthermore, propiverine, M-5 and M-14, but not M-6, reduced the atropine-resistant component of contraction elicited by EFS, suggesting additional effects besides being antimuscarinic. We had assumed that the non-antimuscarinic activity of propiverine and the metabolites M-5 and M-14 might be related to blocking of Ca2+ influx via an L-type Ca2+ current (ICa,L) because it had been shown previously in transgenic mice that knock-out of L-type Ca2+ channels completely abolished detrusor contractions mediated via stimulation of muscarinic receptors (Wegener et al. 2004). While propiverine and M-14 did in fact block ICa,L in detrusor smooth muscle cells, M-5 was ineffective and M-6 impaired ICa,L only at concentrations higher than 10 μM, i.e. concentrations at which atropine-sensitive EFS contractions were mostly abolished (Wuest et al. 2005b). These results did not support a simple relationship between the effects on atropine-resistant EFS contractions and blocking of ICa,L. In addition, our previous study revealed marked differences in the effective concentration range between propiverine and its metabolites (Wuest et al. 2005b).
To gain more insight into the pharmacology of the propiverine metabolites M-5, M-6 and M-14, we investigated their binding to five human muscarinic receptor subtypes (hM1–hM5) stably expressed in Chinese hamster ovary (CHO) cells. In addition, we investigated the potency and efficacy of these compounds in different models of detrusor function. The aim of our study was to provide further experimental evidence for the contribution of these metabolites to the therapeutic action of propiverine.
Methods
Radioligand binding studies
Cell culture
CHO cell lines, stably transfected with one of the five human muscarinic receptor subtypes (hM1–hM5), were obtained from N. Buckley (University College, London, UK), and were maintained in Dulbecco’s modified Eagle’s medium (DMEM) enriched with 10% fetal calf serum (FCS), 200 IU/ml penicillin and 100 μg/ml streptomycin. The stock culture medium also contained geniticin (0.5 mg/ml). Subcultures prepared for muscarinic receptor binding studies were prepared in a medium without geniticin. The cells were harvested with a rubber scraper, centrifuged, rinsed in phosphate buffered saline (PBS) enriched with 1 mM EDTA, homogenised in a 20 mM Tris-HCl buffer enriched with 250 mM sucrose and stored in liquid nitrogen until use.
[3H]-NMS binding
Experiments were carried out using standard protocols as described in the literature (Borchert et al. 2004; Schneider et al. 2005; Maruyama et al. 2006). CHO cell homogenates (at a concentration equivalent to ∼0.075 nM muscarinic receptors) were incubated at 25°C in the presence of the indicated concentrations of the unlabeled drug and [3H]-N-methylscopolamine ([3H]-NMS; specific activity 80 Ci/mmol), for a time sufficient to achieve equilibrium. The radioactive compound was dissolved in 1.2 ml 50 mM sodium-phosphate buffer (pH 7.4), enriched with 2 mM MgCl2. Non-specific binding was defined as binding in the presence of 10 μM atropine. The tracer concentrations used were 0.10 nM for competition binding curves with M2 and M5 receptors. To measure the tracer affinities for these receptors, the [3H]-NMS concentration was varied between 0.05 and 1.5 nM. The incubation period was 2 h for M1, M2 and M4 receptors, and 4 h for M3 and M5 receptors. The incubation was terminated by filtration over Gelman A/C filters (Gelman Sci, Ann Arbor, MI) soaked in 0.01% polyethyleneimine (Sigma, St. Louis, MO), to reduce non-specific binding. The filters were rinsed four times with 2 ml ice cold 50 mM sodium-phosphate buffer (pH 7.4), and the radioactivity was counted by liquid scintillation counting.
Data analysis
The competition curves for propiverine and its metabolites M-5, M-6 and M-14 were repeated at least three times in duplicate. The experimental data were analysed by non linear curve fitting using GraphPad Prism. The unlabeled drugs pK i values were calculated assuming competitive inhibition of tracer binding, using the Cheng and Prusoff equation. Two competition curves using reference selective antagonists were performed in the same cell homogenates to control the identity of the muscarinic receptor subtype studied.
Measurement of intracellular calcium concentration [Ca2+]i
Isolation of human detrusor smooth muscle cells (hDSMC)
Human detrusor tissue was obtained from patients undergoing transurethral tumour resection (TUR-BT). All patients had given informed written consent in accordance with the regulations of the local ethical committee. Samples from tumour-free parts of the bladder wall were taken, immediately placed in cold buffer solution and transported to the laboratory within 1 h. After removal of the mucosal layer, the tissue was cut into small pieces, placed in buffer solution [in mM: 135 NaCl, 6 KCl, 10 HEPES, 2 MgCl2, 1.8 CaCl2, 10 glucose, 1 mg/ml bovine serum albumin (BSA), pH 7.3 adjusted with NaOH] and incubated with 1,000 units collagenase type 2 (218 U/mg) and 0.05% papain (70 U/mg) under continuous stirring for 30 min at 37°C. The tissue was filtered, the solution discarded and the detrusor pieces incubated again using the same buffer containing only 1,000 units collagenase type 2 and stirred for another 10 min. The resulting cell suspension was centrifuged (1,200 rpm for 5 min) and washed twice with buffer solution prior to use for cell culture.
Cell culture and immunofluorescence staining of hDSMC
The hDSMC were cultured in DMEM. The medium contained 10% FCS, 1% glutamax, 1% non essential amino acids and 1% penicillin G/streptomycin. Prior to the [Ca2+]i experiments, cells were grown to near confluence in 175 cm3 culture flasks.
Cells grown on CultureSlides (BD Bioscience; Belgium) were fixed with methanol for 5 min. After rinsing with PBS, cells were incubated with primary antibodies [1:100 (anti-myosin) or 1:400 (anti-α-actin) dilution in PBS] for 60 min. After washing with PBS they were counterstained with 5 μg/ml 4,6-diamidino-2-phenylindole (DAPI).
Cell culture of HEK-293 cells
HEK-293 cells, stably expressing the human muscarinic receptor subtype M3, were obtained from M. Schmidt (Universitatsklinikum Essen, Germany). HEK-293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM/F-12) containing 10% FCS, 1% penicillin G/streptomycin and 1% geniticin-418.
[Ca2+]i measurements
HEK-293 cells and hDSMC were trypsinised, resuspended in a HEPES-containing buffer solution (in mM: 55 NaCl, 6 KCl, 2 MgCl2, 1.8 CaCl2, 10 HEPES, 10 glucose, 1 mg/ml BSA at pH 7.3) and loaded with Fura-2 by incubation with 5 μM Fura-2/AM (dissolved in DMSO) at 37°C for 30 min. Following loading, the cells were washed twice and resuspended in the HEPES buffer solution at a density of about 2×105 cells/0.5 ml. The fluorescence of the cell suspensions was measured within the next 1.5 h in 500 μl quartz glass microcuvettes at the excitation wavelengths of 340/380 nm and an emission wavelength of 505 nm with a dual-wavelength fluorometer (LS50B, Perkin Elmer, Boston, MA). During the measurement cell suspensions were maintained at 37°C. At the end of each experiment, digitonin (final concentration 0.02%) and EGTA (final concentration 7.5 mM) were added to obtain maximum and minimum fluorescence (R max and R min), respectively. [Ca2+]i was calculated from the ratio of fluorescence (R) at 340 nm and 380 nm as described by Grynkiewicz et al. (1985).
Detrusor contraction experiments
Material
The majority of the contractile experiments were done in strips from biopsies obtained during transurethral tumour resections. All patients had given informed written consent in accordance with the regulations of the institutional ethical committee. The mucosa and all tissue obviously damaged by the high voltage forceps were removed, but only one or two muscle strips could be dissected from each specimen. For comparative purposes, some muscle strips from cystectomy material were also used. After removal of serosa and mucosa (see Wuest et al. 2005c), four muscle strips (10–15 mm long, 4–5 mm wide) were dissected from each sample.
Detrusor contraction
Muscle strips were mounted in 5-ml organ baths containing carbogen-gassed Tyrode’s solution maintained at 37°C. Tension generated was measured with an isometric force transducer (GM 2, Föhr Medical Instruments, Seeheim/Ober Beerbach, Germany), amplified and recorded with a data and recording system (Chart 4.0, ADInstruments, Sydney, Australia). Resting load was set to 10 mN and was readjusted after 30 min. During the equilibration period of 60 min, the bath solution was changed once. After 20 min of stabilisation, muscle strips were subjected to EFS (Malysz et al. 2004; Wuest et al. 2005a–c; stimulator, Föhr Medical Instruments) with the following parameters: pulse duration 1 ms at 40 Hz (biopsy strips) or 30 Hz (cystectomy samples) with 90 mA, with trains of stimuli for 10 s (biopsies) or 5 s (cystectomies) every 2 min. The compounds under investigation were added in cumulatively increasing concentrations with 30 min between increments. To estimate the non-neuronally mediated portion of muscle contraction under our stimulation conditions, nerve conduction was completely blocked by adding the neurotoxin tetrodotoxin (TTX, 1 μM) at the end of each experiment. Experimental data were analysed using GraphPad Prism 3.02 (GraphPad Software, San Diego, CA). Average values for the EFS-induced muscle contraction amplitudes were obtained from the last five contractions before the next concentration increase. The magnitude of drug effect is given in percent inhibition of the electrically evoked contraction amplitude before any substance addition (=100 %).
Chemicals and solutions
[3H]-NMS was obtained from Amersham (Little Chalfont, UK). The following drugs were gifts: pirenzepine and AF-DX 116, from Boehringer-Ingelheim (Germany); hexahydro-sila-difendiol (HHSiD), from G. Lambrecht (Frankfurt University, Germany), himbacine, from Dr. Taylor (Melbourne University, Australia) and 4-diphenyl-acetoxy-N-methyl-piperidine methiodide (4-DAMP), from R.B. Barlow (Bristol University, UK). Cell culture media (DMEM, DMEM/F-12), FCS and flasks were obtained either from GIBCO Life Technologies (Gent, Belgium) or SIGMA-Aldrich (St. Louis, MO). Fura-2/AM and Pluronic F-127 were from Calbiochem (EMD Biosciences, Merck, Darmstadt, Germany). The monoclonal smooth muscle specific anti-myosin (smooth) and anti-α-actin (clone 1A4) antibodies (both from mouse) were from Sigma. Propiverine and its metabolites M-5, M-6 and M-14 were obtained from APOGEPHA Arzneimittel GmbH (Dresden, Germany). All other chemicals were purchased from Sigma-Aldrich.
Results
Binding of propiverine and its metabolites to muscarinic receptors
Binding of propiverine and its metabolites to the muscarinic receptor subtypes expressed in CHO cells revealed that the compounds bound to all receptor subtypes in the following order of affinity: M-6 > propiverine > M-14 > M-5 (Table 1). The reference compounds 4-DAMP and AF-DX 116 had the expected selectivity for hM1 / hM3 and hM2, respectively. Propiverine and its metabolites showed similar orders of affinity within their characteristic concentration range of receptor binding: All compounds exhibited higher affinities for hM3 and the remaining receptor subtypes than for hM2. These results suggest that neither propiverine nor its metabolites are selective for hM3 receptor binding.
Effects on [Ca2+]i
In HEK-293 cells expressing hM3, a single application of carbachol (1 μM) induced an increase in [Ca2+]i (Fig. 2), with the increase occurring over a similar concentration range as described previously (Schmidt et al. 1995; Ikeda et al. 2002). Atropine (1 μM) fully inhibited the carbachol-induced [Ca2+]i elevation (data not shown). Exposure of the cells to 100 μM propiverine completely prevented the carbachol-induced effect (Fig. 2b), while 100 μM M-14 merely attenuated the effect (Fig. 2c). The traces also show that addition of 100 μM propiverine alone caused a small increase in [Ca2+]i. A similar effect was observed with M-14. Figure 3 summarises the effects of propiverine, M-5, M-6 and M-14 on the carbachol-induced [Ca2+]i elevation in HEK-293 cells expressing hM3. Increasing concentrations of propiverine and its metabolites impaired the [Ca2+]i response. The −log IC50 [M] values estimated from fitting a sigmoidal function to the mean data points yielded the following order of potency: M-6 [5.47] ≥ propiverine [5.27] > M-14 [4.53] > M-5 [<4.00]. The intrinsic [Ca2+]i elevating effects of propiverine and M-14 (100 μM each) amounted to 49±7 nmol/l [Ca2+]i (n=5) with 100 μM propiverine, and 58±7 nmol/l [Ca2+]i (n=6) with 100 μM M-14 (Fig. 4). This phenomenon was not observed after addition of M-5 or M-6.
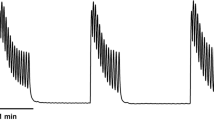
Original recordings for the carbachol-induced elevation in intracellular Ca2+ ([Ca2+]i) using 1 μM carbachol in a the absence of test drugs, b the presence of 100 μM propiverine, and c the presence of 100 μM M-14 in HEK-293 cells stably transfected with hM3 receptors. Maximum Ca2+ concentration was determined after adding 0.02 % digitonin and minimum Ca2+ after addition of 20 mM EGTA. Data are presented as the ratio of the two measured wavelengths (F 340 /F 380)
Concentration-dependent effects of a propiverine and b its metabolite M-14 on the cytosolic [Ca2+]i concentration in HEK-293 cells stably transfected with hM3 receptors. Data are presented as mean ± SEM of n experiments. The insets in both diagrams show representative original tracings of either propiverine or M-14 during electric-field stimulation (EFS) on human detrusor strips
Figure 5a shows cultivated hDSMC in light transmission micrographs and fluorescence micrographs of cells positively stained for smooth muscle α-actin. These cells were used to verify drug effects on [Ca2+]i in native cells; concentration-response curves for the carbachol-induced Ca2+ elevation in hDSMC are shown as the change in ratio from the two fluorescence wavelengths and the increase in [Ca2+]i (Fig. 5b). The maximum rise in [Ca2+]i from 130±13 to 347±32 nmol/l (n=8) occurred with 10 μM carbachol. Addition of 100 μM propiverine reduced the maximum and shifted the concentration-response curve by about 2 log units to higher concentrations.
a Primary culture of human detrusor smooth muscle cells (hDSMC) (left). Immunofluorescence staining with monoclonal anti-myosin clone HSM-V (1:250) and anti-mouse FITC conjugate (1:100; right). The inset on the right side shows a single cell stained with anti-α smooth muscle actin clone 1A4 FITC conjugate. b Concentration-response curves for carbachol in the absence and presence of 100 μM propiverine in cultivated hDSMC. Data are presented as the change in ratio of the two measured wavelengths (F340 /F380) or as cytosolic [Ca2+]i concentration in nmol/l. Data represent the mean ± SEM of n experiments
Effects of propiverine and its metabolites on electrically induced contractions
In human detrusor strips from biopsies, EFS-induced contractions spontaneously declined to 72 ± 10% of the control after 3.5 h of regular stimulation. Like atropine, propiverine and the three metabolites reduced contraction amplitudes, and the concentration-response curve for propiverine and M-14 appeared to be biphasic (see Discussion). The order of potency for the major decline was: atropine > M-6 > propiverine ≈ M14 > M-5 (Fig. 6, Table 2). All compounds impaired contractile responses with a similar efficacy. Addition of the neurotoxin tetrodotoxin (1 μM) did not further reduce electrically induced contractions.
Concentration-dependent effects of propiverine and its metabolites M-5, M-6 and M-14 on electrically induced detrusor contraction in human tissue from biopsy samples in comparison to the effect of atropine and time-matched control experiments without any drugs added. Data represent the mean ± SEM of n experiments
To compare the order of potency of the investigated compounds with the order of affinity, we plotted the −log IC50 values of the effects on hM3 receptors of EFS-induced contractions according to the respective pK i values obtained from the binding experiments (Fig. 7). The concentrations of half maximum force reduction for propiverine and the metabolites correlate well with the binding affinities to hM3 receptors, which are thought to play the key role in mediating muscarinic receptor-induced contractions in human urinary bladder.
Correlation of potency (pEC50) and binding affinity (pK i) on hM3 receptors of the standard antimuscarinic drugs tolterodine, oxybuytnin, propiverine and the propiverine metabolites M-5, M-6 and M-14 in comparison to atropine. Drug potency was estimated as the pEC50 value for suppression of EFS-elicited detrusor contractions (Fig. 5). Binding affinities to hM3 receptor subtype are expressed as pK i values for propiverine, M-5, M-6 and M-14. For comparative purposes, the respective values for tolterodine, oxybutynin and atropine were taken from the literature as indicated (pK i values from Nilvebrant 1997; pEC50 values from Naerger et al. 1995)
Detrusor contractions in strips from biopsy versus cystectomy samples
The detrusor tissue used in the experiments described so far were obtained from biopsies, whereas in our previous studies of antimuscarinic drug effects, we used muscle strips from cystectomy patients (Wuest et al. 2005a,b). Since differences in tissue handling and preparation may directly affect force development, we compared contractile responses to standard stimuli in preparations from both sources. The amplitudes of muscarinic receptor-mediated contractions induced by EFS or by 10 μM carbachol were significantly lower in biopsy samples compared to cystectomy tissue. However, the contraction amplitudes induced by 85 mM KCl were not significantly different in both types of tissue (Fig. 8).
Mean contraction amplitudes of human biopsy samples in comparison to tissue preparations from cystectomy patients after EFS or stimulation with either 10 μM of carbachol or 85 mM KCl. The numbers in the columns represent the number of strips versus the number of patients. Note that the whole biopsy sample was used for mounting one or two muscle strips, whereas three to four strips were obtained from each cystectomy sample
Discussion
The main findings of our study were: (1) propiverine and its metabolites bind to stably expressed human muscarinic receptors in the following order of affinity: M-6 > propiverine > M-14 > M-5; (2) all compounds reduce the carbachol-induced [Ca2+]i elevation mediated via hM3 receptors; and (3) inhibition of electrically induced contractions in human detrusor from biopsies occurs in the same order of potency as binding.
After oral administration, propiverine undergoes an extensive first-pass effect resulting in a number of different metabolites (Guay 2003; Haustein and Hüller 1988). One major metabolic pathway involves oxidation of the tertiary nitrogen in the piperidinyl moiety leading to the production of N-oxides. The main metabolite found in serum and urine is the propiverine N-oxide M-5 (Haustein and Hüller 1988; Siepmann et al. 1998). Twenty-four hours after oral application of a single dose, the concentrations recovered in human urine were (in percent of the original dose): 2–3% propiverine, 20% M-5, 5% M-6 and 1 % M-14 (Haustein and Hüller 1988). The maximum serum concentrations after 5 days of treatment with the standard dose of 15 mg propiverine hydrochloride three times daily (median; range) were: 155 (96–240) ng ml−1 for propiverine, corresponding to 0.33 μM, and 645 (385–955) ng ml−1 for M-5, corresponding to 1.68 μM (Siepmann et al. 1998). In view of the extensive metabolism, it was proposed that the metabolites may contribute to the clinical effects of propiverine. Indeed, we found that M-5, M-6 and M-14 inhibited carbachol-induced Ca2+ release and also reduced electrically stimulated contractions in biopsy strips, as previously reported for strips from cystectomy samples (Wuest et al. 2005b). The order of potency for these two pharmacological effects was the same as the order of binding affinity to hM1–hM5 receptors (see below).
Propiverine and the three metabolites bound to all hM receptor subtypes. Each compound exhibited lowest affinity for hM2 receptors and similar affinity for the other subtypes. The differences in pK i values for binding to hM2 and hM3 receptors varied between 0.5 and 1.5 log units; nevertheless, the order of binding affinity to hM1– hM5 was the same, i.e. M-6 > propiverine > M-14 > M-5.
The hM3 subtype is the most important subtype involved in detrusor contraction (Chess-Williams et al. 2001; Fetscher et al. 2002; Yamanishi et al. 2000). Propiverine bound to hM3 receptors with lower affinity than the selective hM3 receptor antagonist 4-DAMP (see Table 2) and other drugs used in the therapy of OAB (i.e. tolterodine, pK i 8.5, oxybutynin, 8.5–9.2, darifenacin, 8.9–9.1, atropine, 9.0–9.7; Nilvebrant et al. 1997; Chapple 2004; Mansfield et al. 2005). Oxidation of the nitrogen without any change in the aliphatic side chain, as in M-5, dramatically reduced binding affinity to hM3 receptors, and this corresponded well with the observed low functional potency. In fact, M-5 was less potent than the parent compound with respect to preventing carbachol-induced elevation of [Ca2+]i or with respect to impairment of detrusor contractions induced by muscarinic receptor stimulation. In our previous work, M-5 did not block L-type Ca2+ channels even at the highest concentration studied (100 μM), although it reduced EFS-induced force completely at 1 mM (Wuest et al. 2005b). M-5 had only a small effect on maximum carbachol-induced contractions in human detrusor, although it had a larger effect in pig and mouse. This correlates well with the reduction in maximum carbachol contractions in the presence of the selective L-type Ca2+-channel blocker nifedipine, which was found to be significantly lower in human than in other species (M. Wuest et al. unpublished results; Schneider et al. 2004a,b). Therefore, we cannot exclude that M-5 may show some inhibition of ICa,L at very high concentrations.
Converting the tertiary amine into a secondary amine like M-14 lowered the binding affinity to hM3 receptors only slightly in comparison with the parent compound. This corresponds with the general differences in affinity of secondary and tertiary amines to muscarinic receptors, as for example reported for the tricyclic antidepressants desipramine and imipramine (Rehavi et al. 1987). In functional studies, M-14 reduced the maximum effect of the concentration-response curve for carbachol without any shift to higher concentrations (Wuest et al. 2005b). Like propiverine, M-14 reduced hM3 receptor-mediated [Ca2+]i release in HEK cells, albeit at higher concentrations. In line with the small difference in binding affinity to hM3 receptors, M-14 and propiverine show comparable potency and efficacy in reducing EFS detrusor contraction (cf. Fig. 6). Binding of M-14 to muscarinic receptors would support the idea that the metabolite causes a receptor-mediated pharmacological effect. M-14 also blocks L-type Ca2+ channels (Wuest et al. 2005b), and this effect may contribute to reduction of detrusor contractions since activation of calcium-influx via L-type calcium-channels is associated with muscarinic receptor-mediated detrusor contraction (Wegener et al. 2004; Schneider et al. 2004a,b).
Loss of the aliphatic side chain, as in the molecular structure of M-6, is associated with a higher binding affinity to hM3 receptors than the parent compound propiverine (see Table 1). Our data confirm recently published binding affinities of propiverine and M-6 in homogenates of human bladder, with pK i values of 6.81 versus 6.32, respectively (Maruyama et al. 2006). In hM3 receptors expressed in CHO-K1 cells, the differences in pK i values were smaller: 6.76 for propiverine versus 6.94 for M-6 (same reference). In the two functional models of [Ca2+]i elevation mediated by hM3 receptor stimulation and EFS-elicited detrusor contractions, M-6 was more than one order of magnitude more potent than propiverine (this study), but did not affect atropine-resistant contractions (Wuest et al. 2005b). Interestingly, M-6 neither reduced the maximum responses to carbachol nor blocked L-type Ca2+ calcium currents at ≤10 μM (Wuest et al. 2005b). The concentration ranges of binding characteristics and pharmacological effects in different models of detrusor function are strikingly similar (this study; Maruyama et al. 2006; Wuest et al. 2005b), therefore M-6 is likely to be involved in the drug effect. It must be emphasized, however, that M-6 was not detected in serum but was found in urine only (Haustein and Hüller 1988). If M-6 does in fact contribute to the therapeutic effect of propiverine it must be assumed that it reaches its site of action via the urothelium, which also contains muscarinic receptors (Andersson and Arner 2004; Gillespie et al. 2003; Hawthorn et al. 2000).
The paradoxical increases in baseline tension recorded with high concentrations of propiverine and M-14 were similar to our previous observation with atropine and propiverine in cystectomy samples (Wuest et al. 2005a). Although the phenomenon was not found in all preparations, we suggest that it may be related to an unselective, direct effect of the compounds on the Ca2+ release mechanism, since propiverine and M-14 elevated [Ca2+]i in HEK-293 cells (cf. Fig. 4). M-6 did not directly increase [Ca2+]i, which was not surprising because it is the only propiverine metabolite without the aliphatic side chain and probably acts exclusively as a muscarinic antagonist. Conversely, all propiverine metabolites with an unchanged aliphatic side chain were associated with an unsurmountable antagonism in guinea-pig ileum (Siegmund et al. 1990). M-5 on the other hand, like propiverine and M-14, does possess the aliphatic side chain and also depresses carbachol-induced detrusor contraction in an unsurmountable manner. We suggest that the lack of a direct effect on [Ca2+]i may be due to its generally low affinity and potency.
In this study the effects of propiverine and its metabolites were measured in human detrusor biopsies in which absolute force development in response to high KCl was similar as in cystectomy samples, suggesting that depolarisation-dependent contractile function does not differ between the two preparations. However, contractions elicited by electric field- or carbachol were significantly lower in biopsy specimens than in cystectomy samples even when corrected for smaller sample size (Wuest et al. 2005b). This suggests some impairment of muscarinic receptor-mediated signal transduction in the biopsies. Nevertheless, potency and efficacy of the individual spasmolytic compounds were comparable in both types of tissue (see Table 2 and Wuest et al. 2005b). These findings confirm and extend previous reports in the literature where biopsies were found to be suitable preparations for experimenting with porcine or human detrusor contractions (Harrison et al. 1988; O’Reilly et al. 2002).
In summary, our results suggest that the main metabolite of propiverine, M-5, probably contributes little to the overall effect of propiverine because of its low affinity to hM3 receptors. Conversely, because of the high affinity to muscarinic receptors and high potency in different models of detrusor contractions, M-6 should contribute significantly despite the fact that it is not detected in plasma but in urine only. The intermediate position of M-14 with respect to binding affinity and potency suggests some limited contribution to the therapeutic action of propiverine.
References
Andersson KE, Yoshida M (2003) Antimuscarinics and the overactive detrusor – which is the main mechanism of action? Eur Urol 43:1–5
Andersson KE, Arner A (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84:935–986
Andersson KE, Appell R, Cardozo LD, Chapple C, Drutz HP, Finkbeiner AE, Haab F, Vela Navarrete R (1999) The pharmacological treatment of urinary incontinence. BJU Int 84:923–947
Andersson KE, Appell R, Awad S, Chapple C, Drutz HP, Finkbeiner AE, Fourcroy J, Haab F, Wein A (2002) Pharmacological treatment of urinary incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A (eds) Incontinence, 2nd International Consultation on Incontinence, Plybridge, UK, pp 481–511
Borchert VE, Czyborra P, Fetscher C, Goepel M, Michel MC (2004) Extracts from Rhois aromatica and Solidaginis virgaurea inhibit rat and human bladder contraction. Naunyn Schmied Arch Pharmacol 369:281–286
Chapple CR (2004) Darifenacin: a novel M3 muscarinic selective receptor antagonist for the treatment of overactive bladder. Expert Opin Investig Drugs 13:1493–500
Chess-Williams R, Chapple CR, Yamanishi T, Yasuda K, Sellers DJ (2001) The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro. J Auton Pharmacol 21:243–248
Fetscher C, Fleichman M, Schmidt M, Krege S, Michel MC (2002) M3 muscarinic receptors mediate contraction of human urinary bladder. Br J Pharmacol 136:641–644
Gillespie JI, Harvey IJ, Drake MJ (2003) Agonist- and nerve-induced phasic activity in the whole isolated bladder of the guinea pig: evidence for two types of bladder activity. Exp Physiol 88:343–357
Grynkiewicz G, Poenie M, Tsein RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3340–3350
Guay DR (2003) Clinical pharmacokinetics of drugs used to treat urge incontinence. Clin Pharmacokinet 42:1243–1285
Harrison SC, Sibley GN, Doyle PT, Ferguson DR (1988) The use of endoscopic detrusor muscle biopsies for in vitro muscle strip studies. Br J Urol 61:210–212
Haruno A (1992) Inhibitory effects of propiverine hydrochloride on the agonist-induced or spontaneous contractions of various isolated muscle preparations. Arzneim-Forsch/Drug Res 42:815–817
Haustein KO, Hüller G (1988) On the pharmacokinetics and metabolism of propiverine in man. Eur J Drug Metab Pharmacokinet 13:81–90
Hawthorn MH, Chapple CR, Cock M, Chess-Williams R (2000) Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol 129:416–419
Hedge S (2006) Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol 147:S80–S87
Ikeda K, Kobayashi S, Suzuki M, Miyata K, Takeuchi M, Yamada T, Honda K (2002) M(3) receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland. Naunyn Schmied Arch Pharmacol 366:97–103
Madersbacher H, Mürtz G (2001) Efficacy, tolerability and safety profile of propiverine in the treatment of the overactive bladder (non-neurogenic and neurogenic). World J Urol 19:324–335
Malysz J, Buckner SA, Daza AV, Milicic I, Perez-Medrano A, Gopalakrishnan M (2004) Functional characterization of large conductance calciumactivated K+ channel openers in bladder and vascular smooth muscle. Naunyn Schmied Arch Pharmacol 369:481–489
Mansfield KJ, Liu L, Mitchelson FJ, Moore KH, Millard RJ, Burcher E (2005) Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in ageing. Br J Pharmacol 144:1089–1099
Maruyama S, Oki T, Otsuka A, Shinbo H, Ozono S, Kageyama S, Mikami Y, Araki I, Takeda M, Masuyama K, Yamada S (2006) Human muscarinic receptor binding characteristics of antimuscarinic agents to treat overactive bladder. J Urol 175:365–369
Naerger H, Fry C, Nilvebrant L (1995) Effect of tolterodine on electrically induced contractions of isolated human detrusor muscle from stable and unstable bladders [abstract]. Neurourol Urodyn 14:524–526
Nilvebrant L, Andersson KE, Gillberg PG, Stahl M, Sparf B (1997) Tolterodine - a new bladder-selective antimuscarinic agent. Eur J Pharmacol 327:195–207
Oki T, Sato S, Miyata K, Yamada S (2005) Muscarinic receptor binding, plasma concentration and inhibition of salivation after oral administration of a novel antimuscarinic agent, solifenacin succinate in mice. Br J Pharmacol 145:219–227
O’Reilly BA, Kosaka AH, Knight GF, Chang TK, Ford APDW, Rymer JM, Popert R, Burnstock G, McMahon S (2002) P2X receptors and their role in female idiopathic detrusor instability. J Urol 167:157–164
Ouslander JG (2004) Management of overactive bladder. N Engl J Med 350:786–799
Rehavi M, Weiss H, Nissenkorn I, Rubinstein R, Cohen S (1987) A comparative study of the affinities of some tricyclic antidepressants for the muscarinic cholinergic receptor in human and guinea-pig bladder, ileum and brain in relation to differential drug potency. Life Sci 40:1819–1827
Schmidt M, Bienek C, van Koppen CJ, Michel MC, Jakobs KH (1995) Diferential calcium signalling be M2 and M3 muscarinic acethylcholine receptors in a single cell type. Naunyn Schmied Arch Pharmacol 352:469–476
Schneider T, Fetscher C, Krege S, Michel MC (2004a) Signal transduction underlying carbachol-induced contraction of human urinary bladder. J Pharmacol Exp Therap 309:1148–1153
Schneider T, Hein P, Michel MC (2004b) Signal transduction underlying carbachol-induced contraction of rat urinary bladder. I. Phospholipases and Ca2+ sources. J Pharmacol Exp Therap 308:47–53
Schneider T, Hein P, Michel-Reher MB, Michel MC (2005) Effects of ageing on muscarinic receptor subtypes and function in rat urinary bladder Naunyn Schmied Arch Pharmacol 372:71–78
Sellers DJ, Chapple CR, Chess-Williams R (2001) Potential therapeutic targets for treatment of the overactive bladder. World J Urol 19:307–311
Siegmund W, Niguissie M, Talahun K, Aitenfissu H, Franke G, Wengler A (1990) Anticholinergic properties of propiverine and its metabolites. Pharmazie 45:67–68
Siepmann M, Nokhodian A, Thümmler D, Kirch W (1998) Pharmacokinetics and safety of propiverine in patients with fatty liver disease. Eur J Clin Pharmacol 54:767–771
Tokuno H, Chowdhury JU, Tomita T (1993) Inhibitory effects of propiverine on rat and guinea-pig urinary bladder muscle. Naunyn-Schmied Arch Pharmacol 348:659–662
Wada Y, Yoshida M, Kitani K, Kikukawa H, Ichinose A, Takahashi W, Gotoh S, Inadome A, Machida J, Ueda S (1995) Comparison of the effects of anticholinergic drugs on human isolated urinary bladder. Arch Int Pharmacodyn 330:76–79
Wegener JW, Schulla V, Lee TS, Koller A, Feil S, Feil R, Kleppisch T, Klugbauer N, Moosmang S, Welling A, Hofmann F (2004) An essential role of Cav1.2 L-type calcium channel for urinary bladder function. FASEB J 18:1159–1161
Wuest M, Averbeck B, Reif S, Bräter M, Ravens U (2002) Different responses to drugs against overactive bladder in detrusor muscle of pig, guinea pig and mouse. Eur J Pharmacol 454:59–69
Wuest M, Morgenstern K, Graf EM, Braeter M, Hakenberg OW, Wirth MP, Ravens U (2005a) Cholinergic and purinergic responses in isolated human detrusor in relation to age. J Urol 173:2182–2189
Wuest M, Hecht J, Christ T, Braeter M, Schoeberl C, Hakenberg OW, Wirth MP, Ravens U (2005b) Pharmacodynamics of propiverine and three of its main metabolites on detrusor contraction. Br J Pharmacol 145:608–619
Wuest M, Kaden S, Hakenberg OW, Wirth MP, Ravens U (2005c) Effect of rilmakalim on detrusor contraction in the presence and absence of urothelium. Naunyn-Schmied Arch Pharmacol 372:203–212
Yamanishi T, Chapple CR, Yasuda K, Chess-Williams R (2000) The role of M2 muscarinic receptors in mediating contraction of the pig urinary bladder in vitro. Br J Pharmacol 131:1482–1488
Yono M, Yoshida M, Wada Y, Kikukawa H, Takahashi W, Inadome A, Seshita H, Uead S (1999) Pharmacological effects of tolterodine on human isolated urinary bladder. Eur J Pharmacol 368:223–230
Acknowledgements
The authors would like to thank Sabine Kirsch for her technical assistance. This project was supported in part by a grant from the “Inno-Regio-Project” BioMet (BMBF, project number 05) from the German Bundesministerium für Bildung und Forschung.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wuest, M., Weiss, A., Waelbroeck, M. et al. Propiverine and metabolites: differences in binding to muscarinic receptors and in functional models of detrusor contraction. Naunyn-Schmied Arch Pharmacol 374, 87–97 (2006). https://doi.org/10.1007/s00210-006-0103-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-006-0103-0