Abstract
The induction of endoplasmic reticulum (ER) stress has been reported as a key contributor to the cardiotoxicity of doxorubicin. Previous in vitro and in vivo studies suggest that sacubitril/valsartan, a novel angiotensin receptor–neprilysin inhibitor, could be effective against doxorubicin-induced cardiotoxicity. However, the precise mechanisms are not fully understood. Therefore, we investigated whether the cardioprotective effects of sacubitril/valsartan are associated with ER stress modulation in a rat model of doxorubicin-induced cardiotoxicity. Male Sprague–Dawley rats were treated with intraperitoneal injections of doxorubicin (15 mg/kg; cumulative) or saline for 3 weeks. From the day before the first treatment, control animals were gavaged daily with water (n = 8), whereas doxorubicin-treated animals were gavaged daily with water (n = 8) or sacubitril/valsartan (60 mg/kg/day; n = 8) for 6 weeks. Echocardiography was performed 6 weeks after the initiation of doxorubicin. In addition, serum troponin I and N-terminal brain natriuretic peptide levels were determined, and the extent of apoptosis and protein levels related to ER stress in the cardiac tissue and doxorubicin-treated H9c2 cardiomyocytes were analyzed. Sacubitril/valsartan significantly reduced doxorubicin-induced cardiac dysfunction and apoptosis in the myocardium. In addition, sacubitril/valsartan significantly downregulated the expression levels of proteins related to apoptosis and ER stress, including BAX, caspase 3, GRP78, PERK, IRE-1α, ATF-6, eIF-2α, ATF-4, and CHOP, in the myocardium of a rat model of doxorubicin-induced cardiotoxicity in vivo and doxorubicin-treated H9c2 cardiomyocytes in vitro. Sacubitril/valsartan significantly alleviated doxorubicin-induced cardiotoxicity, which may be associated with the reduction of ER stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxorubicin (Dox) has commonly been used as an effective chemotherapeutic drug for the treatment of a wide range of cancers, including both solid and hematogenous cancers, since 1969 (Renu et al. 2018). However, the utility of this drug is limited, because it induces cardiotoxicity and results in irreversible degenerative cardiomyopathy and heart failure (Yeh and Bickford 2009). Several strategies have been used to alleviate Dox-induced cardiotoxicity, which include reducing the cumulative Dox dose and the application of dexrazoxane (Chow et al. 2015; van Dalen et al. 2010). However, there are concerns about mitigating the anticancer effect of Dox and the increased incidence of secondary malignancies following dexrazoxane use (Tebbi et al. 2007). Conventional heart failure treatments, such as β-blockers and angiotensin-converting enzyme (ACE) inhibitors, are partially effective against Dox-induced cardiotoxicity (Bosch et al. 2013; Cardinale et al. 2015). Therefore, it is important to identify novel approaches to alleviate Dox-induced cardiotoxicity without reducing its anticancer effects.
The underlying mechanisms of Dox-induced cardiotoxicity still need to be elucidated. Reactive oxygen species play an important role in Dox-induced cardiotoxicity. Changes in iron homeostasis, mitochondrial dysfunction, calcium dysregulation, inflammation, endothelial dysfunction, autophagy, and cell death also contribute to Dox-induced cardiotoxicity (Renu et al. 2018). Recently, it has been demonstrated that endoplasmic reticulum (ER) stress plays an important role in Dox-induced cardiotoxicity (Fu et al. 2016). However, the detailed mechanisms of ER stress in Dox-induced cardiotoxicity have not been completely elucidated. Sacubitril/valsartan (Sac/Val) is a novel angiotensin receptor–neprilysin inhibitor that reduces cardiovascular events in patients with heart failure (McMurray et al. 2014). Previous studies have shown that Sac/Val could reduce myocardial injury and improve cardiac function in Dox-induced cardiotoxicity (Boutagy et al. 2020; Xia et al. 2017). However, the molecular mechanisms underlying the beneficial effects of Sac/Val in Dox-induced cardiotoxicity are not fully understood. In this study, we investigated whether the cardioprotective effects of sacubitril/valsartan are associated with ER stress modulation in a rat model of Dox-induced cardiotoxicity.
Materials and methods
Animals
The experiments were performed in compliance with the ARRIVE guidelines on animal research (Percie du Sert et al. 2020) and the research protocol was approved by the Hanyang University Institutional Animal Care and Use Committee. Male Sprague–Dawley (SD) rats (Koatech, Kyungki-do, Republic of Korea) aged 7 weeks and weighing 210–230 g were used. The rats were maintained in a specific pathogen-free facility at the Hanyang University Medical School Animal Experiment Center under controlled temperature (23 ± 2 °C) and humidity (55 ± 5%) conditions with an alternating 12-h light/dark cycle.
Experimental design
The rats were randomly assigned to three groups as follows: control group (n = 8)—injection of an equal volume of saline (weekly injection for 3 weeks); Dox group (n = 8)—intraperitoneal injection of Dox at a cumulative dose of 15 mg/kg (weekly injection of Dox at 5 mg/kg for 3 weeks); Dox + Sac/Val group (n = 8)—oral gavage of Sac/Val (60 mg/kg/day; Selleck, Houston, TX, USA) dissolved in normal saline beginning on the day before Dox injection for 6 weeks (in addition to Dox treatment). This dose of Sac/Val was chosen based on prior preclinical studies defining the pharmacokinetics and pharmacodynamics of Sac/Val in rats and other studies demonstrating beneficial effects of Sac/Val on myocardial remodeling in rats (Boutagy et al. 2020; Gu et al. 2010; Xia et al. 2017). Both the control and Dox groups received daily oral gavage of water in the same manner as the Dox + Sac/Val group. At week 6 of the experiment, all rats were euthanized for analysis (Fig. 1).
Schematic of the experimental protocol. Male SD rats aged 7 weeks were randomized into the control group (n = 8, saline injection with normal diet), Dox group (n = 8, Dox injection at 15 mg/kg, cumulative with normal diet), and Dox + Sac/Val group (n = 8, Dox injection at 15 mg/kg, cumulative with oral gavage of Sac/Val at 60 mg/kg/day). At 6 weeks, transthoracic echocardiography was performed under anesthesia, followed by euthanasia for analysis
Echocardiography
At week 6 of the experiment, transthoracic echocardiography was performed before euthanizing the rats by an intramuscular injection of a mixture of zoletil 50 (30 mg/kg; Virbac SA, Carros, France) and rompun (10 mg/kg; Bayer Korea, Seoul, Republic of Korea) (Park et al. 2021). The left side of the chest was shaved to obtain clear images. Echocardiographic examinations (IE33; Philips Healthcare, Andover, MA, USA) of the rats in the left lateral decubitus position were performed by a single sonographer. Left-ventricular ejection fraction (LVEF) and left-ventricular fractional shortening (LVFS) were measured. All measurements were used to generate a mean of five consecutive cardiac cycles, and the mean values were used in the subsequent analyses.
Histological analysis
After the rats were euthanized, the hearts were immediately isolated and weighed, and the ventricle was divided into two parts. One-half of each heart was fixed in formalin, embedded in paraffin, and cut into 4 µm-thick sections. The other half was frozen in liquid nitrogen and stored at − 80 °C for western blot analysis. Apoptotic cardiomyocytes were evaluated by terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay in paraffin sections using the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany). The stained sections were photographed using a light microscope (Leica DM 4000 B; Leica, Wetzlar, Germany). Five regions from each digitized image were selected at random, and the numbers of healthy and TUNEL-positive (apoptotic) nuclei were quantified. The apoptotic index was calculated as the number of TUNEL-positive nuclei/total number of nuclei (Shin et al. 2014). All data were evaluated by an independent, blinded investigator.
Cell culture
The H9c2 rat cardiomyocyte cell line (ATCC, Manassas, VA, USA) was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) containing 5.5 mM glucose, 10% fetal bovine serum (FBS; Gibco), and 1% penicillin and streptomycin (Gibco) at 37 °C in a humidified incubator with 5% CO2. Experiments were performed using cells between passages 10 and 14 at the time of the experiments, which were performed in cell cultures with 70–80% confluence. The cells were divided into three groups for treatment as follows: (1) control group; (2) Dox group—the cells were treated with 500 nM Dox; (3) Dox + Sac/Val group—the cells were treated with 500 nM Dox and 20 μM Sac/Val. After 48 h, the cells were harvested for further analysis. All tests were repeated at least three times.
Biochemical analysis
Blood samples were collected from the rats through the posterior vena cava and centrifuged at 2000 ×g for 15 min at 4 °C to separate the serum. All samples were stored at − 80 °C until analysis. Serum N-terminal pro-brain natriuretic peptide (NT-proBNP) and troponin I levels were measured according to the manufacturer’s instructions by enzyme-linked immunosorbent assay (ELISA) using the Mouse NT-pro BNP ELISA Kit and Rat Cardiac Troponin I ELISA Kit (MyBioSource, San Diego, CA, USA).
Fluorescence-activated cell sorting (FACS) analysis
Fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI) were used to identify apoptotic cells using an FITC-annexin V apoptosis detection kit (BD Biosciences, San Diego, CA, USA) according to the manufacturer’s instructions (Lee et al. 2012). H9c2 cells were harvested and washed twice with PBS. The cells were resuspended in binding buffer, and FITC-annexin V and PI were added. The mixture was incubated for 15 min in the dark at room temperature. The resulting fluorescence was measured by flow cytometry using an FACS flow cytometer (BD Biosciences).
Western blotting
Heart halves were homogenized, and total protein was extracted using protein lysis buffer (PRO-PREP; iNtRON, Seongnam, Republic of Korea). Cardiac tissue samples containing 60 μg total protein were boiled for 20 min and loaded onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (8% stacking and 10% or 15% separating gels). Separated proteins were transferred to nitrocellulose membranes (0.45 μm pore size; Bio-Rad, Hercules, CA, USA) or Immobilon-P polyvinylidene fluoride (PVDF) membranes (0.45 μm pore size; Millipore, Billerica, MD, USA). After blocking in 5% bovine serum albumin solution (Sigma-Aldrich) or 5% skim milk solution (BD Biosciences, San Diego, CA, USA) for 60 min, the membranes were incubated with primary antibodies overnight at 4 °C. The primary antibodies used are listed in Supplementary Table S1. Blots were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (1:2000; Jackson ImmunoResearch, West Grove, PA, USA) or anti-mouse antibody (1:2000; Jackson ImmunoResearch) for 1 h at room temperature. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Positive protein bands were visualized using an enhanced chemiluminescence detection kit (GenDEPOT, Barker, NY, USA), and the results were quantified using an image analyzer (Image Lab 3.0, Bio-Rad).
Statistical analyses
Statistical Package for the Social Sciences (SPSS) software (IBM SPSS Statistics for Windows, version 26; IBM Corp., Armonk, NY, USA) was used for statistical analyses. All data are expressed as the mean ± standard deviation (SD). Data were analyzed using one-way analysis of variance (ANOVA) for multiple comparisons, and post hoc multiple comparisons were performed with Tukey’s test (equal variances assumed) or Dunnett’s T3 test (equal variances not assumed). Statistical significance was set at P < 0.05.
Results
Sac/Val improves biochemical markers and systolic function in a rat model of Dox-induced cardiotoxicity
At the end of the experiments, there were no significant differences in the heart-to-body weight ratio between the groups. The Dox group showed significantly increased troponin I and NT-proBNP levels compared with those of the control group (Table 1). In addition, LVEF and LVFS measured by echocardiography after 6 weeks of treatment with Dox were significantly reduced compared with those of the control group (Fig. 2). These results confirmed the successful development of a rat model of Dox-induced cardiotoxicity.
Protection against Dox-induced cardiotoxicity in rats by Sac/Val. A Representative images of echocardiography. B Changes in left-ventricular systolic function with Sac/Val treatment in Dox-induced cardiotoxicity. All data are expressed as the mean ± SD. LVEF left-ventricular ejection fraction, LVFS left-ventricular fractional shortening
In comparison with treatment with Dox only, cotreatment with Sac/Val resulted in significantly lower levels of troponin I and NT-proBNP. Moreover, LVEF and LVFS were significantly higher in the Dox + Sac/Val group than in the Dox group.
Sac/Val attenuates Dox-induced cardiac apoptosis in rats
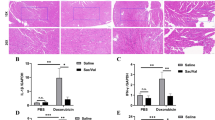
Apoptotic cardiomyocytes in the three groups were detected by TUNEL staining. Compared with the control group, the Dox group had a significantly increased apoptotic index (22.63 ± 3.17% vs. 52.62 ± 5.67%, P < 0.05), which was significantly alleviated in the Dox + Sac/Val group (52.62 ± 5.67% vs. 37.02 ± 1.34%, P < 0.05) (Fig. 3A, B). The levels of the pro-apoptotic proteins bcl-2-associated X (BAX) and cysteine-aspartic proteases 3 (caspase 3) in the cardiac tissue were measured by western blotting (Fig. 3C, D). The results showed that the expression of pro-apoptotic proteins, such as BAX and caspase 3, was significantly higher in the Dox group than in the control group (P < 0.05). However, pro-apoptotic protein expression in the Dox + Sac/Val group was significantly alleviated compared with that in Dox group (P < 0.05).
Attenuation of apoptosis in rats with Dox-induced cardiotoxicity by Sac/Val. A Representative images of TUNEL staining of the myocardium for each group. Scale bar = 100 μm. Apoptotic nuclei are stained brown, and non-apoptotic nuclei are stained blue. B Quantitative analysis of apoptotic cells in the myocardium of each group. C The protein expression of BAX and caspase 3 in the cardiac tissue was detected by western blotting. GAPDH was used as a loading control. D Quantitative western blot analysis of BAX and caspase 3. Expression levels were normalized to the GAPDH expression level
Sac/Val suppresses Dox-induced ER stress in rats
To evaluate the effects of Sac/Val on Dox-induced ER stress, the cardiac expression of ER stress-associated proteins was examined, which included glucose-regulated protein 78 (GRP78), protein kinase R-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme-1α (IRE-1α), activating transcription factor-6 (ATF-6), eukaryotic initiation factor-2α (eIF-2α), activating transcription factor-4 (ATF-4), X-box binding protein 1 (XBP1), apoptosis signal-regulating kinase 1 (ASK1), c-Jun NH2-terminal kinase (JNK), and C/EBP homologous protein (CHOP) (Fig. 4). Compared with the control group, the Dox group showed significantly increased expression levels of GRP78, PERK, IRE-1α, ATF-6, eIF-2α, ATF-4, and CHOP (P < 0.05), which were significantly normalized in the Dox + Sac/Val group (P < 0.05). There was no difference in the protein expression of XBP1, ASK1, and JNK between the groups.
Alleviation of ER stress in rats with Dox-induced cardiotoxicity by Sac/Val. A Western blotting of the expression of ER stress-related proteins such as GRP78, PERK, IRE-1α, ATF-6, eIF-2α, ATF-4, XBP1, ASK1, JNK, and CHOP in the cardiac tissue. GAPDH was used as a loading control. B Quantitative western blot analysis of GRP78, PERK, IRE-1α, ATF-6, eIF-2α, ATF-4, XBP1, ASK1, JNK, and CHOP. Expression levels were normalized to the GAPDH expression level
Sac/Val attenuates Dox-induced apoptosis and ER stress in H9c2 cardiomyocytes
An in vitro experiment was performed to clarify the effects of Sac/Val on ER stress in Dox-induced cardiotoxicity. First, the effects of Dox and Sac/Val on the viability of H9c2 cardiomyocytes were evaluated by MTT assay. Treatment with Dox at concentrations of 10 nM, 100 nM, 500 nM, 1 μM, and 10 μM for 24 h showed concentration-dependent cytotoxic effects (Supplementary Fig. S1A), whereas treatment with Sac/Val at concentrations of 1 μM, 5 μM, 10 μM, and 20 μM showed no cytotoxicity (Supplementary Fig. S1B). To evaluate the effects of Sac/Val on Dox-induced cardiotoxicity, H9c2 cardiomyocytes were treated with 20 μM Sac/Val and 500 nM Dox.
To examine the effects of Sac/Val on Dox-induced apoptosis in cardiomyocytes, H9c2 cardiomyocytes were treated with Dox with or without Sac/Val, and the percentage of apoptotic cells was determined by annexin V/PI staining (Fig. 5A, B). Compared with the control group, the Dox group showed significantly increased cardiomyocyte apoptosis (P < 0.05), which was significantly decreased by cotreatment with Sac/Val (P < 0.05). Pro-apoptotic proteins were detected by western blotting (Fig. 5C, D). Dox administration significantly increased the expression of pro-apoptotic proteins, such as BAX and caspase 3 (P < 0.05). Cotreatment with Sac/Val effectively inhibited the expression of both BAX and caspase 3 (P < 0.05). To investigate the anti-apoptotic mechanisms of Sac/Val in Dox-induced cardiotoxicity, the expression of ER stress-associated proteins was further evaluated (Fig. 5E, F). The Dox group showed significantly elevated protein expression levels of GRP78, PERK, IRE-1α, ATF-6, eIF-2α, ATF-4, and CHOP (P < 0.05), which were significantly normalized in the Dox + Sac/Val group (P < 0.05).
Attenuation of Dox-induced apoptosis and ER stress in H9c2 cardiomyocytes by Sac/Val. A Apoptosis detection by annexin V staining in flow cytometry. B Quantitative analysis of apoptotic cells detected in flow cytometry. C Western blotting of the protein expression of BAX and caspase 3 in H9c2 cardiomyocytes. GAPDH was used as a loading control. D Quantitative western blot analysis of BAX and caspase 3. Expression levels were normalized to the GAPDH expression level. E Expression of ER stress-related proteins such as GRP78, PERK, IRE-1α, ATF-6, eIF-2α, ATF-4, and CHOP. GAPDH was used as a loading control. F Quantitative western blot analysis of GRP78, PERK, IRE-1α, ATF-6, eIF-2α, ATF-4, and CHOP. Expression levels were normalized to the GAPDH expression level
Discussion
In this study, we showed that Sac/Val attenuated cardiac systolic dysfunction and reduced cardiomyocyte apoptosis in a rat model of Dox-induced cardiotoxicity. Moreover, Sac/Val downregulated the expression levels of apoptosis-related proteins, including BAX and caspase 3, and proteins related to ER stress, including GRP78, PERK, IRE-1α, ATF-6, eIF-2α, ATF-4, and CHOP, in the myocardium of a rat model of doxorubicin-induced cardiotoxicity in vivo and doxorubicin-treated H9c2 cardiomyocytes in vitro. These findings provide a molecular explanation, suggesting that the anti-apoptotic effects of Sac/Val may be mediated by the modulation of ER stress in Dox-induced cardiotoxicity.
Dox is one of the most effective anticancer drugs. However, Dox-induced cardiotoxicity remains a major obstacle in the clinical use of Dox (Singal and Iliskovic 1998). The mechanisms of Dox-induced cardiotoxicity are diverse and complicated. Dox contributes to DNA damage by inhibiting topoisomerase II, which leads to cardiotoxicity (Zhang et al. 2012). In addition, reactive oxygen species play a major role in Dox-induced cardiotoxicity (Songbo et al. 2019), and the dysregulation of mitochondrial dynamics and function leads to cardiotoxicity (Osataphan et al. 2020). The precise mechanisms of Dox-induced cardiotoxicity remain elusive; however, the ultimate outcome is the apoptosis of cardiomyocytes. The apoptosis of cardiomyocytes is related to ER stress, and emerging evidence shows that ER stress might play a key role in Dox-induced cardiotoxicity (Yarmohammadi et al. 2021). The ER orchestrates the folding and translocation of secretory and transmembrane proteins. Disruption in the ER protein folding capacity leads to a cell stress response, known as the unfolded protein response or ER stress, which aims to restore protein homeostasis. When ER stress is chronically prolonged and the protein load on the ER greatly exceeds its fold capacity, cellular dysfunction and cell death often occur (Ren et al. 2021; Sano and Reed 2013). CHOP plays an important role in ER stress-induced apoptosis (Hu et al. 2018). CHOP is not expressed under normal conditions (Ron and Habener 1992). However, prolonged ER stress can activate PERK, and activation of PERK leads to the phosphorylation of eIF-2α, promoting ATF-4 transcription and thereby increasing the expression of CHOP (Rozpedek et al. 2016). In addition, the ATF6 and IRE-1α pathways can activate CHOP (Hu et al. 2018). Activated CHOP can downregulate the expression of anti-apoptotic genes and increase the expression of pro-apoptotic genes, leading to mitochondrial-dependent apoptosis (Iurlaro and Muñoz-Pinedo 2016). In this study, we showed that the levels of ER stress-related proteins (GRP78, PERK, IRE-1α, ATF-6, eIF-2α, ATF-4, and CHOP) were increased after Dox treatment, which was accompanied by an increase in cardiomyocyte apoptosis, indicating that ER stress may play a role in Dox-induced cardiotoxicity. Moreover, findings associated with cardiotoxicity were significantly abrogated by cotreatment with Sac/Val. These findings were consistently observed in the myocardial tissue and H9c2 cells. However, an interesting finding in our study was that although the expression levels of IRE-1α (one of the three major pathways of ER stress) and BAX (a pro-apoptotic protein associated with the IRE-1α pathway) were increased in Dox-induced cardiotoxicity, downstream signaling molecules of the IRE-1α pathway (XBP1, ASK1, and JNK) were not activated. A possible explanation for these findings is ER stress response failure, which is of considerable interest to researchers recently (Bhattarai et al. 2020). In ER stress response failure, despite ER stress activation (as evidenced by enhanced upstream proteins), downstream signaling molecules fail to be fully activated. In addition, the findings suggest the presence of another molecular pathway that activates BAX. Further investigation on the detailed mechanisms of the ER stress response in Dox-induced cardiotoxicity is needed.
In recent practical guidelines for the treatment of heart failure, treatment with an ACE inhibitor and a beta-blocker is recommended during anthracycline chemotherapy for cancer patients with LV systolic dysfunction, defined as a 10% or more decrease in LVEF to a value lower than 50% (McDonagh et al. 2021). In addition, considering the evidence of the PARADIGM-HF trial demonstrating that Sac/Val was superior to ACE inhibitors in the treatment of chronic heart failure with reduced ejection fraction, the superiority of Sac/Val in the treatment of Dox-induced cardiotoxicity can be expected (McMurray et al. 2014). Martín-Garcia et al. recently reported that Sac/Val improves the echocardiographic parameters and clinical status of patients with cancer therapy-related cardiac dysfunction based on an analysis of a retrospective multicenter registry database containing 47 patients treated with anthracycline (Martín-Garcia et al. 2020). Despite its clinical potential, preclinical studies that have elucidated the therapeutic mechanisms of Sac/Val in Dox-induced cardiotoxicity are limited. Xia et al. reported that Sac/Val attenuated Dox-induced cardiotoxicity, which was associated with the alleviation of dynamin-related protein 1 (Drp1)-mediated mitochondrial dysfunction (Xia et al. 2017). More recently, Boutagy et al. reported that Sac/Val maintained cardiac function in a rodent model of Dox-induced cardiotoxicity, which was mediated by a reduction in myocardial matrix metalloproteinase activity (Boutagy et al. 2020). In addition to previous studies, this study provides a novel molecular mechanism associated with ER stress modulation by Sac/Val in ameliorating Dox-induced cardiotoxicity. These results are in agreement with those of a previous study in which natriuretic peptides protected cells from various types of injury by inhibiting ER stress (Chang et al. 2019; Courreges et al. 2019; ZHAO et al. 2020). The increase in natriuretic peptides induced by sacubitril, which inhibits natriuretic peptide degradation, effectively modulates ER stress and may have protective effects against Dox-induced cardiotoxicity.
This study has several limitations. First, we provide a new mechanistic understanding of Sac/Val in terms of its anti-apoptotic effects and ER stress reduction in Dox-induced cardiotoxicity; however, we do not provide evidence on a causal relationship between apoptosis and ER stress. Further studies on the detailed mechanisms are needed to determine whether the alleviation of cardiomyocyte apoptosis is related to the ER stress-induced apoptosis pathway. Second, we cannot rule out the possibility that the anti-apoptotic effect of Sac/Val is associated with any other previously postulated mechanism such as oxidative stress, mitochondrial dysfunction, impairment of progenitor cells, activation of immune, and autophagy. Further studies regarding the precise mechanism of the anti-apoptotic effect of Sac/Val are also worth exploring. Third, we did not include a group treated with only Sac/Val. In addition, we did not conduct experiments which compare the effectiveness of Sac/Val with that of valsartan. Therefore, we could not verify the changes when Sac/Val was treated without Dox, and it is not possible to conclude whether the beneficial effects of Sac/Val are attributed to valsartan only or the additional effects of sacubitril. Finally, we evaluated the preventive effects of Sac/Val against Dox-induced cardiotoxicity. Further studies are needed to determine whether Sac/Val can reverse Dox-induced cardiotoxicity when administered after toxicity has occurred.
In conclusion, our study demonstrated that Sac/Val treatment significantly attenuated Dox-induced cardiotoxicity, which may be associated with the alleviation of ER stress. We believe that our findings provide new insights into the potential molecular mechanisms of Dox-induced cardiotoxicity.
Data availability
The interpreted and analyzed data from this study are available from the corresponding author upon reasonable request.
References
Bhattarai KR, Chaudhary M, Kim H-R, Chae H-J (2020) Endoplasmic reticulum (ER) stress response failure in diseases. Trends Cell Biol 30(9):672–675. https://doi.org/10.1016/j.tcb.2020.05.004
Bosch X, Rovira M, Sitges M et al (2013) Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 61(23):2355–2362. https://doi.org/10.1016/j.jacc.2013.02.072
Boutagy NE, Feher A, Pfau D et al (2020) Dual angiotensin receptor-neprilysin inhibition with sacubitril/valsartan attenuates systolic dysfunction in experimental doxorubicin-induced cardiotoxicity. JACC CardioOncol 2(5):774–787. https://doi.org/10.1016/j.jaccao.2020.09.007
Cardinale D, Colombo A, Bacchiani G et al (2015) Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 131(22):1981–1988. https://doi.org/10.1161/circulationaha.114.013777
Chang P, Zhang M, Zhang X et al (2019) B-type natriuretic peptide attenuates endoplasmic reticulum stress in H9c2 cardiomyocytes underwent hypoxia/reoxygenation injury under high glucose/high fat conditions. Peptides 111:103–111. https://doi.org/10.1016/j.peptides.2018.04.016
Chow EJ, Asselin BL, Schwartz CL et al (2015) Late mortality after Dexrazoxane treatment: a report from the Children’s Oncology Group. J Clin Oncol 33(24):2639–2645. https://doi.org/10.1200/JCO.2014.59.4473
Courreges AP, Najenson AC, Vatta MS, Bianciotti LG (2019) Atrial natriuretic peptide attenuates endoplasmic reticulum stress in experimental acute pancreatitis. Biochim Biophys Acta 2:485–493. https://doi.org/10.1016/j.bbadis.2018.12.004
Fu HY, Sanada S, Matsuzaki T et al (2016) Chemical endoplasmic reticulum chaperone alleviates doxorubicin-induced cardiac dysfunction. Circ Res 118(5):798–809. https://doi.org/10.1161/circresaha.115.307604
Gu J, Noe A, Chandra P et al (2010) Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol 50(4):401–414. https://doi.org/10.1177/0091270009343932
Hu H, Tian M, Ding C, Yu S (2018) The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol 9:3083. https://doi.org/10.3389/fimmu.2018.03083
Iurlaro R, Muñoz-Pinedo C (2016) Cell death induced by endoplasmic reticulum stress. FEBS J 283(14):2640–2652. https://doi.org/10.1111/febs.13598
Lee KM, Kang HA, Park M et al (2012) Interleukin-24 attenuates β-glycerophosphate-induced calcification of vascular smooth muscle cells by inhibiting apoptosis, the expression of calcification and osteoblastic markers, and the Wnt/β-catenin pathway. Biochem Biophys Res Commun 428(1):50–55. https://doi.org/10.1016/j.bbrc.2012.09.145
Martín-Garcia A, López-Fernández T, Mitroi C et al (2020) Effectiveness of sacubitril-valsartan in cancer patients with heart failure. ESC Heart Failure 7(2):763–767. https://doi.org/10.1002/ehf2.12627
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. https://doi.org/10.1093/eurheartj/ehab368
McMurray JJ, Packer M, Desai AS et al (2014) Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371(11):993–1004. https://doi.org/10.1056/NEJMoa1409077
Osataphan N, Phrommintikul A, Chattipakorn SC, Chattipakorn N (2020) Effects of doxorubicin-induced cardiotoxicity on cardiac mitochondrial dynamics and mitochondrial function: Insights for future interventions. J Cell Mol Med 24(12):6534–6557. https://doi.org/10.1111/jcmm.15305
Park IH, Shen GY, Song YS et al (2021) Granulocyte colony-stimulating factor reduces the endoplasmic reticulum stress in a rat model of diabetic cardiomyopathy. Endocr J. https://doi.org/10.1507/endocrj.EJ21-0016
Percie du Sert N, Hurst V, Ahluwalia A et al (2020) The ARRIVE guidelines 20: updated guidelines for reporting animal research. PLoS Biol 18(7):e3000410. https://doi.org/10.1371/journal.pbio.3000410
Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y (2021) Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol 18(7):499–521. https://doi.org/10.1038/s41569-021-00511-w
Renu K, A VG, Tp PB, Arunachalam S (2018) Molecular mechanism of doxorubicin-induced cardiomyopathy—an update. Eur J Pharmacol 818:241–253. https://doi.org/10.1016/j.ejphar.2017.10.043
Ron D, Habener JF (1992) CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev 6(3):439–453. https://doi.org/10.1101/gad.6.3.439
Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I (2016) The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med 16(6):533–544. https://doi.org/10.2174/1566524016666160523143937
Sano R, Reed JC (2013) ER stress-induced cell death mechanisms. Biochem Biophys Acta 1833(12):3460–3470. https://doi.org/10.1016/j.bbamcr.2013.06.028
Shin JH, Lim YH, Song YS et al (2014) Granulocyte-colony stimulating factor reduces cardiomyocyte apoptosis and ameliorates diastolic dysfunction in Otsuka Long-Evans Tokushima Fatty rats. Cardiovasc Drugs Ther 28(3):211–220. https://doi.org/10.1007/s10557-014-6519-8
Singal PK, Iliskovic N (1998) Doxorubicin-induced cardiomyopathy. N Engl J Med 339(13):900–905. https://doi.org/10.1056/nejm199809243391307
Songbo M, Lang H, Xinyong C, Bin X, Ping Z, Liang S (2019) Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett 307:41–48. https://doi.org/10.1016/j.toxlet.2019.02.013
Tebbi CK, London WB, Friedman D et al (2007) Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol 25(5):493–500. https://doi.org/10.1200/jco.2005.02.3879
van Dalen EC, Michiels EM, Caron HN (2010) Kremer LC (2010) Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev 5:CD005006. https://doi.org/10.1002/14651858.CD005006.pub4
Xia Y, Chen Z, Chen A et al (2017) LCZ696 improves cardiac function via alleviating Drp1-mediated mitochondrial dysfunction in mice with doxorubicin-induced dilated cardiomyopathy. J Mol Cell Cardiol 108:138–148. https://doi.org/10.1016/j.yjmcc.2017.06.003
Yarmohammadi F, Rezaee R, Haye AW, Karimi G (2021) Endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity may be therapeutically targeted by natural and chemical compounds: a review. Pharmacol Res 164:105383. https://doi.org/10.1016/j.phrs.2020.105383
Yeh ET, Bickford CL (2009) Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 53(24):2231–2247. https://doi.org/10.1016/j.jacc.2009.02.050
Zhang S, Liu X, Bawa-Khalfe T et al (2012) Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 18(11):1639–1642. https://doi.org/10.1038/nm.2919
Zhao X, Kong Y, Li S, Liang B, Li C, Wang W (2020) Atrial natriuretic peptide prevented lipid-induced kidney injuries by inhibiting endoplasmic reticulum stress. FASEB J 34(S1):1–1. https://doi.org/10.1096/fasebj.2020.34.s1.02690
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: JHS and YHL. Methodology: BSK and IHP. Formal analysis: AHL and HJK. Investigation: IHP and AHL. Data curation: BSK and HJK. Writing—original draft preparation: BSK and IHP. Writing—review and editing: JHS and YHL. Supervision: JHS and YHL.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The research protocol was approved by the Hanyang University Institutional Animal Care and Use Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

204_2022_3241_MOESM2_ESM.jpg
Supplementary Fig. S1 Effects of Dox and Sac/Val on the viability of H9c2 cardiomyocytes The cells were treated with increasing concentrations of (a) Dox (10 nM to 10 μM) or (b) Sac/Val (1 μM to 20 μM). MTT assay was performed to measure cell viability (JPG 166 KB)
Rights and permissions
About this article
Cite this article
Kim, B.S., Park, IH., Lee, AH. et al. Sacubitril/valsartan reduces endoplasmic reticulum stress in a rat model of doxorubicin-induced cardiotoxicity. Arch Toxicol 96, 1065–1074 (2022). https://doi.org/10.1007/s00204-022-03241-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-022-03241-1