Abstract
Heterocyclic aromatic amines (HAAs) are primarily produced during the heating of meat or fish. HAAs are mutagenic and carcinogenic, and their toxicity in model systems depend on metabolic activation. This activation is mediated by cytochrome P450 (CYP) enzymes, in particular CYP1A2. Some studies have indicated a role of human sulfotransferase (SULT) 1A1 and N-acetyltransferase (NAT) 2 in the terminal activation of HAAs. In this study, we conducted a metabolism/genotoxicity relationship analysis for 16 HAAs and related heterocyclics. We used the γH2AX genotoxicity assay in V79 cells (deficient in CYP, SULT and NAT) and V79-derived cell lines genetically engineered to express human CYP1A2 alone or in combination with human SULT1A1 or NAT2. Our data demonstrated genotoxic properties for 13 out of the 16 compounds tested. A clear relationship between metabolic bioactivation and genotoxicity allowed to distinguish four groups: (1) Trp-P-1 genotoxicity was linked to CYP1A2 bioactivation only—with negligible effects of phase II enzymes; (2) Glu-P-2, Glu-P-1, Trp-P-2, APNH, MeAαC and AαC were bioactivated by CYP1A2 in combination with either phase II enzyme tested (NAT2 or SULT1A1); (3) IQ, 4-MeIQ, IQx, 8-MeIQx, and 4,8-DiMeIQx required CYP1A2 in combination with NAT2 to be genotoxic, whereas SULT1A1 did not enhance their genotoxicity; (4) PhIP became genotoxic after CYP1A2 and SULT1A1 bioactivation—NAT2 had not effect. Our results corroborate some previous data regarding the genotoxic potency of seven HAAs and established the genotoxicity mechanism for five others HAAs. This study also permits to compare efficiently the genotoxic potential of these 13 HAAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterocyclic aromatic amines (HAAs) are dietary carcinogens formed in some foodstuffs, but also arise in tobacco smoke (Turesky and Le Marchand 2011). HAAs are divided into two major classes. The pyrolysis HAAs (Fig. 1a) arise during high-temperature pyrolysis. These compounds contain a five-membered ring (imidazole or pyrrole) fused with two six-membered rings at separate sites. HAAs of the second class (Fig. 1b) contain a 2-aminoimidazo moiety fused to additional aromatic elements. They are formed in meats that are cooked at lower temperatures, more commonly used in household kitchens (150 °C) (Skog et al. 1998).
Several HAAs have been shown to be carcinogenic in rodents, inducing tumors in multiple organs and tissues (Kato et al. 1988; Ohgaki et al. 1991). Whereas the liver is a major target organ for the carcinogenicity of many HAAs in rodents, only PhIP was found to induce tumors at other sites. Based on current evidence, the International Agency for Research on Cancer (IARC) classified 2-amino-3-methyl-3H-imidazo[4,5-f]quinoline (IQ) as a probable human carcinogen (class 2 A) and nine other HAAs as possible human carcinogens (class 2B) (IARC 2016). HAAs are pro-carcinogens and have to be metabolically activated to intermediates that form DNA adducts, leading to mutations and carcinogenesis. HAAs bioactivation pathway is initiated by the hydroxylation of the exocyclic amine group by cytochrome P450 enzymes (CYPs). In general, CYP1A2 is involved, but other CYPs are also able to N-hydroxylate some HAAs (Boobis et al. 1994; Hammons et al. 1997; Schut and Snyderwine 1999). N-Hydroxy-HAA metabolites are substrates for phase II enzymes, such as N-acetyltransferases (NAT) or sulfotransferases (SULT), and form unstable esters that can transfer a resonance stabilized arylnitrenium/carbonium ion to nucleophilic sites of DNA and other cellular molecules (Turesky and Le Marchand 2011).
The carcinogenicity of HAAs does not reflect the extraordinarily high mutagenicity of some congeners in the Ames test (Turesky and Le Marchand 2011). This discrepancy may be attributed in part to the observed interspecies differences in the metabolic fate of these compounds. The expression of biotransformation processes that can produce reactive intermediates varies widely among species (Martignoni et al. 2006), notably for HAAs (Turesky et al. 1999). On the other hand, the exceptionally efficient activation of some HAAs in the Ames test, owed to the expression of an acetyltransferase in the target cells, may be not reflecting the in vivo situation. In striking contrast to the Ames test, HAAs have often been found negative or only weakly positive in standard mutagenicity tests in mammalian cells in culture using external activation by S9 mix (Mizota et al. 2011; Westerink et al. 2010, 2011). This modest response in mammalian cell tests may be explained by the low expression, or lack, of NATs and SULTs in standard target cells. To better mimic the in vivo situation, potential activating enzymes (CYPs and SULTs, NATs) were expressed in Chinese hamster V79 cells. Glatt and co-workers (Glatt et al. 2004; Glatt 2006) tested seven HAAs in these models for the induction of gene mutations at the Hprt locus (Glatt 2006; Glatt et al. 2004). Recently, we developed and validated a high-throughput genotoxicity assay, named γH2AX in-cell western (ICW), in human cells (Audebert et al. 2010). However, some human cells were unable to detect the genotoxicity of a limited number of genotoxic chemicals, notably IQ or PhIP. We suspected that these HAAs require specific bioactivation process not sufficiently covered by the cell lines used (Khoury et al. 2013, 2016a, b).
The aim of this study was to compare the genotoxic potential of 16 HAAs and related heterocyclics with the γH2AX ICW assay in parental V79 cells using three cell lines genetically engineered to express human CYP1A2 alone or together with human SULT1A1 or NAT2, as previously employed in the Hprt gene mutation assay (Glatt 2006; Glatt et al. 2004). The objective was to answer the following questions: (1) are HAAs tested positive using the Hprt assay also positive in the γH2AX ICW assay in V79-derived cell lines? (2) What is the relative sensitivity of Hprt and γH2AX ICW? (3) Are the enzymatic requirements for the individual HAAs the same in both cases? If γH2AX ICW gives credible answers for the seven HAAs for which Hprt mutagenicity data are available, then γH2AX ICW tests could be conducted with additional HAAs to which humans are exposed.
Materials and methods
Caution
Heterocyclic aromatic amines are potential human carcinogens, and they should be handled carefully.
Chemicals and reagents
All compounds used were of analytical grade. 9-(4′-aminophenyl)-9H-pyrido[3,4-b]indole (APNH), 2-amino-9H-pyrido[2,3-b]indole (AαC), 2-amino-3-methyl-9H-pyrido[2,3-b]indole (MeAαC), 2-amino-6-methyldupyrido[1,2-a:3′,2′-d]imidazole (Glu-P-1), 2-aminodipyrido[1,2-a:3′,2′-d]imidazole (Glu-P-2), 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1), 3-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp-P-2), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), 2-amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline (4-MeIQ), 2-amino-3-methyl-3H-imidazo[4,5-f]quinoxaline (IQx), 2-amino-3,8-dimethyl-3H-imidazo[4,5-f]quinoxaline (8-MeIQx), 2-amino-3,4,8-trimethyl-3H-imidazo[4,5-f]quinoxaline (4,8-DiMeIQx), 2-amino-1,6-dimethyl-1H-imidazo[4,5-b]pyridine (DMIP), 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), harman and norharman, were purchased from Toronto Research Chemicals (North York, ON, Canada). All stock solutions of the tests compounds were prepared in 100% dimethyl sulfoxide (DMSO). From the stocks, tenfold dilution series were prepared.
Penicillin, Streptomycin, trypsin, PBS, RNAse A, and Triton X-100 were purchased from Sigma–Aldrich (Saint Quentin Fallavier, France). The phosphatase inhibitor cocktail tablets (“PHOSSTOP”) were purchased from Roche (France) and the blocking solution (MAXblock Blocking Medium) was purchased from Active Motif (Belgium). CF770 antibody and RedDot2 were purchased from Biotium (Hayward, CA, USA).
Cell culture
The V79 clone used in our laboratory (V79-Mz) has been investigated for many xenobiotic-metabolizing activities (Glatt et al. 1990). These cells do not show any endogenous CYP, SULT or NAT activities. V79-hCYP1A2, V79-hCYP1A2-hNAT2 and V79-hCYP1A2-hSULT1A1 cells were generated from V79-Mz cells by introduction of appropriate expression vectors as described previously (Glatt et al. 2004; Schmalix et al. 1993). Clones stably expressing the corresponding enzymes were selected. The expression of CYP1A2, measured by immunoblotting and mutagenicity tests with a compound whose activation only requires CYP activity (benzo[a]pyrene-trans-7,8-dihydrodiol), is known to be equal in V79-hCYP1A2, V79-hCYP1A2-hNAT2 and V79-hCYP1A2-hSULT1A1 cells (Glatt et al. 2004). The level of SULT1A1 protein in V79-hCYP1A2-hSULT1A1 cells is in the high hepatic physiological range, whereas the level of NAT2 in V79-hCYP1A2-hNAT2 is higher (20-fold above the hepatic in a subject with high expression) (Glatt et al. 2004). The cells were grown in DMEM medium supplemented with 5% fetal bovine serum, 100 U ml−1 penicillin and 100 µg ml−1 streptomycin (Glatt 2006; Glatt et al. 2004). Cultures were maintained in a humidified atmosphere with 5% CO2 at 37 °C and the medium was refreshed every 2–3 days during sub-culturing.
γH2AX in-cell western (ICW) assay
The γH2AX in-cell western technique was performed as previously described (Audebert et al. 2010, 2011, 2012; Graillot et al. 2012a, b; Jamin et al. 2013; Khoury et al. 2013, 2016a, b; Quesnot et al. 2016). Briefly, cells (3.2 × 104 cells per well) were grown in 96-well plates containing 200 µl of medium. Sixteen hours later, cells were treated in duplicate with the model compounds or vehicle in serum free medium. For each plate, DMSO (0.2% v/v final dose) was used as negative control. The positive control used in each treatment was 1 µM etoposide. 24 h after the treatment, cells were analyzed by the ICW technique. For the determination of genotoxicity, relative fluorescent units for γH2AX per cell (as determined by γH2AX divided by DNA content) were divided by the respective controls (vehicle only) to determine the change in phosphorylation of H2AX level compared with the control cells. To determine cytotoxicity, the DNA content (related to the number of cells) recorded in the treated cells was compared to the DNA content in control cells. All experiments were performed at least in triplicate, independently.
Data analysis
Results are presented as mean ± SEM (standard error of the mean) of at least three separate experiments. Statistically significant increases in H2AX phosphorylation after treatment were compared with the vehicle (DMSO) control using two-sided Student’s t test; *p < 0.05; **p < 0.01. Genotoxicity was considered positive when a compound induced a statistically significant 1.3-fold γH2AX histone phosphorylation at a level of cytotoxicity below 50% compared to the control DMSO. These parameters were based on our previous studies (Khoury et al. 2013, 2016a, b) and are similar to those used by other groups who use γH2AX quantification for genotoxicity determination (Ando et al. 2014; Bryce et al. 2014; Smart et al. 2011).
Results
In a first step, we performed the γH2AX ICW assay with 16 HAAs and related heterocyclics in the parental V79 cell line. None of these 16 compounds demonstrated any genotoxicity in this cell line devoid of functional human enzymatic activities (Fig. 2). Then we tested the same chemicals in three cell lines genetically engineered from V79 cells to express specific human xenobiotic-metabolizing enzymes (CYP1A2 alone, CYP1A2 plus SULT1A1 and CYP1A2 plus NAT2). Out of the 16 compounds tested, three (harman, norharman and DMIP) did not exert any genotoxicity whatever the cell line used and the concentration tested (data not shown).
For Trp-P-1, we observed almost the same concentration–response genotoxicity curves whatever the cell line used (V79-hCYP1A2, V79-hCYP1A2-hSULT1A1 and V79-hCYP1A2-hNAT2), with a LEC of 0.01 µM (Fig. 3; Table 1). AαC, Glu-P-1, MeAαC, Glu-P-2 and Trp-P-2 were not genotoxic in V79-hCYP1A2 cells (Figs. 2c, e, f, 4b, d). On the contrary, positive results were obtained with all these HAAs in V79-hCYP1A2-hSULT1A1 as well as in V79-hCYP1A2-hNAT2 cells with a LEC of 0.1 µM for AαC, Glu-P-1 and MeAαC, 1 µM for Trp-P-2, respectively, and a LEC of 10 µM for Glu-P-2. APNH was tested positive in V79-hCYP1A2 cells at the 0.1 and 1 µM concentrations, but its effects were drastically enhanced, with a LEC of 0.001 µM, when SULT1A1 or NAT2 were co-expressed with CYP1A2 (Fig. 4a). The concentration–response curves were found to be nearly equal in the latter cell lines for all these HAAs.
In vitro genotoxicity of Trp-P-1 tested with the γH2AX ICW assay in V79-hCYP1A2, V79-hCYP1A2-hSULT1A1 and V79-hCYP1A2-hNAT2 cell lines. Each value represents the mean ± SEM (n ≥ 3) after 24 h of treatment. Significant differences were observed between controls and matched group (*p ≤ 0.05, **p ≤ 0.01)
In vitro genotoxicity of APNH, AαC, MeAαC, Trp-P-2, Glu-P-2 and Glu-P-1 tested with the γH2AX ICW assay in V79-hCYP1A2, V79-hCYP1A2-hSULT1A1 and V79-hCYP1A2-hNAT2 cell lines. a APNH, b AαC, c Glu-P-1, d MeAαC, e Glu-P-2, f Trp-P-2. Each value represents the mean ± SEM (n ≥ 3) after 24 h of treatment. Significant differences were observed between controls and matched group (*p ≤ 0.05, **p ≤ 0.01)
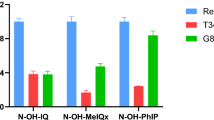
For the five other compounds (IQ, IQx, 4-MeIQ, 8-MeIQx and 4,8-DiMeIQx) we observed only a minimal genotoxicity when using V79-hCYP1A2 or V79-hCYP1A2-hSULT1A1 cell lines, and this only for the two highest concentrations tested (1 and 10 µM, Fig. 5). Conversely, these chemicals were found to be strongly genotoxic in V79-hCYP1A2-hNAT2 cells, with a LEC of 0.01 µM for IQ, 4-MeIQ and 8-MeIQx, and a LEC of 0.1 µM for IQx and 4,8-DiMeIQx. For PhIP (Fig. 6), we observed similar genotoxicity results in V79-hCYP1A2 and V79-hCYP1A2-hNAT2 cells, with a LEC of 1 µM. Nevertheless, the genotoxicity of PhIP was increased by a factor of 10 in the V79-hCYP1A2-hSULT1A1 cell line, with a LEC of 0.1 µM.
In vitro genotoxicity of IQ, IQx, 4-MeIQ, 8-MeIQx and 4,8-diMeIQx tested with the γH2AX ICW assay in V79-hCYP1A2, V79-hCYP1A2-hSULT1A1 and V79-hCYP1A2-hNAT2 cell lines. a IQ, b IQx, c 4-MeIQ, d 8-MeIQx, e 4,8-diMeIQx. Each value represents the mean ± SEM (n ≥ 3) after 24 h of treatment. Significant differences were observed between controls and matched group (*p ≤ 0.05, **p ≤ 0.01)
Discussion
We investigated 16 HAAs and heterocyclics in four cell lines (V79 and three V79 derived cell lines engineered for expressing specific human enzymes) with the γH2AX ICW genotoxicity assay. This experimental work required approximately six person-months, much less than Hprt assays in the same cell lines (over 12 person-months for only seven compounds) (Glatt et al. 2004; Glatt 2006). For seven HAAs it is possible to compare the results of the γH2AX ICW assay (current study) with those of the Hprt mutagenicity assay previously performed in the same cell lines (Table 1). None of these seven HAAs was found to be genotoxic with either assays in parental V79 cells. The remaining 21 situations (seven compounds in three cell lines) can be subdivided as follows: five HAAs were negative in parental V79 cells with both assays; in three situations, both endpoints gave positive test results with a similar LEC (AαC and MeAαC in V79-hCYP1A2-hSULT1A1; IQ in V79-hCYP1A2-hNAT2); in five situations both assays gave positive test results, but the LEC was lower for γH2AX ICW than for gene mutations (8-MeIQx, PhIP, AαC Glu-P-1 in hCYP1A2-hNAT2; PhIP in V79-hCYP1A2-hSULT1A1); in eight situations, positive results were only obtained when using the γH2AX ICW assay (IQ and PhIP in V79-hCYP1A2; Trp-P-2 and MeAαC in V79-hCYP1A2-hNAT2; IQ, in V79-hCYP1A2-hSULT1A1 and Glu-P-1 in V79-hCYP1A2-hSULT1A1). This comparison demonstrates that γH2AX ICW was more sensitive than the gene mutation assay. However, some differences cannot be solely explained by differences in sensitivity. NAT2 enhanced the mutagenicity of PhIP, but not the γH2AX response in CYP1A2 expressing cells; NAT2, unlike SULT1A1, failed to enhance the mutagenicity of N-hydroxy-PhIP in S. typhimurium TA1538/1,8-DNP-derived strains (devoid of endogenous acetyltransferase) (Muckel et al. 2002). Conversely, NAT2 enhanced the γH2AX response to MeAαC similar to SULT1A1, whereas SULT1A1 was required for a mutagenic effect in the Hprt assay. Finally, SULT1A1 enhanced the mutagenicity of MeAαC and N-hydroxy-MeAαC in S. typhimurium TA1538/1,8-DNP-derived strains, although not as strongly as NAT2 (Glatt et al. 2004). Thus, few results obtained with the γH2AX ICW assay in recombinant V79 cells do not exactly match Hprt results in the same cells, but are consistent with mutagenicity findings in recombinant S. typhimurium strains. Incubation conditions (such as cell density) may affect the expression levels and impact of metabolizing enzymes. Putting aside these subtle differences, our findings regarding the role of phase II enzymes in the response returned by the γH2AX ICW test clearly match the results returned by the Hprt assay for the same cellular model, as well as that of various other experimental models (Glatt 2006; Glatt et al. 2004; Muckel et al. 2002; Schut and Snyderwine 1999; Turesky and Le Marchand 2011; Wild et al. 1995). All these data may be helpful to improve structure–mutagenicity relationship for HAAs (Ripa et al. 2014; Shamovsky et al. 2011, 2012).
Selective or preferential terminal activation by either SULT1A1 or NAT2 may be owed to two factors. The first factor is the substrate specificity of enzymes. This is illustrated by the observation that mouse’s Sult1a1, unlike its human orthologue, is not able to activate PhIP (in genetically recombinant S. typhimurium strains as well as genetically modified mouse lines) and that rat Nat1 (the orthologue of human NAT2), unlike human NAT1 and NAT2, expressed in S. typhimurium, is capable of activating N-hydroxy-PhIP (Meinl et al. manuscript in preparation), although much less efficiently than SULT enzymes. The second factor is the chemical reactivity of acetic acid versus sulfuric acid esters. Sulfate is a better leaving group than acetate, as also reflected by the higher acidity of sulfuric acid as compared to acetic acid. There is no information available so far on the respective half-life times of acetic and sulfuric esters derived from heterocyclic amines. Yet, it has been shown that 1-acetoxymethylpyrene is only marginally hydrolyzed after a 70 h stay in water at 37 °C, whereas 1-sulfooxymethylpyrene exhibits a half-life time of 2.8 min under the same conditions (Landsiedel et al. 1996).
Three out of the 16 compounds tested did not exert any genotoxicity in the recombinant cells. Two of these compounds, harman and norharman, are heterocyclics lacking an exocyclic amino group. All previous genotoxicity tests experiments with these compounds have returned negative results (Chang et al. 1978; Holme et al. 1985). These negative findings underline the importance of the exocyclic amino group in the biological activity of HAAs. DMIP was the only HAA showing no genotoxic activity in this study, whatever the cellular model used. It differs from PhIP by the substitution of a methyl group for a phenyl group (Fig. 1). Therefore, the resonance stabilization of a nitrenium/carbonium ion formed from DMIP is expected to be weaker, as compared to PhIP. Then, even an excellent leaving group, such as sulfate, may not provide enough reactivity to produce a genotoxic effect, simply because the intermediate metabolite is not stable enough. APNH was found to be the most genotoxic HAA among the congeners tested in this study, exhibiting a LEC of 1 nM. Interestingly, in previous long-term carcinogenicity studies, lower in vivo doses were required to observe a carcinogenic effect of APNH (Husain et al. 2007) than of other HAAs (Sugimura et al. 2004). APNH differs from the other HAAs tested by the presence of a fourth aromatic ring. This additional ring may enhance the resonance stabilization of nitrenium/carbonium ions, and consequently, the chemical reactivity of N-sulfoxy and N-acetoxy metabolites. Furthermore, we found that both human NAT2 and SULT1A1 were able to strongly enhance the genotoxic activity of APNH. Taken together, these findings may suggest a high carcinogenic activity of APNH in humans. Further studies, like APNH quantification in human urine (Nishigaki et al. 2007) should be carried out to clarify the extent of human exposure to APNH. As well, the conditions in which APNH is formed should be further investigated.
Only Trp-P-1 demonstrated a high genotoxic activity linked with CYP1A2 bioactivation, independently of the expression of SULT1A1 and NAT2 enzymes. Yamazoe et al. reported that seryl-tRNA synthetase from yeast and prolylyl-tRNA synthetase from rat are able to stimulate the covalent binding of N-OH-Trp-P-2 to DNA in cell-free systems (Yamazoe et al. 1981, 1985). Likewise, Saito et al. observed the formation of a semistable glutathione conjugate from N-OH-Trp-P-2, which exhibited higher mutagenic activity in S. typhimurium than N-OH-Trp-P-2 itself. Thus, unusual conjugation reactions may be involved in the activation of Trp-P-2 (Saito and Kato 1984).
In animal models, the main target organs of carcinogenesis differ between HAAs. It is probable that differences in bioactivation requirements are important factors underlying these organotropisms and species-dependent differences. In this study, we demonstrated that the final activation step of many HAAs is highly dependent upon conjugation reactions. One might expect that tissues expressing high levels of the appropriate phase II enzyme(s) are potential targets for adverse effects of HAAs, provided they are sufficiently exposed to N-hydroxy-HAAs, via local activation of HAAs by CYPs or via the circulation. In humans, SULT1A1 is expressed in many different tissues, the levels being particularly high in liver and gut (Teubner et al. 2007). It appears that few human tissues express NAT2, primarily the large intestine (Husain et al. 2007). It has to be emphasized that expression sites and substrate specificity of orthologous enzymes can substantially vary between species. This is true in particular for SULTs (Dobbernack et al. 2011; Glatt et al. 1998; Meinl et al. 2013). The tissue distribution of DNA adduct formation by PhIP in wild-type mice and in mice transgenic for the human SULT1A1–SULT1A2 gene cluster was investigated by in animals orally exposed to PhIP (Dobbernack et al. 2011; Hoie et al. 2016). Whereas the liver demonstrated the lowest level of DNA adducts in wild-type mice, it was the tissue exhibiting the highest adduct levels in transgenic mice. This selective influence of transgenic SULT1A1–SULT1A2 in the liver contrasted with the high expression of the transgene in many extrahepatic tissues. We suspect that PhIP is primarily converted to N-hydroxy-PhIP in the liver; in transgenic mice, it is expected that this metabolite is immediately converted into a DNA reactive, short-lived ester by human SULT1A1 and SULT1A2, explaining the high hepatic adduct levels. Since in wild-type mouse, SULT1A1 does not activate N-hydroxy-PhIP, much N-hydroxy-PhIP may escape the liver and be further activated in other tissues (Meinl et al. manuscript in preparation). This example illustrates that knowledge of critical phase II enzymes may be useful to explain target sites of HAAs. Nevertheless, broad knowledge on the toxicokinetics, including the role of many phase I and phase II enzymes, will be required for the prediction of target sites in humans. High-throughput genotoxicity assays, such as γH2AX ICW, in combination with recombinant cell lines should be useful tools for obtaining the relevant information.
References
Ando M, Yoshikawa K, Iwase Y, Ishiura S (2014) Usefulness of monitoring gamma-H2AX and cell cycle arrest in HepG2 cells for estimating genotoxicity using a high-content analysis system. J Biomol Screen 19(9):1246–1254
Audebert M, Riu A, Jacques C et al (2010) Use of the gammaH2AX assay for assessing the genotoxicity of polycyclic aromatic hydrocarbons in human cell lines. Toxicol Lett 199(2):182–192
Audebert M, Dolo L, Perdu E, Cravedi JP, Zalko D (2011) Use of the gammaH2AX assay for assessing the genotoxicity of bisphenol A and bisphenol F in human cell lines. Arch Toxicol 85(11):1463–1473
Audebert M, Zeman F, Beaudoin R, Pery A, Cravedi JP (2012) Comparative potency approach based on H2AX assay for estimating the genotoxicity of polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol 260(1):58–64
Boobis AR, Lynch AM, Murray S et al (1994) CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res 54(1):89–94
Bryce SM, Bemis JC, Mereness JA et al (2014) Interpreting in vitro micronucleus positive results: simple biomarker matrix discriminates clastogens, aneugens, and misleading positive agents. Environ Mol Mutagen 55(7):542–555
Chang CC, Castellazzi M, Glover TW, Trosko JE (1978) Effects of harman and norharman on spontaneous and ultraviolet light-induced mutagenesis in cultured Chinese hamster cells. Cancer Res 38(12):4527–4533
Dobbernack G, Meinl W, Schade N et al (2011) Altered tissue distribution of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-DNA adducts in mice transgenic for human sulfotransferases 1A1 and 1A2. Carcinogenesis 32(11):1734–1740
Glatt H (2006) Metabolic factors affecting the mutagenicity of heterocyclic amines. Book Acryl Other Hazard Compd Heat Treat Foods:358–404
Glatt H, Gemperlein I, Setiabudi F, Platt KL, Oesch F (1990) Expression of xenobiotic-metabolizing enzymes in propagatable cell cultures and induction of micronuclei by 13 compounds. Mutagenesis 5(3):241–249
Glatt H, Davis W, Meinl W, Hermersdorfer H, Venitt S, Phillips DH (1998) Rat, but not human, sulfotransferase activates a tamoxifen metabolite to produce DNA adducts and gene mutations in bacteria and mammalian cells in culture. Carcinogenesis 19(10):1709–1713
Glatt H, Pabel U, Meinl W, Frederiksen H, Frandsen H, Muckel E (2004) Bioactivation of the heterocyclic aromatic amine 2-amino-3-methyl-9H-pyrido [2,3-b]indole (MeAaC) in recombinant test systems expressing human xenobiotic-metabolizing enzymes. Carcinogenesis 25(5):801–807
Graillot V, Takakura N, Hegarat LL, Fessard V, Audebert M, Cravedi JP (2012a) Genotoxicity of pesticide mixtures present in the diet of the French population. Environ Mol Mutagen 53(3):173–184
Graillot V, Tomasetig F, Cravedi JP, Audebert M (2012b) Evidence of the in vitro genotoxicity of methyl-pyrazole pesticides in human cells. Mutat Res 748(1–2):8–16
Hammons GJ, Milton D, Stepps K, Guengerich FP, Tukey RH, Kadlubar FF (1997) Metabolism of carcinogenic heterocyclic and aromatic amines by recombinant human cytochrome P450 enzymes. Carcinogenesis 18(4):851–854
Hoie AH, Monien BH, Glatt H, Hjertholm H, Husoy T (2016) DNA adducts induced by food mutagen PhIP in a mouse model expressing human sulfotransferases 1A1 and 1A2. Toxicol Lett 248:34–38
Holme JA, Soderlund E, Aune T (1985) Effects of harman and norharman on the metabolism and genotoxicity of 2-acetylaminofluorene in cultured rat hepatocytes. Cell Biol Toxicol 1(3):223–239
Husain A, Zhang X, Doll MA, States JC, Barker DF, Hein DW (2007) Identification of N-acetyltransferase 2 (NAT2) transcription start sites and quantitation of NAT2-specific mRNA in human tissues. Drug Metab Dispos 35(5):721–727
IARC (2016) International Agency for Research on Cancer, Lyon. http://monographs.iarc.fr/ENG/Classification/latest_classif.php
Jamin EL, Riu A, Douki T et al (2013) Combined genotoxic effects of a polycyclic aromatic hydrocarbon (B(a)P) and an heterocyclic amine (PhIP) in relation to colorectal carcinogenesis. PLoS One 8(3):e58591
Kato T, Ohgaki H, Hasegawa H, Sato S, Takayama S, Sugimura T (1988) Carcinogenicity in rats of a mutagenic compound, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline. Carcinogenesis 9(1):71–73
Khoury L, Zalko D, Audebert M (2013) Validation of high-throughput genotoxicity assay screening using gammaH2AX in-cell western assay on HepG2 cells. Environ Mol Mutagen 54(9):737–746
Khoury L, Zalko D, Audebert M (2016a) Complementarity of phosphorylated histones H2AX and H3 quantification in different cell lines for genotoxicity screening. Arch Toxicol 90(8):1983–1995
Khoury L, Zalko D, Audebert M (2016b) Evaluation of four human cell lines with distinct biotransformation properties for genotoxic screening. Mutagenesis 31(1):83–96
Landsiedel R, Engst W, Scholtyssek M, Seidel A, Glatt H (1996) Benzylic sulphuric acid esters react with diverse functional groups and often form secondary reactive species. Polycycl Aromat Compd 11:341–348
Martignoni M, Groothuis GM, de Kanter R (2006) Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol 2(6):875–894
Meinl W, Tsoi C, Swedmark S, Tibbs ZE, Falany CN, Glatt H (2013) Highly selective bioactivation of 1- and 2-hydroxy-3-methylcholanthrene to mutagens by individual human and other mammalian sulphotransferases expressed in Salmonella typhimurium. Mutagenesis 28(5):609–619
Mizota T, Ohno K, Yamada T (2011) Validation of a genotoxicity test based on p53R2 gene expression in human lymphoblastoid cells. Mutat Res 724(1–2):76–85
Muckel E, Frandsen H, Glatt HR (2002) Heterologous expression of human N-acetyltransferases 1 and 2 and sulfotransferase 1A1 in Salmonella typhimurium for mutagenicity testing of heterocyclic amines. Food Chem Toxicol 40(8):1063–1068
Nishigaki R, Totsuka Y, Kataoka H et al (2007) Detection of aminophenylnorharman, a possible endogenous mutagenic and carcinogenic compound, in human urine samples. Cancer Epidemiol Biomark Prev 16(1):151–156
Ohgaki H, Takayama S, Sugimura T (1991) Carcinogenicities of heterocyclic amines in cooked food. Mutat Res 259(3–4):399–410
Quesnot N, Rondel K, Audebert M et al (2016) Evaluation of genotoxicity using automated detection of gammaH2AX in metabolically competent HepaRG cells. Mutagenesis 31(1):43–50
Ripa L, Mee C, Sjo P, Shamovsky I (2014) Theoretical studies of the mechanism of N-hydroxylation of primary aromatic amines by cytochrome P450 1A2: radicaloid or anionic? Chem Res Toxicol 27(2):265–278
Saito K, Kato R (1984) Glutathione conjugation of arylnitroso compound: detection and monitoring labile intermediates in situ inside a fast atom bombardment mass spectrometer. Biochem Biophys Res Commun 124(1):1–5
Schmalix WA, Maser H, Kiefer F et al (1993) Stable expression of human cytochrome P450 1A1 cDNA in V79 Chinese hamster cells and metabolic activation of benzo[a]pyrene. Eur J Pharmacol 248(3):251–261
Schut HA, Snyderwine EG (1999) DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis 20(3):353–368
Shamovsky I, Ripa L, Borjesson L et al (2011) Explanation for main features of structure-genotoxicity relationships of aromatic amines by theoretical studies of their activation pathways in CYP1A2. J Am Chem Soc 133(40):16168–16185
Shamovsky I, Ripa L, Blomberg N et al (2012) Theoretical studies of chemical reactivity of metabolically activated forms of aromatic amines toward DNA. Chem Res Toxicol 25(10):2236–2252
Skog KI, Johansson MA, Jagerstad MI (1998) Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence and intake. Food Chem Toxicol 36(9–10):879–896
Smart DJ, Ahmedi KP, Harvey JS, Lynch AM (2011) Genotoxicity screening via the gammaH2AX by flow assay. Mutat Res 715(1–2):25–31
Sugimura T, Wakabayashi K, Nakagama H, Nagao M (2004) Heterocyclic amines: mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci 95(4):290–299
Teubner W, Meinl W, Florian S, Kretzschmar M, Glatt H (2007) Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem J 404(2):207–215
Turesky RJ, Le Marchand L (2011) Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem Res Toxicol 24(8):1169–1214
Turesky RJ, Constable A, Fay LB, Guengerich FP (1999) Interspecies differences in metabolism of heterocyclic aromatic amines by rat and human P450 1A2. Cancer Lett 143(2):109–112
Westerink WM, Stevenson JC, Horbach GJ, Schoonen WG (2010) The development of RAD51C, Cystatin A, p53 and Nrf2 luciferase-reporter assays in metabolically competent HepG2 cells for the assessment of mechanism-based genotoxicity and of oxidative stress in the early research phase of drug development. Mutat Res 696(1):21–40
Westerink WM, Schirris TJ, Horbach GJ, Schoonen WG (2011) Development and validation of a high-content screening in vitro micronucleus assay in CHO-k1 and HepG2 cells. Mutat Res 724(1–2):7–21
Wild D, Feser W, Michel S, Lord HL, Josephy PD (1995) Metabolic activation of heterocyclic aromatic amines catalyzed by human arylamine N-acetyltransferase isozymes (NAT1 and NAT2) expressed in Salmonella typhimurium. Carcinogenesis 16(3):643–648
Yamazoe Y, Tada M, Kamataki T, Kato R (1981) Enhancement of binding of N-hydroxy-TRP-P-2 to DNA by seryl-tRNA synthetase. Biochem Biophys Res Commun 102(1):432–439
Yamazoe Y, Shimada M, Shinohara A, Saito K, Kamataki T, Kato R (1985) Catalysis of the covalent binding of 3-hydroxyamino-1-methyl-5H-pyrido[4,3-b]indole to DNA by a l-proline- and adenosine triphosphate-dependent enzyme in rat hepatic cytosol. Cancer Res 45(6):2495–2500
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chevereau, M., Glatt, H., Zalko, D. et al. Role of human sulfotransferase 1A1 and N-acetyltransferase 2 in the metabolic activation of 16 heterocyclic amines and related heterocyclics to genotoxicants in recombinant V79 cells. Arch Toxicol 91, 3175–3184 (2017). https://doi.org/10.1007/s00204-017-1935-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-1935-8