Abstract
Nanosized titanium dioxide (nano-TiO2) has tremendous potential for a host of applications, and TiO2 nanoparticles (NP) possess different physicochemical properties compared to their fine particle analogs, which might alter their bioactivity. Their adverse effects on living cells have raised serious concerns recently for their use in health care and consumer sectors such as sunscreens, cosmetics, pharmaceutical additives and implanted biomaterials. Many researches have demonstrated that the physicochemical properties including shape, size, surface characteristics and inner structure of nano-TiO2 particles have different degrees of toxicity to different organism groups under different conditions. Some former reports have demonstrated that nano-TiO2 materials could enter into human body through different routes such as inhalation, dermal penetration and ingestion. After being taken by human body, NP might induce oxidative stress, cytotoxicity, genotoxicity, inflammation and cell apoptosis ultimately in mammal organs and systems. Here, we summarized the update about toxicity of nano-TiO2 and aimed to supply a safety usage guideline of this nanomaterial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the fast development of nanotechnology, there are more and more nanomaterial applications in daily life, such as electronics, commercial products, medical devices and drugs (Emerich and Thanos 2003). Therefore, human exposure to nanoparticles is becoming increasingly frequent. Nanoparticles are considered to be a threat to environment and human heath (Nel et al. 2006). Many evidences have shown that some widely used nanoparticles in many fields impaired human health (Han et al. 2012; Khan et al. 2012; Xu et al. 2012a, b). Therefore, we should take the safety use seriously when kinds of nanomaterials are introduced to the market.
Ordinary speaking, TiO2 is considered as a low toxicity particles. However, this view was changed after The International Agency for Research on Cancer (IARC) classified TiO2 as a Group 2B carcinogen (IARC 2006). Compared to regular TiO2 particles (including fine nano-TiO2 and microsize nano-TiO2), nano-TiO2 particles are more specific due to their stronger catalytic activity. During these few years, nano-TiO2 has become the most widely used nanoparticle and has been produced in large industrial scale. It could be used as additives in pharmaceuticals and cosmetics, as fillers in polymeric materials used to improve bone prostheses and as scaffolds in biomedicine (Bernier et al. 2012; Chen and Mao 2007; Jia et al. 2014).
TiO2 occurs in nature in the form of three well-known crystallographic structure: anatase, rutile and brookite. A lot of studies reported that anatase showed higher activity than the rutile style so that it used more widespread in commercial. Rutile is more stable than the other two structures. Anatase and brookite phase both convert to rutile upon heating (Chen and Mao 2007; Park et al. 2014a). The crystal phases of rutile and anatase TiO2 are tetragonal, whereas that of brookite is orthorhombic. Besides that, brookite is difficult to synthesize, which has been reported to have higher photocatalytic activity (Warheit et al. 2007). Nano-TiO2 could synthesize via different ways. Vijayalakshmi et al. have reported two main routes: sol–gel route and hydrothermal route. They tried to analyze the two on the basis of their crystallinity, crystallite size, band gap and structural properties (Vijayalakshmi and Rajendran 2012). The TiO2 nanoparticles prepared via sol–gel route are highly crystalline and have smaller crystallite size (~7 nm) as compared to the one prepared by hydrothermal method (~17 nm). The band gap of the synthesized nanoparticles is found to be size dependent (Vijayalakshmi and Rajendran 2012). Figure 1 shows that transmission electron microscope (TEM) image of nano-TiO2 synthesized via sol–gel route (a) and hydrothermal method (b).
SEM images of the nanoparticles prepared via sol–gel route (a) and hydrothermal method (b) (Vijayalakshmi and Rajendran 2012)
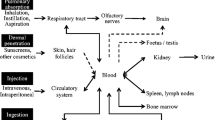
The extensive usage of nano-TiO2 particles is a double-edged sword to human health and ecosystem. Nano-TiO2 materials have several different ways to enter human body, such as injection (blood circulation), inhalation (respiratory tract), ingestion (gastrointestinal tract) and dermal penetration (skin) (Oberdörster et al. 2005). It has been reported that nano-TiO2 could induce inflammation, cytotoxicity, genotoxicity and phototoxicity in mammals and other experiment animals. The fate of the TiO2 nanoparticles in the body may differ according to the size and surface charge of TiO2 nanoparticles even when their shapes are the same. The major target organs for accumulation of nanomaterials may be liver, kidney and spleen even at a low level (Fabian et al. 2008; Liu et al. 2009; Park et al. 2014b). Even though titanium dioxide induces apoptotic cell death through reactive oxygen species-mediated Fas up-regulation and Bax activation, their degrees in different cells are varied (Park et al. 2014a; Yoo et al. 2012). The toxicity of TiO2 nanoparticles depends on the biological system used in the experiment, which indicates that the degrees of toxic and bioaccumulation were different. For instance, expanded simple tandem repeat (ESTR) loci in mice are sensitive markers of mutagenic effects on male germ cells resulting from nano-TiO2 exposures; however, female germ cells show no increased ESTR mutation rates in F1 females exposed in utero to UV-Titan nanoparticles (Boisen et al. 2012). Thus, there is a necessity to summarize and contrast the different degrees of toxicity of different organs and systems, especially the research findings in recent years as an update.
Toxicity of nano-TiO2
Toxicity of nano-TiO2 on skin
Skin is the largest organ of the body and could serve an important portal route for the entry of nanoparticles into mammals. With the special properties and functions, nano-TiO2 was added into some sunscreen formulations to absorb and deflect ultraviolet (UV) radiation. So this nanomaterial, as an additive in cosmetic and sunscreen, had great opportunity to get exposed to skin directly. The assessment with respect to skin absorption and toxicity made by the Scientific Committee on Consumer Safety (SCCS) tended to consider the use of nano-TiO2 in dermal application could not pose significant risk to human (Scientific Committee on Consumer Safety (SCCS) 2013), and they recommended not to use TiO2 with substantially high photocatalytic activity in sunscreen formulations. Other TiO2 nanomaterials that have a relatively lower but still significant level of photocatalytic activity may be used. On the other hand, several studies were conducted and published in order to study all-sided and dig the potential toxicity of nano-TiO2 on skin depending on different conditions. In order to make the effect of nano-TiO2 on skin overall understood by researches, we present some results hereinbelow.
Human keratinocyte (HaCaT) cells were the most common in vitro model used in toxicology studies of skin. Jaeger et al. reported that HaCaT cells exposed to nano-TiO2 induced the mitochondrial “common deletion.” Moreover, this nanomaterial displayed a ROS-mediated cytotoxic and genotoxic potential in human keratinocytes (Jaeger et al. 2012). But this paper did not refer to the phototoxicity of nanosized titanium dioxides in HaCaT keratinocytes. Yin et al. (2012) have demonstrated that nano-TiO2 is phototoxic to human skin keratinocytes, and this phototoxicity is mediated by reactive oxygen species (ROS) generated during UVA irradiation. Under UVA irradiation, electrons in the TiO2 valence band absorbed the photon energy and jumped to the conduction band, leaving valence band holes that extracted electrons from water or hydroxyl ions and generated hydroxyl radicals (·OH). Formation of other ROS, including superoxide (O ·−2 ) and singlet oxygen (1O2), by different mechanisms has also been reported. So the production of ROS is the key process in the generation of phototoxicity under UVA irradiation. We could regard this paper as a supplement of the former one. Another paper reported that N-acetylcysteine (NAC), a sulfhydryl-containing antioxidant, could prevent nano-TiO2-induced oxidative stress and apoptosis in HaCaT cells. The protective effects of NAC on nano-TiO2 induced apoptosis were related to modulation of ROS and the intracellular nitric oxide (NO) levels. These results suggest that NAC has some potential as an antidote for nano-TiO2 phototoxicity (Xue et al. 2011). Furthermore, Ghiazza et al. (2014) found that the ROS-mediated cytotoxicity and genotoxicity of nano-TiO2 toward human keratinocyte cells could be inhibited by iron doping. They suggest that impregnation with iron salts might be a promising strategy to reduce this kind of cytotoxicity and genotoxicity. On the other hand, Park et al. (2011) took out opposite results about the phototoxicity of nano-TiO2, and their results demonstrated that nano-TiO2 particles induced no phototoxicity, acute cutaneous irritation or skin sensitization. Their findings might be a little segmentary because the nanomaterial they used was just one kind of numerous nano-TiO2 and the diameter was less than 25 nm. It was worth mentioning that Tucci et al. (2013) reported that after treatment with 100 mg/ml TiO2 for 24 h, HaCaT cells showed the activation of cellular stress and reduced metabolic capacity. In addition, some other cell types were also employed in the dermal toxicity experiments. Shukla et al. investigated the genotoxicity of human epidermal cells (A431) and suggested that ROS and oxidative stress lead to oxidative DNA damage and micronucleus formation, which was the probable mechanism of genotoxicity (Shukla et al. 2011). Tay et al. focused on the human oral epithelium, they compared nanohydroxyapatite and nanotitanium dioxide, and they found that these two nanomaterials showed different subcellular distribution; nanohydroxyapatite displayed a higher preference to accumulate near the cell membrane compared to nano-TiO2. An elevated ROS level and expression of inflammatory transcripts were observed when the cells were exposed to both two nanomaterials. They further showed that nano-TiO2-mediated cell death was independent of the classical p53-Bax apoptosis pathway. Their findings provide insights into the potential cellular fates of human oral epithelial cells as they interface with industrial-grade nanohydroxyapatite and nano-TiO2 (Tay et al. 2014). A human lens epithelial cell line (HLE B-3, ATCC) was used in another study, and it was indicated that UVB irradiation could efficiently inhibit the cell proliferation in vitro, generated excessive cellular ROS and elevated the intracellular Ca2+, thereby disrupted the intracellular calcium homeostasis, suggesting that UVB irradiation and TiO2 nanomaterial could exert synergistically inhibitory effect on HLE B-3 cell proliferation. Moreover, TiO2 nanomaterial had great potential for the application of posterior capsular opacification (PCO) treatment under UVB irradiation in clinical practice (Wu et al. 2014). Wu et al. (2009) studied the penetration profile and potential toxicity of titanium dioxide nanoparticles not only in vitro but also in vivo via a dermal route. Their results showed that nano-TiO2 particles could penetrate the skin and damage different organs in animals. The most seriously damaged organs were skin and liver of mice. This was a direct evidence that dermal exposure-induced tissue damaged in other organs.
Toxicity of nano-TiO2 on respiratory system
In the process of production, distribution, use and recycle, nano-TiO2 is easy to spread into the air. Therefore, industrial or commercial titanium dioxide particles might become one regular component of indoor or outdoor atmosphere. As inhalation was a common route of TiO2 nanoparticles to enter human body, there was a risk that nano-TiO2 did harm to respiratory system. The respiratory tract became the primary target organ system for the inhaled nanoparticles. So there were lots of studies that paid attention to this significant problem. Some of them used A549, a widely used human lung cancer cell line for inhalation or pulmonary toxicity. Srivastava et al. (2012) reported that exposure to nano-TiO2 could induce oxidative stress, apoptosis and genotoxicity. They also found that expressional changes in apoptosis markers were having good correlation with endpoints of oxidative stress and phenotypic presentation of apoptotic/genotoxic events. Another similar paper suggested that TiO2 nanoparticles (NPs) caused an oxidative stress and exhibited genotoxicity to A549 cells. In addition, they confirmed that the smallest and spherical NPs exert the more pronounced toxic effects, but had no relationship with their crystalline phase. They also observed that the DNA damage caused by nano-TiO2 was single-strand breaks and 8-oxodGuo, but not double-strand breaks or chromosomal breaks or losses. Furthermore, the nanoparticles inactivated both nucleotide excision repair abilities (NER) and base excision repair (BER) pathways leading to the losing of cell ability to repair the damaged DNA (Jugan et al. 2012). Two more recent papers presented similar conclusion. Wang et al. demonstrated that TiO2 NPs inhibited proliferation and caused DNA damage in A549 cells. Moreover, this study indicated that TiO2 NPs induced apoptosis in A549 cells via the mitochondrion-mediated pathway (Wang et al. 2014). Kansara et al. (2015) put forward that the generation of oxidative stress including ROS leading to DNA damage, but they maintained that this damage was double-strand break that was correlated with cell cycle arrest in G2/M phase. On the other hand, Toyooka’ study showed distinct results in spite of the same usage of cell line. They found that smaller TiO2 particles had the potential to cause genotoxicity, which was confirmed by a sensitive DNA damaging marker, phosphorylated histone H2AX (γ-H2AX). The generation of γ-H2AX in dependent of cell cycle phases suggested the direct formation of double-strand breaks (DSBs) by TiO2 particles. However, ROS did not contribute to the generation (Toyooka et al. 2012). Tedja et al. (2011) compared A549 cell line with another pulmonary cell line H1299. The results indicated that A549 cell line was showed to be relatively resistant to the total uptake of TiO2 particles, as measured by cell viability and metabolic assays, while H1299 had a much higher capacity to ingest TiO2 particles and aggregates, with consequent evidence of impact at concentrations as low as 30–150 μg/ml. From another angle, Zhang et al. (2012) detected the cytotoxicity of different-sized TiO2 nanoparticles and found that the 25-nm anatase particles induced stronger cytotoxicity and oxidative stress than those of 5- and 100-nm anatase particles. Both 5- and 100-nm anatase particles had similar toxicity. Rutile particles caused lower toxicity than anatase particles. They drew the conclusion that the toxicity of TiO2 nanoparticles could mainly depend on the structural characteristics. Figure 2 shows that cell viability of Ana-1 cells exposed to different-sized TiO2 particles (Zhang et al. 2012). Lagopati et al. (2014) also declared that the cytotoxicity of TiO2 nanoparticles of similar size but different crystal structure gradually decreased as their composition changed from pure anatase to anatase–rutile mixtures. They found that pure anatase structure induced apoptosis specifically in MDA-MB-468 cells. The molecular mechanism involved increased proapoptotic gene Bax expression, caspase-mediated PARP cleavage and DNA fragmentation, thus resulting in cell apoptosis. Both these two papers confirmed that anatase structure showed more severe cytotoxicity. Xiong et al. (2013b) found the size-dependent cytotoxicity of TiO2 nanoparticles could be due to the fact that smaller particles with larger specific surface area could absorb more biomolecules such as proteins in the environment and so that the smaller particles brought greater damage to organism. Another paper they have published supported this view. Furthermore, they provided the mechanism behind the phototoxicity of TiO2 nanoparticles and clued on how to alleviate such toxicity. They suggested that using the surface coating of TiO2 nanoparticles with poly (ethylene-alt-maleic anhydride) (PEMA) or chitosan could decrease their phototoxicity (Xiong et al. 2013a). Trevor et al. concerned that the toxicity of human nasal septum carcinoma RPMI 2650 cell line by reasons of nasal cavity was a key part of the respiratory system. Their results demonstrated that microfluidic dispersion influenced the in vitro toxicity of TiO2 nanofilaments. Well-dispersed TiO2 nanomaterials processed by the microfluidic device for TiO2 were nontoxic in nasal cells as they did not cause inflammation, alter cellular morphology, or reduce the cellular viability. This result suggests that TiO2 nanomaterials could be applied to drug delivery and bioimaging (Tilly et al. 2014).
Cell viability of Ana-1 cell exposed to TiO2 particles. Control cells treated without TiO2 particles are considered to have 100 % activity. *P < 0.05 versus control cells. TiO2: titanium oxide (Zhang et al. 2012)
Jeon et al. (2011) took in vivo experiment to prove the toxicity of respiratory system. In this study, local histopathological changes, including alveolar septal thickening, neutrophil infiltration, and hyperplastic epithelial changes were observed after accumulation of TiO2 NPs in lung. Collectively, these changes probably resulted in the damage of lung function. Moreover, they also thought that oxidative stress and inflammation were involved in this process. There was an oncogenic risk because the expression of cancer-related proteins (pyruvate kinase, l-lactate dehydrogenase A chain, moesin and heat shock protein 84b) was shown to be altered. Porter et al. (2012) suggested that both nanospheres and long nanobelts resulted in the lung deposition of 135 μg TiO2. At 112-day after exposure, the lung burden was significantly lower in nanosphere-exposed mice than that in nanobelt-exposed mice. Leppänen et al. (2011) set up acute and repeated TiO2 exposure model on outbred Crl:OF1 male mice, finding nano-TiO2 mainly accumulated in the pulmonary macrophages but did not cause nasal or pulmonary inflammation. Tang et al. (2013) investigated toxicity of nano-TiO2 to lung in two aspects, both in vitro and in vivo. They found mitochondrial injury might be the potential mechanism during the damaged process. Besides that, Husian et al. (2013) stated that a small fraction of nano-TiO2 particles induced alterations in the expression of several genes associated with ion homeostasis, and muscle function may potentially interfere with calcium, ion and lipid homeostasis, and affect pulmonary smooth muscle contraction. They also discuss the nano-TiO2 translocation from lungs to blood and extra-pulmonary organs and then toxicity to liver and heart tissue (Husain et al. 2015).
Toxicity of nano-TiO2 on liver and kidney
Once the nanoparticles entered animal body, they could be distributed throughout body. The liver is the major distributed site, followed by kidney. What is more, nano-TiO2 could accumulate in these two organs even though the exposed level was low. Researchers all over the world put attention to this issue and did lots of work about it. Firstly, they used the human hepatocellular liver carcinoma cell line (HepG2) as a model and discussed the mechanism behind the cell death after treatment with nano-TiO2. Shukla et al. considered that the apoptosis of HepG2 was due to ROS-mediated DNA damage via mitochondrial intrinsic pathway (Shukla et al. 2013). Prasad et al. (2014) gave out similar results, which indicated that exposure for 24 h of HepG2 to nano-TiO2 resulted in increased cellular interaction as measured by side scatter using flow cytometry, DNA damage in the comet assay, micronuclei induction and transcriptional activation of NF-κB but not activator protein 1 (AP1). El-Said et al. (2014) suggested more detailed mechanism about the generation of ROS and apoptosis. They demonstrated that the exposure to TiO2 NPs caused oxidative stress, with increased H2O2 and ·OH levels leading to DNA damage and p53 activation, and induced apoptosis by releasing cytochrome C into the cytoplasm and activating caspase-3. Over-expression of toll-like receptor 3 (TLR3) protected against oxidative stress-induced damage in response to TiO2 NP exposure, but over-production of toll-like receptor 4 (TLR4) enhanced the oxidative stress mediated by TiO2 NPs. Moreover, they found TiO2 NPs induced the expression of DNA damage marker genes, especially the ataxia telangiectasia mutated (ATM) and inositol hexakisphosphate kinase 3 (IP6K3) genes, which indicated that the type of DNA damage was double-strand break as well as chromatin condensation, nuclear fragmentation and apoptosis. Figure 3 shows caspase-3 activities which indicate the apoptosis in TiO2 NP-exposed HepG2 cells with and without TLR3 or TLR4 transfection.
Caspase-3 activities in TiO2 NP-exposed HepG2 cells with and without TLR3 or TLR4 transfection. The transfected cells were exposed to 10 μg/ml TiO2 NPs for 48 h. Each plot was produced from at least 3 replicate measurements. All values are presented as mean ± SD (n ≥ 3), (*P < 0.05) (El-Said et al. 2014)
Meanwhile, some in vivo experiments were carried out to prove the toxicity of liver. Attia et al. (2013) suggested that TiO2 NP had health hazard on liver as it affected the architecture of the hepatic cords. The addition of N-acetylcysteine (NAC) had hepatoprotective and ameliorative effect on the biohazard caused by TiO2 NP. We can infer from the results that the hepatic injury has immediate connection with oxidative stress. Hong et al. (2014) proved that hepatotoxicity was closely associated with increased expression of some inflammatory factors, such as interleukin (IL)-4, IL-5, IL-12, interferon (IFN)-γ, GATA-binding domain (GATA)3, GATA4, T-bet, transcription factor (STAT)3, STAT6, eotaxin, MCP-1 and MIP-2 and decreased STAT1 expression due to TiO2 NP exposure in the mouse liver. Therefore, TiO2 NP-induced liver injury might be via the alteration of Th2 cytokine expression and/or a possible IL-4-mediated pathway in mice. Nano-TiO2 also induced hepatotoxicity in rats, and hepatic tissues were altered after intraperitoneal injections (Younes et al. 2015). Vasantharaja et al. studied on serum biochemical changes in adult male Wistar rats. The changes between levels of total protein, glucose, aspartase transaminase, alanine transaminase and alkaline phosphatase indicated that TiO2 NPs induced liver damage. One prior research reported that the intraarticular injected anatase TiO2 nanoparticles had a potential toxicological effect on major organs and knee joints of rats. The severe pathological injury of major organs including liver was induced in the rats after exposure to middle and high-dose TiO2, which was consistent with the changes in serum biochemical parameters (Wang et al. 2009).
The significant increase in the blood urea nitrogen and uric acid indicated the renal damage in the TiO2 NP-treated rats. These results indicated that titanium dioxide particles were accumulated not only in liver, but also in kidney. Further, renal function was impaired by these NPs. Human embryonic kidney cells (HEK-293) were treated with titanium dioxide nanoparticles (TiO2 anatase, <25 nm). Nano-TiO2 inhibited the proliferation of HEK-293 cells by inducing cell apoptosis in a time- and dose-dependent manner. Moreover, nano-TiO2 might induce oxidative stress-mediated DNA damage, which led to the activation of p53 gene and the up-regulation of Bax and caspase-3 (Meena et al. 2012). Hong et al. reported that administration of nano-TiO2 resulted in significant changes in nephrotoxicity biomarkers in mice. Furthermore, the Wnt pathway was directly activated after the administration of nano-TiO2 showing performance in directly increasing the levels of expression of Wnts, Frizzled receptors and the epithelial-to-mesenchymal transition markers (EMT), and decreasing the levels of expression of Wnt antagonists, thus resulting in renal inflammation and fibrosis. Another paper showed similar result but different mechanism of nanoparticle toxicity on kidney. Gui et al. showed that the significant increase in NF-κB, tumor necrosis factor-α (TNF-α), macrophage migration inhibitory factor (MIF), IL-2, IL-4, IL-6, IL-8, IL-10, IL-18, IL-1β, cross-reaction protein (CRP), transforming growth factor-β (TGF-β), INF-γ, cytochrome p450 1A (CYP1A) expressions and significant decrease in heat shock protein 70 (HSP70) expression after being exposed with different concentrations of nano-TiO2. These changes led to the increase in kidney indices, inflammatory responses and cell necrosis in mouse kidney (Gui et al. 2011). Furthermore, the same group concluded that nephrotoxicity was closely associated with the decreased nuclear factor erythroid-2-related factor (Nrf2) expression using the same experiment animal model. It contributed to the pathogenesis of oxidative stress and inflammation and amplified their damaging effects on kidneys caused by TiO2 NP exposure. They suggested that the application of TiO2 NPs should be carried out cautiously, especially in humans (Gui et al. 2013).
From what we have listed above, as two major organs easily accumulated nano-TiO2 particles, liver and kidney had a risk of injury both in vitro and in vivo.
Toxicity of nano-TiO2 on reproductive system and embryo
There was evidence showed that absorbed TiO2 particles might be able to move across the placenta into fetal tissue and caused reproductive and developmental toxicity. The exposed zebrafish to TiO2 particles showed that nanoparticles can impair the normal development of zebrafish embryo. Vicario-Parés et al. (2014) suggested that comparing nano-TiO2 with CuO and ZnO NPs, the toxicity of nano-TiO2 was the least in these three metal NPs. Their research result reminded us when we assessed NPs toxicity using the zebrafish embryo model, it was important to consider not only mortality, but also the sublethal effects produced by the exposures; otherwise, the NP-toxicity could be under estimated. Another paper about zebrafish embryo detected the effect of several metal-doped TiO2 nanoparticles. They demonstrated that the Fe–TiO2 NPs exhibited the highest toxic effects. Among the metals, the Mn–TiO2 NPs demonstrated the improved photocatalysis compared to the other samples except for the Fe–TiO2 NPs along with the lowest toxic effects. For these reasons, the most suitable doping metal was the Mn–TiO2 NPs considering its energy activity and environmental impacts (Park et al. 2014c). Jia et al. (2014) used male Kunming mice as their experiment subject and observed the effect of pubertal nano-TiO2 exposure on testosterone synthesis and spermatogenesis in mice. The results demonstrated that the exposure of NPs could cause adverse effects on male reproductive system. Their data indicated that the decreased serum testosterone (T) levels resulted not only from the decreased expression of P450 17α-hydroxysteroid dehydrogenase (P450-17α) and 17β-hydroxysteroid dehydrogenase (17β-HSD) involved in T synthesis, but also from increased expression of cytochrome P450-19 (Cyp19), which could convert T to estradiol. Meena et al. (2014) reported that intravenous administration of higher doses of TiO2-NPs caused apoptosis during spermiogenesis or sperm maturation, and sperm caspase-3 activity seemed to affect the physiology of reproduction. They also found that the generation of oxidative stress might be the reason of DNA damage and apoptosis. Huang et al. (2014) paid attention to the ability of photocatalysis of nano-TiO2, and they investigated the photocatalytic oxidation properties of caffeine and isocaffeine in the presence of nanostructured TiO2 particles and UV irradiation in different aqueous or organic solvents, including dH2O, PBS and ethanol. The resulting oxidized products of caffeine or isocaffeine were shown to have higher cytotoxicity as well as genotoxicity on A2780 ovarian cancer cells than their unoxidized counterparts.
Toxicity of nano-TiO2 on central nervous system
Generally speaking, brain is under the protection of blood–brain barrier (BBB). The BBB is a highly selective permeability barrier that separates the circulating blood from cerebrospinal fluid and limits the entry of many substances into brain. But nanomaterials could easily penetrate body barrier such as BBB relatively unimpeded because of their particular physical and chemical property. Many studies have unequivocally showed that the treatment of nano-TiO2 could be transported to the central nervous system (CNS) and damage brain neurons and tissue in vitro and in vivo. Márquez-Ramírez et al. (2012) reported the uptake and internalization of TiO2 NPs by glial cells, induced an inhibition in their proliferation. Strong morphological changes were found, which were associated with depolymerization of F-actin and apoptotic cell death. This result suggested that the exposure of brain cells to TiO2 NPs could cause brain injury and contribute to the development of neurodegenerative diseases. Sheng et al. (2014) also reported that nano-TiO2 could induce oxidative stress, destabilization of MMP and the intracellular Ca2+ elevation, and increase the expression of apoptotic proteins in rat primary cultured hippocampal neurons. The neuron apoptosis being involved in mitochondria-mediated signal pathway and ER-mediated signal pathway led to the impairment of neuron development, decreasing the ability of learn and memory. Liu et al. (2010) suggested that oxidative stress was the potential mechanism of cellular apoptosis and revealed that nano-TiO2 could induce a significant cytotoxicity in PC12 cells in a dose-dependent and time-dependent manner. Figure 4 shows the TEM images (a) and dynamic light scattering (DLS) images (b) of the nano-TiO2 particles (Liu et al. 2010). The in vivo experiments mostly focused on learning and memory of animals. Meena’s study aimed to find out the effect of i.v. injected nano-TiO2 on brain of rats. They found that nano-TiO2 was smoothly transported to brain and crossed BBB after injection intravenously through caudal vein. After that, oxidative stress, inflammatory, DNA damage and apoptosis were detected in brain tissue, which might hamper the ability of learning and memory (Meena et al. 2015). Younes et al. (2015) suggested that TiO2 NPs altered the neurobehavioral performance of adult Wistar rats along with the damage of hepatic tissue. Ze et al. (2014) studied the mechanism of TiO2 particle-induced neurotoxicity. They showed that TiO2 NPs could cause an increase in phosphate-activated glutaminase (PAG) activity and a decrease in glutamine synthetase (GS) activity in mouse hippocampus, which also called “glutamate (Glu)–glutamine (Gln)” cyclic pathway. Furthermore, the imbalance of Glu metabolism triggered the inhibitions of N-methyl-d-aspartate receptor subunits (NR)1, NR2A, NR2B and mGluR2 expressions in the TiO2 NP-exposed hippocampus. Their findings might apply theoretical basis, which could improve the ability of learning and memory impaired by nano-TiO2 exposure. Besides that, some researchers compared nano-sized SiO2 and TiO2 to discuss the different neurological effects after direct exposure into the brain. Their findings indicated that exposure to TiO2 and SiO2 NPs could possibly impair the locomotor ability associated with microglial activation, and this deficit may be possibly attributed at least to an inflammatory process (Balvay et al. 2013).
Dispersion and characterization of the TiO2 nanoparticles were characterized by TEM (a) and DLS (b). TEM images showed that the size of the nano-TiO2 was distributed from 20 to 50 nm. And the DLS assay (b) stated that the particle size distribution had a wide range from 24 to 697 nm due to the aggregation, and the hydrodynamic diameter was 368.1 nm (Liu et al. 2010)
Toxicity of nano-TiO2 on peripheral blood cells and spleen
To extend the knowledge of the toxicity of nanosized TiO2 particles, researchers tested nano-TiO2 particles on peripheral blood cells. First of all, Kang et al. (2008) showed that nano-TiO2 had a genotoxic effect in both micronucleus and Comet assays. Furthermore, p21 and Bax were not induced by nano-TiO2-induced genotoxicity, but p53 DNA damage check point signal. After that, Ghosh et al. demonstrated the genotoxicity and cytotoxicity potential of nano-TiO2. A reduction in mitochondrial dehydrogenase activity and a reduction in membrane potential (MMP) were observed in human lymphocyte cells. DNA damage induced by this nanoparticle led to apoptosis. Meanwhile, they found that hemolytic property of erythrocyte cells was broken by titanium dioxide (Ghosh et al. 2013). Takaki et al. (2014) noticed nuclear condensation, chromosomal DNA damage, giant DNA fragmentation followed by ladder-like DNA fragmentation and caspase-3 activation. Thus, they concluded that nanosized TiO2 particles induced caspase-dependent apoptosis, and engulfment was not involved in this effect of TiO2 particles. Finally, some in vivo experiments were carried out. Aziz et al. gave the treatment of nano-TiO2 on adult male albino rat and studied splenocytes in rats. They observed that apoptosis of splenocytes as well as milk thistle seeds extract could help in the protection of spleen against the toxic effect (Aziz and Awaad 2014). Wang et al. (2011b) reported that nanoparticulate TiO2 caused congestion and lymph nodule proliferation of spleen tissue of mice by intragastric administration, and a significant increase in ROS productions in spleen, and subsequently led to a strong induction of heme oxygenase-1 (HO-1) via the p38-Nrf-2 signaling pathway. Similar conclusion could be drawn from another article, Fu et al. also observed congestion and lymph node alternations. Furthermore, increased proliferation of spleen-derived T cells and B cells following mitogen stimulation and enhanced NK cell killing activity were found by repeated instillation of nano-TiO2. Sang et al. (2013) paid more attention to the molecular mechanism in splenic injury induced by repeated administration of nano-TiO2. They found that increasing expression of cyclooxygenase (COX)-2 had a vital role in splenic injury. Both these results showed that nano-TiO2 might be one of triggers to be responsible for the systemic immune response and harmful to spleen and relevant cells.
Conclusion
As one of the most broad-spectrum nanoparticle, nano-TiO2 is related to our daily life compactly. Although this nanomaterial has been studied intensively in recent years, there are totally different conclusions about toxicity of different organs and systems depending on the different experimental conditions. Some papers present that there is no evidence that TiO2 nanosized particles pose a mutagenic/genotoxic, phototoxic or photomutagenic/genotoxic risk to humans, but protect human skin against UV-induced adverse effects, including DNA damage and skin cancer (Schilling et al. 2010). Another paper reports that combined TiO2 and UVA treatment can significantly reduce glioma growth and prolong survival in an animal model (Wang et al. 2011a). Although the usage of such nanomaterial brings our society lots of advantages, we should pay close attention to the toxicity induced by nano-TiO2 due to the diversified administration methods, doses and experimental animals. Some presented studies and theories demonstrated that nano-TiO2 induced oxidative stress, cytotoxicity, genotoxicity and phototoxicity in kinds of cells. If the conditions were deteriorated, the nanoparticles might cause histopathological change and impair the function of skin, lung, liver, kidney, brain, spleen and other important organs in mammal. We could infer that long-term exposure would induce more serious damage. Figure 5 shows the summarization of potential toxicity of nano-TiO2 to organs in mammals. There are some advices concerning this nanomaterial possible health effects to support risk assessment and management. At first, we should pay attention to the biosafety of TiO2 nanoparticle carriers for drug delivery application because these NPs are unimpedingly entered into living body. Second, the researches should value the long-term animal studies to illuminate the toxicities and oncogenicity of different structures of nano-TiO2. At last, catabolism after nano-TiO2 entering body should also become a research hotspot area. Besides that, the molecular mechanism of nano-TiO2 toxicity needs more meticulous discussion.
In summary, this review concludes the up-to-date studies about toxicity of nano-TiO2 particles on different organs and systems in vivo and in vitro. This would help us to realize the risk of the NPs in daily life and make the application of nano-TiO2 safety use.
References
Attia HF, Soliman MM, Abdel-Rahman GH, Nassan MA, Ismail SA, Farouk M, Solcan C (2013) Hepatoprotective effect of N-acetylcystiene on the toxic hazards of titanium dioxide nanoparticles. Am J Pharmacol Toxicol 8:141
Aziz HOA, Awaad A (2014) Titanium dioxide (TiO2) nanoparticles induced apoptosis of splenocytes in adult male albino rat and the protective role of Milk thistle seeds extract. Int J 2:732–746
Balvay A, Thieriet N, Lakhdar L, Bencsik A (2013) Comparative study of neurologic effects of nano-TiO2 versus SiO2 after direct intracerebral exposure in mice. In: Journal of physics: conference series, vol 1. IOP Publishing, p 012027
Bernier M-C, El Kirat K, Besse M, Morandat S, Vayssade M (2012) Preosteoblasts and fibroblasts respond differently to anatase titanium dioxide nanoparticles: a cytotoxicity and inflammation study. Colloids Surf B 90:68–74
Boisen A, Shipley T, Jackson P, Hougaard KS, Wallin H, Yauk CL, Vogel U (2012) NanoTIO2 (UV-Titan) does not induce ESTR mutations in the germline of prenatally exposed female mice. Part Fibre Toxicol 9:19
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959
El-Said KS, Ali EM, Kanehira K, Taniguchi A (2014) Molecular mechanism of DNA damage induced by titanium dioxide nanoparticles in toll-like receptor 3 or 4 expressing human hepatocarcinoma cell lines. J Nanobiotechnol 12:48
Emerich DF, Thanos CG (2003) Nanotechnology and medicine. Expert Opin Biol Ther 3:655–663
Fabian E, Landsiedel R, Ma-Hock L, Wiench K, Wohlleben W, Van Ravenzwaay B (2008) Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch Toxicol 82:151–157
Ghiazza M et al (2014) Inhibition of the ROS-mediated cytotoxicity and genotoxicity of nano-TiO2 toward human keratinocyte cells by iron doping. J Nanopart Res 16:1–17
Ghosh M, Chakraborty A, Mukherjee A (2013) Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. J Appl Toxicol 33:1097–1110
Gui S et al (2011) Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J Hazard Mater 195:365–370
Gui S et al (2013) Renal injury and Nrf2 modulation in mouse kidney following chronic exposure to TiO2 nanoparticles. J Agric Food Chem 61:8959–8968
Han Y, Xu J, Li Z, Ren G, Yang Z (2012) In vitro toxicity of multi-walled carbon nanotubes in C6 rat glioma cells. Neurotoxicology 33:1128–1134
Hong J et al (2014) Th2 factors may be involved in TiO2 NP-Induced hepatic inflammation. J Agric Food Chem 62:6871–6878
Huang X et al (2014) Nanostructured TiO2 catalyzed oxidations of caffeine and isocaffeine and their cytotoxicity and genotoxicity towards ovarian cancer cells. BioNanoScience 4:27–36
Husain M et al (2013) Pulmonary instillation of low doses of titanium dioxide nanoparticles in mice leads to particle retention and gene expression changes in the absence of inflammation. Toxicol Appl Pharmacol 269:250–262
Husain M et al (2015) Intratracheally instilled titanium dioxide nanoparticles translocate to heart and liver and activate complement cascade in the heart of C57BL/6 mice. Nanotoxicology 1–10. doi:10.3109/17435390.2014.996192
IARC (2006) Cobalt in hard-metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide, vol 86. IARC Scientific Publication
Jaeger A, Weiss DG, Jonas L, Kriehuber R (2012) Oxidative stress-induced cytotoxic and genotoxic effects of nano-sized titanium dioxide particles in human HaCaT keratinocytes. Toxicology 296:27–36. doi:10.1016/j.tox.2012.02.016
Jeon Y-M, Park S-K, Kim W-J, Ham J-H, Lee M-Y (2011) The effects of TiO2 nanoparticles on the protein expression in mouse lung. Mol Cell Toxicol 7:283–289
Jia F, Sun Z, Yan X, Zhou B, Wang J (2014) Effect of pubertal nano-TiO2 exposure on testosterone synthesis and spermatogenesis in mice. Arch Toxicol 88:781–788
Jugan M-L, Barillet S, Simon-Deckers A, Herlin-Boime N, Sauvaigo S, Douki T, Carriere M (2012) Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology 6:501–513
Kang SJ, Kim BM, Lee YJ, Chung HW (2008) Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ Mol Mutagen 49:399–405
Kansara K, Patel P, Shah D, Shukla RK, Singh S, Kumar A, Dhawan A (2015) TiO2 nanoparticles induce DNA double strand breaks and cell cycle arrest in human alveolar cells. Environ Mol Mutagen 56:204–217
Khan MI, Mohammad A, Patil G, Naqvi S, Chauhan L, Ahmad I (2012) Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 33:1477–1488
Lagopati N, Tsilibary E-P, Falaras P, Papazafiri P, Pavlatou EA, Kotsopoulou E, Kitsiou P (2014) Effect of nanostructured TiO2 crystal phase on photoinduced apoptosis of breast cancer epithelial cells. Int J Nanomed 9:3219
Leppänen M et al (2011) Nanosized TiO2 caused minor airflow limitation in the murine airways. Arch Toxicol 85:827–839
Liu H, Ma L, Zhao J, Liu J, Yan J, Ruan J, Hong F (2009) Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol Trace Elem Res 129:170–180
Liu S, Xu L, Zhang T, Ren G, Yang Z (2010) Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology 267:172–177
Márquez-Ramírez SG, Delgado-Buenrostro NL, Chirino YI, Iglesias GG, López-Marure R (2012) Titanium dioxide nanoparticles inhibit proliferation and induce morphological changes and apoptosis in glial cells. Toxicology 302:146–156
Meena R, Rani M, Pal R, Rajamani P (2012) Nano-TiO2-induced apoptosis by oxidative stress-mediated DNA damage and activation of p53 in human embryonic kidney cells. Appl Biochem Biotechnol 167:791–808
Meena R, Kajal K, Paulraj R (2014) Cytotoxic and genotoxic effects of titanium dioxide nanoparticles in testicular cells of male Wistar rat. Appl Biochem Biotechnol 175(2):825–840
Meena R, Kumar S, Paulraj R (2015) Titanium oxide (TiO2) nanoparticles in induction of apoptosis and inflammatory response in brain. J Nanopart Res 17:1–14
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627
Oberdörster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113(7):823–839
Park YH et al (2011) Analysis for the potential of polystyrene and TiO2 nanoparticles to induce skin irritation, phototoxicity, and sensitization. Toxicol In Vitro 25:1863–1869. doi:10.1016/j.tiv.2011.05.022
Park EJ, Lee GH, Shim HW, Kim JH, Cho MH, Kim DW (2014a) Comparison of toxicity of different nanorod-type TiO2 polymorphs in vivo and in vitro. J Appl Toxicol 34:357–366
Park EJ et al (2014b) Time-dependent bioaccumulation of distinct rod-type TiO2 nanoparticles: comparison by crystalline phase. J Appl Toxicol 34:1265–1270
Park H-G, Kim JI, Kang M, Yeo M-K (2014c) The effect of metal-doped TiO2 nanoparticles on zebrafish embryogenesis. Mol Cell Toxicol 10:293–301
Porter DW et al (2012) Differential mouse pulmonary dose-and time course-responses to titanium dioxide nanospheres and nanobelts. Toxicol Sci. doi:10.1093/toxsci/kfs261
Prasad RY et al (2014) Cellular interactions and biological responses to titanium dioxide nanoparticles in HepG2 and BEAS-2B cells: role of cell culture media. Environ Mol Mutagen 55:336–342
Sang X et al (2013) Toxicological mechanisms of nanosized titanium dioxide-induced spleen injury in mice after repeated peroral application. J Agric Food Chem 61:5590–5599
Schilling K et al (2010) Human safety review of “nano” titanium dioxide and zinc oxide. Photochem Photobiol Sci 9:495–509
Scientific Committee on Consumer Safety (SCCS) (2013) Revision of the opinion on titanium dioxide, nano form. doi:10.2772/70108
Sheng L et al (2014) Mechanisms of TiO2 nanoparticle‐induced neuronal apoptosis in rat primary cultured hippocampal neurons. J Biomed Mater Res A 103(3):1141–1149
Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S, Dhawan A (2011) ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro 25:231–241. doi:10.1016/j.tiv.2010.11.008
Shukla RK, Kumar A, Gurbani D, Pandey AK, Singh S, Dhawan A (2013) TiO2 nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology 7:48–60
Srivastava R et al (2012) Nano-titanium dioxide induces genotoxicity and apoptosis in human lung cancer cell line, A549. Hum Exp Toxicol. doi:10.1177/0960327112462725
Takaki K, Higuchi Y, Hashii M, Ogino C, Shimizu N (2014) Induction of apoptosis associated with chromosomal DNA fragmentation and caspase-3 activation in leukemia L1210 cells by TiO2 nanoparticles. J Biosci Bioeng 117:129–133
Tang Y, Wang F, Jin C, Liang H, Zhong X, Yang Y (2013) Mitochondrial injury induced by nanosized titanium dioxide in A549 cells and rats. Environ Toxicol Pharmacol 36:66–72
Tay CY, Fang W, Setyawati MI, Chia SL, Tan KS, Hong CH, Leong DT (2014) Nano-hydroxyapatite and nano-titanium dioxide exhibit different subcellular distribution and apoptotic profile in human oral epithelium. ACS Appl Mater Interfaces 6:6248–6256. doi:10.1021/am501266a
Tedja R, Marquis C, Lim M, Amal R (2011) Biological impacts of TiO2 on human lung cell lines A549 and H1299: particle size distribution effects. J Nanopart Res 13:3801–3813
Tilly TB, Kerr LL, Braydich-Stolle LK, Schlager JJ, Hussain SM (2014) Dispersions of geometric TiO2 nanomaterials and their toxicity to RPMI 2650 nasal epithelial cells. J Nanopart Res 16:1–15
Toyooka T, Amano T, Ibuki Y (2012) Titanium dioxide particles phosphorylate histone H2AX independent of ROS production. Mutat Res Genet Toxicol Environ Mutagen 742:84–91
Tucci P et al (2013) Metabolic effects of TiO2 nanoparticles, a common component of sunscreens and cosmetics, on human keratinocytes. Cell Death Dis 4:e549. doi:10.1038/cddis.2013.76
Vicario-Parés U et al (2014) Comparative toxicity of metal oxide nanoparticles (CuO, ZnO and TiO2) to developing zebrafish embryos. J Nanopart Res 16:1–16
Vijayalakshmi R, Rajendran V (2012) Synthesis and characterization of nano-TiO2 via different methods. Arch App Sci Res 4:1183–1190
Wang J-X, Fan Y-B, Gao Y, Hu Q-H, Wang T-C (2009) TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials 30:4590–4600
Wang C, Cao S, Tie X, Qiu B, Wu A, Zheng Z (2011a) Induction of cytotoxicity by photoexcitation of TiO2 can prolong survival in glioma-bearing mice. Mol Biol Rep 38:523–530
Wang J et al (2011b) P38-Nrf-2 signaling pathway of oxidative stress in mice caused by nanoparticulate TiO2. Biol Trace Elem Res 140:186–197
Wang Y, Cui H, Zhou J, Li F, Wang J, Chen M, Liu Q (2014) Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ Sci Pollut Res 22(7):5519–5530
Warheit DB, Webb TR, Reed KL, Frerichs S, Sayes CM (2007) Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicology 230:90–104
Wu J et al (2009) Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol Lett 191:1–8
Wu Q, Guo D, Du Y, Liu D, Wang D, Bi H (2014) UVB irradiation enhances TiO2 nanoparticle-induced disruption of calcium homeostasis in human lens epithelial cells. Photochem Photobiol 90:1324–1331. doi:10.1111/php.12322
Xiong S et al (2013a) Size of TiO2 nanoparticles influences their phototoxicity: an in vitro investigation. Arch Toxicol 87:99–109
Xiong S, George S, Yu H, Damoiseaux R, France B, Ng KW, Loo JS-C (2013b) Size influences the cytotoxicity of poly (lactic-co-glycolic acid)(PLGA) and titanium dioxide (TiO2) nanoparticles. Arch Toxicol 87:1075–1086
Xu J, Xu P, Li Z, Huang J, Yang Z (2012a) Oxidative stress and apoptosis induced by hydroxyapatite nanoparticles in C6 cells. J Biomed Mater Res A 100:738–745
Xu P, Xu J, Liu S, Yang Z (2012b) Nano copper induced apoptosis in podocytes via increasing oxidative stress. J Hazard Mater 241:279–286
Xue C, Liu W, Wu J, Yang X, Xu H (2011) Chemoprotective effect of N-acetylcysteine (NAC) on cellular oxidative damages and apoptosis induced by nano titanium dioxide under UVA irradiation. Toxicol In Vitro 25:110–116. doi:10.1016/j.tiv.2010.09.014
Yin JJ, Liu J, Ehrenshaft M, Roberts JE, Fu PP, Mason RP, Zhao B (2012) Phototoxicity of nano titanium dioxides in HaCaT keratinocytes—generation of reactive oxygen species and cell damage. Toxicol Appl Pharmacol 263:81–88. doi:10.1016/j.taap.2012.06.001
Yoo K-C et al (2012) Titanium dioxide induces apoptotic cell death through reactive oxygen species-mediated Fas upregulation and Bax activation. Int J Nanomed 7:1203
Younes NRB et al (2015) Subacute toxicity of titanium dioxide (TiO2) nanoparticles in male rats: emotional behavior and pathophysiological examination. Environ Sci Pollut Res 22(11):8728–8737
Ze X, Su M, Zhao X, Jiang H, Hong J, Yu X, Liu D, Xu B, Sheng L, Zhou Q, Zhou J, Cui J, Li K, Wang L, Ze Y, Hong F (2014) TiO2 nanoparticle‐induced neurotoxicity may be involved in dysfunction of glutamate metabolism and its receptor expression in mice. Environ Toxicol. doi:10.1002/tox.22077
Zhang J et al (2012) Cytotoxicity of different sized TiO2 nanoparticles in mouse macrophages. Toxicol Ind Health 0748233712442708
Acknowledgments
This work was supported by grant from the National Natural Science Foundation of China (31271074, 81571804) and Tianjin Research Program of Application Foundation and Advanced Technology (14JCZDJC35000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Li, W. & Yang, Z. Toxicology of nanosized titanium dioxide: an update. Arch Toxicol 89, 2207–2217 (2015). https://doi.org/10.1007/s00204-015-1594-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1594-6