Abstract
Lonomia obliqua caterpillar envenomation causes acute kidney injury (AKI), which can be responsible for its deadly actions. This study evaluates the possible mechanisms involved in the pathogenesis of renal dysfunction. To characterize L. obliqua venom effects, we subcutaneously injected rats and examined renal functional, morphological and biochemical parameters at several time points. We also performed discovery-based proteomic analysis to measure protein expression to identify molecular pathways of renal disease. L. obliqua envenomation causes acute tubular necrosis, which is associated with renal inflammation; formation of hematic casts, resulting from intravascular hemolysis; increase in vascular permeability and fibrosis. The dilation of Bowman’s space and glomerular tuft is related to fluid leakage and intra-glomerular fibrin deposition, respectively, since tissue factor procoagulant activity increases in the kidney. Systemic hypotension also contributes to these alterations and to the sudden loss of basic renal functions, including filtration and excretion capacities, urinary concentration and maintenance of fluid homeostasis. In addition, envenomed kidneys increase the expression of proteins involved in cell stress, inflammation, tissue injury, heme-induced oxidative stress, coagulation and complement system activation. Finally, the localization of the venom in renal tissue agrees with morphological and functional alterations, suggesting also a direct nephrotoxic activity. In conclusion, the mechanisms of L. obliqua-induced AKI are complex involving mainly glomerular and tubular functional impairment and vascular alterations. These results are important to understand the mechanisms of renal injury and may suggest more efficient ways to prevent or attenuate the pathology of Lonomia’s envenomation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accidents caused by venomous animals (mainly snakes, spiders, scorpions, bees, wasps and caterpillars) are a costly and critically important public health problem. Despite of this, public health authorities, nationally and internationally, have given little attention to this problem worldwide (Warrell 2010; Williams et al. 2010). Consequentially, the morbidity and mortality associated with envenomation cases produce a great impact on the population and healthcare systems. One of the most important and lethal effects of these animal venoms is nephrotoxicity, and a broad clinical spectrum of renal function impairment has been reported in human and experimental models of envenomation (Sitprija 2006; Berger et al. 2012). As kidneys are highly vascularized organs and have the ability to concentrate substances into urine, they are particularly susceptible to venom toxins. The most common clinical renal manifestation seen in human patients is acute tubular necrosis, but all renal structures may be involved. Thus, the occurrences of acute tubulointerstitial nephritis, renal cortical necrosis, mesangiolysis, vasculitis, glomerulonephritis, proteinuria, hematuria, hemoglobinuria and myoglobinuria have also been described (Sitprija 2006).
Lonomia obliqua caterpillars are well known in southern Brazil where they cause severe hemorrhagic syndrome characterized by perturbed coagulation, ecchymosis, acute kidney injury (AKI) and generalized hemorrhage. Since the 1980s, there has been a considerable increase in the number of hemorrhagic incidents in rural areas of the southernmost Brazilian states of Rio Grande do Sul, Santa Catarina and Paraná. The origin of this epidemic is not clear, but can be partially attributed to recent deforestation, as well as to a progressive reduction in the number of natural predators. Usually, accidents occur when the victim unknowingly leans against a tree trunk containing hundreds of caterpillars and comes into contact with the caterpillar’s venomous bristles, which are chitinous evaginations of cuticule. Often, the caterpillar is crushed, the bristles are broken and venomous secretions, including hemolymph, penetrate the human skin (Veiga et al. 2001). The venom is composed of several active constituents with procoagulant, fibrinogenolytic, proteolytic and hemolytic activities (Pinto et al. 2010). Although consumptive coagulopathy secondary to intravascular disseminated coagulation is commonly observed in human and experimental animals, AKI is the leading cause of death from L. obliqua envenomation (Zannin et al. 2003; Gamborgi et al. 2006; Berger et al. 2010).
Early in the 1980s, the first registered cases of L. obliqua-induced hemorrhagic syndrome indicated that 18 % of envenomed patients had developed AKI. The mortality rate in these patients reached 50 % (Duarte et al. 1990, 1994). However, a lower incidence (5.2 %) was observed in the Brazilian state of Rio Grande do Sul from 1989 to 1995, when only 15 of 286 envenomed patients developed AKI (Duarte 1997). Another study analyzing a larger group of 2,067 envenomed patients in the Santa Catarina state in Brazil (from 1989 to 2003) reported that 39 victims (1.9 %) developed AKI (with serum creatinine levels ≥1.5 mg/dL). Eleven (32 %) of these patients were treated with dialysis and four (10.3 %) developed chronic renal injury (CRI). All victims with AKI presented concomitantly coagulation disturbances and hematuria and/or hemoglobinuria. Seven deaths (4 %) occurred during this period (Gamborgi et al. 2006). An important conclusion of this work is that, even after the introduction of antivenom therapy (with antilonomic serum) in 1995, there was no reduction in the incidence of AKI, despite the significant decrease in the number of deaths and patients who developed CRI (Gamborgi et al. 2006). In fact, recently, we observe that antivenom treatment was able to reduce creatinine and urea levels of rats only if administered 2 h post-venom injection. Serotherapy after 6 h of envenomation fails to neutralize the rising in biochemical markers of renal injury (Berger et al. 2013). Since the average time elapsed between the contact of a person with caterpillars and an appropriate medical care can vary from 19 to 37 h (Zannin et al. 2003), it seems imperative to achieve a better understanding of the mechanisms involved in venom-induced AKI. It is clear that having such knowledge available, it will then make possible to develop new efficient treatments in order to avoid or at least to reduce the progression of renal disease in Lonomia’s and other kind of animal envenoming.
The risk of conducting early renal biopsies in human patients, due to coagulation disturbances inherent to the envenomation, has made it difficult to analyze the acute kidney pathological alterations. There are only two case reports in the literature describing alterations of Lonomia-induced AKI. The main findings were oliguria, high levels of serum creatinine, thickening of the Bowman’s capsule, focal tubular atrophy and acute tubular necrosis (Burdmann et al. 1996; Fan et al. 1998). Since no experimental studies were available until now, the contribution of several factors possibly associated with AKI, such as hemodynamic changes, vascular permeability alterations, hemolysis, tubular obstruction, glomerular fibrin deposition and even a direct venom nephrotoxicity, remains obscure in Lonomia-induced AKI.
In an attempt to better understand the progression of renal disease commonly observed after the contact with L. obliqua caterpillars, we have focused on the action of venom in the kidney. Therefore, an experimental rat model was used in order to characterize changes in renal function, tubular hydroelectrolytic transport, histopathology and hemodynamics.
Materials and methods
Reagents
Evans blue dye, purified coagulation factors (VII, IX and X) and molecular weight standards used in SDS-PAGE and Western blot were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Chromogenic substrate for factor Xa (S2222, Bz-Ile-Glu-Gly-Arg-pNa) was obtained from Chromogenix (Milano, Italy). Ketamine and xylazine were from Syntec, São Paulo, Brazil. L. obliqua antivenom (antilonomic serum—ALS), provided by the Butantan Institute (São Paulo, Brazil), was used as primary antibody for the detection of toxins in urine and renal tissue. ALS is a horse-derived concentrate of purified polyclonal antibodies (IgG) that had been raised against L. obliqua bristle extract (Rocha-Campos et al. 2001). Non-specific background staining in immunohistochemical reactions was blocked using Ultra V Block reagent (Thermo Fisher Scientific, Waltham, MA, USA).
Venom
Lonomia obliqua caterpillars were kindly provided by the Centro de Informações Toxicológicas (CIT), Porto Alegre, Rio Grande do Sul, Brazil. The specimens used in this study were collected in the cities of Bom Princípio and Progresso, both located in Rio Grande do Sul, Brazil. L. obliqua venom was obtained by homogenizing the bristles in cold phosphate buffered saline (PBS), pH 7.4, as previously described (Berger et al. 2010). The venom obtained following this procedure was designated as L. obliqua bristle extract (LOBE). The protein content of the LOBE samples was determined using a BCA assay kit (Pierce, Rockford, USA), and the aliquots were stored at −80 °C prior to use. The total number of caterpillars used for bristle extract preparation was 124 specimens, and the protein concentration of the LOBE samples was 4.10 mg/mL. The total amount of venom extracted per caterpillar was 2.4 mg. All of the LOBE samples had similar in vitro procoagulant activities, and the protein pattern for each sample as monitored by SDS-PAGE and gel filtration chromatography (Pinto et al. 2006; Berger et al. 2010) was also similar.
Ethical statements
All procedures involving animals were carried out in accordance with the Guiding Principles for the Use of Animals in Toxicology (International Society of Toxicology, http://www.toxicology.org) and the Brazilian College of Animal Experimentation (COBEA). The experimental protocol was approved by the ethical committee on research animal care of the Federal University of Rio Grande do Sul, Brazil (register number 2008177/2009), and by the Institute’s Animal Ethics Committee of the Federal University of Minas Gerais, Brazil (protocol 177/2008).
Experimental protocol
Animals
Adult male Wistar rats, weighing 250–300 g, were supplied by the central animal facility of our institution. They were housed in standard conditions within a temperature controlled room (22–23 °C, on a 12-h light/dark cycle, with the lights on at 7:00 am) and had free access to water and food.
Selection of the venom dose
The severity of the natural envenoming is related mainly to the number of caterpillars involved as well as to the intensity of the exposure, since the venom is present not only in the caterpillar’s bristles but also in their skin and hemolymph (Veiga et al. 2001). Considering that accidents with medical importance involve contact with a colony containing at least 40 to 50 caterpillars (Gamborgi et al. 2006) and that during venom extraction, after removal of all spicules, each caterpillar produces approximately 2.4 mg of venom, the total amount of venom injected in an individual weighing 70 kg can reach up to 1.4–1.7 mg/kg. In fact, these doses were calculated based on an artificial method of venom extraction, in which the caterpillar’s bristle was macerated in a solution buffer. Thus, in a real envenomation situation, the total amount of venom transferred is probably lower than the amount calculated. In an attempt to reproduce the clinical conditions observed in a real envenomation, we selected doses of 1.0 and 1.5 mg/kg injected subcutaneously into rats. These doses were also in accordance with the amount of venom used in other studies to induce coagulopathy and test the efficacy of antilonomic serum (Berger et al. 2010; Dias da Silva et al. 1996; Rocha-Campos et al. 2001).
Venom administration
To follow the time course of kidney pathophysiological alterations, we used an experimental model of envenomation in rats. For this purpose, animals were divided into three groups (n = 6/group): The control animals (CTRL) were injected subcutaneously (s.c.) with 100 µL of sterile PBS solution and the experimental animals received a s.c injection containing 1.0 or 1.5 mg of LOBE per kg of body weight in a final volume of 100 µL. Immediately after treatments, the animals were distributed individually into metabolic cages, allowing quantitative urine collections and measurement of water intake. At several time points, post-venom injection (2, 6, 12, 24, 48 and 96 h), blood, urine and kidneys were obtained for biochemical, histopathological and immunohistochemical analyses.
Sample preparation
Blood was collected in conscious rats through the caudal vein in 1:10 (v/v) 3.8 % trisodium citrate. Plasma was obtained by centrifugation at 1,500×g for 10 min and stored at −80 °C prior to use. Urine samples were also centrifuged at 2,500×g for 5 min, and the supernatants were stored at the same conditions. After blood collection, animals from the different groups were anesthetized by intraperitoneal (i.p.) injection of a mixture of ketamine (75 mg/kg) and xylazine (10 mg/kg). Then, an intracardiac perfusion was performed through the left ventricle with PBS solution, and a circulatory circuit was opened by an incision in the right atrium, to ensure the elimination of intravascular blood. Immediately after perfusion, kidneys were quickly removed. One of them was fixed for histological analysis and the other was frozen in liquid nitrogen and stored at −80 °C for the measurement of tissue factor activity and proteomic analysis.
Biochemical measurements
Urinary and plasma levels of creatinine, urinary γ-glutamyl transferase (γ-GT) activity and proteinuria were determined by spectrophotometry (Turner SP-830 plus Barnstead, Dubuque, Iowa, USA) using commercially available kits (BioClin/Quibasa, Belo Horizonte, Brazil). Plasma and urinary concentrations of Na+ and K+ were measured by flame photometry (CELM 180; Belo Horizonte, Minas Gerais, Brazil). Osmolality was determined in plasma and urine samples by cryoscopic osmometery using a Micro-Osmometer 3320 (Advanced Instruments, Norwood, Massachusetts, USA). Urine proteins were also analyzed by gel electrophoresis which was performed according to Laemmli (1970). Urine samples from animals of different times post-venom injection were diluted (10×) and aliquots of 10 µL were submitted to SDS-PAGE on 8–20 % gradient gels under reducing conditions. Toxins excreted in urine were detected by Western blot as previously described (Pinto et al. 2006). Aliquots containing 50 µg of protein were separated by SDS-PAGE and transferred to PVDF membranes. Toxins were recognized using as a primary antibody an equine anti-LOBE IgG (ALS) diluted 1:100 and as a secondary antibody a peroxidase-labeled anti-horse IgG diluted 1:1,000.
Renal function parameters
At the time intervals mentioned above, the following renal function parameters were determined: glomerular filtration rate (GFR), osmolar clearance (C osm), water-free clearance (C H2O), fractional water excretion (FEH2O), fractional sodium excretion (FE +Na ) and fractional potassium excretion (FE +K ). GFR (expressed as mL/min/100 g of body weight) was estimated by the creatinine clearance (C cr), using the standard formula: C cr = U cr. V/P cr, where U cr is the urinary creatinine concentration, V is the urinary output and P cr is the plasma creatinine concentration. C osm (expressed as mL/min) was calculated as C osm = U osm/P osm. V, whereas U osm and P osm are the urinary and plasma osmolalities, respectively. Values of CH2O (mL/min) and FEH2O (%) were obtained, respectively, from the equations: C H2O = V − C osm and FEH2O = V/GFR.100. FE +Na and FE +K (expressed as %) were calculated according to the equation: FE = UE/PF.100. UE represents the urinary excretion of each ion, and PF is the amount filtered in plasma (both expressed as nmol/min).
Hemodynamic parameters
Systemic arterial pressure was measured in conscious rats by an indirect tail-cuff method using an electrosphygmomanometer (LE 5001, Harvard Apparatus, Holliston, Massachusetts, USA) combined with a pneumatic pulse transducer/amplifier, which provides output signals proportional to cuff pressure and amplified Korotkoff sounds. Three consecutive readings of mean arterial pressure and heart rate were recorded before blood collection for each animal in each time post-venom injection.
Renal vascular permeability
The extravasation of Evans blue dye into the kidney was used as an index of increased vascular permeability (Pompermayer et al. 2005). Rats received Evans blue dye (30 mg/kg) intravenously (1 mL/kg) via caudal vein 10 min prior to LOBE (1.5 mg/kg, s.c.) or PBS (100 µL, s.c.) injection. After 12 or 24 h, animals were anesthetized and perfused as described above to remove the intravascular Evans blue. Then, the kidneys were quickly removed, weighed and allowed to dry for 24 h at 40 °C. The dry weight was determined and Evans blue dye extracted in 2.5 mL of 1 % formamide (48 h at 40 °C). The absorbance of extracted solution was measured in triplicate using a microplate reader spectrophotometer (SpectraMAX, Molecular Devices Co., Sunnyvale, USA), and the amounts of Evans blue dye were calculated by a standard curve made with known concentrations of Evans blue. Results are presented as the amount of Evans blue dye extravasated (μg) per 100 mg of kidney tissue.
Renal tissue factor activity
Renal tissue factor (TF) was measured indirectly based on its ability to form a complex with factor VIIa (TF/FVIIa) to activate factor IX and X (Morrissey 1995). Briefly, the kidneys were collected as described above, homogenized in cold PBS solution containing 1 % Triton X-100 and centrifuged at 9,500×g for 15 min. Samples of supernatants (with 10 µg of protein) were incubated with a concentrate mixture of FVII + FIX + FX (total of 15 µg) in 20 mM Tris–HCl, pH 7.4 containing 10 mM of CaCl2 for 10 min at 37 °C. Activated factor Xa (FXa) produced during the reaction was detected by the addition of a specific chromogenic substrate (0.2 mM S2222). The kinetics of p-nitroaniline release was monitored at 405 nm for 30 min in a final volume of 100 µL using a microplate reader spectrophotometer (SpectraMAX, Molecular Devices Co., Sunnyvale, USA). Each sample was measured in triplicate and results expressed as µmol of FXa generated per min per mg of kidney tissue.
Histology and immunohistochemistry
For renal histopathology, kidneys were collected as described above, sectioned sagitally and fixed in 10 % buffered formaldehyde, pH 7.2. After processing in alcohol and xylol, the organs were included in paraffin, and 4-μm-thick sections were obtained and stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS) or picrosirius reagent.
For immunohistochemical analysis of venom distribution in renal tissue, the kidney sections were deparaffinized and treated with 10 % hydrogen peroxide solution in methanol for 15 min to block endogenous peroxidase activity. Non-specific binding sites were blocked with Ultra V Block reagent followed by incubation overnight at 4 °C with an equine anti-LOBE IgG (ALS) diluted 1:250 in PBS. After a washing step, the sections were incubated with the secondary antibody (a biotinylated anti-horse IgG produced in goat, diluted 1:100) and streptavidin diluted 1:100. The chromogenic reaction was developed by incubating the sections with 0.05 % diaminobenzidine solution and 0.2 % hydrogen peroxide. The progress of the reaction was monitored by light microscopy and stopped by washing the slides. Finally, the sections were counterstained with diluted Harris’s hematoxylin. Control reactions were done by incubating kidney sections from non-envenomed rats with equine anti-LOBE IgG (ALS) under the same conditions as described above. No positive reactions were observed in these sections. Some sections were also used as negative controls. In these cases, the primary antibody was substituted by PBS.
Morphometric analysis
Glomerular morphological alterations were quantified by computer-assisted morphometric analysis using a method previously described (Caliari 1997). Briefly, images with 40× magnification of 30 glomeruli from each animal of different groups were randomly digitalized using a JVC TK1270/RGB microcamera (Tokyo, Japan). The KS300 software coupled to a Carl Zeiss image analyzer (Carl Zeiss, Oberkochen, Germany) was used to measure the total area (μm2) of each glomerulus, glomerular tuft and Bowman’s space. The pixels of whole glomerulus and those corresponding to the glomerular tuft were selected and used for the generation of a binary image and subsequent calculation of the corresponding areas. The area of Bowman’s space was obtained by the difference between the whole glomerular area and tuft area. The regions of positive immunohistochemical reaction (expressed as μm2) were also measured and used as a parameter to quantify venom distribution in the renal tissue.
Renal tissue proteomics
Sample preparation for mass spectrometry
The kidneys from controls and envenomed animals (at 24 h post-venom injection) were collected as described above, homogenized in cold PBS solution containing 1 % Triton X-100 with protease inhibitor cocktail (Halt protease inhibitor cocktail, Thermo, Rockford, USA) and centrifuged at 9,500×g for 15 min. The resulting supernatants were completely lyophilized and stored at −80 °C until use. Lyophilized samples were resuspended in water and precipitated using methanol/chloroform protocol. After precipitation, samples were dried at room temperature and resuspended in 8 M urea. Each sample containing 100 µg of protein was reduced with 5 mM tris-2-carboxyethyl-phosphine (TCEP) at room temperature for 20 min and alkylated with 10 mM iodoacetamide at room temperature in the dark for 20 min. After reduction and alkylation, proteins were digested with 2 μg of trypsin (Promega, Madison, WI) by incubation at 37 °C during 16 h. Samples were freeze-dried at −80 °C and, after thaw, formic acid to a final concentration of 5 % was added. Samples were centrifuged at 14,000 rpm for 20 min, and the supernatant was collected and stored at −80 °C.
MudPIT
The protein digest was pressure-loaded into a 250-μm i.d capillary packed with 2.5 cm of 5-µm Luna strong cation exchanger (SCX) (Whatman, Clifton, NJ) followed by 2 cm of 3-µm Aqua C18 reversed phase (RP) (Phenomenex, Ventura, CA) with a 1-µm frit. The column was washed with buffer containing 95 % water, 5 % acetonitrile and 0.1 % formic acid. After washing, a 100-µm i.d. capillary with a 5-µm pulled tip packed with 11 cm of 3-µm Aqua C18 resin (Phenomenex, Ventura, CA) was attached via a union according to Klein et al. (2012). The entire split-column was placed in line with an Agilent 1100 quaternary HPLC (Palo Alto, CA) and analyzed using a modified 11-step separation as described previously (Washburn et al. 2001). The buffer solutions used were 5 % acetonitrile, 0.1 % formic acid (buffer A), 80 % acetonitrile, 0.1 % formic acid (buffer B), and 500 mM ammonium acetate, 5 % acetonitrile and 0.1 % formic acid (buffer C). Step 1 consisted of a 70 min gradient from 0 to 100 % (vol/vol) buffer B. Steps 2–10 had a similar profile with the following changes: 5 min in 100 % (vol/vol) buffer A, 3 min in X % (vol/vol) buffer C, a 6 min gradient from 0 to 15 % (vol/vol) buffer B and a 85 min gradient from 15 to 100 % (vol/vol) buffer B. The 3 min buffer C percentages (X) were 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 % (vol/vol), respectively, for the 10-step analysis.
LTQ-Orbitrap
As peptides eluted from the microcapillary column, they were electrosprayed directly into a LTQ-Orbitrap (Thermo Fisher) with the application of a distal 2.4-kV spray voltage. Full MS spectra were acquired in profile mode, with a mass range of 400–1,600 in the Orbitrap analyzer with resolution set at 60,000 followed by continuous repetition of 10 data-dependent MS/MS spectra at 35 % normalized collision energy was throughout each step of the multidimensional separation. Minimal signal for fragmentation was set to 1,000. Dynamic exclusion was enabled with a repeat count of 1, duration of 30.00 s, list size of 500, exclusion duration of 180.00 s and exclusion mass with high/low of 1.5 m/z. Application of mass spectrometer scan functions and HPLC solvent gradients was controlled by the Xcalibur data system.
Analysis of tandem mass spectra
MS/MS spectra were analyzed using the following software analysis protocol. Protein identification and quantification analysis were done with Integrated Proteomics Pipeline (IP2, Integrated Proteomics Applications, Inc. www.integratedproteomics.com/). Tandem mass spectra were extracted into ms2 files from raw files using RawExtract 1.9.9 (McDonald et al. 2004) and were searched using ProLuCID algorithm (Xu et al. 2006). MS/MS spectra remaining after filtering were searched with the ProLuCID algorithm against the EBI-IPI_rat_3.30_06-28-2007 concatenated to a decoy database in which the sequence for each entry in the original database was reversed (Peng et al. 2003). Searches were performed with cysteine carbamidomethylation as a fixed modification. ProLuCID results were assembled and filtered using the DTASelect program (Tabb et al. 2002) using two SEQUEST (Eng et al. 1994) defined parameters: the cross-correlation score (XCorr) and normalized difference in cross-correlation scores (DeltaCN) to achieve a false discovery rate of 1 %. The following parameters were used to filter the peptide candidates: −p 1 −y 1 –trypstat –fpf 0.01 –dm. Also, we used 50 ppm as precursor tolerance, fragment mass tolerance of 600 ppm, three as number of isotopic peaks and unlimited missed cleavages were allowed.
Assessing differential expression and exclusive proteins/bioinformatic tools
The software PatternLab (Carvalho et al. 2008, 2012) was used to identify exclusive proteins found in the control and treated conditions. PatternLab’s Approximately Area Proportional Venn Diagram (AAPVD) module was used for pinpointing proteins uniquely identified in each condition. The following parameter was used: proteins that were not detected in at least 2 out of 3 runs per condition were not considered. G test spectral counting quantitation was performed in a pair-wise comparison between the two groups, as previously reported (Ambatipudi et al. 2009). Proteins are considered differentially expressed with p < 0.1. Ingenuity Pathway Analysis tool (Ingenuity Systems; http://www.ingenuity.com) was used to generate functional annotations of identified proteins in known molecular pathways and/or biologic function in disease. The significance of the canonical pathways and biologic function defined by identified proteins was measured in two ways: (1) The number of proteins identified from the data set that map to a known pathway or function in disease and (2) a p value (Fisher exact test) determining the probability that the association between the proteins in the data set and the canonical pathway or function in disease is explained by chance alone.
Statistical analyses
Results are expressed as mean ± SE. When appropriate, statistical comparisons were done by using one- or two-way analysis of variance followed by the Bonferroni’s test. A p value of less than 0.05 was chosen to establish significance. Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA).
Results
Renal function
To follow renal alterations in rats, several physiological parameters were measured at different times post Lonomia obliqua venom injection. The results are presented in Fig. 1. Between 2 and 6 h after LOBE administration (doses of 1 mg/kg or 1.5 mg/kg, s.c.), the animals showed signs of acute toxicity, including progressive weakness, lethargy and dyspnea. Compared to PBS-treated animals, there was a reduction in body weight mainly at 6 h in rats that received the higher dose of LOBE (1.5 mg/kg, s.c.) (Fig. 1a). The weight loss was accompanied by a significant reduction in water intake between 6 and 48 h and by an increase in urinary output at 6 and 12 h for both tested doses (Fig. 1b). The polyuria was maximal at 6 h, when urinary output increased from 0.58 ± 0.08 mL/2 h in PBS-treated animals to 1.80 ± 0.16 mL/2 h (p < 0.05) in rats injected with LOBE (1.5 mg/kg, s.c.). At the same time, animals that received a dose of 1.0 mg/kg had a urinary output significantly lower (0.9 ± 0.2 mL/2 h, p < 0.05) compared to those treated with 1.5 mg/kg (Fig. 1c).
Functional parameters during L. obliqua-induced AKI. Rats were injected subcutaneously with PBS (controls—CTRL) or LOBE (1.5 or 1.0 mg/kg). After different times post-administration, the following parameters were determined: a body weight, b water intake, c urine output, d glomerular filtration rate (GFR), e urinary density, f urine osmolality (urine osm), g plasma osmolality (plasma osm), h osmolar clearance (C osm), i water-free clearance (C H2O), j fractional water excretion (FEH2O), k fractional sodium excretion (FE +Na ) and l fractional potassium excretion (FE +K ). Data are presented as mean ± SE (n = 6/group). Significant differences: *p < 0.05 versus CTRL and § p < 0.05 versus LOBE (1.0 mg/kg, s.c)
Important glomerular and tubular functions related to control of fluid filtration, water and electrolyte balance were severely impaired. Despite the polyuria, L. obliqua-induced AKI is associated with a marked reduction in the glomerular filtration rate (GFR). In the first 24 h of envenomation, a rapid decrease in GFR was observed in animals treated with both doses of LOBE. At 48 h, envenomed rats (injected with 1.5 mg/kg of LOBE) presented values of GFR 16 times lower than controls at the same time. This remarkable effect of the venom on GFR was observed for as long as 96 h (Fig. 1d). Similarly, the kidney’s ability to concentrate urine (a primordial tubular function) was also impaired, since the density and osmolality of urine had a reduction in values between 2 and 48 h (Fig. 1e, f). The decrease in urinary osmolality was accompanied by a significant increase in the plasma osmolality observed mainly in rats injected with the higher dose of LOBE (Fig. 1g). Consistent with these results, the osmolar clearance (C osm) was lower in envenomed animals, while the free water clearance (C H2O) increased, which imply that the kidney is producing dilute urine through the excretion of solute-free water (Fig. 1h, i). As expected, with the loss of ability to retain water, the fractional excretion of water (FEH2O) increased from 0.33 ± 0.03 % in PBS-treated animals to 29.1 ± 4.9 % (p < 0.05) in rats injected with LOBE (1.5 mg/kg, s.c.) at 48 h (Fig. 1j). Likewise, tubular ability to conserve and maintain the electrolytic balance was lost throughout the envenoming period. The fractional excretion of sodium and potassium (FENa+ and FEK+) was high between 24 and 48 h, indicating a marked impairment in the tubular reabsorption of filtered Na+ and K+ (Fig. 1k, l). At 96 h, most of the renal function parameters (excepting GFR) tend to return to normal levels (similar from that obtained to non-envenomed rats).
Proteinuria
Most of envenomed animals produced dark brown-colored urine in the period of 6–12 h, indicating the occurrence of hematuria and/or hemoglobinuria. The presence of intact and fragmented erythrocytes, epithelial cells and leukocytes was observed in the urinary sediment by light microscopy (not shown). Consistent with these observations, L. obliqua venom-induced massive acute proteinuria, which was maximal at 6 and 12 h post-venom injection (Fig. 2a). As expected, the group treated with 1.5 mg/kg of LOBE had more severe proteinuria compared to animals treated with 1.0 mg/kg, but for both groups, urinary protein excretion decreased progressively between 12 and 48 h, reaching levels similar to controls at 96 h. Analysis of urine by SDS-PAGE revealed the presence of several proteins with molecular weights ranging from 10 to 75 kDa (Fig. 2b). A greater variety of bands were evident mainly at 6 and 12 h after envenomation, confirming this time interval as crucial to the development of glomerular injury. The two most prominent bands (observed around of 70 and 15 kDa) have molecular weights that match to serum albumin (68 kDa) and subunits of hemoglobin (16 kDa). The presence of venom excreted in urine was verified by Western blot. As shown in Fig. 2c, we detected at least 5 bands in urine from venom-treated rats at 6 and 12 h, which specifically react with antibodies raised against L. obliqua toxic proteins. These bands have molecular weights around of 70–60, 50, 37, 25 and 20 kDa (arrows in Fig. 2c) and were not recognized in urine from non-envenomed animals (controls). Several toxins in this range of molecular weights have already been identified through transcriptomic and proteomic analysis of LOBE. Some of them include lectins and c-type lectin-like proteins (70–60 kDa), serine proteinases (50 kDa), cysteine proteinases (37 kDa) and lipocalins (25–20 kDa) (Ricci-Silva et al. 2008; Veiga et al. 2005).
Proteinuria. a Rats were injected subcutaneously with PBS (controls—CTRL) or LOBE (1.5 or 1.0 mg/kg). After different times post-administration, the protein levels in urine were measured. Data are presented as mean ± SE (n = 6/group). Statistical differences of *p < 0.05 versus CTRL and § p < 0.05 versus LOBE (1.0 mg/kg, s.c) were considered significant. b Representative urine samples from CTRL and rats treated with LOBE (1.5 mg/kg, s.c.) were analyzed by SDS-PAGE (8–20 %) under reducing conditions. c Toxins excreted in urine were detected by Western blot. Samples of urine from CTRL and rats treated with LOBE (1.5 mg/kg, s.c.) at 6 and 12 h post-venom injection were separated by SDS-PAGE, and different toxins were detected by immunoreaction with polyclonal antibodies against LOBE. Toxins present in crude bristle extract are also showed (LOBE). The arrows indicate bands detected in urine samples at 6 and 12 h of envenomation. Molecular weight (MW) standards were shown on the left of figure b and c
Renal histopathological alterations
Light microscopy of kidney biopsies from PBS-injected rats revealed a normal renal parenchyma (Fig. 3a). In contrast, envenomed animals showed progressive degenerative lesions compatible with acute tubular necrosis (ATN) (Fig. 3b–h). Increased acidophilia and dilation of renal tubules were observed between 6 and 48 h after venom administration (Fig. 3c–f). Loss of proximal brush border, cytoplasm vacuolation, and in some tubules, degeneration and desquamation of necrotic cells occurred between 12 and 48 h (Fig. 3d–f). The nuclei of the various proximal tubular cells at 24 and 48 h often showed pyknosis with clumping of chromatin material (Fig. 3e, f). In several tubules at 48 h, the renal epithelium was completely necrotic, whereas the basement membrane was either intact or disrupted by tubular necrosis (Fig. 3f). Consistent with the histological signs of tubular injury, γ-GT urinary activity—which is considered an efficient biomarker for early diagnosis of ATN (Guder and Ross 1984)—increased in a dose- and time-dependent manner up to 48 h. At 96 h, γ-GT activity decreased, but remained significantly high in animals treated with 1.5 mg/kg of LOBE. Histologically, the signs of lesions were much less marked, and the proximal and post-proximal tubular epithelia assumed a normal appearance at 96 h (Fig. 3g, h).
L. obliqua envenomation induces acute tubular necrosis. Representative kidney sections from control (CTRL) or envenomed animals (injected with 1.5 mg/kg, s.c.) are presented (a–g). Note the normal morphology of kidney from CTRL animal (a) in comparison with the progressive degenerative lesions of venom-treated rats (b–g). Increased acidophilia, dilation of renal tubules, loss of proximal brush border, cytoplasm vacuolation, nuclear pyknosis and desquamation of necrotic cells can be observed (d–f). Hyaline (arrowheads in c, d and g) and hematic (arrows in d) casts are also present inside renal tubules. Arrowheads in E indicate necrotic cells. Asterisks in d and f indicate the presence of a hyaline material within the Bowman’s space and an inflammatory cell infiltrate and edema, respectively. All sections were stained with H&E. Magnification: ×10. h Levels of urinary γ-glutamyl transferase (γ-GT) activity were measured in rats injected with PBS or LOBE (1.5 or 1.0 mg/kg) at different times post-administration. Data are presented as mean ± SE (n = 6/group). Statistical differences of *p < 0.05 versus CTRL and § p < 0.05 versus LOBE (1.0 mg/kg, s.c) were considered significant
Besides the evidence of degenerative lesions, proximal and distal tubules also had swollen lumens. Hyaline and hematic casts and cellular debris were found within tubules, obstructing their lumens (Figs. 3c–f, 4). Hyaline casts (predominantly formed by a protein-rich material) were more prevalent at 6 and 12 h (Figs. 3c, d, 4a), while hematic casts (predominantly formed by fragmented or intact erythrocytes) and cellular debris have appeared commonly at 12, 24 and 48 h (Figs. 3d–f, 4a, b).
L. obliqua envenomation induces renal tubular obstruction. Representative kidney sections from animals injected with LOBE (1.5 mg/kg, s.c.) showing details of tubular obstruction by hyaline and hematic casts and cellular debris at 12 h (a) and 24 h (b) post-venom injection. Hyaline casts (black arrowheads) are formed by a protein-rich material (predominantly serum albumin and hemoglobin), while hematic casts (black arrows) are formed by fragmented or intact erythrocytes. Due to tubular necrosis, the basement membrane in some tubules is disrupted, resulting in detachment of necrotic cells into the lumen (white arrowheads). All sections were stained with H&E. Magnification: ×4 (a) and ×20 (b)
An intense inflammatory response characterized by edema, cellular infiltration and fibrosis was observed mainly at 48 and 96 h (Figs. 3f, 5). Inflammatory cell infiltrate and edema were detected at 48 h in regions of extensive necrosis (Fig. 3f) and within glomeruli (Fig. 5c). At 96 h, despite signs of tubular regeneration, the inflammatory infiltrate increased significantly, probably to help in tissue repair (Fig. 5b). Light microscopy of sections stained with picrosirius revealed extensive peritubular collagen deposition at 96 h after administration of L. obliqua venom (Fig. 5e) compared to PBS-treated rats (Fig. 5d), indicating fibrosis. Foci of inter-glomerular collagen deposition were also observed in the renal cortex at the same time (Fig. 5f).
L. obliqua envenomation induces renal inflammation and fibrosis. Light micrographs showing a marked inflammatory cell infiltrate (arrowheads) in the tubulointerstitial region at 96 h (b) and glomerulus at 48 h (c) after LOBE injection (1.5 mg/kg, s.c.). There were no signs of inflammation in control (CTRL) animals (a). It was also observed an extensive peritubular (e) and inter-glomerular (f) collagen deposition at 96 h (regions stained in red), indicating fibrosis. Arrowheads in these panels indicate inflammatory infiltrate. There were no signs of fibrosis in CTRL rats (d). Stain: H&E (a–c) and picrosirius (d–f). Magnification: ×10 (a, b, d and e), ×20 (f) and ×40 (c)
Glomerular morphometric alterations and renal tissue factor activity
Glomerular alterations in envenomed animals were associated with lobulation of the capillary tufts, dilation of glomeruli, deposition of a hyaline material inside the Bowman’s space and distention of Bowman’s space (Figs. 3d, 6a, 7b). Nodules that formed a dense, strongly stained mesangial matrix evidenced by the presence of a PAS-positive stain in the capillary tufts were also observed between 2 and 12 h post-venom (Fig. 6a). Consistent with histological observations, computer-assisted morphometric analysis has indicated a significant increase in glomerular area at all-time intervals examined. There was also an increase in the areas of glomerular tuft and Bowman’s space, which confirms that the capillaries and Bowman’s space are dilated (Fig. 6b). The enlargement of Bowman’s space observed at 12 h possibly is related to the presence of a hyaline material (rich in plasma proteins) found in several glomeruli at this time (details in Figs. 3d, 7b).
Glomerular alterations. a Light micrographs showing a time-dependent increase in the deposition of a PAS-positive material in glomerular capillaries of LOBE-injected animals (1.5 mg/kg, s.c.) in comparison with controls. Also note the increase in glomerular size. All sections were stained with PAS. Magnification: ×40. b Thirty glomeruli from each animal injected with the dose of 1.5 mg/kg were used to quantify the mean area of glomerulus, glomerular tuft and Bowman’s space of animals treated with the dose of 1.5 mg/kg. Data are presented as mean ± SE (n = 6/group). Statistical differences of *p < 0.05 in comparison with the respective control (C) were considered significant. c Renal tissue factor activity was measured in control (C) and envenomed (1.5 mg/kg, s.c.) animals by generation of factor Xa (FXa). Data are presented as mean ± SE (n = 6/group). Statistical differences of *p < 0.05 were considered significant in comparison with the respective control
Renal vascular permeability. Representative micrographs of a kidney blood vessel (a) and glomerulus (b) from animals injected with LOBE (1.5 mg/kg, s.c.) after 12 h of envenomation. Note the vascular leakage and edema (asterisks) and migration of inflammatory cells to damaged tissue (arrowheads). Also, the presence of a hyaline material inside the Bowman’s space (asterisk in b) was associated with the increase in glomerular area observed at this time. All sections were stained with H&E. Magnification: ×10 (a) and ×40 (b). c Evaluation of changes in renal vascular permeability were assessed by Evans blue dye extravasation. Results are expressed as μg Evans blue dye per 100 mg of renal tissue from control (CTRL) and LOBE-treated (1.5 mg/kg, s.c.) rats at 12 and 24 h post-venom administration. Data are presented as mean ± SE (n = 6/group). Statistical comparisons are indicated
Since intra-glomerular fibrin deposition can impair renal function and L. obliqua venom is able to activate the coagulation system both in vitro and in vivo (Berger et al. 2010), we decide to measure the levels of renal tissue factor (TF) activity during envenomation. TF is a transmembrane enzyme activator that triggers the coagulation cascade, generating activated factor X and fibrin. As showed in Fig. 6c, renal TF activity increased rapidly from the first 2 h, reaching levels twice higher than control values at 6 h. At 12 and 24 h post-venom injection, this level remained significantly high, but decreased progressively thereafter (Fig. 6c). Interestingly, the rise in renal TF activity was coincident with the presence of PAS-positive deposits (which stain specifically glycoproteins) within the capillary tufts at 2–12 h (Fig. 6a).
Hemodynamics and renal vascular permeability
Mean arterial blood pressure was lower in animals that received LOBE compared to basal levels in controls (Table 1). Sustained hypotension was detected between 24 and 96 h in rats treated with the both doses of venom. The maximum decrease occurred at 24 h. In contrast, the heart rate increased significantly at 12 and 96 h in animals injected with 1 mg/kg of LOBE and at 48 h in animals injected with 1.5 mg/kg. Hypotension was accompanied by an increase in renal vascular permeability (Fig. 7). Kidney blood vessels were hyperemic, and signs of plasma leakage, migration of inflammatory cells and interstitial edema were also evident after 12 h of venom administration (Fig. 7a). As mentioned above, several glomeruli at 12 h have their Bowman’s space filled with a protein-rich material that had extravasated from glomerular capillaries (Fig. 7b). Confirming these observations, a marked increase in vascular permeability in the kidney, as measured by the extravasation of Evans blue dye, was detected at 12 and 24 h after LOBE injection (1.5 mg/kg, s.c) (Fig. 7c).
Immunohistochemical detection of venom in the renal tissue
Venom distribution and its binding to renal structures was investigated by submitting kidney biopsies from venom-treated and control rats to immunohistochemistry using anti-LOBE IgG that reacts specifically with venom toxins (Fig. 8). Positive immunohistochemical reaction was found in cortical and medullar regions of kidneys from rats injected with 1.5 mg/kg of LOBE (Fig. 8d, c). Venom was detected in glomerular capillaries, Bowman’s capsule, in proximal and distal tubules and in intra-tubular casts. Staining for venom was intense in tubular brush border at 2 and 6 h and was also present in cells of tubules in degeneration at 48 h (Fig. 8b–d). Generally, tubules stained more than glomeruli. There was no immunoreactivity for venom in the renal structures of PBS-treated rats (controls) (Fig. 8a). The highest levels of venom (estimated by the area of positive immunohistochemical reaction) were detected in the renal tissue at 2 h (Fig. 8f). After that, venom immunoreactivity decreased progressively until 96 h when the staining was weak (Fig. 8e, f). L. obliqua toxins were also detected in other kidney structures such as in perivascular connective tissue of blood vessels, endothelium and smooth muscle of arteries and veins (Fig. 9a, b).
Immunohistochemical detection of L. obliqua venom in renal tissue. Positive immunohistochemical reaction was found in cortical and medullar regions of kidneys from rats injected with 1.5 mg/kg of LOBE (b–e). Venom was detected in glomerular capillaries (arrows in b), Bowman’s capsule (arrow in the inset B), tubular brush border (arrows in the inset C), in intra-tubular casts (arrowheads in c) and also was present in cells of tubules in degeneration (arrowheads in the insets c and d). After 96 h, the immunoreactivity for venom was weak and mainly localized in tubules (arrows in e). There was no immunoreactivity in the renal structures of control (CTRL) rats (a). Magnification: ×10 (a–e) and ×40 (insets in b–d) f. The amounts of venom detected in renal tissue were estimated by the area of positive immunohistochemical reaction. Thirty sections per rat were analyzed as described in “Materials and methods.” Data are presented as mean ± SE (n = 6/group)
Immunohistochemical detection of L. obliqua venom in renal vascular tissue. Positive immunohistochemical reaction was detected in renal arteries (a) and veins (b) of rats injected with LOBE (1.5 mg/kg, s.c.) at 6 h of envenomation. Note the presence of venom in perivascular connective tissue (arrows) and endothelium and smooth muscle cells (arrowheads). Magnification: ×10 (b) and ×40 (a)
Kidney proteins differentially expressed during envenomation
To gain further mechanistic insight which drive venom-induced kidney disease, we applied a semi-quantitative discovery-based shotgun proteomic approach to identify the proteins differentially expressed in the kidney of envenomed animals. For this purpose, the proteomic data were acquired by tandem mass spectrometry with subsequent quantification, analysis of differential protein expression, validation and functional annotations in order to identify the involved molecular pathways. The proteins identified consist of two classes: (1) those that were differentially expressed and met our significance criteria and (2) proteins that were uniquely identified in kidneys of rats injected with PBS or LOBE (1.5 mg/kg) after 24 h. Figure 10a represents differentially expressed and unique proteins via a Venn diagram. Overlaps between PBS- and LOBE-treated animals represent significant differentially expressed proteins, and non-overlapping portions of the diagram represent unique protein identifications. A total of 779 proteins were identified. Twenty-four proteins were exclusively identified in envenomed animals; 169 were exclusively identified in control rats, and 586 were common proteins in both treatments. Among these 586 proteins, 138 (23.5 %) were identified as being differentially expressed.
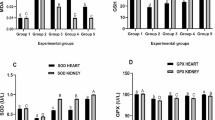
Kidney proteins differentially expressed during L. obliqua envenomation. Unique or differentially expressed kidney proteins from control and envenomed (1.5 mg/kg, s.c.) animals were identified at 24 h post-venom injection by proteomic analysis. Exclusive and common proteins in each condition, as well as the total number of proteins identified are showed via a Venn diagram (a). Top canonical pathways of differentially expressed and unique proteins identified in control (CTRL) (b) and LOBE-treated (c) kidneys are shown. Those proteins functionally related to renal disease were also categorized accordingly to their roles in different types of renal pathologies (d). Each functional annotation is assigned to a significance score represented as p value (Fisher exact test) determining the probability that the association between the proteins in the data set and the canonical pathway or function in disease is explained by chance alone. The number of identified proteins (NIP) that belong to a particular canonical pathway or play a role in renal pathology is shown
Ingenuity pathway analysis was used for functional annotations and revealed several key protein categories and pathways significantly enriched in the differentially expressed proteins. Thus, the sets of proteins were assigned to either biologic function in disease or canonical signaling pathways. Through these analyses, it was evident that the set of proteins identified in control kidneys displayed healthy biological functions such amino acid metabolism and small-molecule biochemistry associated with normal renal metabolic pathways (Fig. 10b). On the other hand, protein expression shifts toward “cellular distress” functions in the kidney of envenomed rats (Fig. 10c). In this case, the expression profiles were considerably enriched for proteins that belong to acute-phase inflammatory response signaling, LXR/RXR activation (involved in retinoic acid-mediated gene activation triggered by inflammatory stimulus), oxidative stress response pathways such as that mediated by the nuclear erythroid-related factor 2 (NRF2) and coagulation and complement systems (involved in thrombosis, fibrosis, inflammation and vascular alterations) (Fig. 10c).
Accordingly to our functional and histopathological results, several novel proteins related to renal disease were identified in the kidney of LOBE-treated animals. Proteins associated with tubular and glomerular injury, necrosis, inflammation and fibrosis were up-regulated or unique in envenomed rats (Fig. 10d). Some of these molecules, mainly those that were related to renal disease or identified in canonical pathways, are listed in Table 2. A complete list with differentially expressed and unique proteins of envenomed and control animals are shown in supplemental Table 1. It is worth mentioning that proteins functionally linked to tissue injury (markers of necrosis and/or apoptosis), osmotic and oxidative stress, electrolytic imbalance, acute-phase inflammatory response, fibrosis and thrombosis were expressed in the kidneys of LOBE-treated rats (Table 2). Proteins of the kallikrein-kinin and complement systems which are related to hypotension and control of vascular permeability were also found to be up-regulated in envenomed animals (Table 2). Our proteomic data also confirm that an important mechanism of Lonomia-induced AKI is mediated by hemoglobin and the release of its degradation products: free heme and iron. In fact, hemoglobin, hemopexin and ferritin were up-regulated, and the expression of heme oxygenase-1 was uniquely induced in the kidneys of envenomed rats (Table 2). As a result of heme and iron release, several antioxidant enzymes were found to be up-regulated, indicating the generation of reactive oxygen species (Table 2).
Discussion
AKI is frequently described and is life threatening in several cases of snake and arthropod envenomation (Sitprija 2006; Berger et al. 2012). Particularly in Lonomia obliqua envenomation, AKI is the main cause of death and its mechanisms are completely unknown until now. In this work, we use an in vivo experimental model to characterize the L. obliqua-induced AKI. According to our results, the pathophysiological mechanism seems to be complex and multifactorial involving four main issues: (1) vascular abnormalities; (2) tubular and glomerular alterations; (3) renal inflammation and (4) a direct venom cytotoxic activity.
Vascular abnormalities
Rats injected with L. obliqua venom presented important hemodynamic alterations characterized by systemic hypotension and increased heart rate. The time of maximal decrease in blood pressure was coincident with the reduction in GFR and the impairment of renal function. In addition, an increase in renal vascular permeability and edema was also observed at the same period of envenomation. These findings are important because systemic vasodilation is associated with the decrease in GFR and can lead to renal hypoperfusion and ischemia in different pathological conditions (Schrier et al. 2004). Specifically in this type of envenomation, one mechanism that could contribute to vasodilation is the activation of kallikrein-kinin system (KKS). It is known that L. obliqua venom has toxins (kallikrein activators and kininogenases) able to activate plasma pre-kallikrein and directly release bradykinin (BK) from low molecular weight kininogen (LMWK) (Pinto et al. 2010; Bohrer et al. 2007). Interestingly, our results indicated that the expression of LMWK (the main substrate of tissue kallikrein and venom kininogenases) is up-regulated in the kidneys of envenomed animals, which may favor the generation of BK. The immediate consequence of intravascular activation of KKS is a fall in systemic blood pressure and in peripheral tissues is edema and erythema formation (Bohrer et al. 2007). The participation of BK was already confirmed, since the hypotensive and edematogenic responses elicited by LOBE were inhibited by HOE-140, a B2 receptor antagonist (Bohrer et al. 2007). In agreement with these experimental observations, hypotension and reduced plasma levels of pre-kallikrein are common features observed in patients (Zannin et al. 2003), which support the evidence that KKS is activated during envenomation and is clinically relevant. Moreover, the data presented here also suggest that KKS activation may be involved in venom-induced AKI.
Tubular and glomerular alterations
Despite the scarce clinical case reports, the main pathological finding obtained from kidney biopsies is acute tubular necrosis (ATN). Experimental animals also showed histological alterations compatible with ATN such as loss of proximal brush border, cytoplasm vacuolation, pyknotic nuclei, degeneration and desquamation of necrotic cells. These necrotic cells exfoliating into the lumen due to either cell death or defective cell-to-cell or cell-to-basement membrane adhesion can obstruct the flow of filtrated fluid and give rise to a back pressure limiting glomerular filtration (Trof et al. 2006). Accordingly, in this work, markers of ATN were detected in urine (urinary γ-GT), and several proteins related to tubule injury were found to be up-regulated (Orm-1, YBX1, Alb, CK-B, FABP3 and NHE-RF1) or uniquely expressed (NGAL) in the kidneys of envenomed rats. Proteins linked to apoptosis (CytC, S100-A8 and MFP-1) were also up-regulated. The occurrence of glomerular dysfunction was evident, because envenomed rats presented massive proteinuria; serum Alb had a 2.1-fold increase in the kidneys, and a band corresponding to the molecular weight of Alb was detected in urine. In addition, our results confirm that hematuria and hemoglobinuria are predominant characteristics of L. obliqua-induced AKI. Indeed, the venom has strong in vitro and in vivo hemolytic activity and a phospholipase A2 responsible for this effect was already isolated (Seibert et al. 2004, 2006). Intact erythrocytes were found within the tubules forming intra-tubular casts and, as a result of intravascular hemolysis, different Hb subunits were detected in urine and kidneys. It is known that the formation of Hb deposits may be toxic to renal tubules due to heme cytotoxicity (Zager 1996). Once reabsorbed by the proximal tubular cells the heme porphyrin ring is rapidly catabolized by Hmox-1 yielding equimolar amounts of free iron, biliverdin and carbon monoxide (Camara and Soares 2005). Free iron then up-regulates intracellular ferritin expression, a key defense mechanism against iron-induced tissue damage. However, in the presence of large amounts of Hb, the levels of released iron also increase, saturating the binding capacity of ferritin. Thus, the iron not removed by ferritin binding is able to readily accept and donate electrons and greatly facilitates free radical production (Zager 1996; Khan 2009; Zager et al. 2012). As proteins related to canonical pathways of oxidative stress were identified exclusively in the kidneys of LOBE-treated rats, we believe that heme cytotoxicity plays a significant role in L. obliqua-induced AKI. Consistent with this, several antioxidant enzymes (Prdx-5, Prdx-2, GST, DJ-1 and SOD) and proteins associated with heme and iron metabolism (Hmox-1, ferritin, Hpx and Cygb) were up-regulated or uniquely expressed during envenomation, suggesting that heme is effectively metabolized, generating free radicals and inducing oxidative damage to proteins, lipids and DNA. In fact, in previous experiments, we demonstrated that LOBE induces kidney DNA damage leading to double-strand breaks and formation of oxidized purines and pyrimidines (Berger et al. 2013). Probably this is associated with the increased expression of Rad50, a double-strand break repair protein, detected in the present work.
As a consequence of ATN, the excretion of Na+ and K+ increased significantly during envenomation. Injured proximal tubule cells have alterations in the actin and microtubule cytoskeletal networks that lead to a redistribution of Na+/K+ ATPase from the basolateral to the apical membrane, contributing to a decrease in Na+ transport and reabsorption (Thadhani et al. 1996). It was reported that FENa+ and FEK+ also increase in envenomation caused by Bothrops snakes (Boer-Lima et al. 1999; Linardi et al. 2011). Despite the redistribution of Na+/K+ ATPase to the apical membrane, Linardi et al. (2011) reported an increase in expression and activity of Na+/K+ ATPase during envenomation by Bothrops alternatus and suggest that it is a protective mechanism triggered in response to natriuresis with the aim to preserve renal function during acute damage. In agreement with this observation, we found an increase in expression of type-3 Na+/H+ exchanger regulatory cofactor, which is important in Na+ and HCO3 − reabsorption in proximal tubule cells (Trof et al. 2006). Likewise Na+/K+, water excretion increased significantly, and envenomed animals had polyuria. Several parameters measured, such as urinary density and osmolality, C osm, C H2O and FEH2O, indicate that kidneys from envenomed rats are producing dilute urine through the excretion of solute-free water. Possibly, the presence of intra-tubular Na+ not reabsorbed by proximal tubule cells may contribute to the increased water excretion and both, Na+ and water rejection, may also be associated with the fall in blood pressure.
Regarding the glomerular dysfunctions, a valuable hypothesis that should be considered is the deposition of fibrin clots in glomerular capillaries. Indeed, the most potent activity of LOBE in vitro is the procoagulant activity (Donato et al. 1998; Veiga et al. 2003). In vivo, LOBE also causes activation of coagulation and fibrinolysis leading to a consumptive coagulopathy characteristic of this type of envenomation. Two enzymes responsible for the venom procoagulant activity, activators of prothrombin and factor X, have already been isolated (Alvarez-Flores et al. 2006; Reis et al. 2006). Besides the direct effect on coagulation factors, LOBE is also able to induce a procoagulant profile in endothelial cells in culture through an up-regulation of TF expression (Pinto et al. 2008). Confirming these results obtained in endothelial cells, envenomed kidneys showed an increase in TF procoagulant activity. In addition, the expression of known markers of disseminated intravascular coagulation and thrombosis (serpin 1 alpha-1 antiproteinase, PAI-1 and HC-2) increased in the kidneys of venom-treated rats. The gamma and beta chains of fibrinogen were detected solely in envenomed kidneys, which are probably related to the PAS-positive stain observed in glomeruli and are suggestive of fibrin formation.
Renal inflammation
Intense inflammatory response is a common feature in L. obliqua envenomation. Pain and edema are the most characteristic initial symptoms observed at the local site of contact (Zannin et al. 2003). Usually signs of systemic inflammation with neutrophilic leukocytosis, cell infiltrate and edema have also been described in lungs, kidney and heart of experimental animals (Berger et al. 2013). The inflammatory response is accompanied by the production of several cytokines (TNF, IL-1β IL-8, IL-6, CCL2 and CXCL1), vasoactive mediators (BK, histamine, prostaglandins and nitric oxide), adhesion molecules (E-selectin, VCAM-1 and ICAM-3) and an increase in leukocyte rolling and adhesion to the endothelium (Alvarez-Flores et al. 2006; Bohrer et al. 2007; Pinto et al. 2008; Berger et al. 2010; Nascimento-Silva et al. 2012). Specifically in the kidney, it was observed an up-regulation of several proteins related to acute-phase inflammatory signaling, nephritis, inflammatory cell infiltration, increase in vascular permeability, glomerular dilation, distention of Bowman’s space and interstitial edema. Kidney sections stained with picrosirius revealed extensive collagen deposition in cortical periglomerular and peritubular regions and proteins involved with fibrosis, such as Cygb, PAI-1, complement C3 and complement factor D were identified. Enhanced deposition of extracellular matrix (ECM) proteins in renal tissue has been observed in response to a variety of stimuli, including TGF-β, TNF-α, IL-1, several adhesion molecules and chemoattractants. These stimuli can also increase the levels of tissue inhibitors of matrix metalloproteinases, thereby attenuating ECM turnover, and thus, favoring the deposition of collagen and other matrix proteins (Eddy 1996; Pawluczyk and Harris 1998).
Besides fibrosis, activated complement components (C3 and factor D) may also contribute to the alterations in vascular permeability and acts as a chemoattractant for neutrophils (Turnberg et al. 2006). Moreover, complement activation by albumin is a powerful underlying mechanism of tubular and interstitial injury via cytotoxic, proinflammatory and fibrogenic effects, which often occur in renal diseases where proteinuria is present (Portella et al. 2013). In an experiment conducted in proximal tubular cells incubated with serum proteins, in vitro complement activation was observed, which could be associated with changes in the cytoskeleton, production of superoxide anion, hydrogen peroxide and proinflammatory cytokines, such as IL-6 and TNF-α (Abbate et al. 2006).
Direct venom cytotoxic activity
As the kidneys are highly vascularized organs, they are particularly susceptible to direct venom toxicity (Sitprija 2006). Renal epithelial cells in culture and isolated perfused kidneys have been used to characterize the direct cytotoxic effects of different venoms including bee, snake and spider venoms. The most common isolated toxins, which are nephrotoxic, belong to the classes of metalloproteinases, serine proteinases, C-type lectins, phospholipases A2, sphingomyelinases D and L-amino acid oxidases (Berger et al. 2012). In the case of L. obliqua, some toxins belonging to these classes have already been isolated (Pinto et al. 2010); however, their effects on renal cells are unknown. Our results provide some evidence that the whole venom probably has a direct nephrotoxic effect, since immunohistochemical staining confirmed the presence of venom in renal tissue, with stronger staining in the initial 6 h after venom administration followed by a progressive decrease thereafter. The venom was rapidly excreted in urine, because at least 5 bands which specifically react with antibodies raised against L. obliqua toxins were detected in urine. Interestingly, the positive immunohistochemical reaction for venom in different kidney structures agreed with the morphological and histological damage caused by the venom in these anatomical regions and indicated that there was a close correlation between the sites of venom localization and subsequent tissue injury. Previous studies are consistent with our observations. Using immunochemical and radiolabeling methods to analyze venom biodistribution in rats, the highest quantities of LOBE were detected in kidneys, blood and urine (Rocha-Campos et al. 2001; Da Silva et al. 2004).
Conclusion
In this work, a rat experimental model was used to study the progression of renal disease during Lonomia obliqua envenomation. According to our results, the pathophysiological mechanism involved in L. obliqua-induced AKI seems to be multifactorial where events such as systemic hypotension and fibrin deposition contribute to renal hypoperfusion, tubular necrosis and the sudden loss of basic renal functions, including filtration and excretion capacities, urinary concentration and maintenance of body fluid homeostasis. In addition, when compared to control rats, the kidneys from envenomed animals showed to be increasingly enriched for stress-related proteins, which are commonly associated with inflammation, tissue injury, heme-induced oxidative stress, coagulation and complement systems activation. Finally, the localization of the venom in renal tissue agreed with morphological and functional alterations, suggesting a close correlation between venom tissue levels and renal damage. Thus, the different mechanisms as well as the renal injury biomarkers identified here can be useful and guide further experiments to the discovery of alternative forms of treatment to L. obliqua-induced AKI.
References
Abbate M, Zoja C, Remuzzi G (2006) How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17:2974–2984
Alvarez-Flores MP, Fritzen M, Reis CV, Chudzinski-Tavassi AM (2006) Losac, a factor X activator from Lonomia obliqua bristle extract: its role in the pathophysiological mechanisms and cell survival. Biochem Biophys Res Commun 343:1216–1223
Ambatipudi KS, Lu B, Hagen FK, Melvin JE, Yates JR (2009) Quantitative analysis of age specific variation in the abundance of human female parotid salivary proteins. J Proteome Res 8:5093–5102
Basnakian AG, Kaushal GP, Shah SV (2002) Apoptotic pathways of oxidative damage to renal tubular epithelial cells. Antioxid Redox Signal 4(6):915–924
Berger M, Reck J Jr, Terra RM, Pinto AF, Termignoni C, Guimarães JA (2010) Lonomia obliqua caterpillar envenomation causes platelet hypoaggregation and blood incoagulability in rats. Toxicon 55(1):33–44
Berger M, Vieira MAR, Guimaraes JA (2012) Acute Kidney Injury Induced by Snake and Arthropod Venoms. In: Polenakovic M (ed) Renal failure—the facts. ISBN: 978953-51-0630-2, InTech-Open, Rijeka, Croatia, pp 157–186
Berger M, Beys-da-Silva W, Santi L, de Oliveira IM, Jorge PM, Henriques JAP, Driemeier D, Vieira MAR, Guimarães JA (2013) Acute Lonomia obliqua caterpillar envenomation-induced physiopathological alterations in rats: Evidence of new toxic venom activities and the efficacy of serum therapy to counteract systemic tissue damage. Toxicon 74:179–192
Boer-Lima PA, Gontijo JA, da Cruz-Höfling MA (1999) Histologic and functional renal alterations caused by Bothrops moojeni snake venom in rats. Am J Trop Med Hyg 61(5):698–706
Bohrer CB, Reck J Jr, Fernandes D, Sordi R, Guimarães JA, Assreuy J, Termignoni C (2007) Kallikrein-kinin system activation by Lonomia obliqua caterpillar bristles: involvement in edema and hypotension responses to envenomation. Toxicon 49:663–669
Bowler RP, Duda B, Chan ED, Enghild JJ, Ware LB, Matthay MA, Duncan MW (2004) Proteomic analysis of pulmonary edema fluid and plasma in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 286(6):L1095–L1104
Brocker C, Lassen N, Estey T, Pappa A, Cantore M, Orlova VV, Chavakis T, Kavanagh KL, Oppermann U, Vasiliou V (2010) Aldehyde dehydrogenase 7A1 (ALDH7A1) is a novel enzyme involved in cellular defense against hyperosmotic stress. J Biol Chem 285(24):18452–18463
Burdmann EA, Antunes I, Saldanha LB, Abdulkader RC (1996) Severe acute renal failure induced by the venom of Lonomia caterpillars. Clin Nephrol 46(5):337–339
Caliari MV (1997) Princípios de Morfometria Digital: KS300 para iniciantes. Ed. UFMG
Camara NO, Soares MP (2005) Heme oxygenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic Biol Med 38(4):426–435
Caplin B, Wang Z, Slaviero A, Tomlinson J, Dowsett L, Delahaye M, Salama A, Wheeler DC, Leiper J (2012) Alanine-glyoxylate aminotransferase-2 metabolizes endogenous methylarginines, regulates NO, and controls blood pressure. Arterioscler Thromb Vasc Biol 32(12):2892–2900
Carvalho PC, Fischer JS, Chen EI, Yates JR 3rd, Barbosa VC (2008) PatternLab for proteomics: a tool for differential shotgun proteomics. BMC Bioinform 9:316
Carvalho PC, Fischer JS, Xu T, Yates JR 3rd, Barbosa VC (2012) PatternLab: from mass spectra to label-free differential shotgun proteomics. Curr Protoc Bioinform Chapter 13(Unit13):19
Chandler RJ, Zerfas PM, Shanske S, Sloan J, Hoffmann V, DiMauro S, Venditti CP (2009) Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J 23(4):1252–1261
Chang SH, Phelps PC, Berezesky IK, Ebersberger ML Jr, Trump BF (2000) Studies on the mechanisms and kinetics of apoptosis induced by microinjection of cytochrome c in rat kidney tubule epithelial cells (NRK-52E). Am J Pathol 156(2):637–649
Chen G, Paka L, Kako Y, Singhal P, Duan W, Pillarisetti S (2001) A protective role for kidney apolipoprotein E: regulation of mesangial cell proliferation and matrix expansion. J Biol Chem 276:49142–49147
Chinnaiyan AM, Huber-Lang M, Kumar-Sinha C, Barrette TR, Shankar-Sinha S, Sarma VJ, Padgaonkar VA, Ward PA (2001) Molecular signatures of sepsis: multiorgan gene expression profiles of systemic inflammation. Am J Pathol 159(4):1199–1209
Da Silva GH, Panunto PC, Hyslop S, Da Cruz-Höfling MA (2004) Immunochemical detection of Lonomia obliqua caterpillar venom in rats. Microsc Res Tech 65:276–281
Devarajan P, Krawczeski CD, Nguyen MT, Kathman T, Wang Z, Parikh CR (2010) Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. Am J Kidney Dis 56(4):632–642
Dias da Silva W, Campos CM, Gonçalves LR, Sousa-e-Silva MC, Higashi HG, Yamagushi IK, Kelen EM (1996) Development of an antivenom against toxins of Lonomia obliqua caterpillars. Toxicon 34:1045–1049
Donato JL, Moreno RA, Hyslop S, Duarte A, Antunes E, Le Bonniec BF, Rendu F, de Nucci G (1998) Lonomia obliqua Caterpillar Spicules Trigger Human Blood Coagulation via Activation of Factor X and Prothrombin. Thromb Haemost 79:539–542
Duarte AC (1997) Síndrome Hemorrágica causada por larvas de mariposa do gênero Lonomia: estudo clínico-epidemiológico [Dissertação]. Universidade Federal do Rio Grande do Sul. Porto Alegre
Duarte AC, Caovilla JJ, Lorini JD, Mantovani G, Sumida J, Manfre PC, Silveira RC, de Moura SP (1990) Insuficiência renal aguda por acidentes com lagartas. J Bras Nefrol 12:184–187
Duarte AC, Crusius PS, Pires CAL (1994) Insuficiência renal aguda nos acidentes com lonomia obliqua. Nefrol Lat Am 1(1):38–40
Eng JK, Mccormack AL, Yates JR (1994) An approach to correlate tandem mass-spectral data of peptides with amino-acid-sequences in a protein database. J Am Soc Mass Spectrom 5(11):976–989
Eddy AA (1996) Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol 7:2495–2508
Eddy AA (2004) Proteinuria and interstitial injury. Nephrol Dial Transpl 9(2):277–281
Fan HW, Cardoso JL, Olmos RD, Almeida FJ, Viana RP, Martinez AP (1998) Hemorrhagic syndrome and acute renal failure in a pregnant woman after contact with Lonomia caterpillars: a case report. Rev Inst Med Trop São Paulo 40(2):119–120
Feng MG, Navar LG (2010) Afferent arteriolar vasodilator effect of adenosine predominantly involves adenosine A2B receptor activation. Am J Physiol Renal Physiol 299(2):F310–F315
Gamborgi GP, Metcalf EB, Barros EJG (2006) Acute renal failure provoked by toxin from caterpillars of the species Lonomia obliqua. Toxicon 47:68–74
Godoy JR, Oesteritz S, Hanschmann EM, Ockenga W, Ackermann W, Lillig CH (2011) Segment-specific overexpression of redoxins after renal ischemia and reperfusion: protective roles of glutaredoxin 2, peroxiredoxin 3, and peroxiredoxin 6. Free Radic Biol Med 51(2):552–561
Guder WG, Ross BD (1984) Enzyme distribution along the nephron. Kidney Int 26:101–111
Khan FY (2009) Rhabdomyolysis: a review of the literature. Neth J Med 67(9):272–283
Klein MI, Xiao J, Lu B, Delahunty CM, Yates JR 3rd, Koo H (2012) Streptococcus mutans protein synthesis during mixed-species biofilm development by high-throughput quantitative proteomics. PLoS One 7:e45795
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassú AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepúlveda N, Smith A, Soares MP (2010) A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 2(51):51ra71
Linardi A, Rocha e Silva TA, Miyabara EH, Franco-Penteado CF, Cardoso KC, Boer PA, Moriscot AS, Gontijo JA, Joazeiro PP, Collares-Buzato CB, Hyslop S (2011) Histological and functional renal alterations caused by Bothrops alternatus snake venom: expression and activity of Na+/K+-ATPase. Biochim Biophys Acta 1810(9):895–906
McDonald WH, Tabb DL, Sadygov RG, MacCoss MJ, Venable J, Graumann J, Johnson JR, Cociorva D, Yates JR (2004) MS1, MS2, and SQT-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun Mass Spectrom 18(18):2162–2168
Morrissey JH (1995) Tissue factor modulation of factor VIIa activity: use in measuring trace levels of factor VIIa in plasma. Thromb Haemost 74(1):185–188
Mimura I, Nangaku M, Nishi H, Inagi R, Tanaka T, Fujita T (2010) Cytoglobin, a novel globin, plays an antifibrotic role in the kidney. Am J Physiol Renal Physiol 299(5):F1120–F1133
Nascimento-Silva V, Rodrigues da Silva G, Moraes JA, Cyrino FZ, Seabra SH, Bouskela E, Almeida Guimarães J, Barja-Fidalgo C (2012) A pro-inflammatory profile of endothelial cell in Lonomia obliqua envenomation. Toxicon 60(1):50–60
Nishi H, Inagi R, Kawada N, Yoshizato K, Mimura I, Fujita T, Nangaku M (2011) Cytoglobin, a novel member of the globin family, protects kidney fibroblasts against oxidative stress under ischemic conditions. Am J Pathol 178(1):128–139
Pawluczyk IZA, Harris KPG (1998) Cytokine interactions promote synergistic fibronectin accumulation by mesangial cells. Kidney Int 54:62–70
Peng J, Elias JE, Thoreen CC, Licklider LJ, Gygi SP (2003) Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis. The yeast proteome. J Proteome Res 2:43–50
Pinho FM, Zanetta DM, Burdmann EA (2005) Acute renal failure after Crotalus durissus snakebite: a prospective survey on 100 patients. Kidney Int 67(2):659–667
Pinto AFM, Silva KRLM, Guimaraes JA (2006) Proteases from Lonomia obliqua venomous secretions: comparison of procoagulant, fibrin(ogen)olytic and amidolytic activities. Toxicon 47:113–121
Pinto AFM, Dragulev B, Guimarães JA, Fox JW (2008) Novel perspectives in the pathogenesis of Lonomia obliqua caterpillar envenomation based on assessment of host response by gene expression analysis. Toxicon 51:1119–1128
Pinto AFM, Berger M, Reck-Jr J, Terra RM, Guimarães JA (2010) Lonomia obliqua venom: In vivo effects and molecular aspects associated with the hemorrhagic syndrome. Toxicon 56(7):1103–1112
Portella VG, Silva-Filho JL, Landgraf SS, de Rico TB, Vieira MA, Takiya CM, Souza MC, Henriques MG, Canetti C, Pinheiro AA, Benjamim CF, Caruso-Neves C (2013) Sepsis-surviving mice are more susceptible to a secondary kidney insult. Crit Care Med 41(4):1056–1068
Pompermayer K, Souza DG, Lara GG, Silveira KD, Cassali GD, Andrade AA, Bonjardim CA, Passaglio KT, Assreuy J, Cunha FQ, Vieira MA, Teixeira MM (2005) The ATP-sensitive potassium channel blocker glibenclamide prevents renal ischemia/reperfusion injury in rats. Kidney Int 67(5):1785–1796
Reis CV, Andrade SA, Ramos OH, Ramos CR, Ho PL, Batista IF, Chudzinski-Tavassi AM (2006) Lopap, a prothrombin activator from Lonomia obliqua belonging to the lipocalin family: recombinant production, biochemical characterization and structure-function insights. Biochem J 398(2):295–302
Revelo MP, Federspiel C, Helderman H, Fogo AB (2005) Chronic allograft nephropathy: expression and localization of PAI-1 and PPAR-gamma. Nephrol Dial Transpl 20:2812–2819
Ricci-Silva ME, Valente RH, León IR, Tambourgi DV, Ramos OH, Perales J, Chudzinski-Tavassi AM (2008) Immunochemical and proteomic technologies as tools for unravelling toxins involved in envenoming by accidental contact with Lonomia obliqua caterpillars. Toxicon 51:1017–1028
Rocha-Campos AC, Gonçalves LR, Higasshi HG, Yamagushi IK, Fernandes I, Oliveira JE, Ribela MT, Sousa-e-Silva MC, Dias da Silva W (2001) Specific heterologous F(ab’)2 antibodies revert blood incoagulability resulting from envenoming by Lonomia obliqua caterpillars. Am J Trop Med Hyg 64:283–289
Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD (2007) The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 71(6):504–513
Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, Liebhaber SA, Cooke NE (1999) Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest 103(2):239–251
Schrier RW, Wang W, Poole B, Mitra A (2004) Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 114(1):5–14
Seibert CS, Oliveira MRL, Gonçalves LRC, Santoro ML, Sano-Martins IS (2004) Intravascular hemolysis induced by Lonomia obliqua caterpillar bristle extract: an experimental model of envenomation in rats. Toxicon 44:793–799
Seibert CS, Tanaka-Azevedo AM, Santoro ML, Mackessy SP, Torquato RJS, Lebrun I, Tanaka AS, Sano-Martins IS (2006) Purification of a phospholipase A2 from Lonomia obliqua caterpillar bristle extract. Biochem Biophys Res Commun 342:1027–1033
Shashidharamurthy R, Mahadeswaraswamy YH, Ragupathi L, Vishwanath BS, Kemparaju K (2010) Systemic pathological effects induced by cobra (Naja naja) venom from geographically distinct origins of Indian peninsula. Exp Toxicol Pathol 62(6):587–592
Sitprija V (2006) Snakebite nephropathy. Nephrology (Carlton) 11(5):442–448
Son D, Kojima I, Inagi R, Matsumoto M, Fujita T, Nangaku M (2008) Chronic hypoxia aggravates renal injury via suppression of Cu/Zn-SOD: a proteomic analysis. Am J Physiol Renal Physiol 294(1):F62–F72
Stefanska B, Huang J, Bhattacharyya B, Suderman M, Hallett M, Han ZG, Szyf M (2011) Definition of the landscape of promoter DNA hypomethylation in liver cancer. Cancer Res 71(17):5891–5903
Tabb DL, McDonald WH, Yates JR 3rd (2002) DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res 1:21–26
Takatsuka H, Nakajima T, Nomura K, Wakae T, Toda A, Itoi H, Okada M, Misawa M, Hara H (2006) Heparin cofactor II as a predictor of thrombotic microangiopathy after bone marrow transplantation. Hematology 11(2):101–103
Thadhani R, Pascual M, Bonventre JV (1996) Acute renal failure. N Engl J Med 334(22):1448–1460
Thukral SK, Nordone PJ, Hu R, Sullivan L, Galambos E, Fitzpatrick VD, Healy L, Bass MB, Cosenza ME, Afshari CA (2005) Prediction of nephrotoxicant action and identification of candidate toxicity-related biomarkers. Toxicol Pathol 33(3):343–355
Tofovic SP, Kusaka H, Li P, Jackson EK (1998) Effects of adenosine deaminase inhibition on blood pressure in old spontaneously hypertensive rats. Clin Exp Hypertens 20(3):329–344
Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, Yoshida H, Doi T, Mizoguchi A, Matsuura N, Niho Y, Nishimune Y, Nishikawa SI, Takai Y (1999) Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene 18(39):5373–5380
Trof RJ, Di Maggio F, Leemreis J, Groeneveld AB (2006) Biomarkers of acute renal injury and renal failure. Shock 26(3):245–253
Turnberg D, Lewis M, Moss J, Xu Y, Botto M, Cook HT (2006) Complement activation contributes to both glomerular and tubulointerstitial damage in adriamycin nephropathy in mice. J Immunol 177:4094–4102
Veiga ABG, Blochtein B, Guimarães JA (2001) Structures involved production, secretion and injection of the venom produced by the caterpillar Lonomia obliqua (Lepidoptera, saturniidae). Toxicon 39:1343–1351
Veiga ABG, Pinto AFM, Guimarães JA (2003) Fibrinogenolytic and procoagulant activities in the hemorrhagic syndrome caused by Lonomia obliqua caterpillars. Thromb Res 111(1–2):95–101
Veiga ABG, Ribeiro JM, Guimarães JA, Francischetti IM (2005) A catalog for the transcripts from the venomous structures of the caterpillar Lonomia obliqua: identification of the proteins potentially involved in the coagulation disorder and hemorrhagic syndrome. Gene 355:11–27
Warrell DA (2010) Snake bite. Lancet 375(9708):77–88
Washburn MP, Wolters D, Yates JR 3rd (2001) Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol 19:242–247
Williams D, Gutiérrez JM, Harrison R, Warrell DA, White J, Winkel KD, Gopalakrishnakone P (2010) Global snake bite initiative working group; international society on toxinology. The global snake bite initiative: an antidote for snake bite. Lancet 375(9708):89–91
Xu T, Venable JD, Park SK, Cociorva D, Lu B (2006) ProLuCID, a fast and sensitive tandem mass spectra-based protein identification program. Mol Cell Proteomics 5:S174
Zager RA (1996) Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 49(2):314–326
Zager RA, Johnson AC, Becker K (2012) Renal cortical hemopexin accumulation in response to acute kidney injury. Am J Physiol Renal Physiol 303(10):F1460–F1472
Zannin M, Lourenço DM, Motta G, Costa LRD, Grando M, Gamborgi GP, Noguti MA, Chudzinski-Tavassi AM (2003) Blood coagulation and fibrinolytic factors in 105 patients with hemorrhagic syndrome caused by accidental contact with Lonomia obliqua caterpillar in Santa Catarina, Southern Brazil. Thromb Haemost 89:355–364
Acknowledgments
This work was supported by funding and fellowships from the Brazilian Agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Ministério da Educação, Brazil (CAPES-MEC)—Edital Toxinologia (Processo: 23038.006277/2011-85) and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Ministério da Ciência e Tecnologia, Brazil (CNPq-MCT). This work was also supported by NIH Grant P41 GM103533 (to J.R.Y.). We thank Dr. Jeffrey N. Savas for his advice on the mass spectrometric analysis and for a critical reading of the manuscript.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Berger, M., Santi, L., Beys-da-Silva, W.O. et al. Mechanisms of acute kidney injury induced by experimental Lonomia obliqua envenomation. Arch Toxicol 89, 459–483 (2015). https://doi.org/10.1007/s00204-014-1264-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1264-0