Abstract
Hepatotoxicity of the antidiabetic drug metformin has been reported, but the underlying mechanisms remain unclear. We here investigated the effect of metformin in immune-mediated liver damage. While not hepatotoxic alone, metformin (200 mg/kg) aggravated concanavalin A (Con A, 12 mg/kg)-induced hepatitis, an experimental model of T cell-mediated liver injury, in both relatively resistant BALB/c and highly susceptible C57Bl/6 mice. Metformin + Con A-treated mice had elevated serum levels of pro-inflammatory cytokines TNF-α and IFN-γ, accompanied by a massive mononuclear cell infiltration in the liver. This was associated with the higher numbers of CD4+ T cells producing TNF-α, IFN-γ and IL-17, CD4+ T cells expressing chemokine receptor CXCR3 and activation marker CD27, CD4+CD62L−CCR7− and CD8+CD62L−CCR7− effector memory cells, IFN-γ producing NK cells, IL-4 and IL-17 producing NKT cells and IL-12 producing macrophages/dendritic cells. The percentage of CD4+CXCR3+Tbet+IL-10+ and CD4+CD69+CD25− regulatory T cells was reduced. Metformin stimulated inducible nitric oxide synthase (iNOS) expression in the liver and spleen, and genetic deletion of iNOS attenuated the hepatotoxicity of metformin. Metformin increased the autophagic light chain 3 conversion and mRNA expression of important autophagy-inducing (beclin-1, Atg5 and GABARAP) and pro-apoptotic (p21, p27, Puma, Noxa, Bax, Bad, Bak1, Bim and Apaf1), but not anti-apoptotic molecules (Bcl-xL, survivin and XIAP), which correlated with the apoptotic caspase-3/PARP cleavage in the liver. The autophagy inhibitor chloroquine (20 mg/kg) prevented liver injury and apoptotic changes induced by metformin. Therefore, metformin aggravates immune-mediated hepatitis by promoting autophagy and activation of immune cells, affecting effector, as well as liver-specific regulatory T cells and iNOS expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metformin is the most commonly prescribed oral antidiabetic medication (Miralles-Linares et al. 2012). The hepatic uptake mediated by organic cation transporters and the subsequent inhibition of gluconeogenesis are apparently required for its pharmacological activity (Graham et al. 2011). Although hepatotoxicity is not considered as a major side effect of metformin, several cases of metformin-induced liver injury have been reported recently (Cone et al. 2010; de la Poza et al. 2008; Miralles-Linares et al. 2012; Olivera-Gonzalez et al. 2010). Liver damage was documented by elevated liver enzymes and the improvement in liver function occurred after a week of discontinuation of the drug. However, the mechanism by which metformin induces liver injury remains unclear. Having in mind the rare occurrence of metformin hepatotoxicity, it is conceivable to assume that metformin alone does not induce liver injury, but may act in concert with some other insult, such as inflammation, as it has previously been proposed for other hepatotoxic drugs (Prandota 2005). Although potentially important for treating hyperglycemic patients with liver inflammation, this possibility has not been investigated so far.
To address the possible hepatotoxicity of metformin during liver inflammation, we used concanavalin A (Con A)-induced hepatitis as a well-established experimental model of T/NKT cell-mediated fulminant hepatitis in humans (Santodomingo-Garzon and Swain 2011; Tiegs et al. 1992; Wang et al. 2012). Here, we provide the first evidence that metformin aggravates inflammation-induced liver injury by enhancing activation of immune cells, affecting effector and liver-specific T regulatory cells and nitric oxide (NO) production, as well as autophagy and apoptosis in the liver tissue.
Materials and methods
Animals
Male 6–8-week-old BALB/c, C57Bl/6, CBA and iNOS deficient (iNOS−/−) mice were used. All experiments were approved by the Animal Ethics Committee of Faculty of Medicine (Kragujevac, Serbia) and performed according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23 revised 1985).
Con A-induced hepatitis and treatment
For hepatitis induction, mice were given a single intravenous injection of Con A (Sigma-Aldrich, St. Louis, MO, USA) at 12 mg/kg of body weight in 250 μl of saline. Because of the fast clearance rate, metformin (99.9 %, Galenika a.d., Belgrade, Serbia) was given i.p. three times a day in a 200 μL of saline (total dose 200 mg/kg), starting 24 h before Con A administration. The chosen dose was not toxic to experimental animals (Yuan et al. 2012). According to the formula: animal dose (mg/kg) × animal Km/human Km, where Km is a correction factor reflecting the relationship between body weight and body surface area (Km = 37 for an adult human of 60 kg, and Km = 3 for a mouse of 20 g), the human equivalent dose is 970 mg for a person of 60 kg, which is 2.6 times lower than the maximum safe dose of 2,550 mg/day recommended in the Physician’s Desk Reference. Autophagy inhibitor chloroquine (Sigma-Aldrich, St. Louis, MO, USA) was administered i.p. (20 mg/kg) simultaneously with the first metformin injection. The livers and spleens for the flow cytometry, immunoblot and RT-PCR analysis, as well as sera for cytokine measurement, were collected 8 h after Con A injection, while the liver tissue for histological analysis was collected 24 h after Con A administration. Serum levels of alanine aminotransaminase (ALT) and aspartate aminotransferase (AST) were measured 24 h after Con A administration using the automated biochemistry analyzer Olympus AU 400 (Olympus Diagnostica GMBH, Hamburg, Germany).
Histological analysis of liver injury
The histological analysis of liver tissue was performed as previously described (Volarevic et al. 2012b). Briefly, the livers, isolated from individual mice, were cut longitudinally, fixed in 10 % phosphate-buffered formalin and embedded in paraffin. Consecutive 4-μm tissue sections were cut at various depths and mounted on slides. Sections were stained with hematoxylin and eosin (H&E) and examined under low-power (100×) light microscope (Zeiss Axioskop 40, Jena, Germany) equipped with digital camera. Sections were examined by two independent investigators in a blind manner.
Isolation and flow cytometry analysis of hepatic mononuclear cells (MNC) and splenocytes
The isolation and flow cytometry analysis of liver-infiltrating MNC and splenocytes was conducted as previously described (Volarevic et al. 2012a, b). Briefly, the inferior vena cava was cut above the diaphragm, and the liver was flushed with cold phosphate-buffered saline (PBS, Sigma-Aldrich, St. Louis, MO, USA) until it became pale. The connective tissue and the gall bladder were removed, and the liver was minced into small pieces and forced gently through a 200-mm-gauge stainless steel mesh using a sterile syringe plunger and than suspended in 50 ml RPMI-1640 medium containing GlutaMAX™-1 (Life Technologies Carlsbad, CA, USA), 25 mM HEPES and 10 % fetal calf serum (FCS, pH 7.4) (both from Sigma-Aldrich, St. Louis, MO, USA). The cell suspension was centrifuged at 60g with the off-brake setting for 1 min. The obtained supernatant was transferred to a new tube and centrifuged at 480g with the high-brake setting for 8 min. The pellet was resuspended in 10 ml 37.5 % Percoll in Hanks’ balanced salt solution containing 100 U/ml heparin (all from Sigma-Aldrich, St. Louis, MO, USA) and then centrifuged at 850g with the off-brake setting for 30 min. The pellet was resuspended in 2 ml NH4Cl/Tris–Cl (pH 7.2) (erythrocyte lysing buffer) for 5 min and centrifuged at 480g with the high-brake setting for 8 min at 8 °C. Finally, the pellet was resuspended in 1 ml PBS containing 0.1 % NaN3 (FACS buffer) and prepared for flow cytometry. The spleens were minced in RPMI 1640 (Sigma-Aldrich, St. Louis, MO, USA) and forced gently through a 40-mm cell-strainer nylon mesh using a sterile syringe plunger and centrifuged at 400g for 5 min. Pelleted spleen cells were incubated in 2 ml NH4Cl/Tris–Cl (pH 7.2) for 5 min, supplemented with 1 ml FBS, centrifuged at 400g for 5 min and then resuspended in RPMI 1640 with 10 % FCS. MNC derived from the liver and spleens were stained for the presence of CD3, CD4, CD8, CD25, NKp46, NK1.1, CD11c, CD11b, CD19, CD5, CD62L, CXCR3, CCR7, CD69, F4/80, CD80 and CD86, and concomitantly for the intracellular content of TNF-α, IFN-γ, IL-17, IL-10, IL-12, IL-4, Foxp3 and T-bet by using the fixation/permeabilization kit and anti-mouse monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP) and allophycocyanin (APC) (all from BD Biosciences, San Jose, CA, USA) following manufacturer’s instructions. The stained cells were counted using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA), and the results were analyzed with WinMDI software.
Hepatocyte isolation
Mouse hepatocytes were isolated as previously described (Li et al. 2010). Briefly, cannula was inserted in the portal vein, and liver was perfused for 15–20 min with T1 solution (0.9 % NaCl, 0.05 % KCl, HEPES 0.2 %, 0.08 mg/ml EGTA, pH 7.4) (all from Sigma-Aldrich, St. Louis, MO, USA). As soon as the perfusion is started, the hepatic vein was immediately cut to allow perfusate to run as a waste. Then, liver was perfused with T2 solution (0.6 % NaCl, 0.05 % KCl, 1.2 % HEPES, 0.07 % CaCl2, 3 g/ml collagenase Type I, pH 7.4) (all from Sigma-Aldrich, St. Louis, MO, USA). The disaggregated liver tissue was collected with a curved spatula and transferred into a plate where the disruption of the tissue was mechanically disrupted with a scalpel. Minced tissue was collected with 2 ml Dulbecco`s Modified Eagle Medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA), filtered through a 70-μm cell strainer and centrifuged at 600 rpm for 4 min. The supernatant was removed, pellet was resuspended in 3 ml DMEM, cells were passed on 37.5 % Percoll cushion (30 ml), centrifuged at 1,050 rpm for 3 min and resuspended in 2 ml of DMEM, and viable hepatocytes were counted.
Cytokine measurement
The serum levels of TNF-α and IFN-γ were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Apoptosis assay
The phosphatidylserine exposure on apoptotic cells was examined by flow cytometry using Annexin V-FITC Detection Kit (BD Pharmingen, San Jose, CA, USA) according to the manufacturer’s protocol. Briefly, cells were washed twice with PBS and resuspended in annexin-binding buffer (0.01 M HEPES/NaOH pH 7.4, 0.14 M NaCl, 2.5 mM CaCl2) at concentration 1 × 106/ml. Annexin-FITC and propidium iodide (PI) were added to the 100 μl of cell suspension and incubated for 15 min at room temperature (25 °C) in the dark. Afterward, 400 μl of binding buffer was added to each tube, and stained cells were analyzed within 1 h using FACSCalibur flow cytometer (BD Biosciences, San Jose, USA) and WinMDI software.
Immunoblotting
Tissues/cells were lysed in lysis buffer (30 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 % NP-40) containing 1 mM phenylmethylsulfonyl fluoride, 1 mM Na vanadate, 10 mM NaF and protease/phosphatase inhibitor cocktail (both from Sigma-Aldrich, St. Louis, MO, USA) on ice for 30 min, centrifuged at 14,000g for 15 min at 4 °C, and the supernatants were collected. Equal amounts of protein from each sample were separated by SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hemel Hempstead, UK). Following incubation with primary rabbit antibodies against microtubule-associated protein 1 light chain 3β (LC3β), phosphor-AMP-activated protein kinas (AMPK)α (Thr172), phospho-Akt (Ser473), phospho-extracellular signal-regulated kinase (ERK) (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182) mitogen-activated protein kinase (MAPK), phospho-c-Jun N-terminal kinase (JNK) (Thr183/Tyr185), caspase 3, poly (ADP ribose) polymerase (PARP), p62/SQSTM1, inducible nitric oxide synthase (iNOS) or actin (all from Cell Signaling Technology, Beverly, MA, USA) and peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) as the secondary antibody, specific protein bands were visualized using the enhanced chemiluminescence reagent (GE Healthcare, Pollards Wood, UK). The results were quantified by densitometry using Image J software, and the results are presented relative to actin.
Real-time RT-PCR
Total RNA was extracted using TRIZOL and reverse transcribed with MuLV reverse transcriptase and random hexamers (all from Life Technologies, Carlsbad, CA, USA). The real-time RT-PCR analysis was performed in a Realplex2 Mastercycler (Eppendorf, Hamburg, Germany) using mouse TaqMan Master Mix and the following TaqMan primers and probes from Life Technologies (Carlsbad, CA, USA): autophagy-related (Atg) 4b (00558047_m1), Atg5 (00504340_m1), Atg7 (00512209_m1), Atg12 (00503201_m1), gamma-aminobutyric acid receptor-associated protein (GABARAP, 00490678_m1), beclin-1 (01265461_m1), p62/Sqstm1 (00448091_m1), p21/Waf1 (00432448_m1), p27/Kip1 (00438168_m1), p53-upregulated modulator of apoptosis (Puma, 00519268_m1), Noxa (00451763_m1), B cell lymphoma 2 (Bcl-2, 00477631_m1), Bcl-2-associated X protein (Bax, 00432051_m1), Bcl-2-associated death promoter (Bad, 00432042_m1), Bcl-2 homologous antagonist killer 1 (Bak1, 00432045_m1), Bim (00437796_m1), p53 (01731287_m1), phosphatase and tensin homolog (Pten, 00477208_m1), apoptotic protease activating factor 1 (Apaf1, 01223702_m1), Bcl-xL (00437783_m1), X-linked inhibitor of apoptosis protein (XIAP, 00776505_m1), survivin (00599749_m1), 18 s ribosomal RNA (03928990_g1) and β2-microglobulin (00437762_m1). The geometric mean of threshold cycle (Ct) values of housekeeping genes (18 s RNA and β2-microglobulin) was subtracted from Ct values of target genes to obtain ΔCt, and the relative gene expression was determined as 2−ΔCt (Vandesompele et al. 2002). The results are presented relative to the control values, arbitrarily set to 1.
Statistical analysis
Results were analyzed using the Student’s t test or one-way ANOVA. All data were expressed as the mean ± SEM. The values of p < 0.05 were considered as statistically significant.
Results
Metformin enhances Con A-induced liver injury
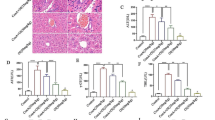
In preliminary experiments, we found that metformin increased transcription of IL-23/IL-12p40, IL-23p19, IL-17 and ROR-γT, which was reflected in the enhanced capacity of peripheral leukocytes to produce IL-17 and IL-23 (Suppl. Figure 1). As these cytokines were involved in Con A-induced T/NKT cell-mediated fulminant hepatitis in mice (Tiegs et al. 1992; Volarevic et al. 2012a, b), we tested the ability of metformin to modulate Con A-induced liver injury. Metformin did not induce liver damage as evaluated by liver function test (Fig. 1a) and histology (data not shown). However, when relatively resistant BALB/c (Fig. 1a) and highly susceptible C57Bl/6 mice (Suppl. Fig. 2A) were treated with metformin before Con A, serum AST and ALT levels significantly increased compared to treatment with Con A only. Histological analysis of liver tissue sections in metformin + Con A-treated BALB/c mice showed widespread areas of necrosis characterized by standard morphologic criteria (loss of architecture, cytoplasmic swelling and vacuolization, increased eosinophilia) (Fig. 1b). Nuclear chromatin condensation was also frequently found, indicating hepatocyte apoptosis. The increase in liver damage by metformin was associated with the extensive infiltration of MNC within the liver lobules and around the central veins and portal tracts (Fig. 1b). The flow cytometric analysis confirmed a significant increase in the total number of infiltrating MNC in the liver of Con A-treated mice exposed to metformin (Fig. 1c), thus indicating an ongoing inflammatory process. Accordingly, metformin pretreatment in Con A hepatitis caused a significant elevation of serum concentrations of the pro-inflammatory cytokines TNF-α and IFN-γ (Fig. 1d). Results similar to those presented in Fig. 1a–d were obtained in highly susceptible C57Bl/6 mice (Suppl. Fig. 2A-D).
Metformin aggravates Con A-induced liver damage. AST and ALT levels show significant enhancement of liver cell damage in metformin + Con A group compared to mice treated with Con A only (a). Histology (H&E ×400) shows more intense liver parenchymal damage and cell infiltration in metformin + Con A-treated mice (b). Total numbers of liver-infiltrating MNC (c) and serum levels of pro-inflammatory cytokines TNF-α and IFN-γ (d) were significantly higher in metformin + Con A than in Con A only group. Combined results of 2 experiments with at least 8 BALB/c mice per group, 24 h (a, b) or 8 h (c, d) after Con A administration (mean ± SEM; *p < 0.05; **p < 0.01)
Metformin promotes liver infiltration of T, NK, NKT, B2 cells, macrophages and dendritic cells
Further analysis revealed the increase in the total numbers of liver-infiltrated CD4+ T cells, CD8+ T cells and NK1.1+CD3− NK cells (Suppl. Fig. 2E, F, G, also observed in BALB/c mice—data not shown), accompanied by the increase in the number of TNF-α, IFN-γ and IL-17 producing CD4+ cells (Fig. 2a and Suppl. Fig. 2E), as well as IFN-γ producing NK cells, IL-4 producing NKT cells and IL-17 producing NKT cells (Fig. 2b and Suppl. Fig. 2G). No significant difference was observed in the numbers of IL-4 and IL-10 producing CD4+ cells, or IFN-γ producing CD8+ T lymphocytes and NKT cells (data not shown). The total numbers of CD19+CD11b−CD5− B2 cells (Fig. 1c, Suppl. Fig. 2H), CD80+CD86+F4/80+ activated macrophages, CD11c+ dendritic cells, as well as IL-12 producing macrophages and dendritic cells were also increased by metformin treatment (Fig. 2d and Suppl. Fig. 2I).
Metformin increases numbers of liver-infiltrating CD4+T, NK, NKT, B2, dendritic cells and macrophages. Total numbers of CD4+ T cells containing TNF-α, IFN-γ and IL-17 (a), IFN-γ producing NK and IL-4 producing NKT cells (b), CD11b−CD5−CD19+ inflammatory B2 cells (c), (CD80+CD86+) F4/80+ activated macrophages and CD11c+ dendritic cells, as well as IL-12 producing macrophages/dendritic cells (Fig. 1d), were significantly higher in the liver of mice treated with metformin and Con A compared to mice treated with Con A only. Combined results of 2 experiments with at least 8 BALB/c mice per group, 8 h after Con A administration (mean ± SEM; *p < 0.05; **p < 0.01)
Metformin stimulates infiltration of activated and effector memory T cells, but downregulates liver-specific regulatory cells in Con A hepatitis
The livers of Con A-treated mice that received metformin displayed a striking increase in the total numbers of activated CD4+CD27+ T cells, and CD4+CD62L−CCR7− and CD8+CD62L−CCR7− effector memory cells (Fig. 3a). We also noticed that metformin + Con A-treated mice had a significantly higher number of liver-infiltrated CD4+ cells expressing CXCR3 receptor (Fig. 3a), an important Th1 chemokine receptor involved in Th1 trafficking in liver inflammatory conditions (Erhardt et al. 2011). This was accompanied by the changes in cellular make up of the spleen (Suppl. Fig. 3), suggesting the release of inflammatory cells from the spleen and their infiltration to the liver. Compared to mice treated with Con A alone, the spleens from mice preexposed to metformin had significantly lower numbers of MNC, B2 cells, activated CD80+CD86+ macrophages and DCs, NK and NKT cells, TNF-α producing CD4+ T lymphocytes and NKT cells, IFN-γ producing CD4+, CD8+ T lymphocytes, NK and NKT cells and IL-17 producing NKT cells (Suppl. Fig. 3). Finally, we examined the influence of metformin pretreatment on regulatory T cells involved in the pathogenesis of Con A hepatitis. As shown in Fig. 3b, there was no difference in the percentage of CD25+Foxp3+ CD4+ and CD8+ T regulatory cells. However, there was a significant decrease in the percentage of two populations of regulatory cells recently described in the liver pathology (Han et al. 2009; Zhu et al. 2011), CD4+CXCR3+Tbet+IL-10+ and CD4+CD69+CD25− T cells.
Metformin promotes influx of activated CD4+ and effector memory T cells, but reduces liver-specific Tregs in the liver of Con A-treated mice. Total numbers of CD4+ cells expressing activation marker CD27 or liver-specific chemokine receptor CXCR3, as well as effector memory CD4+ and CD8+ T cells (CD4+CD62−CCR7− and CD8+CD62L−CCR7−) (a), were significantly higher in the liver of mice treated with metformin and Con A compared to mice treated with Con A only. Metformin did not affect the percentage of CD4+CD25+Foxp3+ and CD8+CD25+Foxp3+ T regulatory cells in Con A hepatitis, but significantly downregulated the participation of the two other regulatory CD4+T cells in the liver: CD4+CXCR3+Tbet+IL-10+ and CD4+CD69+CD25− T cells (b). Combined results of 2 experiments with at least 8 BALB/c mice per group, 8 h after Con A administration (mean ± SEM; *p < 0.05)
iNOS is involved in metformin-mediated exacerbation of Con A hepatitis
Nitric oxide generated by iNOS contributes to Con A-induced liver injury by potentiating intrahepatic TNF-α production and subsequent inflammation (Sass et al. 2001). As we noticed the increase in serum level of TNF-α and total number of TNF-α producing infiltrating cells in livers of metformin + Con A-treated mice, we further examined the effect of metformin on iNOS expression. We observed a significant increase in iNOS protein levels both in the liver and spleen of metformin only and metformin + Con A-treated mice compared to untreated controls or treatment with Con A alone (Fig. 4a). In order to further demonstrate the importance of NO in aggravation of liver injury by metformin, we attempted to induce disease in iNOS−/− and WT CBA mice. Metformin alone did not affect AST or ALT levels in either WT or iNOS−/− animals (Fig. 4b). Deletion of iNOS attenuated both Con A-induced disease and effects of metformin, as evaluated by biochemical parameters (Fig. 4b) and cellular make up of the liver (Fig. 4c–e). CBA WT mice develop Con A-induced hepatitis which was significantly enhanced by metformin, but iNOS deletion reduced the susceptibility to disease in WT mice and completely abrogated its potentiation by metformin. There was no difference between AST and ALT levels (Fig. 4b), or the total number of liver-infiltrating MNC, CD4+ cells, dendritic cells and macrophages (Fig. 4c, d) between iNOS−/− mice treated with Con A alone and in combination with metformin. Accordingly, the percentage of regulatory CD4+CD69+CD25− and CD4+CXCR3+Tbet+IL-10+ cells was significantly decreased in metformin + Con A-treated WT mice, but not affected in iNOS−/− mice (Fig. 4e).
Metformin increases iNOS expression, and iNOS deletion attenuates metformin effects in Con A hepatitis. Immunoblot analysis demonstrated that in Con A-treated CBA mice, metformin enhanced expression of iNOS (a). CBA WT mice develop Con A-induced hepatitis that was enhanced by metformin, but iNOS deletion completely abrogated the susceptibility to disease as evaluated by liver enzymes (b), total numbers of infiltrated MNC, CD4+ T cells (c), CD11c+ dendritic cells producing IL-12 and F4/80+ macrophages (d). Percentage of regulatory CD4+CD69+CD25− and CD4+CXCR3+Tbet+IL-10+ cells was significantly decreased in WT mice, but not affected in iNOS−/− mice (e). Combined results of 2 experiments with at least 6 (a) or 8 (b–e) animals per group, 8 h (a, c–e) or 24 h (b) after Con A administration (mean ± SEM; *p < 0.05 compared to control; # p < 0.05 compared to Con A)
Metformin induces autophagy and apoptosis in the liver of Con A-treated animals
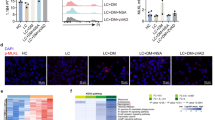
As both apoptosis (programmed cell death type I) and autophagy (programmed cell death type II) are responsible for liver damage in Con A hepatitis (Rautou et al. 2010; Torisu et al. 2008; Zhou et al. 2005), we analyzed whether metformin could influence apoptosis or autophagy in Con A-treated animals. The Con A-induced conversion of LC3-I to its lipidated, autophagosome-associated form LC3-II, indicating the induction of autophagy, was further augmented by metformin (Fig. 5a). Accordingly, the expression of mRNA encoding important autophagy-inducing proteins beclin-1, Atg5 and GABARAP was significantly increased in the liver tissue of metformin + Con A-treated animals compared to treatment with Con A only (Fig. 5b). The expression of Atg4b, Atg12, Atg7 and autophagic cargo receptor p62 was also increased by metformin in livers of Con A-treated mice, but the difference did not reach statistical significance (Fig. 5b). No increase in LC3 conversion or expression of autophagy-related genes was observed in the splenocytes or liver-infiltrated MNC, respectively, of metformin + Con A-treated mice (Fig. 5c, d). Also, there was no change in apoptotic phosphatidylserine externalization (Annexin+ cells) in liver-infiltrated MNC of metformin + Con A compared to Con A-treated mice (Fig. 5e). However, in agreement with the ability of metformin to aggravate Con A-induced hepatitis (Fig. 1 and Suppl. Fig. 2), the drug significantly augmented the liver expression of nine pro-apoptotic genes (p21, p27, Puma, Noxa, Bax, Bad, Bak1, Bim and Apaf1), while only two pro-apoptotic genes (p53 and Pten) did not display a noticeable increase (Fig. 5b). On the other hand, only mRNA for Bcl-2, but not for other anti-apoptotic genes (Bcl-xL, survivin, XIAP) was significantly up-regulated by metformin in Con A-exposed animals (Fig. 5f).
Metformin stimulates autophagy and apoptosis in the liver of Con A-treated mice. Autophagic LC3 conversion and expression of mRNA encoding autophagy-inducing proteins (beclin-1, Atg5, GABARAP) were increased in the liver (a, b), but not spleen (c) or liver-infiltrated MNC (d) of metformin + Con A-treated animals compared to those treated with Con A only. There was no difference in the percentage of the late apoptotic annexin+PI+ MNC in the liver of metformin + Con A versus Con A only-treated mice (e). Real-time RT-PCR analysis revealed that metformin increased the expression of several pro-apoptotic genes (p21, p27, Puma, Noxa, Bax, Bad, Bak1, Bim and Apaf1) but not anti-apoptotic genes (Bcl-xL, survivin and XIAP) in the liver tissue of Con A-treated mice. Combined results of 2 experiments with at least 8 BALB/c mice per group, 8 h after Con A administration (mean ± SEM; *p < 0.05 compared to control; # p < 0.05 compared to Con A)
Autophagy inhibitor chloroquine prevents metformin-mediated exacerbation of Con A-induced hepatitis by reducing hepatocyte apoptosis
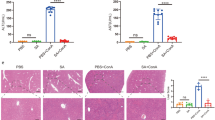
To assess the role of autophagy in metformin-mediated aggravation of Con A hepatitis, we used chloroquine, a lysosomal inhibitor that blocks autophagic proteolysis (Kimura et al. 2013). The treatment of Con A-injected mice with chloroquine reduced serum and completely prevented the increase in AST and ALT levels upon administration of metformin (Fig. 6a). To assess the ability of chloroquine to inhibit autophagic proteolysis in our experimental model, we analyzed the hepatocyte expression of p62, an LC3-binding protein selectively degraded by autophagy (Ichimura and Komatsu 2010). The levels of p62 were lower in hepatocytes of Con A-treated and even further reduced in those of metformin + Con A-treated mice (Fig. 6b), which is consistent with the induction of autophagy by Con A and its potentiation by metformin. Administration of chloroquine completely restored p62 levels in hepatocytes of metformin + Con A-treated animals, thus confirming the blockade of autophagic proteolysis. The activation of the key apoptosis-executing enzyme caspase-3 and subsequent cleavage of the DNA-repairing protein poly (ADP ribose) polymerase (PARP), which were increased in hepatocytes of Con A-treated mice and even more pronounced upon metformin administration, were significantly downregulated in mice that received chloroquine (Fig. 6b).
Autophagy inhibitor chloroquine prevents metformin-mediated exacerbation of Con A-induced hepatitis by reducing hepatocyte apoptosis. Chloroquine attenuated metformin-mediated exacerbation of Con A-induced liver damage, as evaluated by liver enzymes (a). Autophagic proteolysis of p62, caspase-3 activation and PARP cleavage were increased by chloroquine in the livers of metformin + Con A-treated mice (b). Combined results of 2 experiments with at least 6 BALB/c mice per group, 8 h after Con A administration (mean ± SEM; *p < 0.05 compared to untreated control, # p < 0.05 compared to Con A, § p < 0.05 compared to metformin + Con A)
Metformin activates Akt and inhibits AMPK and p38 MAPK in Con A hepatitis
Finally, to get some insight into the intracellular signaling pathways involved in the observed effects of metformin, we used immunoblotting to assess the phosphorylation (activation) status of AMPK), Akt and MAPK family members p38, ERK and JNK in the liver and spleen. The phosphorylation of AMPK (Thr172) was not affected in liver (Fig. 7a) and significantly reduced in splenocytes (Fig. 7b) of metformin + Con A-treated mice compared to Con A only controls. Metformin increased phosphorylation of Akt (Ser473) and reduced phosphorylation of p38 (Thr180/Tyr182) in both liver and spleen of Con A-treated mice (Fig. 7a, b). The phosphorylation of ERK (Thr202/Tyr204) and JNK (Thr183/Tyr185) in Con A-treated animals was not significantly affected by metformin (Fig. 7a, b).
The effects of metformin on the activation of AMPK, Akt, p38 MAPK, ERK and JNK in Con A hepatitis. Compared to treatment with Con A alone, metformin co-administration did not affect the phosphorylation of AMPK in liver (a) and markedly reduced it in the spleen (b). The phosphorylation of Akt was increased and that of p38 MAPK was reduced in both liver (a) and spleen (b). The phosphorylation of ERK and JNK was not affected in either liver (a) or spleen (b) of metformin + Con A-treated compared to Con A-treated mice. Combined results of 2 experiments with at least 6 BALB/c mice per group, 8 h after Con A administration (mean ± SEM; *p < 0.05 compared to untreated control, # p < 0.05 compared to Con A)
Discussion
We here report that metformin aggravates Con A-induced hepatitis, a well-established experimental model of T cell-mediated liver injury (Prandota 2005; Tiegs et al. 1992; Volarevic et al. 2012a, b). It has recently been shown that metformin alleviates fulminant liver injury in lipopolysaccharide/d-galactosamine-challenged mice (Yuan et al. 2012), which may seem to be at variance with our findings. However, this discrepancy could be due to different experimental models and particularly to different effects of metformin on activated T cells and macrophages. In contrast to Con A-induced hepatitis, liver injury induced by LPS/d-Gal is independent of T-cell activation because LPS activates macrophages directly (Yuan et al. 2012).
It is known that IFN-γ, TNF-α and/or IL-17 producing CD4+ T lymphocytes, as well as IL-4 producing NKT cells and IL-12 producing macrophages, are the major effector cells involved in Con A hepatitis (Ajuebor et al. 2003; Kaneko et al. 2000; Kusters et al. 1996; Tiegs et al. 1992; Zhu et al. 2007). Accordingly, we found that metformin treatment significantly increased the numbers of TNF-α, IFN-γ and IL-17 expressing CD4+ T cells (Fig. 2a and Suppl. Fig. 2E), as well as IFN-γ producing NK and IL-4 producing NKT cells (Fig. 2b and Suppl. Fig. 2H). The numbers of aggressive B2 lymphocytes (Fig. 2c) activated (CD80+CD86+) IL-12-producing F4/80+ macrophages, and CD11c+ dendritic cells (Fig. 2d) in hepatic tissue of Con A-treated mice were also increased by metformin, thus presumably contributing to the observed liver damage. Furthermore, metformin markedly stimulated the liver infiltration of activated CD4+CD27+ T cells, CD4+CD62L−CCR7− and CD8+CD62L−CCR7− effector memory cells (Fig. 3a). In line with our results, metformin promoted generation, activity and survival of CD8+ effector memory T cells by activating a metabolic switch that enabled them to cope with the increasing energy demands (Pearce et al. 2009). The increase in IFN-γ production (Fig. 1d) and the expression of chemokine receptor CXCR3 (Fig. 3a), which are important for migration of inflammatory cells from the spleen to the liver (Erhardt et al. 2011), were correlated with the changes in cellular make up of the spleen (Suppl. Fig. 3). These results suggest that the metformin-mediated increase in activation/expansion of the effector immune cells was coupled with their efficient release from the spleen, thus enabling subsequent infiltration to the liver.
Metformin-mediated amelioration of LPS/D-Gal induced hepatitis and encephalomyelitis in mice correlated with the reduced expression of iNOS and NO production in macrophages (Kato et al. 2010; Nath et al. 2009; Yuan et al. 2012). In contrast, we observed that in Con A-induced T cell-dependent liver injury metformin significantly increased iNOS expression both in the liver and in spleen tissue (Fig. 5), while iNOS deletion attenuated both Con A-induced disease and its potentiation by metformin (Fig. 6). This is consistent with the ability of iNOS-derived NO to kill hepatocytes in vitro (Siendones et al. 2003) and to contribute to Con A-induced liver inflammation and injury by stimulating intrahepatic TNF-α production (Zhu et al. 2011). Conversely, TNF-α together with IFN-γ and IL-17 induces iNOS transcription and NO production (Miljkovic and Trajkovic 2004). The observed upregulation of iNOS expression in our study was associated with the increase in serum levels of IFN-γ and TNF-α (Fig. 1d), as well as with the liver infiltration of IFN-γ+, TNF-α+ and IL-17+ T cells (Fig. 2a), thus indicating a detrimental positive feedback between IFN-γ/TNF-α/IL-17 and NO in metformin-mediated liver damage. The production of NO can also be stimulated by IL-12/IL-23 p40 homodimer (Brahmachari and Pahan 2009), and in our experiments, metformin enhanced mitogen-triggered p40 transcription in human PBMC (Suppl. Fig. 1), as well as the numbers of IL-12 producing macrophages and DCs in the liver of Con A-treated mice. Interestingly, NO production mediated by IL-12/IL-23 p40 homodimer has recently been found to suppress the classic FoxP3+ regulatory T cells (Brahmachari and Pahan 2009), which are known to inhibit development of IFN-γ+ Th1 cells in a NO-sensitive manner (Lee et al. 2011). While we did not observe any difference in the liver infiltration of classic CD25+Foxp3+ CD4+ and CD8+ T regulatory cells, the hepatotoxicity of metformin correlated with a significant decrease in the percentage of two populations of Tregs recently described in the liver pathology—CD4+CXCR3+Tbet+IL-10+ and CD4+CD69+CD25− T cells (Fig. 4). The former are generated in the liver in dependence of IFN-γ and after dissemination specifically migrate into the liver, where they limit immune-mediated damage (Erhardt et al. 2011), while the latter principally act in the inflamed liver by suppressing CD4+ T cell proliferation in a cell-to-cell contact manner (Han et al. 2009; Zhu et al. 2011). As iNOS deficiency abrogated the ability of metformin to suppress the induction of CD4+CXCR3+Tbet+IL-10+ and CD4+CD69+CD25− cells, it is tempting to speculate that the downregulation of these liver-specific Tregs by NO contributed to the observed increase in liver injury.
Autophagy is an evolutionary conserved mechanism for lysosomal machinery-dependent digestion of intracellular components (Yang and Klionsky 2010), previously reported to be induced by metformin in different cell types (Tomic et al. 2011; Xie et al. 2011). Autophagic recycling promotes cell survival during energy stress, but when extensive and/or inappropriately activated, autophagy can promote apoptotic or necrotic cell death or act as an alternative cell death mode (Eisenberg-Lerner et al. 2009). By examining the increase in LC3 conversion, autophagic degradation of p62 and expression of several Atg genes, we confirmed that metformin induced autophagy in hepatocytes, but not splenocytes or liver-infiltrated MNC of Con A-injected mice (Figs. 5, 7a). Moreover, the inhibition of autophagic proteolysis by lysosomal inhibitor chloroquine prevented liver injury and caspase-3/PARP cleavage in hepatocytes of metformin + Con A-exposed mice (Fig. 8b), thus indicating a role for autophagy in mediating apoptotic liver damage in these conditions. These data are consistent with the finding that Con A and IFN-γ cooperate in inducing autophagy-dependent hepatocyte death (Chang et al. 2011), indicating that this process could be further augmented by metformin, either directly or through the increase in production of IFN-γ and other autophagy-inducing cytokines. On the other hand, our results obtained on splenocytes and liver MNC (Fig. 6d, e) argue against the autophagy or apoptosis modulation in immune cells as a mechanism for metformin-mediated exacerbation of Con A hepatitis. It should be noted, however, that in addition to apoptotic cell death, the liver tissue from Con A-exposed animals displayed extensive necrotic changes. It therefore remains to be established if metformin plays any role in regulating hepatocyte necrosis and its interplay with apoptosis and/or autophagy during Con A hepatitis.
A schematical representation of the mechanisms underlying metformin-mediated exacerbation of Con A hepatitis. Metformin promotes hepatocyte apoptotic death in Con A-treated mice by enhancing liver infiltration of cytokine-producing immune cells, NO production and hepatocyte autophagy, as well as downregulation of liver-specific regulatory T cells
Finally, with regard to the molecular mechanisms underlying the effects of metformin in Con A hepatitis, we have focused on AMPK, Akt, p38 MAPK, ERK and JNK, which are all targeted by metformin (Bhamra et al. 2008; Boyle et al. 2010; Isakovic et al. 2007; Isoda et al. 2006) and regulate T cell activation (Blagih et al. 2012; Smith-Garvin et al. 2009), iNOS expression (Pilon et al. 2004; Rao 2000; Teshima et al. 2004), autophagy induction (Sridharan et al. 2011) and hepatocyte survival (Cosgrove et al. 2010; Yang et al. 2010). The observed Akt stimulation and AMPK inhibition (Fig. 5b) are consistent with the positive role of Akt and negative role of AMPK in both T cell activation (Blagih et al. 2012; Smith-Garvin et al. 2009) and iNOS induction (Pilon et al. 2004; Teshima et al. 2004). However, neither mechanism could explain autophagy induction by metformin (Figs. 5, 7), as Akt inhibits and AMPK activates autophagic response (Sridharan et al. 2011). Having in mind the prosurvival action of Akt in hepatocytes (Cosgrove et al. 2010), its activation in liver tissue by metformin is probably only a compensatory mechanism not responsible for the hepatotoxicity of the drug. It is also unlikely that the modulation of MAPK signaling contributed to aggravation of Con A hepatitis, as p38, which mainly promotes T cell activation (Smith-Garvin et al. 2009), induction of iNOS (Rao 2000), autophagy (Sridharan et al. 2011) and hepatocyte death (Cosgrove et al. 2010), was inhibited by metformin, while ERK and JNK were not affected. It should be noted the inhibition of splenocyte AMPK by metformin, observed in the present study, seems at odds with the well-known ability of the drug to activate this intracellular energy sensor in various cell types (Boyle et al. 2010). However, some recent findings revealed the ability of metformin to inhibit AMPK phosphorylation in hypothalamic neurons in vitro and in vivo (Chau-Van et al. 2007; Stevanovic et al. 2012), indicating a cell type- and/or context-dependent AMPK modulation by the drug. In our preliminary experiments, the stimulatory effect of metformin on IL-17/IL-23 production in mitogen-activated human leukocytes was surprisingly mimicked by the AMPK inhibitor compound C, while AMPK activation by acadesine actually had the opposite effect (Volarevic et al. unpublished observation). Therefore, the mechanisms underlying the unexpected observed inhibitory effect on splenocyte AMPK, as well as its possible role in exacerbation of Con A hepatitis by metformin, seem worthy of further investigation.
In summing up, metformin aggravates Con A-induced liver injury through multiple, possibly interconnected mechanisms involving modulation of effector and liver-specific regulatory T cell activation, iNOS expression, autophagy and Akt, p38 MAPK and AMPK signaling (Fig. 8). Nevertheless, the temporal and causal relationships between these events remain to be established. As there is a pathogenic link between the presence of type 2 diabetes and liver injury (Hsieh and Hsieh 2011), metformin-induced release of pro-inflammatory mediators and abrogation of regulatory immune mechanisms in the liver may represent a significant therapeutic concern.
References
Ajuebor MN, Hogaboam CM, Le T, Swain MG (2003) C–C chemokine ligand 2/monocyte chemoattractant protein-1 directly inhibits NKT cell IL-4 production and is hepatoprotective in T cell-mediated hepatitis in the mouse. J Immunol 170:5252–5259
Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM (2008) Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol 103:274–284
Blagih J, Krawczyk CM, Jones RG (2012) LKB1 and AMPK: central regulators of lymphocyte metabolism and function. Immunol Rev 249:59–71
Boyle JG, Salt IP, McKay GA (2010) Metformin action on AMP-activated protein kinase: a translational research approach to understanding a potential new therapeutic target. Diabet Med 27:1097–1106
Brahmachari S, Pahan K (2009) Suppression of regulatory T cells by IL-12p40 homodimer via nitric oxide. J Immunol 183:2045–2058
Chang CP, Yang MC, Lei HY (2011) Concanavalin A/IFN-gamma triggers autophagy-related necrotic hepatocyte death through IRGM1-mediated lysosomal membrane disruption. PLoS ONE 6:e28323
Chau-Van C, Gamba M, Salvi R, Gaillard RC, Pralong FP (2007) Metformin inhibits adenosine 5′-monophosphate-activated kinase activation and prevents increases in neuropeptide Y expression in cultured hypothalamic neurons. Endocrinology 148:507–511
Cone CJ, Bachyrycz AM, Murata GH (2010) Hepatotoxicity associated with metformin therapy in treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease. Ann Pharmacother 44:1655–1659
Cosgrove BD, Alexopoulos LG, Hang TC, Hendriks BS, Sorger PK, Griffith LG, Lauffenburger DA (2010) Cytokine-associated drug toxicity in human hepatocytes is associated with signaling network dysregulation. Mol BioSyst 6:1195–1206
de la Poza GomezG, Rivero Fernandez M, Vazquez Romero M, Angueira Lapena T, Arranz de la Mata G, Boixeda de Miquel D (2008) Constitutional syndrome associated to metformin induced hepatotoxicity. Gastroenterol Hepatol 31:643–645
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 16:966–975
Erhardt A, Wegscheid C, Claass B, Carambia A, Herkel J, Mittrucker HW, Panzer U, Tiegs G (2011) CXCR3 deficiency exacerbates liver disease and abrogates tolerance in a mouse model of immune-mediated hepatitis. J Immunol 186:5284–5293
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50:81–98
Han Y, Guo Q, Zhang M, Chen Z, Cao X (2009) CD69 + CD4 + CD25 − T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol 182:111–120
Hsieh PS, Hsieh YJ (2011) Impact of liver diseases on the development of type 2 diabetes mellitus. World J Gastroenterol 17:5240–5245
Ichimura Y, Komatsu M (2010) Selective degradation of p62 by autophagy. Semin Immunopathol 32:431–436
Isakovic A, Harhaji L, Stevanovic D, Markovic Z, Sumarac-Dumanovic M, Starcevic V, Micic D, Trajkovic V (2007) Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci 64:1290–1302
Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schonbeck U, Libby P (2006) Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol 26:611–617
Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, Taniguchi M (2000) Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med 191:105–114
Kato Y, Koide N, Komatsu T, Tumurkhuu G, Dagvadorj J, Kato K, Yokochi T (2010) Metformin attenuates production of nitric oxide in response to lipopolysaccharide by inhibiting MyD88-independent pathway. Horm Metab Res 42:632–636
Kimura T, Takabatake Y, Takahashi A, Isaka Y (2013) Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res 73:3–7
Kusters S, Gantner F, Kunstle G, Tiegs G (1996) Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology 111:462–471
Lee SW, Choi H, Eun SY, Fukuyama S, Croft M (2011) Nitric oxide modulates TGF-beta-directive signals to suppress Foxp3 + regulatory T cell differentiation and potentiate Th1 development. J Immunol 186:6972–6980
Li WC, Ralphs KL, Tosh D (2010) Isolation and culture of adult mouse hepatocytes. Methods Mol Biol 633:185–196
Miljkovic D, Trajkovic V (2004) Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev 15:21–32
Miralles-Linares F, Puerta-Fernandez S, Bernal-Lopez MR, Tinahones FJ, Andrade RJ, Gomez-Huelgas R (2012) Metformin-induced hepatotoxicity. Diabetes Care 35:e21
Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S (2009) Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol 182:8005–8014
Olivera-Gonzalez S, de Escalante-Yanguela B, Velilla-Soriano C, Amores-Arriaga B, Martin-Fortea P, Navarro-Aguilar ME (2010) Metformin-associated hepatotoxicity. Med Intensiva 34:483–487
Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y (2009) Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460:103–107
Pilon G, Dallaire P, Marette A (2004) Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem 279:20767–20774
Prandota J (2005) Important role of proinflammatory cytokines/other endogenous substances in drug-induced hepatotoxicity: depression of drug metabolism during infections/inflammation states, and genetic polymorphisms of drug-metabolizing enzymes/cytokines may markedly contribute to this pathology. Am J Ther 12:254–261
Rao KM (2000) Molecular mechanisms regulating iNOS expression in various cell types. J Toxicol Environ Health B Crit Rev 3:27–58
Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R (2010) Autophagy in liver diseases. J Hepatol 53:1123–1134
Santodomingo-Garzon T, Swain MG (2011) Role of NKT cells in autoimmune liver disease. Autoimmun Rev 10:793–800
Sass G, Koerber K, Bang R, Guehring H, Tiegs G (2001) Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest 107:439–447
Siendones E, Fouad D, Abou-Elella AM, Quintero A, Barrera P, Muntane J (2003) Role of nitric oxide in D-galactosamine-induced cell death and its protection by PGE1 in cultured hepatocytes. Nitric Oxide 8:133–143
Smith-Garvin JE, Koretzky GA, Jordan MS (2009) T cell activation. Annu Rev Immunol 27:591–619
Sridharan S, Jain K, Basu A (2011) Regulation of autophagy by kinases. Cancers 3:2630–2654
Stevanovic D, Janjetovic K, Misirkic M, Vucicevic L, Sumarac-Dumanovic M, Micic D, Starcevic V, Trajkovic V (2012) Intracerebroventricular administration of metformin inhibits ghrelin-induced Hypothalamic AMP-kinase signalling and food intake. Neuroendocrinology 96:24–31
Teshima S, Nakanishi H, Nishizawa M, Kitagawa K, Kaibori M, Yamada M, Habara K, Kwon AH, Kamiyama Y, Ito S, Okumura T (2004) Up-regulation of IL-1 receptor through PI3 K/Akt is essential for the induction of iNOS gene expression in hepatocytes. J Hepatol 40:616–623
Tiegs G, Hentschel J, Wendel A (1992) A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest 90:196–203
Tomic T, Botton T, Cerezo M, Robert G, Luciano F, Puissant A, Gounon P, Allegra M, Bertolotto C, Bereder JM, Tartare-Deckert S, Bahadoran P, Auberger P, Ballotti R, Rocchi S (2011) Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis 2:e199
Torisu T, Nakaya M, Watanabe S, Hashimoto M, Yoshida H, Chinen T, Yoshida R, Okamoto F, Hanada T, Torisu K, Takaesu G, Kobayashi T, Yasukawa H, Yoshimura A (2008) Suppressor of cytokine signaling 1 protects mice against concanavalin A-induced hepatitis by inhibiting apoptosis. Hepatology 47:1644–1654
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Volarevic V, Milovanovic M, Ljujic B, Pejnovic N, Arsenijevic N, Nilsson U, Leffler H, Lukic ML (2012a) Galectin-3 deficiency prevents concanavalin A-induced hepatitis in mice. Hepatology 55:1954–1964
Volarevic V, Mitrovic M, Milovanovic M, Zelen I, Nikolic I, Mitrovic S, Pejnovic N, Arsenijevic N, Lukic ML (2012b) Protective role of IL-33/ST2 axis in Con A-induced hepatitis. J Hepatol 56:26–33
Wang HX, Liu M, Weng SY, Li JJ, Xie C, He HL, Guan W, Yuan YS, Gao J (2012) Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J Gastroenterol 18:119–125
Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH (2011) Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60:1770–1778
Yang Z, Klionsky DJ (2010) Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 22:124–131
Yang YM, Han CY, Kim YJ, Kim SG (2010) AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J Gastroenterol 16:3731–3742
Yuan H, Li L, Zheng W, Wan J, Ge P, Li H, Zhang L (2012) Antidiabetic drug metformin alleviates endotoxin-induced fulminant liver injury in mice. Int Immunopharmacol 12:682–688
Zhou F, Ajuebor MN, Beck PL, Le T, Hogaboam CM, Swain MG (2005) CD154-CD40 interactions drive hepatocyte apoptosis in murine fulminant hepatitis. Hepatology 42:372–380
Zhu R, Diem S, Araujo LM, Aumeunier A, Denizeau J, Philadelphe E, Damotte D, Samson M, Gourdy P, Dy M, Schneider E, Herbelin A (2007) The Pro-Th1 cytokine IL-12 enhances IL-4 production by invariant NKT cells: relevance for T cell-mediated hepatitis. J Immunol 178:5435–5442
Zhu J, Feng A, Sun J, Jiang Z, Zhang G, Wang K, Hu S, Qu X (2011) Increased CD4(+) CD69(+) CD25(−) T cells in patients with hepatocellular carcinoma are associated with tumor progression. J Gastroenterol Hepatol 26:1519–1526
Acknowledgments
This study was supported by Grants ON175069, ON175103 and III41025 from the Ministry of Education, Science and Technology, Republic of Serbia, and MP01/12 from The Faculty of Medical Sciences University of Kragujevac, Serbia. The authors thank Mr. Milan Milojevic for technical support, Dr. Mirjana Sumarac-Dumanovic (Clinic for Endocrinology, Diabetes and Diseases of Metabolism, School of Medicine, University of Belgrade) for useful discussion and Dr. Zeljka Radulovic (Galenika a.d., Belgrade) for providing metformin.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Volarevic, V., Misirkic, M., Vucicevic, L. et al. Metformin aggravates immune-mediated liver injury in mice. Arch Toxicol 89, 437–450 (2015). https://doi.org/10.1007/s00204-014-1263-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1263-1