Abstract
Autoimmune hepatitis (AIH) is a severe immune-mediated inflammatory liver disease that currently lacks feasible drug treatment methods. Our study aimed to evaluate the protective effect of succinic acid against AIH and provide a reliable method for the clinical treatment of AIH. We performed an in vivo study of the effects of succinic acid on concanavalin A (ConA)-induced liver injury in mice. We examined liver transaminase levels, performed hematoxylin and eosin (HE) staining, and observed apoptotic phenotypes in mice. We performed flow cytometry to detect changes in the number of neutrophils and monocytes, and used liposomes to eliminate the liver Kupffer cells and evaluate their role. We performed bioinformatics analysis, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), and western blotting to detect mitochondrial apoptosis-induced changes in proteins from the B-cell lymphoma 2(Bcl-2) family. Succinic acid ameliorated ConA-induced AIH in a concentration-dependent manner, as reflected in the survival curve. HE and TUNEL staining and terminal deoxynucleotidyl transferase dUTP nick end labeling revealed decreased alanine transaminase and aspartate aminotransferase levels, and reduced liver inflammation and apoptosis. RT-qPCR and enzyme-linked immunosorbent assay revealed that succinic acid significantly reduced liver pro-inflammatory cytokine levels. Flow cytometry revealed significantly decreased levels of liver neutrophils. Moreover, the protective effect of succinic acid disappeared after the Kupffer cells were eliminated, confirming their important role in the effect. Bioinformatics analysis, RT-qPCR, and western blotting showed that succinic acid-induced changes in proteins from the Bcl-2 family involved mitochondrial apoptosis, indicating the molecular mechanism underlying the protective effect of succinic acid. Succinic acid ameliorated ConA-induced liver injury by regulating immune balance, inhibiting pro-inflammatory factors, and promoting anti-apoptotic proteins in the liver. This study provides novel insights into the biological functions and therapeutic potential of succinic acid in the treatment of autoimmune liver injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Autoimmune hepatitis (AIH) is a chronic inflammatory disease of the liver caused by an interaction between multiple internal and external factors. AIH is clinically not self-limiting, and if not effectively controlled, it can progress to liver fibrosis, cirrhosis, or cancer [1]. The pathogenesis of AIH is believed to be related to an imbalance in the liver immune tolerance [2]. Under normal circumstances, the liver develops an immune tolerance mechanism to regulate the processing of various food antigens and small amounts of microbial antigens [3, 4]. However, when this immune tolerance mechanism is disrupted by various factors, many immune cells quickly accumulate in the liver and initiate immune attacks, resulting in immunological liver injury that is characterized histologically by dense infiltration of the liver by lymphocytes, macrophages, and plasma cells [2].
Concanavalin A (ConA) is a plant-derived lectin that can cause specific immune damage to the mouse liver [5]. ConA-induced liver injury is characterized by an infiltration of the liver by many immune cell types, including macrophages, T cells, neutrophils, and natural killer (NK) cells [6]. During this process, many inflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ), are produced, leading to widespread liver cell necrosis and apoptosis [7]. A mouse model of ConA-induced liver injury has been widely used to explore the pathogenesis of autoimmune liver diseases as well as in clinical drug screening.
Succinic acid is an intermediate product of the human tricarboxylic acid cycle and an important metabolite of intestinal microbial processes [8]. Recent studies have shown that succinic acid promotes the deposition of skeletal muscle proteins [9], regulates glucose homeostasis [10], promotes angiogenesis, and alleviates ischemic losses [11, 12]. Additionally, it is associated with various immune cell activities. Succinic acid is recognized by cluster cells through its receptor, succinate receptor 1 (SUCNR1), thereby activating type 2 innate lymphoid cells (ILC2) [13, 14]. Moreover, succinic acid plays a role in macrophage polarization [15]. According to a previous report, succinic acid-SUCNR1 signaling can induce migration of dendritic cells (DCs) and synergize with toll-like receptor signaling [16]. Thus, succinic acid exerts an important regulatory effect on the immune system. However, little is known about whether succinic acid is capable of alleviating immune-mediated hepatitis. This study aimed to investigate the effects of succinic acid on ConA-induced liver injury and its potential mechanism of action.
MATERIALS AND METHODS
Animals and Experimental Design
Specific pathogen-free (SPF) grade C57BL/6 male mice (age, 6–8 weeks; weight, 18–24 g) were purchased from the Experimental Animal Center of Xiamen University (Xiamen, China). Chemokine C–C-Motif Receptor 2 (CCR2-/-) mice were generated by Cyagen Biosciences. The mice were raised at the SPF Animal Room of the Experimental Animal Center of Xiamen University at an ambient temperature of 24 ± 2 ℃, a relative humidity of 55 ± 10%, and a 12-h light/dark cycle, and were subjected to adaptive feeding during a week before the start of the experiment. All animal experiments were conducted in accordance with the regulations of the Xiamen University Laboratory Animal Centre. The animal study protocol was approved by the Ethics Committee of Xiamen University (approval no. XMULAC2023052) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (https://www.ncbi.nlm.nih.gov/books/NBK54050/).
The mice were randomly assigned to four groups: a control group, and low-, medium-, and high-dose succinic acid (dissolved in phosphate buffer saline, PBS); Sigma, USA) pretreatment groups. The experimental groups were administered an intraperitoneal injection of succinic acid at a low dose (50 mg/kg), medium dose (100 mg/kg), or high dose (200 mg/kg) 12 h before experimental treatment. The control group was injected with an equal amount of PBS. After pretreatment, the mice were injected with 15 mg/kg of ConA (1 mg/ml in PBS, Solarbio, China) through the tail vein, and sacrificed after 12 h for specimen collection. In addition, we used a lethal dose of ConA (40 mg/kg) to determine the survival curves of the different experimental groups.
For the macrophage depletion assay, the experimental groups received an intraperitoneal injection of clodronate liposomes (200 µL per mouse; Yeasen, China) for macrophage clearance, and the control group received the same volume of PBS. After 24 h, the experimental and control groups were administered succinic acid and PBS, respectively. Thereafter, specimen collection was performed as previously described.
For the Bcl-2 inhibit assay, Venetoclax (20 mg/kg, Fisher Scientific, USA) (Ven) was administered to the mice 2 h before the administration of succinic acid or PBS by intraperitoneal injection. Specimen collection was performed as previously described.
Serum Transaminase Activity
Blood samples from the mice were collected via the retro-orbital bleeding technique and centrifuged at 4 ℃ for 10 min at 4000 rpm to obtain serum. Alanine transaminase (ALT) and aspartate aminotransaminase (AST) levels in the serum samples were measured directly using ALT and AST assay kits (Solarbio, China) according to the manufacturer’s instructions.
Histology and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
Fresh mouse liver tissues were fixed in 4% paraformaldehyde. The fixed liver tissues were dehydrated, embedded in paraffin, and cut into 4–5 µm thick sections. After performing a standard hematoxylin–eosin (HE) staining and neutral gum sealing, pathological changes such as liver inflammation and immune cell infiltration were observed under a microscope. Apoptotic hepatocytes were detected by performing the TUNEL assay using DAB (SA-HRP) TUNEL Cell Apoptosis Detection Kit (Servicebio, China). Images were captured using a digital pathology scanner (Aperio Versa 200; Leica, Germany).
Reverse Transcriptase-Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA from the collected liver tissues was extracted using TRIzol reagent and reverse-transcribed to cDNA using RT-qPCR kits (Yeasen, China). We performed qPCR using the SYBR Green qPCR Mix (Yeasen, China). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal reference gene, and the relative expression levels of gene mRNA in each group were analyzed and calculated using the 2–ΔΔCt method. The primer sequences used were as follows: GAPDH, CATCACTGCCACCCAGAAGACTG (forward) and ATGCCAGTGAGCTTCCCGTTCAG (reverse); transforming growth factor- β (TGF-β), CCACCTGCAAGACCATCGAC (forward) and CTGGCGAGCCTTAGTTTGGAC (reverse); Interleukin(IL)-10 GATTTTAATAAGCTCCAAGACCAAGGT (forward) and CTTCTATGCAGTTGATGAAGATGTCAA (reverse); IL-1β, TGGACCTTCCAGGATGAGGACA (forward) and GTTCATCTCGGAGCCTGTAGTG (reverse); IL-2, GTGCTCCTTGTCAACAGCG (forward) and GGGGAGTTTCAGGTTCCTGTA (reverse); TNF-α, GGGAGTAGACAAGGTACAACCC (forward) and CCTGTAGCCCACGTCGTAG (reverse); IFN-γ, GATGCATTCATGAGTATTGCCAAGT (forward) and GTGGACCACTCGGATGAGCTC (reverse); Bcl-2 interacting mediator of cell death(Bim), CCCCTACCTCCCTACAGACAGA (forward) and TCCAATACGCCGCAACTCTT (reverse); Bcl-2, AAGATTGATGGGATCGTTGC (forward) and GCGGAACACTTGATTCTGGT (reverse); BCL2-associated X protein(Bax), GTGCACCAAGGTGCCGGAAC (forward) and TCAGCCCATCTTCTTCCAGA (reverse).
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of TNF- α, IFN- γ, and IL-1β were determined using an ELISA kit (DAKEWE Beijing, China) according to the manufacturer’s instructions. The absorbance of each well was measured at 450 nm using a microplate reader, and the concentration of each sample was calculated based on a standard curve.
Western Blotting
Total protein was extracted from the collected mouse liver tissue and lysed using radioimmunoprecipitation assay lysis buffer. Protein concentrations were measured using a bicinchoninic acid (BCA) kit for protein detection. Western blotting was performed according to standard procedures. Briefly, equal amounts of the protein were separated using sodium dodecyl-sulfate polyacrylamide gel electrophoresis, and were transferred onto polyvinylidene fluoride membranes (Bio-Rad, USA), blocked with 50 g/L skim milk, and incubated overnight at 4 °C with Bcl-2 (1:1000; #15,071, CST, USA), Bax (1:1000; #2772, CST, USA), and GAPDH (1:1000; #97,166, CST, USA) antibodies. After washing thrice with tris-buffer saline with tween 20, the membranes were incubated with a secondary antibody (horseradish peroxidase-conjugated goat anti-mouse IgG, SA00001-1, 1:5000; Proteintech, USA) for 1 h at room temperature. After the final washes, the density of the target bands was determined using ImageJ (version 1.51; USA) with GAPDH as internal control.
Flow Cytometry
Fresh mouse liver tissue was cut into small pieces and placed in digestive juices containing collagenase IV and DNase I (prepared with D-Hanks’ Balanced Salt Solution). The digestion was carried out at 37 °C and 200 rpm for 40 min. The digested tissues were filtered and centrifuged to obtain a single-cell suspension. The liver immune cells were isolated using the Percoll reagent (Cytiva, USA). After blocking with Fc-block for approximately 30 min, the cells were stained with CD45 (30-F11, BD Bioscience, N.J.), CD11B (M1/70, BD Bioscience, N.J.), LY-6G (1A8, BD Bioscience, N.J.), Ly6C (HK1.4, BD Bioscience, N.J.), F4/80 (BM8, BD Bioscience, N.J.), CD3 (145-2C11, BD Bioscience, N.J.), CD4 (RM4-5, BD Bioscience, N.J.), CD8 (53–6.7, BD Bioscience, N.J.), or NK1.1 (PK135, BD Bioscience, N.J.), and incubated for 30 min. Finally, the stained cells were analyzed using a flow cytometer according to the manufacturer’s instructions.
Identification of Potential Target of Succinic Acid
The potential targets of succinic acid were using the CTD (https://ctdbase.org/), Targetnet (http://targetnet.scbdd.com/, probability > 0.6), SuperPred (https://prediction.charite.de/, probability > 0.6), and Swiss Target Prediction (http://www.swisstargetprediction.ch/, probability > 0.6) databases. The Uniprot database (http://www.uniprot.org/) was used to convert the potential targets identified in the previously mentioned databases into standard gene target names. Then take the intersection of all genes and remove duplicate genes.
Acquisition of AIH-Related Targets
The keyword “autoimmune hepatitis” was used to search the Gene Cards (https://www.genecards.org/, Relevance score > 5.0) and Omim database (https://www.omim.org/) databases for target genes related to the disease. Summarize Genes retrieved from both databases were selected as potential targets related to AIH, and duplicate genes were removed.
Construction of Potential Target Network
A Venn diagram of the target genes of succinic acid and of AIH was constructed using an online tool (http://bioinformatics.psb.ugent.be/webtools/Venn/), and genes located at the intersection were selected as potential targets. A protein–protein interaction (PPI) network of key targets was constructed using the STRING database (version 12.0) and imported into Cytoscape software (version 3.9.0) for visualization.
GO and KEGG Enrichment Analysis
To explore the biological functions of potential targets of succinic acid for AIH treatment, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were conducted by applying the “clusterProfiler” package. GO enrichment analysis includes biological processes (BP), molecular functions (MF), and cell composition (CC).
Statistical Analysis
Data were processed and visualized using GraphPad Prism® 9.0 (GraphPad Software, USA). Data were analyzed using the log-rank test (for survival) and t-tests. All general statistical analyses were calculated with 95% confidence intervals, and P < 0.05 was considered statistically significant.
RESULTS
Succinic Acid Inhibited ConA-Induced Liver Injury
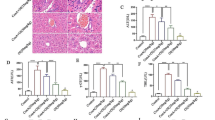
ConA-induced liver injury is characterized by severe liver inflammation and hepatocellular apoptosis. To investigate the effects of succinic acid on ConA-induced liver injury, several doses of succinic acid (50 mg/kg, 100 mg/kg, and 200 mg/kg) were intraperitoneally injected into mice before a lethal dose of ConA (40 mg/kg) was administered. As shown in Fig. 1a, succinic acid significantly increased the survival rate of ConA-treated mice regardless of dosage. To determine the role of succinic acid, 200 mg/kg of succinic acid was administered to the mice before a medium dose of ConA (15 mg/kg) was administered (Fig. 1b). We found that the group pretreated with 200 mg/kg of succinic acid had significantly diminished serum ALT and AST levels compared to the group treated with ConA only (Fig. 1c and d). Similarly, we found no significant change in serum ALT and AST levels in the group pretreated with 200 mg/kg of succinic acid alone compared with the group treated with PBS (Fig. 1c and d), indicating that 200 mg/kg of succinic acid has no toxic effects on the liver. Histological examination of the livers showed massive areas of necrosis and inflammatory infiltration in ConA-treated mice, whereas succinic acid pretreatment attenuated these histopathological changes (Fig. 1e). Similarly, the TUNEL assay showed a significantly decreased number of positive nuclear bodies in the livers of mice pretreated with succinic acid compared with the livers of those treated with ConA only (Fig. 1f), indicating that succinic acid pretreatment has a protective effect against ConA-induced liver injury in mice.
Succinic acid pretreatment alleviates ConA-induced acute liver injury in mice. a Survival curves of ConA-treated with succinic acid (n = 5). Survival rates were analyzed using the log-rank test. b Experimental design and arrangement. c, d ALT and AST serum levels measured 12 h after ConA injection. e Liver tissues stained with HE and determination of histological liver scores are shown. Low magnification: × 40 (Scale bar = 500 µm); high magnification: × 200 (Scale bar = 100 µm). f Apoptosis was detected using the TUNEL assay (apoptotic cells are stained in brown) and followed by quantification of TUNEL-positive area. Low magnification: × 40 (Scale bar = 500 µm); high magnification: × 200 (Scale bar = 100 µm). The data are expressed as the mean ± SEM of three independent experiments. ns, P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. ALT, alanine transaminase; AST, aspartate aminotransferase; HE, hematoxylin and eosin; ConA, concanavalin A; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Succinic Acid Regulated the Expression of Cytokines in ConA-Induced Liver Injury
As cytokines play an important role in the development of liver injury, we extracted total mRNA from the liver tissues and performed RT-qPCR to evaluate the effect of succinic acid on the liver. The fold changes represent difference in mRNA levels according to RT-qPCR. As shown in Fig. 2, the mRNA expressions of TGF-β, IL-10, IL-1β, IL-2, TNF-α, and IFN-γ in the succinic acid alone and PBS-treated groups were not significantly different, indicating that succinic acid alone could not induce a measurable immune response (Fig. 2a). However, the mRNA levels of IL-1β, IL-2, TNF-α, and IFN-γ were significantly decreased in the succinic acid + ConA-treated group compared with the PBS + ConA-treated group (Fig. 2b). Moreover, TGF-β and IL-10 mRNA levels were significantly decreased, which might be beneficial to maintaining immune balance (Fig. 2b). The results showed that succinic acid effectively inhibited the mRNA expression of pro-inflammatory factors. Moreover, ELISA results showed that the levels of the inflammatory cytokines, IL-1β, IFN-γ, and TNF-α were decreased by succinic acid pretreatment compared with those measured after the ConA challenge (Fig. 2c–e). The results showed that succinic acid effectively inhibits the expression of inflammatory factors.
Succinic acid decreases the expression of pro-inflammatory cytokines in ConA-induced liver injury. a, b mRNA expression levels of TGF-β, IL-10, IL-1β, IL-2, TNF-α, and IFN-γ in the liver homogenate were measured after succinic acid pretreatment or succinic acid + ConA challenge. c, d, e Concentrations of TNF-α, IFN-γ, and IL-1β in the serum were measured using ELISA. Results of cytometric bead array immunoassay analysis. Data are expressed as the means ± SDs (n ≥ 3). *P < 0.05; **P < 0.01. ConA, concanavalin A; TGF- β, transforming growth factor-β; IL, interleukin, TNF, tissue necrosis factor-α; IFN, interferon-γ; ELISA, enzyme-linked immunosorbent assay.
Succinic Acid Ameliorated ConA-Induced Liver Injury by Inhibiting the Infiltration of Hepatic Neutrophils
Lymphocyte infiltration of the liver is crucial for the development of ConA-induced liver injury [17]. To determine the main lymphocyte subsets affected by succinic acid, hepatic lymphocytes were isolated and stained with the Fc-block. The cells were stained for the cell markers CD45, CD3, CD4, CD8, NK1.1, Ly-6G, CD11b, F4/80, and Ly6C to study the effects of succinic acid treatment on NK, CD8 + T, CD4 + T, neutrophils, monocytes, and Kupffer cells. The gating strategy used for flow cytometry is shown in Fig. 3a. Flow cytometry analysis revealed no significant differences in the proportions of NK, NKT, CD4 + T, and CD8 + T cells between the succinic acid-pretreated and control mice and those pretreated with succinic acid, which indicates that succinic acid pretreatment did not influence the percentage of NK, NKT and T cells present in the liver (Fig. 3e and i–l). However, the percentage of neutrophils in the liver significantly decreased, and that of monocytes and Kupffer cells increased after succinic acid treatment (Fig. 3b–d and f–h). Taken together, these results indicate that succinic acid regulates immune system balance during ConA-induced liver injury.
Succinic acid ameliorates ConA-induced acute liver injury by inhibiting the infiltration of neutrophils and increasing the infiltration of monocytes. a Gating strategy of CD45 + cells for flow cytometric analysis. b, c, d, e Monocytes, Kupffer cells, neutrophils, NK, NKT, CD4 + T, and CD8 + T cells in the liver were detected using flow cytometry. f–l Quantification of the results. Data are expressed as mean ± SD (n ≥ 3). *P < 0.05; **P < 0.01. ConA, concanavalin A.
Kupffer Cells (KCs) Play an Important Role in the Succinic Acid-Mediated Amelioration of ConA-Induced Liver Injury
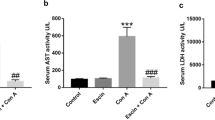
To further determine whether KCs are required for the effect of succinic acid on ConA-induced liver injury, KCs were depleted using clodronate liposomes before PBS and succinic acid were administered (Fig. 4a), and the depletion of macrophages after clodronate liposomes injection was confirmed (Supplementary Fig. 1). Consistently with previous studies [18], ConA-induced liver injury was significantly inhibited in KC-depleted mice, which showed downregulated serum levels of ALT and AST, and reduced severe necrosis and apoptosis (Fig. 4b–g). It is worth noting that the levels of ALT and AST in the PBS + ConA and in the succinic acid + ConA groups were comparable after the depletion of KCs (Fig. 4b and c). In accordance with these results, similar histopathological lesions (Fig. 4d) and TUNEL-positive areas were observed in mice treated with succinic acid and in those treated with PBS after the depletion of KCs (Fig. 4e). Interestingly, a similar percentage of liver neutrophils and similar serum levels of TNF-α, IFN-γ, and IL-1β were found in these two groups (Supplementary Fig. 2–3). In addition, we found that the administration of succinic acid decreased ALT and AST in CCR2-/- mice, which indicates that the effect of succinate acid, does not depend on monocytes (Supplementary Fig. 4). Taken together, these results suggest that KCs are crucial for the protective effects of succinic acid against ConA-induced liver injury.
KCs are crucial for the protective effects of succinic acid on ConA-induced acute liver injury. The mice were treated with succinic acid, with or without clodronate pretreatment, before ConA injection. a Experimental design and arrangement. b, c Serum ALT and AST levels were measured 12 h after the ConA challenge. d Representative histological liver sections stained with HE. e Apoptosis in representative histological liver sections detected using the TUNEL assay (apoptotic cells are stained in brown) and followed by quantification of TUNEL-positive area. Low magnification: × 40 (Scale bar = 500 µm); high magnification: × 200 (Scale bar = 100 µm). The data are expressed as the mean ± SD (n ≥ 3). ns, P ≥ 0.05; ****P < 0.0001. ALT, alanine transaminase; AST, aspartate aminotransferase; HE, hematoxylin and eosin; ConA, concanavalin A; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Succinic Acid Treatment Regulated the Bim, Bcl2, and Bax Expressions in ConA-Treated Mice
A total of 166 targets of succinic acid were obtained and converted into symbols of the corresponding genes using UniProt. A total of 1694 AIH-related targets were obtained through screening using the GeneCards and Omim databases. Figure 5a shows the 47 genes located at the intersection of the Venn plots. Figure 5b shows a network diagram of protein–protein interactions within potential targets.
Succinic acid treatment downregulates the Bim/Bcl-2/Bax signaling pathway in the liver. a Venn plot visualization. b PPI network. c GO enrichment analysis. d KEGG enrichment analysis (the abscissa shows the enrichment factor, the ordinate shows the pathway name, the color represents the p value, and the size of the dot represents the number of genes). e Screening of the apoptotic Bim/Bcl-2/Bax signaling pathway. f mRNA levels of Bim, Bcl-2, and Bax in the liver of mice pretreated with succinic acid or PBS alone before the ConA challenge, measured using RT-qPCR. g Western blot analysis of total liver lysates for the Bcl-2 and Bax. (h, i) ALT and AST serum levels measured 12 h after ConA injection. Data are presented as mean ± SEM of three independent experiments (n = 3). ns, P ≥ 0.05; *P < 0.05; ***P < 0.001; ****P < 0.0001. ConA, concanavalin A; GO, gene ontology; KEGG, Kyoto Encyclopedia of Gene and Genome; PBS, phosphate buffer saline; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
In order to investigate the biological processes involved in the treatment of AIH with succinic acid, we conducted KEGG and GO enrichment analysis on the 47 potential targets identified in the previous step. The KEGG analysis (Fig. 5c) revealed that the identified genes were enriched for cancer (small cell lung cancer and pancreatic cancer), Kaposi sarcoma-associated herpesvirus infection, lipid and atherosclerosis, apoptosis, AGE − RAGE signaling pathway in diabetic complications, HIF − 1 signaling pathway, chemical carcinogenesis − receptor activation, chronic myeloid leukemia and chemical carcinogenesis − reactive oxygen species. The GO enrichment analysis (Fig. 5d) revealed enrichment in the regulation of apoptotic signaling pathways, response to lipopolysaccharides, response to molecules of bacterial origin, and regulation of the response to endoplasmic reticulum stress in biological processes. Moreover, Bcl-2 family protein complex, external side of plasma membrane, RNA polymerase II transcription regulator complex, membrane raft, and caveola are enriched in cellular components. Furthermore, enrichment of transcription coregulator binding, death domain binding, non-membrane spanning protein tyrosine phosphatase activity, transcription coactivator binding, and protein heterodimerization activity were also found in molecular function.
Based on the discovery of significant enrichment in apoptosis-related genes according to both GO and KEGG analyses, the Bim/Bcl-2/Bax signaling pathway was identified as a potential mechanism by which succinic acid protects hepatocytes from apoptosis (Fig. 5e). To further clarify this mechanism, we first determined the mRNA expression of potential anti-apoptotic genes in the livers of ConA-treated mice. Notably, the expressions of Bcl-2 were increased, while those of Bim and Bax were decreased in mice pretreated with succinic acid compared to control mice (Fig. 5f). In addition, western blotting results confirmed that succinic acid pretreatment upregulated the protein expression of Bcl-2 and downregulated that of Bax in the livers of ConA-treated mice (Fig. 5g). To further confirm the function of Bcl-2, venetoclax was administered before succinic acid administration. The result shows that ALT and AST levels were comparable after Bcl-2 inhibition (Fig. 5h and i). This suggests that succinic acid treatment downregulates Bim/Bcl-2/Bax signaling pathway in ConA-treated mice, which might be related to its protective effects on the liver of these mice.
DISCUSSION
The pathogenesis of AIH is unclear, and although it represents a healthcare threat and is potentially fatal, effective pharmacotherapy against it is currently lacking [18, 19]. ConA-induced liver injury is a widely accepted model of acute immune liver injury in humans [20]. ConA induces the infiltration of various immune cell types and mediates gradual necroinflammation and degradation of hepatic parenchyma cells [21]. In this study, we investigated the protective role of succinic acid, an intermediate of the tricarboxylic acid cycle and a metabolite of the gut microbiota, in ConA-induced hepatocyte degeneration. This protection may be related to Kupffer cells and the upregulation of anti-apoptotic proteins.
The liver is a unique metabolic organ that monitors metabolites in the portal and systemic circulation. Succinic acid is a metabolite that helps regulate cellular nutrient metabolism, with potential applications in medical care [8]. Succinic acid decreases hyperglycemia in obese mice by regulating intestinal gluconeogenesis or improving glucose tolerance [10, 22]. Additionally, succinic acid is reportedly a potential therapeutic agent for hematopoiesis [23], sepsis [24], and cancer [25]. However, excessive succinate accumulation contributes to the activation of hepatic stellate cells (HSCs) and increases the risk of hepatic fibrosis and liver cancer [26,27,28]. This study shows that succinic acid at an appropriate dose attenuates ConA-induced autoimmune hepatitis.
ConA triggers an inflammatory cascade first by first stimulating resident KCs in the liver, inducing neutrophil recruitment, and then initiating T cell infiltration (especially CD4 +) [29,30,31]. SUCNR1 (known as GPR91) is a G protein-coupled receptor distributed in several different cell and tissue types, including HSCs, macrophages, DCs, and platelets, and is involved in the regulation of innate immunity, inflammation, and allergic reactions [27, 32]. Our flow cytometry results showed that succinate acid decreases the percentage of neutrophils which promote inflammation and increases that of monocytes that might phagocytose necrotic tissues in the liver. These results indicate that succinic acid regulates immune balance in ConA-induced hepatitis. Notably, no additional benefits were observed in the group pretreated with succinic acid compared with the control group after KCs depletion, which suggests that KCs play an important role in the regulation of immune balance by succinic acid.
The ConA-induced inflammatory response is amplified by the release of various pro-inflammatory cytokines, namely TNF-α, IFN-γ, and IL-1β, which play a crucial role in acute liver injury [33, 34]. It has been reported that ConA-induced liver injury is ameliorated by the downregulation of TNF-α and IFN-γ expression in the mouse [34, 35]. We extracted total mRNA from the liver for RT-qPCR, and the results showed that the levels of the pro-inflammatory factors, TNF-α, IFN-γ, IL-2, and IL-1β, were decreased in the group pretreated with succinic acid. Similarly, the inhibitory factors, TGF-β and IL-10, were decreased, which might be beneficial for immune homeostasis. Moreover, the ELISA results showed that the TNF-α, IFN-γ, and IL-1β levels were decreased after succinic acid treatment. These results indicate that succinic acid could inhibit ConA-induced inflammation to some extent.
The Bcl family plays a significant role in the regulation of mitochondrial apoptosis [36]. Among these, Bcl-2 is an anti-apoptotic gene belonging to the Bcl-2 family located in the mitochondrial outer membrane [37, 38]. Bax induces apoptosis by acting on the mitochondria and regulating caspase activity, whereas Bcl-2 counteracts the action of Bax and consequently inhibits apoptosis. Therefore, the Bax/Bcl-2 protein ratio is of great significance to determine survival or death following apoptotic stimulation. Bioinformatics analysis revealed that the Bim/Bcl-2/Bax signaling pathway may be a potential mechanism by which succinic acid protects the liver cells against apoptosis. RT-qPCR and western blotting revealed a significant increase in the expression of Bcl-2, and a significant decrease in the expression of Bax in the group pretreated with succinic acid, both at the mRNA and protein levels. Therefore, we speculated that succinic acid may have a protective effect against ConA-induced liver cell apoptosis by affecting the expression levels of members of the Bcl-2 family.
As reported previously, clodronate liposomes were used in this study to deplete KCs [29]; however, clodronate liposomes can also deplete many other cell types, including monocytes, macrophages, and DCs. Moreover, SUCNR1 is not only expressed by KCs but also by HSCs and DCs [15, 16]. Our results cannot rule out the possibility that HSCs, DCs, or other cell types might participate in the protective effect of succinic acid against ConA-induced liver injury, which might also involve Bcl-2 and Bax expression in hepatocytes.
CONCLUSION
Our results indicate that the administration of succinic acid ameliorates ConA-induced liver damage by regulating the immune balance, inhibiting pro-inflammatory factors, and having an anti-apoptotic effect. This study provides novel insights into the biological functions and therapeutic potential of succinic acid in the treatment of autoimmune liver injury.
Availability of Data and Materials
The datasets supporting the conclusions of this article are included within the article and its supplementary information files.
References
Goel, A., and P. Kwo. 2024. Treatment of autoimmune hepatitis. Clinics in Liver Disease 28 (1): 51–61.
Beretta-Piccoli, B.T., G. Mieli-Vergani, and D. Vergani. 2022. Autoimmmune hepatitis. Cellular & Molecular Immunology 19 (2): 158–176.
Pabst, O., M.W. Hornef, F.G. Schaap, V. Cerovic, T. Clavel, and T. Bruns. 2023. Gut-liver axis: Barriers and functional circuits. Nature Reviews Gastroenterology & Hepatology 20 (7): 447–461.
Tilg, H., T.E. Adolph, and M. Trauner. 2022. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metabolism 34 (11): 1700–1718.
Sass, G., S. Heinlein, A. Agli, R. Bang, J. Schümann, and G. Tiegs. 2002. Cytokine expression in three mouse models of experimental hepatitis. Cytokine 19 (3): 115–120.
Khan, H.A., M.Z. Ahmad, J.A. Khan, and M.I. Arshad. 2017. Crosstalk of liver immune cells and cell death mechanisms in different murine models of liver injury and its clinical relevance. Hepatobiliary & Pancreatic Diseases International 16 (3): 245–256.
Fujita, T., K. Soontrapa, Y. Ito, K. Iwaisako, C.S. Moniaga, M. Asagiri, et al. 2016. Hepatic stellate cells relay inflammation signaling from sinusoids to parenchyma in mouse models of immune-mediated hepatitis. Hepatology 63 (4): 1325–1339.
Wei, Y.-h, X. Ma, J.-C. Zhao, X.-Q. Wang, and C.-Q. Gao. 2023. Succinate metabolism and its regulation of host-microbe interactions. Gut Microbes. 15 (1).
Yuan, Y., Y. Xu, J. Xu, B. Liang, X. Cai, C. Zhu, et al. 2017. Succinate promotes skeletal muscle protein synthesis via Erk1/2 signaling pathway. Molecular Medicine Reports. 16 (5): 7361–7366.
Wang, K., M. Liao, N. Zhou, L. Bao, K. Ma, Z. Zheng, et al. 2019. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Reports 26 (1): 222.
Moyon, A., P. Garrigue, L. Balasse, S. Fernandez, P. Brige, A. Bouhlel, et al. 2021. Succinate injection rescues vasculature and improves functional recovery following acute peripheral ischemia in rodents: A multimodal imaging study. Cells 10 (4).
Cao, Z., S. Mu, M. Wang, Y. Zhang, G. Zou, X. Yuan, et al. 2023. Succinate pretreatment attenuates intestinal ischemia-reperfusion injury by inhibiting necroptosis and inflammation via upregulating Klf4. International Immunopharmacology 120.
Nadjsombati, M.S., J.W. McGinty, M.R. Lyons-Cohen, J.B. Jaffe, L. DiPeso, C. Schneider, et al. 2018. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49 (1): 33.
Schneider, C., C.E. O’Leary, J. von Moltke, H.-E. Liang, Q.Y. Ang, P.J. Turnbaugh, et al. 2018. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 174 (2): 271.
Macias-Ceja, D.C., D. Ortiz-Masia, P. Salvador, L. Gisbert-Ferrandiz, C. Hernandez, M. Hausmann, et al. 2019. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunology 12 (1): 178–187.
Rubic, T., G. Lametschwandtner, S. Jost, S. Hinteregger, J. Kund, N. Carballido-Perrig, et al. 2008. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nature Immunology 9 (11): 1261–1269.
Erhardt, A., and G. Tiegs. 2010. Tolerance induction in response to liver inflammation. Digestive Diseases 28 (1): 86–92.
Gatselis, N.K., K. Zachou, G.K. Koukoulis, and G.N. Dalekos. 2015. Autoimmune hepatitis, one disease with many faces: Etiopathogenetic, clinico-laboratory and histological characteristics. World Journal of Gastroenterology 21 (1): 60–83.
Sebode, M., J. Hartl, D. Vergani, A.W. Lohse, Int Autoimmune hepatitis Grp I. 2018. Autoimmune hepatitis: From current knowledge and clinical practice to future research agenda. Liver International 38 (1): 15–22.
Wang, H.-X., M. Liu, S.-Y. Weng, J.-J. Li, C. Xie, H.-L. He, et al. 2012. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World Journal of Gastroenterology 18 (2): 119–125.
Neumann, K., K. Karimi, J. Meiners, R. Voetlause, S. Steinmann, W. Dammermann, et al. 2017. A proinflammatory role of type 2 innate lymphoid cells in murine immune-mediated hepatitis. Journal of Immunology 198 (1): 128–137.
Ives, S.J., K.S. Zaleski, C. Slocum, D. Escudero, C. Sheridan, S. Legesse, et al. 2020. The effect of succinic acid on the metabolic profile in high-fat diet-induced obesity and insulin resistance. Physiological Reports 8 (21): e14630-Article No.: e.
Hakak, Y., K. Lehmann-Bruinsma, S. Phillips, T. Le, C. Liaw, D.T. Connolly, et al. 2009. The role of the GPR91 ligand succinate in hematopoiesis. Journal of Leukocyte Biology 85 (5): 837–843.
Liu, H., H. Zhang, X. Zhang, Q. Chen, and L. Xia. 2022. Role of succinic acid in the regulation of sepsis. International Immunopharmacology 110.
Iplik, E.S., T. Catmakas, and B. Cakmakoglu. 2018. A new target for the treatment of endometrium cancer by succinic acid. Cellular and Molecular Biology 64 (1): 60–63.
Chen, H., C. Jin, L. Xie, and J. Wu. 2024. Succinate as a signaling molecule in the mediation of liver diseases. Biochimica Et Biophysica Acta-Molecular Basis of Disease 1870 (2).
Li, Y.H., S.H. Woo, D.H. Choi, and E.-H. Cho. 2015. Succinate causes α-SMA production through GPR91 activation in hepatic stellate cells. Biochemical and Biophysical Research Communications 463 (4): 853–858.
Correa, P.R.A.V., E.A. Kruglov, M. Thompson, M.F. Leite, J.A. Dranoff, and M.H. Nathanson. 2007. Succinate is a paracrine signal for liver damage. Journal of Hepatology 47 (2): 262–269.
Hatano, M., S. Sasaki, S. Ohata, Y. Shiratsuchi, T. Yamazaki, K. Nagata, et al. 2008. Effects of Kupffer cell-depletion on Concanavalin A-induced hepatitis. Cellular Immunology 251 (1): 25–30.
Schümann, J., D. Wolf, A. Pahl, K. Brune, T. Papadopoulos, N. van Rooijen, et al. 2000. Importance of Kupffer cells for T-cell-dependent liver injury in mice. American Journal of Pathology 157 (5): 1671–1683.
Dong, Z., H. Wei, R. Sun, and Z. Tian. 2007. The roles of innate immune cells in liver injury and regeneration. Cellular & Molecular Immunology 4 (4): 241–252.
Trauelsen, M., T.K. Hiron, D. Lin, J.E. Petersen, B. Breton, A.S. Husted, et al. 2021. Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated Gq signaling. Cell Reports 35 (11): 1.
Jakubowski, A., M. Sternak, K. Jablonski, M. Ciszek-Lenda, J. Marcinkiewicz, and S. Chlopicki. 2016. 1-Methylnicotinamide protects against liver injury induced by concanavalin A via a prostacyclin-dependent mechanism: A possible involvement of IL-4 and TNF-α. International Immunopharmacology 31: 98–104.
Mizuhara, H., M. Uno, N. Seki, M. Yamashita, M. Yamaoka, T. Ogawa, et al. 1996. Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology 23 (6): 1608–1615.
Ksontini, R., D.B. Colagiovanni, M.D. Josephs, C.K. Edwards, C.L. Tannahill, C.C. Solorzano, et al. 1998. Disparate roles for TNF-α and Fas ligand in concanavalin A-induced hepatitis. Journal of Immunology 160 (8): 4082–4089.
Llambi, F., and D.R. Green. 2011. Apoptosis and oncogenesis: Give and take in the BCL-2 family. Current Opinion in Genetics & Development 21 (1): 12–20.
Edlich, F. 2018. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochemical and Biophysical Research Communications 500 (1): 26–34.
Chen, H.C., M. Kanai, A. Inoue-Yamauchi, H.C. Tu, Y.F. Huang, D.C. Ren, et al. 2015. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nature Cell Biology 17 (10): 1270.
Funding
This project was supported by Fujian Provincial Natural Science Foundation (Grant No: 2021J05278), The Health Science Foundation of Fujian Youth Program (Grant No: 2021QNB017), National Natural Science Foundation of China (Grant No: 82303109), Natural Science Foundation of Fujian Province, China (Grant No: 2022J05299), and the Cross-Strait Postdoctoral Exchange Funding Program of Fujian Province, China (Grant No: 2021B002).
Author information
Authors and Affiliations
Contributions
YC, ZC, and EC contributed to the design of the study protocol. YC and DZ performed the statistical analysis. YC, TW, and MS drew the picture. YC, ZC, and EC contributed to the writing of the study protocol and made the final corrections to this manuscript. YL finally corrected the manuscript. All authors read and approved the final manuscript. YC, ZC, and EC have contributed equally to this work and share first authorship.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The animal study protocol was approved by the Ethics Committee of Xiamen University (approval no. XMULAC2023052) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (https://www.ncbi.nlm.nih.gov/books/NBK54050/).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Messages
• Succinic acid ameliorated ConA-induced hepatitis
• Succinic acid inhibits the infiltration of liver neutrophils and the production of inflammatory factors
• Macrophages contribute to the protective role of succinic acid against ConA-induced liver injury.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, Y., Chen, Z., Chen, E. et al. Succinic Acid Ameliorates Concanavalin A-Induced Hepatitis by Altering the Inflammatory Microenvironment and Expression of BCL-2 Family Proteins. Inflammation (2024). https://doi.org/10.1007/s10753-024-02021-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-02021-6