Abstract
Chronic arsenic exposure has been linked to endothelial dysfunction and apoptosis. We investigate the involvement of unfolded protein response (UPR) signaling in the arsenic-mediated cytotoxicity of the SVEC4-10 mouse endothelial cells. The SVEC4-10 cells underwent apoptosis in response to As2O3 dose- and time-dependently, accompanied by increased accumulation of calcium, and activation of caspase-3. These phenomena were completely inhibited by α-lipoic acid (LA), which did not scavenge ROS over-production, but were only partially or not ameliorated by tiron, a potent superoxide scavenger. Moreover, arsenic activated UPR, leading to phosphorylation of eukaryotic translation initiation factor 2 subunit α (eIF2α), induction of ATF4, and processing of ATF6. Treatment with arsenic also triggered the expression of endoplasmic reticulum (ER) stress markers, GRP78 (glucose-regulated protein), and CHOP (C/EBP homologous protein). The activation of eIF2α, ATF4 and ATF6 and expression of GRP78 and CHOP are repressed by both LA and tiron, indicating arsenic-induced UPR is mediated through ROS-dependent and ROS-independent pathways. Arsenic also induced ER stress-inducible genes, BAX, PUMA (p53 upregulated modulator of apoptosis), TRB3 (tribbles-related protein 3), and SNAT2 (sodium-dependent neutral amino acid transporter 2). Consistent with intracellular calcium and cell viability data, ROS may not be important in arsenic-induced death, because tiron did not affect the expression of these pro-apoptotic genes. In addition, pretreatment with salubrinal, a selective inhibitor of eIF2α dephosphorylation, enhanced arsenic-induced GRP78 and CHOP expression and partially prevented arsenic cytotoxicity in SVEC4-10 cells. Taken together, these results suggest that arsenic-induced endothelial cytotoxicity is associated with ER stress, which is mediated by ROS-dependent and ROS-independent signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arsenic is a naturally occurring poisonous metalloid element present in environmental sources such as air, water, and soil. It has been estimated that 100 million people worldwide are exposed to arsenic in drinking water (Nordstrom 2002). Chronic arsenic exposure to inorganic arsenic is associated with greater risks of neurotoxicity (Vahidnia et al. 2007), vascular diseases (Simeonova and Luster 2004) and tumors of the bladder (Radosavljevic and Jakovljevic 2008), skin (Hsueh et al. 1995), and lung (Celik et al. 2008). The accumulated literature indicates that arsenic exposure exerts deleterious health effects primarily through the induction of oxidative stress. Arsenic triggers stress responses that counteract protein misfolding, such as induction of heat shock protein chaperones (Del Razo et al. 2001), ubiquitin proteasome system components (Stanhill et al. 2006), and the unfolded protein response (UPR) (Lin et al. 2007; Lu et al. 2011).
The endoplasmic reticulum (ER) is a protein folding factory, responsible for the biosynthesis, folding, assembly, and modification of numerous soluble and membrane proteins (Kaufman 1999). Various stresses, including glucose starvation and oxidative stress, can disrupt ER functioning, leading to the accumulation of misfolded proteins in the organelle, inducing a condition called ER stress, the term given to an imbalance between the cellular demand for ER function and ER capacity (Schroder and Kaufman 2005). Upon ER stress, cells activate a series of complementary adaptive mechanisms to cope with protein folding alterations, which together are known as UPR (Hetz 2012; Lai et al. 2007; Rutkowski and Kaufman 2004). The UPR consists of three main signaling cascades initiated by three prototypical ER localized stress sensors: inositol-requiring kinase/endonuclease 1α (IRE1α), double-stranded RNA-dependent protein kinase-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6). The ER luminal domain of PERK, IRE1α, and ATF6 interacts with the ER chaperone GRP78 (glucose-regulated protein); however, upon accumulation of unfolded proteins, GRP78 dissociates from these molecules, leading to their activation.
Transient global translation is initially stalled during ER stress by the PERK signaling pathway (Lai et al. 2007). PERK is activated by homodimerization and autophosphorylation, allowing PERK to phosphorylate and inactivate the translation initiation factor eIF2α. Phosphorylated eIF2α selectively enhances translation of the ATF4 transcription factor that induces expression of UPR target genes, which are involved in amino acid biosynthesis and transport, the oxidative stress response, and ER-induced apoptosis (Harding et al. 2003; Rutkowski and Kaufman 2004). IRE1α is also activated by homodimerization and autophosphorylation and activated IRE1α causes splicing of X-box-binding protein-1 (XBP1) mRNA. Translation of spliced XBP1 (XBP1s) mRNA produces a transcription factor that activates expression of many genes involved in protein folding, secretion, and ER-associated degradation (ERAD). When ATF6 is free from GRP78, it translocates from the ER to the Golgi where it is proteolytically processed by S1P and S2P, releasing a ~50-kDa cytosolic fragment that directly controls genes encoding ERAD components and XBP1 (Hetz 2012; Lai et al. 2007; Todd et al. 2008; Yamamoto et al. 2007).
In addition to upregulating the genes that support adaptation to and recovery from ER stress, prolonged ER stress induces proapoptotic pathways (Rasheva and Domingos 2009). ER stress-induced apoptosis is mediated largely by CHOP/GADD153, a transcription factor that is homologous to C/EBP (CAAT/enhancer binding protein) and is downstream of the PERK–eIF2α–ATF4 and ATF6 pathways (Ma et al. 2002; Marciniak et al. 2004). Several CHOP-induced pro-apoptotic genes, including tribbles-related protein 3 (TRB3) (Ohoka et al. 2005) and BCL-2 homology 3 (BH3)-only proteins, such as NOXA and p53 upregulated modulator of apoptosis (PUMA) (Li et al. 2006), are transcriptionally activated to regulate the activation of BAX and/or BAK to trigger apoptosis. Together, ATF4, XBP1s, and ATF6 govern the expression of a large range of partially overlapping target genes, the protein products of which modulate adaptation to stress or the induction of cell death under conditions of chronic ER stress (Hetz 2012).
Recent studies reveal that arsenic causes apoptosis through ER stress or UPR in various kinds of cells (Binet et al. 2010a, b, 2011; Bolt et al. 2012; Li et al. 2011; Lu et al. 2011; Naranmandura et al. 2012; Oh et al. 2012; Tang et al. 2009; Yen et al. 2011; Zhang et al. 2007). There is evidence that vascular endothelial cells, which are present in every blood vessel, are the targets for arsenic toxicity (Tseng 2005; Tseng et al. 2007). We previously reported that arsenic dose-dependently caused mouse endothelial cell death and overexpression of heme oxygenase-1 (HO-1), interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and vascular endothelial growth factor (VEGF). These effects were completely inhibited by α-lipoic acid (LA), a thioreductant, but only partially ameliorated by tiron, a potent superoxide scavenger (Wang et al. 2012). In the current research, we aim to further investigate whether induction of UPR by arsenic is implicated in arsenic-mediated endothelial cell death.
Materials and methods
Chemical
Arsenic trioxide, α-lipoic acid (LA), tiron (4,5-dihydroxy-1,3-benzene disulfonic acid), and other chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) unless otherwise indicated. The eIF2α dephosphorylation inhibitor salubrinal (Sal) was purchased from Calbiochem (Darmstadt, Germany).
Cell culture
The SVEC4-10 cell line (ATCC, #CRL-2181) was derived from mouse vascular endothelium. The SVEC4-10 cells were routinely maintained in DMEM medium (Invitrogen Co., Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum, 2.2 g/L sodium bicarbonate, and l-glutamine (0.03 % w/v).
Cell viability analysis
To examine the cell viability of SVEC4-10 cells, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay was used. Briefly, SVEC4-10 cells (2 × 105 cells/well) were seeded into 24-well plates overnight and then changed to serum-free medium containing test reagent. After the indicated period, the supernatant was aspirated and 100 μl MTT solution (0.5 mg/ml in DMEM) was added for a further 3 h incubation at 37 °C. The medium was carefully removed by aspiration, and formazan crystals were dissolved in DMSO. The extent of the reduction of MTT was quantified by measurement of the absorbance at 550 nm.
Measurement of intracellular calcium
Intracellular Ca2+ levels were measured using flow cytometry. Briefly, cultured cells were stained with Fluo 4 AM (Molecular Probe, Invitrogen) at a final concentration of 1 μM for 30 min at 37 °C. The cells were then washed and harvested with phosphate-buffered saline (PBS) for flow cytometry analysis (FACScan, BD Biosciences, San Jose, CA, USA). Data were acquired from 10,000 cells (events), and relative intracellular levels were assessed as mean fluorescence (MFI) intensity in the FL1 channel as calculated by the distribution histogram with cell counts on the vertical axis and fluorescence intensity on a log scale on the horizontal axis.
Cell cycle analysis
After treatment, SVEC4-10 cells were washed twice with PBS and fixed with ice-cold 70 % ethanol overnight. The cells were then centrifuged at 1,500×g for 10 min and washed with PBS twice. Cells were stained by addition of 1 ml DNA-staining solution [20 μg/ml of propidium iodide (PI) and 50 μg/ml of RNase] and incubated in the dark at 4 °C for 15 min before flow cytometry analysis. The cell cycle was analyzed using Modfit LT 3.2 (Verity Software House), and apoptotic cells were indicated by the substantially lower DNA content than those seen in Go/G1 peak shown in the DNA histogram.
Protein extraction and immunoblotting
Protein samples were prepared from SVEC4-10 cells using ice-cold RIPA buffer (Thermo Fisher Scientific, Inc., Rockford, IL, USA). The supernatant (cell lysate) was carefully transferred, and the protein concentration was measured by Bradford method (1976), using bovine serum albumin as a standard. Cell lysate (10 μg) was separated on 12 % SDS-PAGE and transferred onto Hybond-P PVDF membrane (GE Healthcare) at 20 V overnight at 4 °C. The membranes were blocked at room temperature in blocking buffer (5 % BSA in PBS solution) for 6 h. Blots were analyzed with one of the primary antibodies listed in Table 1 overnight at 4 °C. After three washes with PBST, the blots were incubated with suitable horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) at a dilution of 1:10,000 for 1 h. The blots were washed again, the proteins of interest were detected using Amersham ECL™ Prime Western Blotting Detection Reagents (GE Healthcare) according to the manufacturer’s instructions, and the chemiluminescence signal was then visualized with Hyperfilm ECL (GE Healthcare).
RNA extraction, real-time RT-PCR, and semi-quantative RT-PCR
Total cellular RNA was prepared using Illustra RNAspin Mini RNA Isolation Kit (GE Healthcare, Buckinghamshire, UK). Real-time PCR was performed with 2 μl of the cDNA obtained above in 25 μl containing 200 nM primers (Table 2) and Power SYBR® Green PCR Master Mix (Life Technologies). Amplification was conducted in an ABI Prism 7300 sequence detection system. The PCR conditions were as follows: 95 °C for 2 min, 40 cycles at 94 °C for 15 s, and 60 °C for 60 s. Target gene expression was measured and normalized to the respective β-actin expression level. The identity and purity of the amplified product were checked through analysis of the melting curve carried out at the end of amplified product. Relative expression was evaluated with the ΔΔC T method. For the semi-quantative mRNA levels of XBP1s and XBP1u, the cDNA product obtained above was also subjected to 30 cycles of PCR using the forward primer 5′-ACACGCTTGGGAATGGACAC-3′ (Li et al. 2011) and reverse primer 5′-CCATGGGAAGATGTTCTGGG-3′ specific for mouse XBP1, followed by 3 % agarose gel electrophoresis.
Statistical analysis
All experiments were repeated at least three times. The results were analyzed using one-way ANOVA with post hoc Tukey test, and a p value of <0.05 was taken to be significant.
Results
High concentrations of arsenic trioxide-induced SVEC4-10 cell death, calcium accumulation, and caspase-3 activation
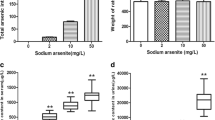
We began this work by investigating the effect of arsenic trioxide on the viability of immortalized mouse lymph node endothelial cells, SVEC4-10 cells. Figure 1a shows that As2O3 exhibited dose- and time-dependent cytotoxicity in SVEC4-10 cells. In high concentrations, such as 20 and 40 μM, it caused ~14 and ~40 % of cell death, respectively, after 4 h incubation. In contrast, in low concentration, such as 1 μM, it did not exhibit cytotoxicity after 24 h treatment (Fig. 1a).
Arsenic induces SVEC4-10 cell death, intracellular calcium accumulation, and caspase-3 activation. a SVEC4-10 cells were incubated with the indicated concentrations of As2O3 for the indicated period at 37 °C. Cell viability was measured by MTT as described in “Materials and methods” section. Data represent the mean ± SD of three independent experiments. *p < 0.05 and **p < 0.01 represent significant differences compared with vehicle control (without arsenic). b, c SVEC4-10 cells were treated with As2O3 for 16 h, and intracellular calcium level was detected by Fluo 4 AM staining as described in “Materials and methods” section. **p < 0.01 represents significant differences compared with vehicle control (without arsenic); ## p < 0.01 represents significant differences compared with As2O3-treated vehicle. d Cell lysates prepared from SVEC4-10 cells, which were treated with the indicated reagent or vehicle (0.1 % DMSO) for 30 min followed by the addition of As2O3 for 16 h, were subjected to SDS-PAGE. Then, immunoblotting was carried out using an anti-cleaved caspase-3 antibody (BioVision) or anti-α-tubulin antibody (Sigma). The blots are representative from one of three independent experiments
Arsenic trioxide-induced alterations in the level of intracellular Ca2+ were measured by Fluo 4 AM staining. Figure 1b shows that incubation of SVEC4-10 cells with 5 or 7.5 μM arsenic trioxide for 16 h both caused about 1.6-fold increases in the mean fluorescence intensity (p < 0.01), indicating increased accumulation of calcium.
To investigate the relationship between arsenic-induced ROS overproduction and calcium accumulation, α-lipoic acid (1,2-dithiolane-3-pentanoic acid; LA) and tiron (4,5-dihydroxy-1,3-benzene disulfonic acid) were added before arsenic insult. Figure 1c shows that LA (50 μM) or tiron (0.25 mM) alone did not affect intracellular Ca2+ levels. LA (50 μM), but not tiron (0.25 mM), significantly attenuated 7.5 μM arsenic trioxide-provoked calcium accumulation to the level of vehicle control (p < 0.01).
To make sure that arsenic caused SVEC4-10 cell apoptosis, the level of activated caspase-3 in the cell lysate after being exposed to arsenic for 16 h was analyzed by Western blotting. Consistent with the cytotoxicity data shown in Fig. 1a, 5 μM arsenic trioxide only slightly induced caspase-3 activation, leading to undetectable cytotoxicity; however, 7.5 μM dramatically increased the level of cleaved caspase-3, resulting in more than 40 % cell death. Furthermore, we found that there was no caspase-3 activation in the cells co-treated with LA (50 mM), but trace level of active caspase-3 was detected for that co-treated with tiron (0.25 mM) (Fig. 1d).
We also analyzed the sub-G0/G1 hypodiploid apoptotic SVEC4-10 cells by staining the cellular DNA with PI. Supplemental Figure 1 shows that when SVEC4-10 cells were cultured in the presence of arsenic trioxide (7.5 μM) for 24 h, the cell population displayed an emergence of the sub-G0/G1 phase (45.2 ± 1.9 %) as compared with those cultured without arsenic (16.8 ± 0.2 %), suggesting induction of apoptotic DNA fragmentation. We further found that 50 μM LA exhibited a greater inhibitory effect on arsenic-induced apoptosis than 0.25 mM tiron, as the sub-G0/G1 population decreased to 26.5 and 37.6 %, respectively. The results all demonstrated that arsenic trioxide induces SVEC4-10 cell apoptosis dose- and time-dependently.
Arsenic trioxide triggers PERK–eIF2α–ATF4 activation
Perturbation of ER calcium homeostasis can disrupt the protein folding process, causing ER stress and the activation of the UPR (Zhang 2010). We first examined the effects of arsenic trioxide on each of the three canonical arms of UPR signaling: PERK–eIF2α–ATF4, ATF6, and XBP1 splicing. To examine how arsenic trioxide affects the PERK–eIF2α–ATF4 pathway, SVEC4-10 cells were incubated with 7.5 μM arsenic trioxide for various periods, and total cell lysate was prepared and subjected to Western blotting. Figure 2a shows that treatment of SVEC4-10 cells with 7.5 μM arsenic trioxide for 1 h led to a marked increase in eIF2 phosphorylation, which remained elevated after 4 h treatment.
Arsenic activates PERK-eIF2α-ATF4 pathway in SVEC4-10 cells. a SVEC4-10 cells were treated with 7.5 μM As2O3 for the indicated period. Cell lysate was prepared, and p-eIF2α and eIF2 were analyzed by Western blotting as described in “Materials and methods” section. b Cell lysates prepared from SVEC4-10 cells, which were treated with the indicated reagent or vehicle (0.1 % DMSO) for 30 min followed by the addition of 7.5 μM As2O3 for 2 h, were subjected to immunoblot with anti-p-eIF2α and anti-eIF2α antibodies. c Cell lysates prepared from SVEC4-10 cells, which were treated with the indicated reagent or vehicle (0.1 % DMSO) for 30 min followed by the addition of As2O3 for 16 h, were subjected to immunoblot with anti-ATF4 and anti-α-tubulin antibodies. The blots are representative from one of three independent experiments. d Changes in ATF4 mRNA expression after being incubated with indicated antioxidant in the presence or absence of arsenic trioxide for 6 h. ATF4 mRNA expression was measured by RT-Q-PCR and normalized to β-actin, as described in “Materials and methods” section. Data represent the mean ratio ± SD of three independent experiments. **p < 0.01 represents significant differences compared with vehicle control (without arsenic); ## p < 0.01 represents significant differences compared with As2O3-treated vehicle
To investigate the role of ROS production by arsenic on the activation of PERK–eIF2α–ATF4, we tested the effects of LA and tiron on the phosphorylation of eIF2α. Figure 2b shows that in the absence of arsenic trioxide, 0.25 mM tiron slightly decreased phosphorylation of eIF2α; however, 50 μM LA did not. In the presence of 7.5 μM arsenic trioxide, both LA (50 μM) and tiron (0.25 mM) significantly decreased arsenic-mediated phosphorylation of eIF2α.
Incubation of SVEC4-10 cells with arsenic trioxide for 16 h also led to a significant and dose-dependent increase in ATF4, the downstream target protein of phospho-eIF2α. The addition of LA (50 μM) and tiron (0.25 mM) significantly reduced arsenic-induced ATF4 protein expression, and the former had a stronger inhibitory effect than the latter. Collectively, these observations suggest that ROS production by arsenic is one of the upstreams of PERK–eIF2α–ATF4 activation in SVEC4-10 cells, and the removal of ROS by tiron could dampen both phosphorylation of eIF2α and the subsequent ATF4 translation.
Moreover, 7.5 μM arsenic trioxide treatment for 6 h also induced mRNA expression of ATF4 as measured by RT-Q-PCR, indicating that ATF4 expression was induced both transcriptionally and translationally by arsenic trioxide treatment (Fig. 2d). However, the addition of LA (50 μM), but not tiron (0.25 mM), could attenuate ATF4 mRNA expression. These data suggest that arsenic-mediated ATF4 mRNA upregulation is ROS-independent and LA may work through chelating arsenic or blocking other signaling pathways (Wang et al. 2012), thereby not only reducing PERK–eIF2α–ATF4 activation, but also inhibiting ATF4 transcription.
Arsenic trioxide triggers ATF6 activation but not XBP1 splicing
To investigate the possible activation of the ATF6 pathway, SVEC4-10 cells were incubated with arsenic trioxide for 16 h, and total cell lysate was prepared and subjected to Western blotting with two anti-ATF6α antibodies, one specific for the N-terminal region and the other for the C-terminal one. Figure 3a shows that treatment with arsenic trioxide for 16 h led to significant increases in the levels of both precursor (~90 kDa) and cleaved forms of ATF6α (~60 and ~30 kDa) dose-dependently. ATF6α (N) (~60 kDa), the active form of ATF6α, is the cleaved N-terminal fragment of the preexisting precursor form, while the ATF6α (C) (~30 kDa) is the cleaved the C-terminal one. The addition of LA (50 μM) and tiron (0.25 mM) decreased the levels of full length and truncated forms of ATF6α. This result indicates that arsenic trioxide induces both the expression and processing of ATF6α protein through both ROS-dependent and ROS-independent pathways.
Effects of arsenic on the processing of ATF6α and XBP-1 in SVEC4-10 cells. SVEC4-10 cells were treated with the indicated antioxidant or vehicle (0.1 % DMSO) for 30 min, followed by the addition of As2O3. a After 16 h of incubation, the cell lysates were prepared and subjected to Western blotting to detect the full length (~90 kDa) and truncated (~60 and ~30 kDa) forms of ATF6α. α-tubulin was used as a loading control. The blots are representative from one of three independent experiments. b After 6 h of incubation with arsenic trioxide, RNA was prepared and mRNA expression of ATF6α was analyzed by RT-Q-PCR by normalized to β-actin as described in “Materials and methods” section. c After 6 h of incubation with arsenic trioxide, RNA was prepared and XBP1s level was analyzed by RT-Q-PCR by normalized to XBP1u, as described in “Materials and methods” section. Data represent the mean ± SD of three independent experiments. *p < 0.05 and **p < 0.01 represent significant differences compared with vehicle control (without arsenic). # p < 0.05 and ## p < 0.01 represent significant differences compared with As2O3-treated vehicle. d After 6 h of incubation, RNA was prepared and semi-quantative RT-PCR was used for the analysis of mRNA levels of XBP1s and XBP1u as described in “Materials and methods” section. Cells treated with tunicamycin (1.5 μg/ml) served as a positive control
Figure 3b further demonstrates that, similar to the results found for ATF4, ATF6α mRNA was significantly induced by arsenic trioxide after 6 h treatment, and only LA, but not tiron, was able to block its transcriptional upregulation. Together, these observations suggest that arsenic trioxide induced the transcription, translation, and processing of ATF6α. Arsenic-mediated ROS formation thus plays a key role in regulating both the translation and processing of ATF6α, but not in mRNA induction.
To examine whether arsenic trioxide increases XBP1 splicing, SVEC4-10 cells were incubated with 7.5 μM arsenic trioxide for 6 h, and the changes in the spliced XBP1 (XBP1s) mRNA were first measured by RT-Q-PCR normalized with those of unspliced XBP1 (XBP1u). Figure 3c shows that arsenic trioxide (7.5 μM) decreased the level of XBP1s; however, the known UPR inducer tunicamycin (1.5 μg/ml) robustly increased its level. Semi-quantative RT-PCR followed by agarose gel analysis was employed to make sure that XBP1 splicing was not induced in arsenic-treated SVEC4-10 cells. Figure 3d shows that arsenic decreased the level of XBP1s slightly, while tunicamycin increased it dramatically. These results indicate that arsenic trioxide induces two of the canonical arms of UPR signaling, namely PERK–eIF2α–ATF4 and ATF6, and both are induced by ROS-dependent and ROS-independent pathways in SVEC4-10 cells.
Arsenic trioxide upregulates GRP78 and CHOP expression
We further investigated the induction of UPR targets, ER resident chaperone BiP/GRP78, and the key UPR-pro-apoptotic player CHOP (GADD153), which is positively controlled by the ATF4 and ATF6 and promotes transcription of pro-apoptotic genes (Hetz 2012). Figure 4 shows that GRP78 and CHOP proteins were dose-dependently induced by arsenic. LA effectively inhibited protein expression of GRP78 and CHOP, while tiron reduced their levels to a less significant degree. These data suggest that in addition to triggering the adaptive phase of the UPR, arsenic also activates the apoptotic UPR response, and that arsenic-mediated ROS production is not sufficient to induce UPR in SVEC4-10 cells.
Effects of arsenic on the expression of GRP78 and CHOP in SVEC4-10 cells. SVEC4-10 cells (1 × 106/ml) were treated with indicated antioxidant or vehicle (0.1 % DMSO) for 30 min followed by the addition of 7.5 μM As2O3. After 16 h of incubation, cell lysate was prepared for Western blotting analysis of GRP78 and CHOP, and α-tubulin was used as a loading control. The blots are representative from one of three independent experiments
Arsenic trioxide induces expression of pro-apoptotic and nutrient sensing genes
We then determined the effects of arsenic trioxide on the expression of pro-apoptotic genes, including BAX, a member of the BCL-2 family that is an effector of apoptotic cell death, BCL-2 homologous 3 (BH3)-only protein PUMA (p53 upregulated modulator of apoptosis), and carbonic tribbles-related protein 3 (TRB3).
The expression levels of both BAX and PUMA were analyzed to explore the involvement of BCL-2 family members in As2O3-induced apoptosis in SVEC4-10 cells. We found that treatment of SVEC4-10 cells with 7.5 μM arsenic trioxide for 6 h resulted in a 2.5-fold increase in the expression of pro-apoptotic BAX mRNA expression (Fig. 5a). LA completely prevented arsenic-upregulated BAX expression (p < 0.01), but tiron did not have any effect.
Arsenic induces mRNA expression of BAX, PUMA, TRB3, and SNAT2 in SVEC4-10 cells. SVEC4-10 cells (1 × 106/ml) were treated with the indicated antioxidant or vehicle (0.1 % DMSO) for 30 min followed by the addition of 7.5 μM As2O3 in serum-free medium for 6 h. RNA was prepared, and the mRNA levels of BAX, PUMA, TRB3, and SNAT2 were analyzed by RT-Q-PCR by normalized to β-actin, as described in “Materials and methods” section. Data represent the mean ± SD of three independent experiments. **p < 0.01 represents significant differences compared with vehicle control (without arsenic). # p < 0.05 and ## p < 0.01 represent significant differences compared with As2O3-treated vehicle
BH3-only proteins are responsible for binding to and activating pro-apoptotic BAX and BAK in response to diverse apoptotic stimuli. Members of the BH3-only family are implicated in mediating apoptosis triggered by ER stress (Rodriguez et al. 2011). A 2.9-fold increase in PUMA mRNA was noted for SVEC4-10 cells treated with 7.5 μM arsenic trioxide (Fig. 5b). LA completely blocked arsenic-upregulated PUMA expression (p < 0.01), but tiron only partially dampened it (p < 0.05).
TRB3 upregulation by arsenite has been reported in HepG2 cells (Ord et al. 2009). Figure 5c shows that treatment of SVEC4-10 cells with 7.5 μM arsenic trioxide for 6 h led to a 5.7-fold increase in TRB3 mRNA expression. Treatment with LA, but not tiron, significantly attenuated arsenic-mediated TRB3 upregulation.
SNAT2 (neutral amino acid transporter), the most broadly expressed subtype of sodium-dependent aliphatic amino acid transporters (Mackenzie and Erickson 2004), was recently reported as a specific arsenite stress response gene and mediated by ATF4 in human embryonic kidney HEK293 cells (Oh et al. 2012). Figure 5d shows that arsenic trioxide dramatically upregulated SNAT2 by 3.7-fold. Similar to the above pro-apoptotic genes, upregulation of SNAT2 was attenuated by LA instead of tiron. All of the above results indicate that the mRNA expression of CHOP-targeted BAX, TRB3, and SNAT2 was upregulated by arsenic in ROS-independent pathways.
Effects of salubrinal on arsenic-induced alteration of ER stress-related protein expression and cellular damage
Salubrinal, a selective inhibitor of cellular complexes that dephosphorylate eukaryotic translation initiation factor 2 subunit α (eIF2α), has been shown to protect the endothelial cells from the deleterious effects of ER stress (Bouvier et al. 2009). However, the effect of salubrinal on arsenic-induced ER stress in endothelial cells remains to be elucidated. Figure 6a shows that pretreatment of SVEC4-10 cells with salubrinal (10 μM) for 16 h slightly enhanced arsenic-mediated GRP78 and CHOP expression possibly due to the enhanced eIF2α phosphorylation-ATF4 signaling. Figure 6b shows that in the absent of arsenic, salubrinal (20 μM) exhibited a modest cytotoxicity as compared with control (p < 0.05), possibly due to sustained global translation inhibition. However, pretreatment with salubrinal (10 and 20 μM) provided significant protection against arsenic trioxide (7.5 μM) insult (p < 0.01). Salubrinal was previously found to inhibit dephosphorylation of eIF2α and to counteract ER stress in cultured cells (Boyce et al. 2005). As shown by our data, the protective effect of salubrinal was robust, although not complete, indicating attenuation of global translation prevented misfolded protein overload caused by arsenic. Furthermore, other pathways not blocked by salubrinal may also be involved in arsenic-mediated cell death.
Salubrinal protects SVEC4-10 cells from arsenic-induced ER stress and cell death. SVEC4-10 cells were grown for 16 h with salubrinal (Sal) or vehicle and then treated with arsenic trioxide in serum-free medium. a After 16 h of incubation, cell lysate was prepared for Western blotting analysis of GRP78 and CHOP, and α-tubulin was used as a loading control. b After 24 h of incubation, cell viability was analyzed by MTT assay. *p < 0.05 and **p < 0.01 represent significant differences compared with vehicle control (without arsenic). ## p < 0.01 represents significant differences compared with As2O3-treated vehicle
Discussion
Arsenic contamination of drinking water remains a serious public health problem, affecting hundreds of millions of people worldwide. The exposure to arsenic can be associated with increased risk of all forms of atherosclerotic disease, including peripheral vascular disease (PVD), ischemic heart disease and stroke. More importantly, subclinical arterial insufficiency and microcirculatory defects can be seen in those with chronic arsenic exposure before clinical diseases come into notice (Tseng 2008). The atherogenicity of arsenic could be associated with its effects of hypercoagulability, endothelial injury, smooth muscle cell proliferation, somatic mutation, oxidative stress, and apoptosis (Tseng 2002, 2005; Tseng et al. 2007; Yang 2006).
A unique PVD called blackfoot disease (BFD) is endemic to the southwestern coast of Taiwan. Epidemiologic studies revealed that BFD was associated with the consumption of inorganic arsenic from the artesian wells (Tseng 2002; Yu et al. 1998). In addition to arsenic, several compounds were also found in this artesian well water, including ergotamine, organic chlorides, and fluorescent substances. Fluorescent compounds known as humic substances were also suggested to initiate the development of BFD (Lu et al. 1990). However, there has been no epidemiologic evidence presented to date to show a correlation between exposure to humic substances and development of PVD (Yang 2006).
Although the potential biological outcomes have been well documented, the pathomechanisms leading from arsenic exposure to occurrence and development of the diseases remain largely unclear. Microvasculature constitutes the vast majority of the human vascular compartment and is the place where most pathophysiologic events take place. It has been found that arsenite had a greater effect on human microvascular endothelial cells (HMEC-1) as compared to human umbilical vein endothelial cells (HUVECs) (Jiang et al. 2002). Herein, we investigated the effects of arsenic on SVEC4-10 microvascular endothelial cells, a cloned SV40-transformed cell line derived from LPS-resistant C3H/HeJ mice (O’Connell and Edidin 1990), to mimic the response of cells under the toxic effect of arsenic. In our study, we demonstrated the involvement of UPR signaling on arsenic-mediated cytotoxicity in endothelial cells and found that arsenic-induced endothelial cytotoxicity is associated with ER stress, which is mediated by ROS-dependent and ROS-independent signaling.
Arsenic-induced SVEC4-10 apoptosis is mediated by ROS-dependent and ROS-independent signaling
We found that arsenic induced cytotoxicity in a concentration- and time-dependent manner in SVEC4-10 endothelial cells, supporting the view that the dose and time involved in arsenic exposure are the keys to controlling intracellular signaling and biological outcomes (Roboz et al. 2000). Furthermore, arsenic treatment altered intracellular Ca2+ homeostasis, which leads to the promotion of endothelial cell dysfunction and apoptosis. The raised levels of Ca2+ also lead to the activation of proteases and changes in protein phosphorylation (Brnjic et al. 2010). Consistent with this, we found that arsenic trioxide increased the activation of caspase-3, which is one of the hallmarks of the apoptotic process and represents both initiators and executors of cell death, in SVEC4-10 cells.
It is generally accepted that arsenic toxicity is mediated through oxidative stress (Lantz and Hays 2006). Current study demonstrated that LA, but not tiron, effectively decreased calcium accumulation; while caspase-3 activation could be reduced by both compounds. Arsenic can interact with thiols, thus depleting the level of cellular glutathione (GSH), which plays a critical role in maintaining cellular redox homeostasis Shi et al. (2004). The cytoprotective effects of LA may relate to their abilities to increase the availability of GSH in cells, serve as a modifier of critical protein thiolates, or chelate arsenic (Spuches et al. 2005; Flora and Pachauri 2010). These results also indicate that arsenic-induced SVEC4-10 apoptosis is mediated by both ROS-dependent and ROS-independent signaling. Similar findings were also reported in other cells, and ROS was concluded to be not important in the induction of the antioxidative response or cellular death by arsenic trioxide (Morales et al. 2009; Wei et al. 2005).
Instead of IRE1α-XBP1, arsenic induces eIF2α–ATF4 and ATF6 signaling
Any chemical that perturbs calcium signaling, induces ROS production, or depletes glutathione has the potential to induce ER stress and the UPR (Jennings et al. 2013). There is growing evidence that arsenic can cause ER stress in various cell types, including human lens epithelial cells (Zhang et al. 2007), cultured osteoblasts (Tang et al. 2009), human neutrophils (Binet et al. 2010b), human lymphoblastoid cells (Bolt et al. 2012), mouse skin (Li et al. 2011), mouse cerebrum (Yen et al. 2011), rat pancreatic beta-cell-derived RIN-m5F cells (Lu et al. 2011), myoblasts (Yen et al. 2012), and rat liver RLC-16 cells (Naranmandura et al. 2012).
We found that treatment of SVEC4-10 cells with arsenic instantly induced the phosphorylation of translation initiation factor eIF2α, which could be attenuated by both LA and tiron. Whereas eIF2α phosphorylation suppresses global protein synthesis to decrease ER load, it selectively enhances the translation of ATF4, encoding a transcription activator of genes important for essential adaptive and apoptotic functions. It is evident that in addition to translational control, ATF4 expression is subject to transcriptional regulation. Stress conditions, such as ER stress, oxidative stress, amino acid deprivation, and glucose starvation, induces both transcription and translation of ATF4, which together enhance expression of ATF4 and its target genes in response to phosphorylation of eIF2α (Baird and Wek 2012; Dey et al. 2010). We found that both the translational and transcriptional upregulation of ATF4 could be attenuated by the treatment with LA. However, the superoxide scavenger tiron only affects the translational stage, but not the transcriptional one. This result indicates that in addition to ROS, arsenic induces other signaling pathways, which can upregulate ATF4 transcription in SVEC4-10 cells.
ATF6 (~90 kDa) is located in the ER membrane and, in the absence of ER stressors, is maintained in an inactive form by binding to GRP78. ATF6α and ATF6β are ER membrane-bound transcription factors (Haze et al. 1999, 2001). Under ER stress conditions, they transit to the Golgi for further processing into truncated N-terminal ATF6 isoforms, which translocates to the nucleus (Hetz 2012; Lai et al. 2007; Todd et al. 2008; Yamamoto et al. 2007). It was reported that ATF6α, but not ATF6β, is responsible for transcriptional induction of ER stress response element (ERSE)-mediated genes (Thuerauf et al. 2004; Yamamoto et al. 2007). In this research, we showed that arsenic induced translation and processing of ATF6α in SVEC4-10 cells, and both stages can be attenuated by LA and tiron, implying the involvement of ROS-dependent and ROS-independent signaling. ER stressors (hypoxia, tunicamycin and thapsigargin) and arsenic trioxide have been reported to upregulate ATF6 mRNA expression (Binet et al. 2010b; Lee et al. 2003; Namba et al. 2007; Terai et al. 2005). In current study, we found that arsenic trioxide also induced ATF6α transcription in SVEC4-10 endothelial cells, and ROS depletion per se did not affect its upregulation as shown by the treatment with tiron.
ER stress also triggers activation of inositol-requiring protein 1α (IRE1α), which processes the mRNA encoding unspliced X-box-binding protein 1 (XBP1u) to produce an active transcription factor, spliced XBP1 (XBP1s). XBP1s controls the transcription of genes encoding proteins involved in protein folding, ERAD, protein quality control, and phospholipid synthesis (Hetz 2012). It was reported that arsenic/arsenite activates all of the three classic UPR pathways (Binet et al. 2010b; Li et al. 2011; Lu et al. 2011). However, we found that arsenic trioxide did not activate IRE1α signaling in SVEC4-10 cells, because no enhanced XBP1 splicing was observed. This implies that the pro-survival adaptive IRE1-XBP1 branch is not triggered by arsenic trioxide in SVEC4-10 endothelial cells.
Arsenic activates ER stress markers, GRP78 and CHOP
Under ER stress, cells activate GRP78 (also known as BiP), which protects cells from lethal conditions, and CHOP (also known as GADD153), which plays major roles in ER stress-induced apoptosis (Schroder and Kaufman 2005). We observed arsenic trioxide induced the expression of GRP78 and CHOP in SVEC4-10 cells. Because UPR-mediated cell survival or death is regulated by the balance of GRP78 and CHOP expression, the preferential induction of CHOP rather than GRP78 in SVEC4-10 cells exposed to arsenic indicated the possible involvement of ER stress in its cytotoxicity. Furthermore, in parallel to the protective effects against arsenic toxicity (Wang et al. 2012), LA completely blocked CHOP over-expression, while tiron only partially suppressed it.
The effect of LA is specific for arsenic but not for tunicamycin-mediated ER stress
Tunicamycin (Tm) prevents the first committed step of N-linked glycosylation of proteins by UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase (DPAGT1) in the ER (Heifetz et al. 1979), which causes extensive protein misfolding and activation of the UPR. We further investigated how LA and tiron affect tunicamycin-induced cytotoxicity and expression of GRP78 and CHOP in SVEC4-10 cells. We found that different from their effects for arsenic-mediated cell death and gene expression, neither LA nor tiron was able to attenuate those for Tm in SVEC4-10 cells (Supplemental Figure 2). These data strongly demonstrate that the cytoprotective effect of LA is specific for arsenic stress response, which is different from Tm-induced ER stress (Oh et al. 2012).
Arsenic elicits ER stress-mediated apoptotic gene expression
It is well known that BCL-2 protein family members play a crucial role in the mitochondria to trigger apoptosis when cells are under chronic or irreversible ER stress (Rodriguez et al. 2011). It was reported that the pro-apoptotic multidomain BAX gene was induced in sodium arsenite-exposed human keratinocyte HaCaT cells (Shin et al. 2011), and BH3-only proteins were upregulated in multiple myeloma cells exposed to cytotoxic levels of arsenic (Morales et al. 2008). In this study, we extended these findings to SVEC4-10 cells and found that BAX and BH3-only PUMA were both augmented in response to arsenic.
TRB3, a member of the tribbles family of pseudokinases, was revealed to be a target of CHOP/ATF4 and seemed to be involved in CHOP-dependent cell death as a second messenger during ER stress (Morse et al. 2010; Ohoka et al. 2005). It has been reported that TRB3 expression is augmented by multiple cellular stressors such as ER stress, fasting, nutrient deprivation, nerve growth factor depletion, hypoxia, and activation of phosphatidylinositol-3-kinase (Bowers et al. 2003; Du et al. 2003; Morse et al. 2010; Ohoka et al. 2005; Ord et al. 2007). Upregulation of TRB3 expression was previously described in HepG2 cells exposed to arsenite (Ord et al. 2009), and a similar finding was observed in endothelial cells in this study. Although it was first regarded as an ER stress-inducible gene and is involved in cell death (Ohoka et al. 2005), TRB3 was also found to be cytoprotective (Ord and Ord 2005). Therefore, the effects of TRB3 on survival or apoptosis are likely cell type and context dependent (Morse et al. 2010).
The nutrient sensing gene SNAT2 was recently identified as a novel arsenite stress response gene, and its transporter activity is required for arsenite-mediated ER stress (Oh et al. 2012). In this research, we also found that SNAT2 was induced by arsenic in SVEC4-10 cells.
The finding that tiron blocked arsenic-mediated ROS production with a concomitant attenuation of both activation of eIF2α–ATF4 and ATF6, but only partially inhibited the expression of their direct downstream CHOP and GRP78 expression, and slightly or without affecting pro-apoptotic BCL-2 family member or nutrient sensing gene expression, suggests that although ROS production is involved in arsenic-mediated UPR, other pathways also participate in arsenic-mediated cytotoxicity.
ATF4 serves as an accessory transcription factor for arsenic-mediated ER stress
It has been known that ATF4 is a transcription factor that induces the expression of GRP78 and CHOP (Rutkowski and Kaufman 2004). Furthermore, it was found that arsenite-induced SNAT2 expression is ATF4-dependent in HEK293T cells (Oh et al. 2012). This work examined the role of ATF4 in arsenic-mediated UPR-related gene expression by knockdown ATF4 using transiently transfecting synthetic small interfering RNA (siRNA). ATF4 siRNA-transfected cells were then treated with 7.5 μM As2O3 in serum-free medium, and the expression of ATF4 was determined. Supplemental Figure 3A shows that we successfully achieved ~40 % knockdown of arsenic induced rather than endogenous ATF4 gene expression. When SVEC4-10 cells were transfected with non-targeting siRNA and then treated with arsenic for 6 h, CHOP and GRP78, the direct targets of ATF4, increased by 4.1- and 2.4-fold, respectively. In ATF4 siRNA-transfected cells, no alternation in their expression was observed (Supplemental Fig. 3B and C). Similarly, expression of PUMA and SNAT2 in arsenic-treated SVEC4-10 cells remained unchanged in ATF4 siRNA-transfected cells (Supplemental Fig. 3D and E). However, TRB3 was significantly attenuated in ATF4 siRNA-transfected cells (Supplemental Fig. 3E). These results imply that ATF4 expression does not seem to be required for inducing ER stress-related mRNA expression but could constitute an accessory pathway that promotes UPR in response to arsenic.
Cytotoxicity of arsenic is in part caused by ER stress in SVEC4-10 cells
Salubrinal, a selective inhibitor of cellular complexes that dephosphorylate eIF2α, has been shown to protect cells from ER stress-induced apoptosis (Boyce et al. 2005). It has been reported that salubrinal induces resistance against arsenic-induced apoptosis in dorsal root ganglion explants (Chou et al. 2008). Our results confirmed that salubrinal treatment enhanced the expression of ER target genes, CHOP and GRP78, and partially protected SVEC4-10 cells from arsenic toxicity, indicating that the cytotoxicity of arsenic is in part caused by ER stress.
In conclusion, the findings of this study show that arsenic causes cytotoxicity, caspase-3 activation, intracellular calcium accumulation, and ER stress, which results in the activation of both the adaptive and apoptotic responses of the UPR in SVEC4-10 mouse vascular endothelium cells. We found that arsenic trioxide activated two of the canonical pathways of the UPR, PERK–eIF2α–ATF4, and ATF6, and induced the expression of target genes GRP78, CHOP, TRB3, BAX, PUMA, and SNAT2. The activation of eIF2α, ATF4, and ATF6 and protein expression of GRP78 and CHOP were suppressed by LA, a potent thioreducant, and tiron, a superoxide scavenger, indicating ROS-dependent and ROS-independent signaling are involved. Pretreatment with salubrinal, a selective inhibitor of eIF2α dephosphorylation, enhanced the protein levels of GRP78 and CHOP and partially protected cells from arsenic-induced cytotoxicity. These results indicate that arsenic-induced SVEC4-10 cytotoxicity is caused at least in part by ER stress.
References
Allagnat F, Christulia F, Ortis F et al (2010) Sustained production of spliced X-box binding protein 1 (XBP1) induces pancreatic beta cell dysfunction and apoptosis. Diabetologia 53(6):1120–1130. doi:10.1007/s00125-010-1699-7
Baird TD, Wek RC (2012) Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv Nutr 3(3):307–321. doi:10.3945/an.112.002113
Binet F, Chiasson S, Girard D (2010a) Arsenic trioxide induces endoplasmic reticulum stress-related events in neutrophils. Int Immunopharmacol 10(4):508–512. doi:10.1016/j.intimp.2010.01.013
Binet F, Chiasson S, Girard D (2010b) Evidence that endoplasmic reticulum (ER) stress and caspase-4 activation occur in human neutrophils. Biochem Biophys Res Commun 391(1):18–23. doi:10.1016/j.bbrc.2009.10.141
Binet F, Chiasson S, Girard D (2011) Interaction between arsenic trioxide (ATO) and human neutrophils. Hum Exp Toxicol 30(5):416–424. doi:10.1177/0960327110372645
Bolt AM, Zhao F, Pacheco S, Klimecki WT (2012) Arsenite-induced autophagy is associated with proteotoxicity in human lymphoblastoid cells. Toxicol Appl Pharmacol 264(2):255–261. doi:10.1016/j.taap.2012.08.006
Bouvier N, Flinois JP, Gilleron J et al (2009) Cyclosporine triggers endoplasmic reticulum stress in endothelial cells: a role for endothelial phenotypic changes and death. Am J Physiol Renal Physiol 296(1):F160–F169. doi:10.1152/ajprenal.90567.2008
Bowers AJ, Scully S, Boylan JF (2003) SKIP3, a novel Drosophila tribbles ortholog, is overexpressed in human tumors and is regulated by hypoxia. Oncogene 22(18):2823–2835. doi:10.1038/sj.onc.1206367
Boyce M, Bryant KF, Jousse C et al (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307(5711):935–939. doi:10.1126/science.1101902
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brnjic S, Olofsson MH, Havelka AM, Linder S (2010) Chemical biology suggests a role for calcium signaling in mediating sustained JNK activation during apoptosis. Mol BioSyst 6(5):767–774. doi:10.1039/b920805d
Celik I, Gallicchio L, Boyd K et al (2008) Arsenic in drinking water and lung cancer: a systematic review. Environ Res 108(1):48–55. doi:10.1016/j.envres.2008.04.001
Chou YH, Chao PL, Tsai MJ et al (2008) Arsenite-induced cytotoxicity in dorsal root ganglion explants. Free Radic Biol Med 44(8):1553–1561. doi:10.1016/j.freeradbiomed.2007.12.014
Del Razo LM, Quintanilla-Vega B, Brambila-Colombres E, Calderon-Aranda ES, Manno M, Albores A (2001) Stress proteins induced by arsenic. Toxicol Appl Pharmacol 177(2):132–148. doi:10.1006/taap.2001.9291
Dey S, Baird TD, Zhou D, Palam LR, Spandau DF, Wek RC (2010) Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem 285(43):33165–33174. doi:10.1074/jbc.M110.167213
Du K, Herzig S, Kulkarni RN, Montminy M (2003) TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300(5625):1574–1577. doi:10.1126/science.1079817
Flora SJ, Pachauri V (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7(7):2745–88. doi:10.3390/ijerph7072745
Harding HP, Zhang Y, Zeng H et al (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11(3):619–633
Haze K, Yoshida H, Yanagi H, Yura T, Mori K (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10(11):3787–3799
Haze K, Okada T, Yoshida H et al (2001) Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J 355(Pt 1):19–28
Heifetz A, Keenan RW, Elbein AD (1979) Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry (Mosc) 18(11):2186–2192
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13(2):89–102. doi:10.1038/nrm3270
Hsueh YM, Cheng GS, Wu MM, Yu HS, Kuo TL, Chen CJ (1995) Multiple risk factors associated with arsenic-induced skin cancer: effects of chronic liver disease and malnutritional status. Br J Cancer 71(1):109–114
Jennings P, Limonciel A, Felice L, Leonard MO (2013) An overview of transcriptional regulation in response to toxicological insult. Arch Toxicol 87(1):49–72. doi:10.1007/s00204-012-0919-y
Jiang SJ, Lin TM, Wu HL, Han HS, Shi GY (2002) Decrease of fibrinolytic activity in human endothelial cells by arsenite. Thromb Res 105(1):55–62
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13(10):1211–1233
Lai E, Teodoro T, Volchuk A (2007) Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology 22(3):193–201. doi:10.1152/physiol.00050.2006
Lantz RC, Hays AM (2006) Role of oxidative stress in arsenic-induced toxicity. Drug Metab Rev 38(4):791–804. doi:10.1080/03602530600980108
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23(21):7448–7459
Li J, Lee B, Lee AS (2006) Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem 281(11):7260–7270. doi:10.1074/jbc.M509868200
Li C, Xu J, Li F et al (2011) Unfolded protein response signaling and MAP kinase pathways underlie pathogenesis of arsenic-induced cutaneous inflammation. Cancer Prev Res (Phila) 4(12):2101–2109. doi:10.1158/1940-6207.CAPR-11-0343
Lin AM, Chao PL, Fang SF, Chi CW, Yang CH (2007) Endoplasmic reticulum stress is involved in arsenite-induced oxidative injury in rat brain. Toxicol Appl Pharmacol 224(2):138–146. doi:10.1016/j.taap.2007.06.016
Lu FJ, Shih SR, Liu TM, Shown SH (1990) The effect of fluorescent humic substances existing in the well water of Blackfoot disease endemic areas in Taiwan on prothrombin time and activated partial thromboplastin time in vitro. Thromb Res 57(5):747–753
Lu TH, Su CC, Chen YW et al (2011) Arsenic induces pancreatic beta-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol Lett 201(1):15–26. doi:10.1016/j.toxlet.2010.11.019
Ma Y, Brewer JW, Diehl JA, Hendershot LM (2002) Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318(5):1351–1365
Mackenzie B, Erickson JD (2004) Sodium-coupled neutral amino acid (system N/A) transporters of the SLC38 gene family. Pflugers Arch 447(5):784–795. doi:10.1007/s00424-003-1117-9
Marciniak SJ, Yun CY, Oyadomari S et al (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18(24):3066–3077. doi:10.1101/gad.1250704
Morales AA, Gutman D, Lee KP, Boise LH (2008) BH3-only proteins Noxa, Bmf, and Bim are necessary for arsenic trioxide-induced cell death in myeloma. Blood 111(10):5152–5162. doi:10.1182/blood-2007-10-116889
Morales AA, Gutman D, Cejas PJ, Lee KP, Boise LH (2009) Reactive oxygen species are not required for an arsenic trioxide-induced antioxidant response or apoptosis. J Biol Chem 284(19):12886–12895. doi:10.1074/jbc.M806546200
Morse E, Schroth J, You YH et al (2010) TRB3 is stimulated in diabetic kidneys, regulated by the ER stress marker CHOP, and is a suppressor of podocyte MCP-1. Am J Physiol Renal Physiol 299(5):F965–F972. doi:10.1152/ajprenal.00236.2010
Namba T, Ishihara T, Tanaka K, Hoshino T, Mizushima T (2007) Transcriptional activation of ATF6 by endoplasmic reticulum stressors. Biochem Biophys Res Commun 355(2):543–548. doi:10.1016/j.bbrc.2007.02.004
Naranmandura H, Xu S, Koike S et al (2012) The endoplasmic reticulum is a target organelle for trivalent dimethylarsinic acid (DMAIII)-induced cytotoxicity. Toxicol Appl Pharmacol 260(3):241–249. doi:10.1016/j.taap.2012.02.017
Nordstrom DK (2002) Public health. Worldwide occurrences of arsenic in ground water. Science 296(5576):2143–2145. doi:10.1126/science.1072375
O’Connell KA, Edidin M (1990) A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J Immunol 144(2):521–525
Oh RS, Pan WC, Yalcin A et al (2012) Functional RNA interference (RNAi) screen identifies system A neutral amino acid transporter 2 (SNAT2) as a mediator of arsenic-induced endoplasmic reticulum stress. J Biol Chem 287(8):6025–6034. doi:10.1074/jbc.M111.311217
Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H (2005) TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24(6):1243–1255. doi:10.1038/sj.emboj.7600596
Ord D, Ord T (2005) Characterization of human NIPK (TRB3, SKIP3) gene activation in stressful conditions. Biochem Biophys Res Commun 330(1):210–218. doi:10.1016/j.bbrc.2005.02.149
Ord D, Meerits K, Ord T (2007) TRB3 protects cells against the growth inhibitory and cytotoxic effect of ATF4. Exp Cell Res 313(16):3556–3567. doi:10.1016/j.yexcr.2007.07.017
Ord T, Ord D, Koivomagi M, Juhkam K, Ord T (2009) Human TRB3 is upregulated in stressed cells by the induction of translationally efficient mRNA containing a truncated 5′-UTR. Gene 444(1–2):24–32. doi:10.1016/j.gene.2009.06.001
Radosavljevic V, Jakovljevic B (2008) Arsenic and bladder cancer: observations and suggestions. J Environ Health 71(3):40–42
Rasheva V, Domingos P (2009) Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 14(8):996–1007. doi:10.1007/s10495-009-0341-y
Roboz GJ, Dias S, Lam G et al (2000) Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood 96(4):1525–1530
Rodriguez D, Rojas-Rivera D, Hetz C (2011) Integrating stress signals at the endoplasmic reticulum: the BCL-2 protein family rheostat. Biochim Biophys Acta 1813(4):564–574. doi:10.1016/j.bbamcr.2010.11.012
Rutkowski DT, Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14(1):20–28
Schroder M, Kaufman RJ (2005) ER stress and the unfolded protein response. Mutat Res 569(1–2):29–63. doi:10.1016/j.mrfmmm.2004.06.056
Shi H, Shi X, Liu KJ (2004) Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem 255(1-2):67–78
Shin SY, Kim CG, Lim Y, Lee YH (2011) The ETS family transcription factor ELK-1 regulates induction of the cell cycle-regulatory gene p21(Waf1/Cip1) and the BAX gene in sodium arsenite-exposed human keratinocyte HaCaT cells. J Biol Chem 286(30):26860–26872. doi:10.1074/jbc.M110.216721
Simeonova PP, Luster MI (2004) Arsenic and atherosclerosis. Toxicol Appl Pharmacol 198(3):444–449
Spuches AM, Kruszyna HG, Rich AM, Wilcox DE (2005) Thermodynamics of the As(III)-thiol interaction: arsenite and monomethylarsenite complexes with glutathione, dihydrolipoic acid, and other thiol ligands. Inorg Chem 44(8):2964–72. doi:10.1021/ic048694q
Stanhill A, Haynes CM, Zhang Y et al (2006) An arsenite-inducible 19S regulatory particle-associated protein adapts proteasomes to proteotoxicity. Mol Cell 23(6):875–885. doi:10.1016/j.molcel.2006.07.023
Tang CH, Chiu YC, Huang CF, Chen YW, Chen PC (2009) Arsenic induces cell apoptosis in cultured osteoblasts through endoplasmic reticulum stress. Toxicol Appl Pharmacol 241(2):173–181. doi:10.1016/j.taap.2009.08.011
Terai K, Hiramoto Y, Masaki M et al (2005) AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol 25(21):9554–9575. doi:10.1128/MCB.25.21.9554-9575.2005
Thuerauf DJ, Morrison L, Glembotski CC (2004) Opposing roles for ATF6alpha and ATF6beta in endoplasmic reticulum stress response gene induction. J Biol Chem 279(20):21078–21084. doi:10.1074/jbc.M400713200
Todd DJ, Lee AH, Glimcher LH (2008) The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 8(9):663–674. doi:10.1038/nri2359
Tseng CH (2002) An overview on peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Angiology 53(5):529–537
Tseng CH (2005) Blackfoot disease and arsenic: a never-ending story. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 23(1):55–74. doi:10.1081/GNC-200051860
Tseng CH (2008) Cardiovascular disease in arsenic-exposed subjects living in the arseniasis-hyperendemic areas in Taiwan. Atherosclerosis 199(1):12–18. doi:10.1016/j.atherosclerosis.2008.02.013
Tseng CH, Chong CK, Tseng CP, Centeno JA (2007) Blackfoot disease in Taiwan: its link with inorganic arsenic exposure from drinking water. Ambio 36(1):82–84
Vahidnia A, van der Voet GB, de Wolff FA (2007) Arsenic neurotoxicity—a review. Hum Exp Toxicol 26(10):823–832. doi:10.1177/0960327107084539
Wang L, Kou MC, Weng CY, Hu LW, Wang YJ, Wu MJ (2012) Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: roles of ROS, NF-kappaB, and MAPK pathways. Arch Toxicol 86(6):879–896. doi:10.1007/s00204-012-0845-z
Wei M, Arnold L, Cano M, Cohen SM (2005) Effects of co-administration of antioxidants and arsenicals on the rat urinary bladder epithelium. Toxicol Sci 83(2):237–245. doi:10.1093/toxsci/kfi033
Yamamoto K, Sato T, Matsui T et al (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13(3):365–376. doi:10.1016/j.devcel.2007.07.018
Yang CY (2006) Does arsenic exposure increase the risk of development of peripheral vascular diseases in humans? J Toxicol Environ Health A 69(19):1797–1804. doi:10.1080/15287390600630237
Yen CC, Ho TJ, Wu CC et al (2011) Inorganic arsenic causes cell apoptosis in mouse cerebrum through an oxidative stress-regulated signaling pathway. Arch Toxicol 85(6):565–575. doi:10.1007/s00204-011-0709-y
Yen YP, Tsai KS, Chen YW, Huang CF, Yang RS, Liu SH (2012) Arsenic induces apoptosis in myoblasts through a reactive oxygen species-induced endoplasmic reticulum stress and mitochondrial dysfunction pathway. Arch Toxicol 86(6):923–933. doi:10.1007/s00204-012-0864-9
Yu HS, Chang KL, Kao YH et al (1998) In vitro cytotoxicity of IgG antibodies on vascular endothelial cells from patients with endemic peripheral vascular disease in Taiwan. Atherosclerosis 137(1):141–147
Zhang K (2010) Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med 3(1):33–40
Zhang H, Duncan G, Wang L et al (2007) Arsenic trioxide initiates ER stress responses, perturbs calcium signalling and promotes apoptosis in human lens epithelial cells. Exp Eye Res 85(6):825–835. doi:10.1016/j.exer.2007.08.018
Acknowledgments
This study was supported by Grant NSC-99-2320-B-041-005-MY3 (to M.-J. Wu) from National Science Council, Taiwan, ROC.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Weng, CY., Chiou, SY., Wang, L. et al. Arsenic trioxide induces unfolded protein response in vascular endothelial cells. Arch Toxicol 88, 213–226 (2014). https://doi.org/10.1007/s00204-013-1101-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1101-x