Abstract
The normally picturesque Cache Valley in northern Utah is frequently reported to have the worst particulate (PM) air pollution in the United States. Numerous epidemiological studies conducted elsewhere have associated PM exposure to a variety of cardiovascular diseases and early mortality. We have previously shown that Cache Valley PM (CVPM) is pro-inflammatory, through a variety of mechanisms involving the release of inflammatory cytokines, unfolded protein response, ER stress, and C-reactive protein (CRP). This study was undertaken to determine whether Cache Valley PM (CVPM) would activate Akt, an upstream mechanism common to these events. Human lung (BEAS-2B) cells were treated with either fine (PM2.5) or coarse (PM10) particles (12.5 and 25 μg/ml) for periods up to 24 h. PM-exposed cells exhibited Akt activation as evidenced by phosphorylation at Thr308 and Ser473. Events downstream of Akt activation such as NF-κB activation were observed at 1 and 24 h, but IκB phosphorylation occurred only at 24 h, indicating that mechanisms of PM-mediated NF-κB activation are time dependent. Akt and NF-κB related inflammatory cytokine IL-1α, and IL-6 and the chemokine IL-8 were upregulated in treated cells at 6 and 24 h. The calpain inhibitor leupeptin limited Akt phosphorylation to Ser473 and reduced release of IL-1α, IL-6, and IL-8, indicating that calpain or similar protease(s) are involved in PM-induced activation of Akt and subsequent release of inflammatory cytokines. Our data indicate that PM activates Akt, which may play a role in the pro-inflammatory response to PM exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The normally picturesque Cache Valley in northern Utah frequently experiences the worst particulate air pollution (PM) in the United States, a situation exacerbated by winter atmospheric inversions that trap and concentrate pollutants (Edgerton et al. 2006; Lurmann et al. 2006; Malek et al. 2006; USA-Today 2005). Similar to PM such as that from the San Joaquin Valley of California, the single largest chemical component of Cache Valley PM (CVPM) is ammonium nitrate (NH4NO3), a secondary pollutant formed by atmospheric reactions between nitrogen oxides from vehicles, and ammonia gas from the excreta of dairy cows and other animals (Malek et al. 2006; Mangelson et al. 1997; National Center for Environmental Assessment (Research Triangle Park N.C.) 1996; Edgerton et al. 2006; Lurmann et al. 2006).
In other locations, exposure to PM is associated with early all-cause mortality, as well as to numerous cardiovascular and cardiopulmonary diseases, including ischemic heart disease, cardiac arrest, hypertensive disease, cerebrovascular disease, pneumonia, influenza, in addition to diabetes, neurodegeneration, and cancer (Peters et al. 2006; Pope et al. 2002; Pope et al. 2004a). Particulates are chemically diverse and elicit inflammatory and pro-oxidative responses in a variety of cell types through differing mechanisms (Tao et al. 2003). U.S. Federal regulations have been implemented following epidemiological studies regulating 2 PM size fractions of which PM2.5 appeared to have greater association with adverse health effects than PM10 (Schwartz et al. 1996; Delfino et al. 2005), although some other studies indicate a significant health threat posed by the larger PM10 (Choi et al. 2004; Osornio-Vargas et al. 2003; Pozzi et al. 2003; Monn and Becker 1999; Soukup and Becker 2001; Jalava et al. 2007; Becker et al. 2005c; Becker and Soukup 2003).
While little is known about the mechanisms by which PM may induce harm (Lippmann et al. 2003; Mossman et al. 2007; NHEERL 2010; National Center for Environmental Assessment (Research Triangle Park N.C.) 2003), the inflammatory response has received the most attention (Delfino et al. 2005; Pope et al. 2004b; Donaldson et al. 2001; Schins et al. 2004). A potential mechanism for the pro-inflammatory action of PM is through activation of nuclear factor-kappa B (NF-κB), a transcription factor stimulated in response to many pro-inflammatory agents (Jimenez et al. 2000; Dagher et al. 2007), which is activated by Akt (formerly known as protein kinase B). Another important component of inflammation is apoptosis suppression in cytokine-releasing cells, a process that also involves Akt (Abraham 2005).
Akt is a cytosolic serine/threonine kinase (Rane et al. 2003) required for cancer cell growth, promoting the survival of cancer cells exposed to chemotherapy and radiation-induced apoptosis (Brognard et al. 2001). Activated Akt is detectible in 90% of non-small cell lung cancer cell lines, and 100% of those derived from smokers (West et al. 2003). Akt contributes to lung inflammation and injury (Abraham 2003), promotes chemotaxis to fight bacterial infection (Abraham 2005), and is a pro-survival adaptation mechanism following stress (Hu et al. 2004). Akt contributes to inflammation activating the pro-inflammatory transcription factor NF-κB playing a central role in survival by altering cellular metabolism and inhibiting apoptosis (Venkatachalam et al. 2008; Newcomb et al. 2008; Amaravadi and Thompson 2005; Chen 2005; Misra et al. 2006). Akt regulation is primarily through posttranslational modification (Amaravadi and Thompson 2005). Prototypical Akt activation occurs though receptor-mediated activation of phosphatidylinositol 3-kinase (PI3K). Activated PI3K produces 3-phosphoinositides (PIP3), which bind to and induce a conformational change in Akt as well its translocation from the cytoplasm to the plasma membrane where it is phosphorylated. Akt is fully activated when phosphorylated at Ser473 and Thr308, a process inhibited by tumor suppressor phosphatase and tensin homolog (PTEN) that dephosphorylates PIP3 (Cantley and Neel 1999). PTEN also limits the action of MDM2 allowing upregulation of p53 activity (Brognard et al. 2001; Hajduch et al. 2001). PTEN is inactivated by phosphorylation of its C-terminal tail (Vazquez et al. 2000), an event associated with a number of human tumors (Meek and Knippschild 2003).
We have previously demonstrated that ambient CVPM activates Stat3, P70S6 kinase, calpain, and Hsp27, and concomitant increases in Hsp90 protein in human airway epithelial (BEAS-2B) cells (Watterson et al. 2007). Because these events are associated with Akt (Watterson et al. 2007; Rane et al. 2003; Chatterjee et al. 2006; Tan et al. 2006; Peterson and Schreiber 1998; Watterson et al. 2009a, 2009b), we hypothesized that CVPM would activate Akt and related proteins leading to the release of Akt-associated inflammatory cytokines.
Materials and methods
Chemicals and reagents
BEAS-2B cells were a kind gift from Dr. Katerine Macé (Nestle Research Centre; Lausanne, Switzerland). Recombinant IL-1 was from R&D Systems (Minneapolis, MN). LHC-9 cell growth media was from Invitrogen (Camarillo, CA). IRAK-1 primary antibody was from Affinity Bioreagents (Golden, CO). All remaining antibodies, CHAPs cell extract buffer, and biotinylated molecular weight ladders were from Cell Signaling Technology (Danvers, MA). The Akt antibodies were not isoform specific but were able to detect total Akt or phosphorylated total Akt. Cytokine antibody array was from Quansys Biosciences (Logan, UT). Lipopolysaccharide (LPS) from Salmonella minnesota was from Alexis Biochemicals (San Diego, CA). Restore Western Blot Stripping buffer was from Pierce (Rockford, IL). CellBIND 6-well cell culture plates were from Corning (Corning, NY). Nitrocellulose membranes were from Bio-Rad (Hercules, CA).
PM collection and extraction, Cell culture, and exposure
PM sampling, extraction, endotoxin detection, and culture of BEAS-2B cells were previously described (Watterson et al. 2007; Watterson et al. 2009a, b). For time course studies, cells from the same passage were seeded onto separate plates and cell numbers adjusted accordingly (approximately 3.6 × 102 cells/well). Cells were grown until ~80% confluent then treated with fine (PM2.5) and coarse (PM10) at “low” (12.5) or “high” (25 μg/ml) concentrations. These concentrations are below a hypothetical “high” exposure of 50 μg/ml (Becker et al. 2005b) and represent potential “real-world” exposures. In all experiments, LPS (10 ng/ml) was also used for comparison. None of the concentrations of PM or LPS used in this study are cytotoxic to BEAS-2B cells (Watterson et al. 2009a, 2009b). Because Akt activation can occur rapidly (West et al. 2003), cells were harvested at 1 and 24 h time points with cytokine release examined at 1, 6, and 24 h.
Western blotting and ELISA
Methods for isolation of cell lysates, western blotting, and luminescent analysis have been previously described in detail (Van Vleet et al. 2006; Watterson et al. 2007) with the exception that PVDF membranes were used in this study rather than nitrocellulose. As PVDF allows for stripping and re-probing with additional primary antibodies, where appropriate, PVDF membranes were stripped for 15 min at room temperature. Membranes were re-incubated in chemiluminescent substrate for 5 min and reexamined to ensure proper stripping. Membranes were then re-probed with primary antibodies, and images were captured using an imaging workstation (UV Products, Upland, CA), and analysis was performed using the histogram function in Adobe PhotoShop CS (San Jose, CA) (Woznicova and Votava 2001). The software provided mean pixel intensity, standard deviations, and total number of pixels sufficient for one-way analysis of variance by Holm Sidak with significance set at p < 0.05. Mean luminescent values of the selected bands obtained from Adobe PhotoShop (San José, CA) were normalized to control and to bands of unmodified protein or to β-actin on the same membrane. Gels were loaded with 10 μg protein/well. Media from treatments was saved for ELISA, which was performed on multiplex chemiluminescent ELISA plates (Quansys Biosciences, Logan, UT) according to the manufacturers’ instructions as previously described (Barnard et al. 2006; Yuan et al. 2007; Gowen et al. 2006). Curve fitting and analysis were performed using SigmaPlot with the curve presenting the lowest PRESS statistic judged to have the best fit for interpolation (Daly et al. 2005).
Statistical analysis
One-way analysis of variance (ANOVA) was performed to examine differences between 3 or more groups, with post hoc Holm–Sidak multiple comparisons analysis (Glantz 2005). Where type II error was suspected from multiple comparisons, the “versus control” function of the Holm–Sidak analysis was utilized. Comparisons between two groups were performed using the t-test. SigmaStat software (SYSTAT, San José, CA) was used for all testing. P ≤ 0.05 was judged to be significant.
Results
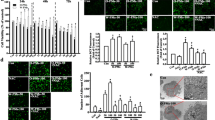
Akt requires phosphorylation at Thr308 and Ser473 for full activation, which was visualized on immunoblots. Akt was constitutively active (Fig. 1a), and cells exposed to PM had significant increases in Akt phosphorylation at both Ser473 and Thr308, although phosphorylation at Thr308 appeared to be affected more than Ser473 (Fig. 1). After 1 h, only low concentrations of both PM size classes provoked significant Akt phosphorylation (P ≤ 0.001 for both Thr308 and Ser473) compared to control (Fig. 1a). However, at 24 h, Akt phosphorylation at both Thr308 and Ser 473 was significantly (P < 0.001) elevated in lysates from all PM treatments compared to control and to LPS-treated cells (Fig. 1b).
PM treatment resulted in marked increases in Akt phosphorylation in BEAS-2B cells. Western immunoblots (a–b) and luminescent analysis (c) examining the activation state of Akt (60 kDa). Blots are of cell lysates treated with 1- (a) or 24-h (b) exposures of 12.5 (low) and 25 (high) μg/ml PM2.5 and PM10 including 10 ng/ml LPS and untreated negative control. Gels were loaded with 10 μg protein. Figures are representative of a minimum of 3 independent experiments. Results are presented as mean intensity normalized to control and unmodified Akt ± SE. a significant from control, b significant from differing concentration of the same PM diameter, c significant from same concentration of differing PM diameter, d significant from LPS (P ≤ 0.05)
Further evidence of Akt activation was determined by observing the effects of PM treatment on two upstream proteins responsible for mediating Akt phosphorylation: phosphoinositide-dependent protein kinase 1 (PDK-1), which phosphorylates Akt at Thr308 (Williams et al. 2000) while in the process is phosphorylated itself at Ser241(Casamayor et al. 1999), and PTEN, a negative effector of Akt phosphorylation. Other proteins affected by activated Akt, glycogen synthase kinase (GSK) that is inactivated by Akt via phosphorylation at Ser9, and p53 whose levels are decreased by activated Akt (Limesand et al. 2006) were also examined. After either 1- (Fig. 2) or 24-h (Fig. 3) exposures to fine and coarse PM, there was heightened PDK phosphorylation compared to control and LPS (P ≤ 0.001), with the greatest responses at 1 h (Fig. 2) seen in cells exposed to low PM2.5 and nearly equivalent responses with the fine PM after 24 (Fig. 3, P = 0.242). At 1 h, the signal for p-PTEN was detectable but too faint to reliably analyze (Fig. 2), but significant (P ≤ 0.001) increases in this protein were observed after 24-h exposures compared to control (Fig. 3). The greatest response for p-PTEN at 24 h was with the high concentration of coarse PM and LPS, which were nearly identical (P = 0.5; Fig. 3).
PM treatment induced alterations of Akt-related proteins in BEAS-2B cells following 1-h exposure. Western immunoblots (a) and luminescent analysis (b) examining the alterations of the phosphorylation states of PDK (58–68 kDa), PTEN (54 kDa), and GSK (46 kDa), and the total levels of p53. Gels were loaded with 10 μg protein. Blots are of lysates from cells receiving 1-h exposure of 12.5 (low) and 25 (high) μg/ml PM2.5 and PM10 including a 10 ng/ml LPS-positive control and untreated negative control. Figures are representative of a minimum of 3 independent experiments. Results presented as mean intensity normalized to control and to β-actin (45 kDa) ±SE. a significant from control, b significant from differing concentration of the same PM diameter, c significant from same concentration of differing PM diameter, d significant from LPS (P ≤ 0.05)
PM treatment induced alterations of Akt-related proteins in BEAS-2B cells following 24-h exposure. Western immunoblots (a) and luminescent analysis (b) examining the alterations of the phosphorylation states of PDK (58–68 kDa), PTEN (54 kDa), and GSK (46 kDa), and the total levels of p53. Blots are of lysates from cells receiving 24-h exposures of 12.5 (low) and 25 (high) μg/ml PM2.5 and PM10 including a 10 ng/ml LPS-positive control and untreated negative control. Gels were loaded with 10 μg protein. Figures are representative of a minimum of 3 independent experiments. Results presented as mean intensity normalized to control and to β-actin (45 kDa) ± SE. a Significant from control, b significant from differing concentration of the same PM diameter, c significant from same concentration of differing PM diameter, d significant from LPS at P ≤ 0.05
Appearance of the inactivated p-GSK was increased in PM2.5, but decreased in cells exposed to PM10 relative to control (P ≤ 0.001) following 1 h (Fig. 2). After 24-h treatments, p-GSK declined to levels below that of control (Fig. 3).
Western blots of p53 showed PM-related increases in p53 from cell lysates exposed to both size fractions of PM compared to control after 1-h exposure (P ≤ 0.001) (Fig. 2). Low PM2.5 caused the greatest increase in p53, which was significantly greater than the high PM2.5 and both concentrations of PM10 (P ≤ 0.001). There was no significant difference between the two PM10 concentrations (P = 0.405) or from the high PM2.5 to either low or high PM10 (P = 0.455 and 0.169, respectively). At 1 h, the signal for the second of the doublet bands often seen by this particular antibody in lysates from BEAS-2B cells (Van Vleet et al. 2006) was visible but not measureable as shown in Fig. 2a. Following 24 h, p53 was observed as doublet bands with no significant alterations in treatment-mediated response (P = 0.97, luminescent analysis not shown Fig. 3b). The signal responses (212–238 luminescent pixels) were below that of saturation (246 luminescent pixels), indicating that the equivalent p53 response was not due to image overexposure. The lower band in the doublet, likely indicating a different phosphorylation state (Van Vleet et al. 2006), was also examined with no significant differences between groups (P = 0.067, luminescent analysis not shown). Akt-mediated phosphorylation of PDK endothelial nitric oxide synthase (eNOS) on the Ser1177 site was examined but not detected following 1- and 24-h exposures (data not shown).
Given the documented ability of Akt to activate NF-κB (Misra et al. 2006), alterations in NFκ-B were examined as a marker of Akt activation. NF-κB is classically activated by phosphorylation and subsequent degradation of the inhibitory protein IκB, although degradation can also be mediated through calpain (Pianetti et al. 2001). Alternative NF-κB activity is through the constitutive processing of p105 to p50 and the inducible processing of p100 to give p52 (Hayden and Ghosh 2004). Therefore, both pathways of NF-κB activation were explored in PM-exposed cells.
Expression of constitutive IκB was clearly evident in all PM-treated cells after either 1- (Fig. 4) or 24-h (Fig. 5) exposures; 1-h exposures to PM did not result in the appearance of p-IκB, a measure of classical NF-κB activity (Fig. 4). Levels of inactive NF-κB p100 were slightly increased from control (P = 0.018 for the high PM2.5, P ≤ 0.001, for the remainder), with PM10-exposed cells giving the greatest response. There was elevated expression of active p52, p50, and p105 (P ≤ 0.001), in PM-exposed cells compared to control (Fig. 4).
PM alters non-classical NF-κB activity in BEAS-2B cells after 1-h incubation. Western immunoblots (a) and luminescent analysis (b) examining alterations of IκB (40 kDa), NF-κB p100/52, and NF-κB p105/p50. Gels were loaded with 10 μg protein/well with the exception of the positive control lane which was a lysate of TNF-α treated HeLa cells provided by the antibody manufacturer loaded at 10 μl/well. Blots are of lysates from cells receiving 1-h exposures of 12.5 (low) and 25 (high) μg/ml PM2.5 and PM10 including a 10 ng/ml LPS-positive control and untreated negative control. Figures are representative of a minimum of 3 independent experiments. Results presented as mean intensity normalized to control and to β-actin (45 kDa) ± SEM. a Significant from control, b significant from differing concentration of the same PM diameter, c significant from same concentration of differing PM diameter d significant from LPS at P ≤ 0.05
PM alters classical NF-κB activity in BEAS-2B cells after 24-h incubation. Western immunoblots (a) and luminescent analysis (b) examining alterations of IκB (40 kDa), NF-κB p100/52, and NF-κB p105/p50. Gels were loaded with 10 μg protein/well with the exception of the positive control lane which was a lysate of TNF-α-treated HeLa cells provided by the antibody manufacturer loaded at 10 μl/well. Blots are of lysates from cells receiving 24-h exposures of 12.5 (low) and 25 (high) μg/ml PM2.5 and PM10 including a 10 ng/ml LPS-positive control and untreated negative control. Figures are representative of a minimum of 3 independent experiments. Results presented as mean intensity normalized to control and to β-actin (45 kDa) ± SEM. a Significant from control, b significant from differing concentration of the same PM diameter, c significant from same concentration of differing PM diameter, d significant from LPS at P ≤ 0.05
At 24 h (Fig. 5), constitutive p-IκB (Ser32) was detected in all cell lysates with significantly elevated levels in lysates from cells that were treated with PM and LPS (P ≤ 0.001). Cells treated with PM10 had the highest levels of p-IκB, but no concentration effect was observed (P = 0.589; Fig. 5). Interestingly, phosphorylation of IκB was not reflected in reductions of total IκB with only the lysates from LPS-treated cells having significantly (P = 0.031) lower levels of the protein than control. Lysates from cells treated with the lowest concentration of PM2.5 had the highest levels of protein (Fig. 5). With the inducible active p52 protein, lysates from cells treated with PM demonstrated significant increases (P ≤ 0.001) from control with the exception of the high PM2.5 (P = 0.43). The LPS-exposed cells expressed lower (P ≤ 0.001) levels of p52 than that from control (Fig. 5). While the inactive p100 dimer was not detectable, there was increased expression of the inactive p105 dimer in PM-treated cells (P ≤ 0.001 for all) compared to control. All PM treatments induced increases (P ≤ 0.001) in the active p50 protein.
Cytokine release is regulated by activities of Akt (Strassheim et al. 2004) and NF-κB (Hayden and Ghosh 2004), so the ability of CVPM to induce cytokine release was examined. IL-1α, IL-6, and IL-8 were detected, and IL-1β, IL-2, IL-4, IL-10, interferon-γ, TNF-α, and TNF-β were not. Cytokine release was detectable but not quantifiable 1 h posttreatment but was measurable after 6 h (Fig. 6). At that time point, the fine (P ≤ 0.004) but not the coarse (P ≥ 0.128) elicited significantly (P = 0.00) greater IL-1α release than control. At 24 h, only the low-fine exhibited significantly (P = 0.002) greater IL-1α release compared to control (Fig. 6). With IL-6 following 6-h exposures, all treatments (P ≤ 0.005) excepting the low-coarse (P = 0.174) induced significantly greater IL-6 levels than control with LPS eliciting a 4× greater response than that of the PM. Following 24 h, all treatments exhibited significant increases from control (P ≤ 0.001) with LPS inducing only a 40% greater response than the nearest PM-induced response (Fig. 6). IL-8 release was not significantly affected by PM treatment after 6 h, and only the high PM2.5 elicited (and LPS) a greater response than control after 24 h (P ≤ 0.001 each).
PM exposure causes the release of NF-κB-related interleukins following 6- and 24-h exposures. Results are a combination of n = 3 independent experiments with six replicates and are given in mean concentration (pg/ml) ± SEM. a Significant from control, b significant from differing concentration of the same PM diameter, c significant from same concentration of differing PM diameter, d significant from LPS at P ≤ 0.05
Previous studies have shown that CVPM treatment results in increases in extracellular Hsp70 (Watterson et al. 2009a, b), which exhibits its signaling capacity through the toll-like receptor (TLR)/IL-1 receptor pathway (Vabulas et al. 2002). As there were PM-mediated IL-1 release and alterations in NF-κB activity, which can also be activated through IL-1R1 activation, we examined the central regulator of TLR-2,4/IL-1R the interleukin-1 receptor-associated kinase 1 (IRAK) (Arcaroli et al. 2006). IRAK phosphorylation was not detected on Thr387, which is required for IRAK activation (Kollewe et al. 2004), following 1- or 24-h of PM and LPS exposure (Fig. 7). IRAK phosphorylation at Ser376, which is indicative of IRAK-4 activation (Koziczak-Holbro et al. 2007) likewise, was not detected (data not shown). Inactive IRAK was detected and was increased in PM-treated cells, which is consistent with PM treatment (Watterson et al. 2007) and indicative of a lack of IRAK degradation (Yamin and Miller 1997). Antibody functionality was verified by treating cells with 25 ng/ml IL-1α, which caused IRAK phosphorylation at both Ser376 and Thr387 sites following 1- and 24-h treatment (data not shown).
PM signaling in BEAS-2B cells is not through an IRAK-1-dependent mechanism. Immunoblots revealed that IRAK-1 is not phosphorylated following treatment after 1- (a) or 24-h (b) treatment. This indicates that the IL-1 receptor pathway is likely not activated by the PM used in this study in BEAS-2B cells. This is despite PM-mediated increases in IRAK levels. Western immunoblots of cell lysates treated with 1- or 24-h exposures of 12.5 (low) and 25 (high) μg/ml Cache Valley PM2.5 and PM10 including a 10 ng/ml LPS-positive control and untreated negative control. Gels were loaded with 10 μg/ml total protein. Figures are representative of a minimum of 3 independent experiments
In order to determine whether calpain activation might be involved in PM-mediated Akt phosphorylation (Tan et al. 2006), cells were co-incubated with a non-cytotoxic concentration of 40 μg/ml leupeptin (Momiyama et al. 2006), a “relatively select” calpain inhibitor (Takahashi et al. 2006) for 24 h. Leupeptin is water soluble so does not need to be used with a vehicle that might itself alter some of the subtle pathways that PM alters such as PEG (Ono et al. 1999), ethanol (Carloni et al. 2004), and DMSO (our laboratory, unpublished findings) altering calpain activity. Leupeptin eliminated detectable Akt phosphorylation at Thr308, PTEN phosphorylation, PDK phosphorylation, and Gsk phosphorylation (data not shown). Leupeptin co-treatment had little effect upon the detection of Akt phosphorylation at Ser473. Leupeptin co-incubations with PM and LPS exhibited significantly greater effects than leupeptin alone control (P ≤ 0.001) except with the low concentration of the fine (P = 0.296) PM (Fig. 8a). Cytokine levels were examined in the conditioned media from the leupeptin plus PM and LPS treatments. For IL-1α, the high PM2.5 and both low and high PM10 plus leupeptin treatments achieved significantly different results from the leupeptin only (P = 0.004, P = 0.001, and P = 0.001, respectively). Leupeptin plus the low concentration of fine PM and leupeptin plus LPS were not significant from leupeptin only (P = 0.140, and P = 0.09). In the case of IL-6, the leupeptin co-treatment eliminated the PM-mediated increase from control of the PM2.5 and the low concentration of the PM10. Only the high (25 μg/ml) and LPS treatments exhibited significantly greater effects than control (P = 0.001 and P ≤ 0.001, respectively). Cells co-treated with both types of PM and leupeptin had slightly less IL-8 release than the cells treated with leupeptin only. This reduction was not significant (P = 0.067 and P = 0.122 for low and high PM2.5-treated cells and P = 0.252 and P = 0.077 for low and high PM10-treated cells). Only the LPS plus leupeptin co-treatment group achieved a significantly greater response (P ≤ 0.001) from the leupeptin-only group (Fig. 8b).
Simultaneous leupeptin co-treatment alters PM-mediated Akt activity and cytokine release. a Co-incubation of the calpain inhibitor leupeptin with PM and LPS completely attenuated the ability to detect Akt phosphorylation at Thr308, p-GSK, p-PTEN, and p-PDK as measured via western immunoblots. Only p-Akt (60 kDa) at Ser473 was detectable (compare with Figs. 1, 2). As phosphorylation of both sites on Akt is needed for full activity, it indicates that calpain is required for PM-mediated Akt activation. Western immunoblots were of cell lysates from BEAS-2B cells following 24-h co-exposures of 12.5 (low) and 25 (high) μg/ml concentrations of Cache Valley PM2.5, PM10, 10 ng/ml LPS plus 40 μg/ml of the calpain inhibitor leupeptin (leu) and leupeptin-only control. Gels were loaded with 10 μg/well. Figures are representative of a minimum of 3 independent experiments. b Calpain inhibition induced changes in PM-mediated cytokine release limiting the ability of most PM treatments, but not LPS to result in significantly elevated levels of IL-6 and IL-8 (compare with Fig. 6). a Significant from control, b significant from differing concentration of the same PM diameter, c significant from same concentration of differing PM diameter, d significant from LPS at P ≤ 0.05
Discussion
To our knowledge, this study is the first to demonstrate that ambient PM activates Akt in vitro with concomitant inactivation of the tumor suppressor PTEN. Previous studies have shown that reserve oil fly ash altered related Akt genes in rat neonatal cardiomyocytes (Knuckles and Dreher 2007) and diesel exhaust particles (DEP) induced Akt activity in human umbilical vein epithelial cells (Sumanasekera et al. 2007). In murine keratinocytes, DEP activated Akt in the absence of PTEN phosphorylation (Ma et al. 2004). In another study, DEP (50 μg/ml) caused downregulation of Akt phosphorylation and concomitant apoptosis in A549 cells, events that were suppressed when these cells were stably transfected with recombinant human thioredoxin-1 (rh-Trx-1), suggesting a protective role of this protein against reactive oxygen species released from DEP (Kaimul Ahsan et al. 2005). Diet-induced obese mice exposed to PM (1.6 mg/kg, intratracheal instillation and inhalation of 13 μg/m3 for 6 h/day, 5 days/week, for 128 days) showed reductions in Akt phosphorylation (Ser473) in intact aorta but no changes in Akt phosphorylation in epithelium-denuded aortic tissues (Sun et al. 2009). Akt directly controls cellular metabolism via hexokinase and phosphofructokinase-2 and is involved in respiratory burst in neutrophils (Chen et al. 2003) likely explaining previous findings of PM-mediated oxidative burst (Soukup et al. 2000) and PM-mediated increases in MTT reduction (Watterson et al. 2007; Pruett and Loftis 1990).
The pro-inflammatory activity of Akt is primarily due to its ability to suppress apoptosis in cytokine-releasing cells (Matute-Bello et al. 1997), a mechanism thought to contribute to acute lung injury (Abraham 2003). Akt suppresses apoptosis through mechanisms involving MDM2 activation and p53 degradation, inactivation of caspase-9 and Bad, stabilization of the anti-apoptotic protein XIAP, Bim downregulation (Amaravadi and Thompson 2005), prevention of mitochondrial cytochrome-c release (Abraham 2003), and forkhead inactivation (Brunet et al. 1999). Akt activation is also a pro-survival compensatory action taken by cells following insult, such as endoplasmic reticulum stress (Hu et al. 2004), ischemia (Mullonkal and Toledo-Pereyra 2007), sepsis, (Li et al. 2004), or ultraviolet light (Mallikarjuna et al. 2004). As we have shown CVPM to trigger ER stress in these cells (Watterson et al. 2009), it is possible that the Akt activation observed here is also a compensatory action.
The signals for NF-κB activation include Akt, TNF, and TLR network (Hazeki et al. 2007; Oda and Kitano 2006). NF-κB activation typically occurs through the phosphorylation and ubiquitin-mediated degradation of the inhibiting protein IκB-α although NF-κB activation via oxidative stress can bypass IκB-α degradation (Pianetti et al. 2001). PM has been demonstrated to activate NF-κB upon contact with cell surfaces via mechanisms that are independent of IκB degradation (Churg et al. 2005; Jimenez et al. 2000). The lack of IκB degradation with concurrent phosphorylation found in this study may be due to inhibition of proteosomal degradation, or to IκB upregulation, a recovery effect observed following LPS stimulation (Velasco et al. 1997). PM-mediated enhancement of IκB-α phosphorylation observed in the present study is possibly associated with Akt (Abraham 2005) or via calpain (Watterson et al. 2009a, 2009b; Pianetti et al. 2001) which activates Akt (Tan et al. 2006) and can be activated by PM (Watterson et al. 2009). The PM-induced appearance of processed p50 (NF-κB1) and p52 (NF-κB2) fragments is consistent with constitutive NF-κB signaling (Senftleben et al. 2001) and increases in the constitutive activation of Stat3 with PM exposure we previously observed (Watterson et al. 2007; Nadiminty et al. 2006). Of interest are the increases in NF-κB p105/p50, an effect observed with cell migration and tumor progression (Gao et al. 2006).
Akt signaling elicits inflammatory cytokine release (Wong et al. 2007) and is likely to be partially responsible for the release of inflammatory cytokines IL-6 and the chemokine IL-8 observed in this study, in agreement with previous studies using this cell type (Veranth et al. 2004; Frampton et al. 1999).
Chronic PM-mediated IL-6 release may lead to atherogenesis via eNOS inhibition (Saura et al. 2006) and may be a possible mechanism for linking PM to cardiovascular mortality (Pope et al. 2004a) although it may require consistent PM exposure that may or may not occur with ambient conditions. IL-6 is constitutively released in these cells and has potential autocrine signaling, its release is enhanced by PM exposure (Watterson et al. 2007), and eNOS is expressed in the lung and lung cells (Higashimoto et al. 2005; Ten Broeke et al. 2006). Our inability to detect eNOS phosphorylation at Ser1177 (Morrow et al. 2003) despite Akt activation, which activates eNOS (Ndiaye et al. 2005), supports these hypotheses of IL-6 inhibiting eNOS and warrants additional study. IL-8 is angiogenic contributing to tumor progression in lung cancer (Yuan et al. 2005; Luppi et al. 2007), but IL-8 autocrine signaling is unlikely here, as evidenced by a lack of observable STAT-1 phosphorylation examined previously (Watterson et al. 2009a, 2009b). While this is not the first study to detect IL-1α release from BEAS-2B cells (Griego et al. 2000), it appears the first to detect PM-mediated IL-1α release from BEAS-2B cells. The contribution of IL-1 to atherogenesis is well documented (Chi et al. 2004), and IL-1 contributes to tumor progression (Lewis et al. 2006; Elaraj et al. 2006). Of the cytokines not detected in this study, TNF-α release from BEAS-2B cells has been documented following exposure to studded-tire wear particles (Lindbom et al. 2006).
This evidence of increases in Akt activation raises additional questions concerning the mechanisms of PM-induced pathogenesis. Akt is well known for its role in insulin signaling (Alessi et al. 1996), and insulin in the growth media (approximately 5 mg/L, Invitrogen Technical support) may be responsible for the basal levels of Akt phosphorylation observed here, as well as in other studies (Zhang et al. 2006). It would be interesting to see whether PM-mediated Akt activation might play a role in the observed association between PM exposure and diabetes prevalence (Pearson et al. 2010). The role of the TLR network in PM-mediated effects has been examined (Becker et al. 2002, 2005a), and the TLR network is implicated in Akt activation (Hazeki et al. 2007; Ha et al. 2008; Li et al. 2004). The current study has demonstrated that PM-mediated Akt and NF-κB activation are through an IL-1R1-independent mechanism as evidenced by the lack of PM-inducible IRAK-1 phosphorylation. This is intriguing given that PM treatment resulted in increases in IRAK-1 protein levels (Fig. 7). TOLLIP may be upregulated resulting in IRAK/MyD88 inhibition, or MyD88 may be activated by other receptors (Hayden and Ghosh 2004). IRAK/dependent signaling represents a small portion of the large TLR signaling network, and many other potential mechanisms of receptor-mediated activation of Akt exist (Oda and Kitano 2006). Akt activation also occurs through receptor tyrosine kinases (RTKs) (Zhang et al. 2007), IL-6 (Chen et al. 1999), the janus kinase (Jak)/Stat pathway (Gross et al. 2006), G-protein-coupled receptors (Kong et al. 2006), peroxisome proliferator–activating receptors, (Amaravadi and Thompson 2005), and calpain (Tan et al. 2006; Pianetti et al. 2001). The leupeptin co-administration experiments demonstrated that the PM-mediated Akt activation is possibly due to calpain or a similar serine/cysteine protease(s). Leupeptin-mediated calpain inhibition appeared to inhibit PDK-1, which phosphorylates Akt at Thr308 (Vanhaesebroeck and Alessi 2000) or promoted its dephosphorylation (Gao et al. 2005), while not affecting PM-mediated increases in integrin-linked kinase (Persad et al. 2001), MTOR (Sarbassov et al. 2005), or other unknown protein(s) with PDK-2 activity that phosphorylate Akt at Ser473 (Vanhaesebroeck and Alessi 2000). The PM-mediated calpain activation and subsequent Akt activation are possibly due to PM-induced ER stress (Watterson et al. 2009).
There were no consistent differences between the responses to fine and coarse PM in this study. The slight differences observed, when present, were possibly due to a slightly different chemical profile generally present in differing PM diameter ranges (National Center for Environmental Assessment (Research Triangle Park N.C.) 2003). In any event, toxicological differences between size categories in vivo are mostly likely a result of differential deposition patterns in the respiratory tract, which are not possible to assess in an in vitro cell-based system. There were time-related differences between coarse and fine PM as evidenced by the 1- and 24-h exposures with pGSK, IκB, pPTEN, and p-Akt (Figs. 2, 3). More rapidly activated pathways may be stimulated by the components of fine PM and the slower by the coarse. Given that the longer 24-h exposure resulted in increased effects, individuals who have compromised clearance may be at greater risk for PM-mediated harm.
The USEPA states “Despite a strong consensus that exposure to PM induces adverse health effects […] relatively little is known about the specific physical or chemical characteristics of the particles that cause these effects or the mechanisms through which the adverse effects are induced” (NHEERL 2010). Thus, the present study may be helpful in revealing molecular and cellular mechanisms by which PM exerts harm.
This study demonstrates that ambient PM enhances activation of Akt in human pulmonary epithelial cells in vitro, and this activation is likely compensatory to ER stress and other events. Activation of Akt involves PTEN inactivation and appears to depend upon calpain or other similar proteases, rather than receptor-mediated MyD88 activation. In total, these studies support the hypothesis that the pro-inflammatory effects of PM may be linked to Akt activation.
References
Abraham E (2003) Neutrophils and acute lung injury. Crit Care Med 31(4 Suppl):S195–199
Abraham E (2005) Akt/protein kinase B. Crit Care Med 33(12 Suppl):S420–422
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15(23):6541–6551
Amaravadi R, Thompson CB (2005) The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest 115(10):2618–2624
Arcaroli J, Silva E, Maloney JP, He Q, Svetkauskaite D, Murphy JR, Abraham E (2006) Variant IRAK-1 haplotype is associated with increased nuclear factor-{kappa}B activation and worse outcomes in sepsis. Am J Respir Crit Care Med 173(12):1335–1341. doi:10.1164/rccm.200603-341OC
Barnard DL, Day CW, Bailey K, Heiner M, Montgomery R, Lauridsen L, Winslow S, Hoopes J, Li JK, Lee J, Carson DA, Cottam HB, Sidwell RW (2006) Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antiviral Res 71(1):53–63
Becker S, Soukup J (2003) Coarse(PM(2.5-10)), fine(PM(2.5)), and ultrafine air pollution particles induce/increase immune costimulatory receptors on human blood-derived monocytes but not on alveolar macrophages. J Toxicol Environ Health A 66(9):847–859
Becker S, Fenton MJ, Soukup JM (2002) Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Respir Cell Mol Biol 27(5):611–618
Becker S, Dailey L, Soukup JM, Silbajoris R, Devlin RB (2005a) TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol Appl Pharmacol 203(1):45–52
Becker S, Dailey LA, Soukup JM, Grambow SC, Devlin RB, Huang YC (2005b) Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ Health Perspect 113(8):1032–1038
Becker S, Mundandhara S, Devlin RB, Madden M (2005c) Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicol Appl Pharmacol 207(2 Suppl):269–275
Brognard J, Clark AS, Ni Y, Dennis PA (2001) Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 61(10):3986–3997
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96(6):857–868
Cantley LC, Neel BG (1999) New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 96(8):4240–4245
Carloni S, Mazzoni E, Balduini W (2004) Caspase-3 and calpain activities after acute and repeated ethanol administration during the rat brain growth spurt. J Neurochem 89(1):197–203
Casamayor A, Morrice NA, Alessi DR (1999) Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem J 342(Pt 2):287–292
Chatterjee M, Jain S, Stuhmer T, Andrulis M, Ungethum U, Kuban RJ, Lorentz H, Bommert K, Topp M, Kramer D, Muller-Hermelink HK, Einsele H, Greiner A, Bargou RC (2006) STAT3 and MAPK signaling maintains overexpression of the heat shock proteins 90 {alpha} and {beta} in multiple myeloma cells, which critically contribute to tumor cell survival. Blood 109(2):720–728
Chen ZJ (2005) Ubiquitin signalling in the NF-kappa B pathway. Nat Cell Biol 7(8):758–765
Chen RH, Chang MC, Su YH, Tsai YT, Kuo ML (1999) Interleukin-6 inhibits transforming growth factor-beta-induced apoptosis through the phosphatidylinositol 3-kinase/Akt and signal transducers and activators of transcription 3 pathways. J Biol Chem 274(33):23013–23019
Chen Q, Powell DW, Rane MJ, Singh S, Butt W, Klein JB, McLeish KR (2003) Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J Immunol 170(10):5302–5308
Chi H, Messas E, Levine RA, Graves DT, Amar S (2004) Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation 110(12):1678–1685
Choi JH, Kim JS, Kim YC, Kim YS, Chung NH, Cho MH (2004) Comparative study of PM2.5—and PM10—induced oxidative stress in rat lung epithelial cells. J Vet Sci 5(1):11–18
Churg A, Xie C, Wang X, Vincent R, Wang RD (2005) Air pollution particles activate NF-kappa B on contact with airway epithelial cell surfaces. Toxicol Appl Pharmacol 208(1):37–45
Dagher Z, Garcon G, Billet S, Verdin A, Ledoux F, Courcot D, Aboukais A, Shirali P (2007) Role of nuclear factor-kappa B activation in the adverse effects induced by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. J Appl Toxicol 27(3):284–290
Daly DS, White AM, Varnum SM, Anderson KK, Zangar RC (2005) Evaluating concentration estimation errors in ELISA microarray experiments. BMC Bioinform 6:17
Delfino RJ, Sioutas C, Malik S (2005) Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect 113(8):934–946
Donaldson K, Stone V, Seaton A, MacNee W (2001) Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect 109(Suppl 4):523–527
Edgerton ES, Hartsell BE, Saylor RD, Jansen JJ, Hansen DA, Hidy GM (2006) The Southeastern Aerosol Research and Characterization Study, part 3: continuous measurements of fine particulate matter mass and composition. J Air Waste Manag Assoc 56(9):1325–1341
Elaraj DM, Weinreich DM, Varghese S, Puhlmann M, Hewitt SM, Carroll NM, Feldman ED, Turner EM, Alexander HR (2006) The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res 12(4):1088–1096
Frampton MW, Ghio AJ, Samet JM, Carson JL, Carter JD, Devlin RB (1999) Effects of aqueous extracts of PM(10) filters from the Utah valley on human airway epithelial cells. Am J Physiol 277(5 Pt 1):L960–967
Gao T, Furnari F, Newton AC (2005) PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell 18(1):13–24
Gao K, Dai DL, Martinka M, Li G (2006) Prognostic significance of nuclear factor-kappa B p105/p50 in human melanoma and its role in cell migration. Cancer Res 66(17):8382–8388
Glantz SA (2005) Primer of biostatistics, 6th edn. McGraw-Hill Medical Pub, New York
Gowen BB, Hoopes JD, Wong MH, Jung KH, Isakson KC, Alexopoulou L, Flavell RA, Sidwell RW (2006) TLR3 deletion limits mortality and disease severity due to Phlebovirus infection. J Immunol 177(9):6301–6307
Griego SD, Weston CB, Adams JL, Tal-Singer R, Dillon SB (2000) Role of p38 mitogen-activated protein kinase in rhinovirus-induced cytokine production by bronchial epithelial cells. J Immunol 165(9):5211–5220
Gross ER, Hsu AK, Gross GJ (2006) The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3 beta. Am J Physiol Heart Circ Physiol 291(2):H827–834
Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C (2008) Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3 K/Akt-dependent mechanism. Cardiovasc Res 78(3):546–553
Hajduch E, Litherland GJ, Hundal HS (2001) Protein kinase B (PKB/Akt)-a key regulator of glucose transport? FEBS Lett 492(3):199–203
Hayden MS, Ghosh S (2004) Signaling to NF-kappa B. Genes Dev 18(18):2195–2224
Hazeki K, Nigorikawa K, Hazeki O (2007) Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull 30(9):1617–1623
Higashimoto Y, Ohata M, Yamagata Y, Iwata T, Masuda M, Ishiguchi T, Okada M, Satoh H, Itoh H (2005) Effect of the adenovirus E1A gene on nitric oxide production in alveolar epithelial cells. Clin Microbiol Infect 11(8):644–650
Hu P, Han Z, Couvillon AD, Exton JH (2004) Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem 279(47):49420–49429
Jalava PI, Salonen RO, Pennanen AS, Sillanpaa M, Halinen AI, Happo MS, Hillamo R, Brunekreef B, Katsouyanni K, Sunyer J, Hirvonen MR (2007) Heterogeneities in inflammatory and cytotoxic responses of RAW 264.7 macrophage cell line to urban air coarse, fine, and ultrafine particles from six European sampling campaigns. Inhal Toxicol 19(3):213–225
Jimenez LA, Thompson J, Brown DA, Rahman I, Antonicelli F, Duffin R, Drost EM, Hay RT, Donaldson K, MacNee W (2000) Activation of NF-kappa B by PM(10) occurs via an iron-mediated mechanism in the absence of Ikappa B degradation. Toxicol Appl Pharmacol 166(2):101–110
Kaimul Ahsan M, Nakamura H, Tanito M, Yamada K, Utsumi H, Yodoi J (2005) Thioredoxin-1 suppresses lung injury and apoptosis induced by diesel exhaust particles (DEP) by scavenging reactive oxygen species and by inhibiting DEP-induced downregulation of Akt. Free Radic Biol Med 39(12):1549–1559
Knuckles TL, Dreher KL (2007) Fine oil combustion particle bioavailable constituents induce molecular profiles of oxidative stress, altered function, and cellular injury in cardiomyocytes. J Toxicol Environ Health A 70(21):1824–1837
Kollewe C, Mackensen AC, Neumann D, Knop J, Cao P, Li S, Wesche H, Martin MU (2004) Sequential autophosphorylation steps in the interleukin-1 receptor-associated kinase-1 regulate its availability as an adapter in interleukin-1 signaling. J Biol Chem 279(7):5227–5236
Kong KC, Billington CK, Gandhi U, Panettieri RA Jr, Penn RB (2006) Cooperative mitogenic signaling by G protein-coupled receptors and growth factors is dependent on G(q/11). Faseb J 20(9):1558–1560
Koziczak-Holbro M, Joyce C, Gluck A, Kinzel B, Muller M, Tschopp C, Mathison JC, Davis CN, Gram H (2007) IRAK-4 kinase activity is required for IL-1 receptor- and toll-like receptor 7-mediated signaling and gene expression. J Biol Chem
Lewis AM, Varghese S, Xu H, Alexander HR (2006) Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med 4:48
Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, Browder W, Williams DL (2004) Modulating toll-like receptor mediated signaling by (1– > 3)-beta-D-glucan rapidly induces cardioprotection. Cardiovasc Res 61(3):538–547
Limesand KH, Schwertfeger KL, Anderson SM (2006) MDM2 is required for suppression of apoptosis by activated Akt1 in salivary acinar cells. Mol Cell Biol 26(23):8840–8856
Lindbom J, Gustafsson M, Blomqvist G, Dahl A, Gudmundsson A, Swietlicki E, Ljungman AG (2006) Exposure to wear particles generated from studded tires and pavement induces inflammatory cytokine release from human macrophages. Chem Res Toxicol 19(4):521–530
Lippmann M, Frampton M, Schwartz J, Dockery D, Schlesinger R, Koutrakis P, Froines J, Nel A, Finkelstein J, Godleski J, Kaufman J, Koenig J, Larson T, Luchtel D, Liu LJ, Oberdorster G, Peters A, Sarnat J, Sioutas C, Suh H, Sullivan J, Utell M, Wichmann E, Zelikoff J (2003) The US Environmental Protection Agency Particulate Matter Health Effects Research Centers Program: a midcourse report of status, progress, and plans. Environ Health Perspect 111(8):1074–1092
Luppi F, Longo AM, de Boer WI, Rabe KF, Hiemstra PS (2007) Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer 56(1):25–33
Lurmann FW, Brown SG, McCarthy MC, Roberts PT (2006) Processes influencing secondary aerosol formation in the San Joaquin Valley during winter. J Air Waste Manag Assoc 56(12):1679–1693
Ma C, Wang J, Luo J (2004) Activation of nuclear factor kappa B by diesel exhaust particles in mouse epidermal cells through phosphatidylinositol 3-kinase/Akt signaling pathway. Biochem Pharmacol 67(10):1975–1983
Malek E, Davis T, Martin RS, Silva PJ (2006) Meteorological and environmental aspects of one of the worst national air pollution episodes (January, 2004) in Logan, Cache Valley, Utah, USA. Atmospheric Research 79(2):108–122
Mallikarjuna G, Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R (2004) Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res 64(17):6349–6356
Mangelson NF, Lewis L, Joseph JM, Cui WX, Machir J, Williams NW, Eatough DJ, Rees LB, Wilkerson T, Jensen DT (1997) The contribution of sulfate and nitrate to atmospheric fine particles during winter inversion fogs in Cache Valley, Utah. Journal Of The Air & Waste Management Association 47(2):167–175
Matute-Bello G, Liles WC, Radella F 2nd, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR (1997) Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 156(6):1969–1977
Meek DW, Knippschild U (2003) Posttranslational modification of MDM2. Mol Cancer Res 1(14):1017–1026
Misra UK, Deedwania R, Pizzo SV (2006) Activation and cross-talk between Akt, NF-kappa B, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem 281(19):13694–13707
Momiyama J, Hashimoto T, Matsubara A, Futai K, Namba A, Shinkawa H (2006) Leupeptin, a calpain inhibitor, protects inner ear hair cells from aminoglycoside ototoxicity. Tohoku J Exp Med 209(2):89–97
Monn C, Becker S (1999) Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10-2.5) in outdoor and indoor air. Toxicol Appl Pharmacol 155(3):245–252
Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP (2003) Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem 278(34):31629–31639
Mossman BT, Borm PJ, Castranova V, Costa DL, Donaldson K, Kleeberger SR (2007) Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Part Fibre Toxicol 4:4
Mullonkal CJ, Toledo-Pereyra LH (2007) Akt in ischemia and reperfusion. J Invest Surg 20(3):195–203
Nadiminty N, Lou W, Lee SO, Lin X, Trump DL, Gao AC (2006) Stat3 activation of NF-{kappa} B p100 processing involves CBP/p300-mediated acetylation. Proc Natl Acad Sci USA 103(19):7264–7269
National Center for Environmental Assessment (Research Triangle Park N.C.) (1996) Air quality criteria for particulate matter. National Center for Environmental Assessment, Office of Research and Development. US Environmental Protection Agency, Research Triangle Park
National Center for Environmental Assessment (Research Triangle Park N.C.) (2003) Air quality criteria for particulate matter. National Center for Environmental Assessment-RTP Office, Office of Research and Development, US Environmental Protection Agency. http://purl.access.gpo.gov/GPO/LPS34686, http://purl.access.gpo.gov/GPO/LPS34687
Ndiaye M, Chataigneau M, Lobysheva I, Chataigneau T, Schini-Kerth VB (2005) Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. Faseb J 19(3):455–457
Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, McHenry CL, Hennrick KT, Tsai WC, Bentley JK, Lukacs NW, Johnston SL, Hershenson MB (2008) Human rhinovirus 1B exposure induces PI 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med
NHEERL (2010) Particulate Matter (PM) Health Effects. http://www.epa.gov/NHEERL/research/pm/. Accessed December 4, 2010 2011
Oda K, Kitano H (2006) A comprehensive map of the toll-like receptor signaling network. Mol Syst Biol 2:15
Ono Y, Sorimachi H, Suzuki K (1999) The Calpain superfamily. In: Wang KW, Yuen P (eds) CALPAIN: pharmacology and toxicology of calcium-dependent protease. Taylor and Francis, Philadelphia, pp 1–24
Osornio-Vargas AR, Bonner JC, Alfaro-Moreno E, Martinez L, Garcia-Cuellar C, Ponce-de-Leon Rosales S, Miranda J, Rosas I (2003) Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ Health Perspect 111(10):1289–1293
Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS (2010) Association between fine particulate matter and diabetes prevalence in the US Diabetes Care 33(10):2196–2201
Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S (2001) Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem 276(29):27462–27469
Peters A, Veronesi B, Calderon-Garciduenas L, Gehr P, Chen LC, Geiser M, Reed W, Rothen-Rutishauser B, Schurch S, Schulz H (2006) Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol 3:13
Peterson RT, Schreiber SL (1998) Translation control: connecting mitogens and the ribosome. Curr Biol 8(7):R248–250
Pianetti S, Arsura M, Romieu-Mourez R, Coffey RJ, Sonenshein GE (2001) Her-2/neu overexpression induces NF-kappa B via a PI3-kinase/Akt pathway involving calpain-mediated degradation of I kappa B-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene 20(11):1287–1299
Pope CA 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD (2002) Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama 287(9):1132–1141
Pope CA 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ (2004a) Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 109(1):71–77
Pope CA 3rd, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, Eatough DJ (2004b) Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect 112(3):339–345
Pozzi R, De Berardis B, Paoletti L, Guastadisegni C (2003) Inflammatory mediators induced by coarse (PM2.5–10) and fine (PM2.5) urban air particles in RAW 264.7 cells. Toxicology 183(1-3):243–254
Pruett SB, Loftis AY (1990) Characteristics of MTT as an indicator of viability and respiratory burst activity of human neutrophils. Int Arch Allergy Appl Immunol 92(2):189–192
Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB (2003) Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 278(30):27828–27835
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307(5712):1098–1101
Saura M, Zaragoza C, Bao C, Herranz B, Rodriguez-Puyol M, Lowenstein CJ (2006) Stat3 mediates interleukin-6 inhibition of human endothelial nitric-oxide synthase expression. J Biol Chem 281(40):30057–30062
Schins RP, Lightbody JH, Borm PJ, Shi T, Donaldson K, Stone V (2004) Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol 195(1):1–11
Schwartz J, Dockery DW, Neas LM (1996) Is daily mortality associated specifically with fine particles? J Air Waste Manag Assoc 46(10):927–939
Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M (2001) Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293(5534):1495–1499
Soukup JM, Becker S (2001) Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicol Appl Pharmacol 171(1):20–26
Soukup JM, Ghio AJ, Becker S (2000) Soluble components of Utah Valley particulate pollution alter alveolar macrophage function in vivo and in vitro. Inhal Toxicol 12(5):401–414
Strassheim D, Asehnoune K, Park JS, Kim JY, He Q, Richter D, Kuhn K, Mitra S, Abraham E (2004) Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of toll-like receptor 2-stimulated neutrophils. J Immunol 172(9):5727–5733
Sumanasekera WK, Ivanova MM, Johnston BJ, Dougherty SM, Sumanasekera GU, Myers SR, Ali Y, Kizu R, Klinge CM (2007) Rapid effects of diesel exhaust particulate extracts on intracellular signaling in human endothelial cells. Toxicol Lett 174(1–3):61–73
Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S (2009) Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119(4):538–546
Takahashi M, Tanonaka K, Yoshida H, Koshimizu M, Daicho T, Oikawa R, Takeo S (2006) Possible involvement of calpain activation in pathogenesis of chronic heart failure after acute myocardial infarction. J Cardiovasc Pharmacol 47(3):413–421
Tan Y, Wu C, De Veyra T, Greer PA (2006) Ubiquitous calpains promote both apoptosis and survival signals in response to different cell death stimuli. J Biol Chem 281(26):17689–17698
Tao F, Gonzalez-Flecha B, Kobzik L (2003) Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radic Biol Med 35(4):327–340
Ten Broeke R, De Crom R, Van Haperen R, Verweij V, Leusink-Muis T, Van Ark I, De Clerck F, Nijkamp FP, Folkerts G (2006) Overexpression of endothelial nitric oxide synthase suppresses features of allergic asthma in mice. Respir Res 7:58
USA-Today (2005) Northern Utah residents choke on bad air. USA Today, March 8, 2005
Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H (2002) HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277(17):15107–15112
Van Vleet TR, Watterson TL, Klein PJ, Coulombe RA Jr (2006) Aflatoxin B1 alters the expression of p53 in cytochrome P450-expressing human lung cells. Toxicol Sci 89(2):399–407
Vanhaesebroeck B, Alessi DR (2000) The PI3 K-PDK1 connection: more than just a road to PKB. Biochem J 346(Pt 3):561–576
Vazquez F, Ramaswamy S, Nakamura N, Sellers WR (2000) Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol 20(14):5010–5018
Velasco M, Diaz-Guerra MJ, Martin-Sanz P, Alvarez A, Bosca L (1997) Rapid Up-regulation of I kappa B beta and abrogation of NF-kappa B activity in peritoneal macrophages stimulated with lipopolysaccharide. J Biol Chem 272(37):23025–23030
Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B (2008) Resveratrol inhibits high glucose-induced PI3 K/AKT/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am J Physiol Heart Circ Physiol 294(5):H2078–2087
Veranth JM, Reilly CA, Veranth MM, Moss TA, Langelier CR, Lanza DL, Yost GS (2004) Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide, and surface-modified particles. Toxicol Sci 82(1):88–96
Watterson TL, Sorensen J, Martin R, Coulombe RA, Jr (2007) Effects of PM2.5 collected from Cache Valley Utah on genes associated with the inflammatory response in human lung cells. J Toxicol Environ Health A 70(20):1731–1744
Watterson TL, Hamilton B, Martin R, Coulombe RA Jr (2009a) Urban particulate matter causes ER stress and the unfolded protein response in human lung cells. Toxicol Sci 112(1):111–122
Watterson TL, Hamilton B, Martin R, Coulombe RA Jr (2009) Urban particulate matter causes ER stress and the unfolded protein response in human lung cells. Toxicol Sci. Submitted for publication
West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA (2003) Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 111(1):81–90
Williams MR, Arthur JS, Balendran A, van der Kaay J, Poli V, Cohen P, Alessi DR (2000) The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol 10(8):439–448
Wong CK, Cheung PF, Ip WK, Lam CW (2007) Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol 37(1):85–96
Woznicova V, Votava M (2001) Western blot determination of IgG avidity in primary and secondary syphilis. Scripta Medica (BRNO) 74(5):353–360
Yamin TT, Miller DK (1997) The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J Biol Chem 272(34):21540–21547
Yuan A, Chen JJ, Yao PL, Yang PC (2005) The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci 10:853–865
Yuan H, Wong LS, Bhattacharya M, Ma C, Zafarani M, Yao M, Schneider M, Pitas RE, Martins-Green M (2007) The effects of second-hand smoke on biological processes important in atherogenesis. BMC Cardiovasc Disord 7:1
Zhang Y, Bhatia D, Xia H, Castranova V, Shi X, Chen F (2006) Nucleolin links to arsenic-induced stabilization of GADD45alpha mRNA. Nucleic Acids Res 34(2):485–495
Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, Griffin JD, Kwiatkowski DJ (2007) PDGFRs are critical for PI3 K/Akt activation and negatively regulated by mTOR. J Clin Invest 117(3):730–738
Acknowledgments
The authors thank Dr. John R. Stevens for the helpful suggestions. This work was supported in part by a generous gift from the Marriner S. Eccles Foundation, a pilot project of the National Children’s Study, and by the Utah Agricultural Experiment Station. Portions of this work were presented at the Society of Toxicology annual conference March 2009 in Baltimore, MD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watterson, T.L., Hamilton, B., Martin, R.S. et al. Urban particulate matter activates Akt in human lung cells. Arch Toxicol 86, 121–135 (2012). https://doi.org/10.1007/s00204-011-0739-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0739-5