Abstract
Selective low (15 mg sodium fluoride (NaF)/L) and relatively high (150 mg NaF/L) doses of in vivo fluoride (F) treatment to Swiss albino mice through drinking water elicited organ-specific toxicological response. All the F-exposed groups showed severe alterations in both liver and kidney architectures, but there was no significant change in the rate of water consumption and body weight. Vacuolar degeneration, micronecrotic foci in the hepatocytes, and hepatocellular hypertrophy were evident in the mice exposed to low dose (15 mg NaF/L for 30 days) while sinusoidal dilation with enlarged central vein surrounded by deep-blue erythrocytes were preponderant when treated with the same dose for a period of 90 days. Blood filled spaces, disintegration of tubular epithelium, and atrophy of glomeruli were also recorded in the kidney of the same treatment group. Change in reduced glutathione level (GSH), glutathione-s-transferase (GST) activity, malondialdehyde (MDA) production in both liver and kidney, disturbances in liver function, induction of heat shock protein 70 (Hsp 70) expression in kidney and its down regulation in liver were positively correlated with histopathological lesion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluoride (F) is ubiquitously present in the environment and is one of the most abundant elements in earth crust and an important environmental toxicant originating from natural and industrial sources (Whitford 1983). The main source of F contamination is through drinking water. Other sources include food, dental products, F dust and fumes from industries using F-containing salt and or hydrofluoric acid (Shulman and Wells 1997). Fluoride crosses cell membranes by simple diffusion, enters soft tissues and causes impairment of soft tissues (Carlson et al. 1960; Jacyszyn and Marut 1986). Fluorosis, an irreversible disease is a major public health hazard in India and several other countries, affects all the age group due to chronic ingestion of F (Susheela and Bhatnagar 2002). In addition to skeletal manifestations, F ingestion over a long period of time affects the soft tissues like muscle, liver, kidney, gastrointestinal tract and several other reproductive and endocrine organs (Chinoy et al. 1991; Susheela et al. 1988; DeCamargo and Merzel 1980; Darmani et al. 2001; Kour et al. 1981; Kour and Singh 1980; Liu et al. 1999; Machalinska et al. 2002; Shashi and Thapar 2000; Shashi et al. 2002; Zhan et al. 2006; Zhang et al. 2006). F remains in drinking water in ionic form and passes through the intestinal mucosa passively and interferes with major metabolic pathways.
We have earlier reported that F is more genotoxic at lower dose (15 mg NaF/L) than the higher dose (150 mg NaF/L) when treated through drinking water for 30 days in mice (Podder et al. 2008a, b). In dilute aqueous solution, 100% of NaF dissociate to sodium (Na) and F ions (Machalinski et al. 2003), thus 15 mg/L NaF yields 6.8 mg F/L. This concentration range of F is reported in some potable water in India and other countries (Kotoky et al. 2008). In Guadiana Valley of Mexico, spontaneous hip fractures and eventually greater proportion of decrease in bone tensile strength was found in the 6 ppm F-exposed human population rather than at lower or higher concentration (Alarcón-Herrera et al. 2001).
There are several reports on hepatic and kidney toxicity of F, but most of the studies were conducted using much higher doses of F (DeCamargo and Merzel 1980; Darmani et al. 2001; Machalinska et al. 2002; Shashi and Thapar 2000; Shashi et al. 2002). There has been no investigation into tissue-specific toxic responses in mammals exposed to low and high dose of F for different time periods. In the present study, we attempted to look into the histopathological changes in liver and kidney as well as tried to understand the underlying mechanism of action. Parameters studied were rate of water consumption, body weight gain, organo-somatic index (OSI), liver function tests and oxidative status (GSH level, GST activity and MDA production) and concurrent response of heat shock protein 70 (Hsp 70) induced by F in Swiss albino mice. In view of the prolonged exposure of F to human population, our study will illustrate the overall toxic potential of F in those endemic areas where the population is exposed naturally to F through drinking water or any other natural entry routes.
Materials and methods
Chemicals
The primary antibody and anti-heat shock protein 70 (raised in mouse) were procured from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Ammonium persulfate, 5-5′-dithiobis (2-nitro benzoic acid) (DTNB), sodium fluoride (NaF, molecular weight 41.99) and all other chemicals used were of analytical grade, purchased from Sisco Research Laboratories (Mumbai, India) and E. Merck (Mumbai, India). Glutamate oxaloacetate transaminase GOT (AST) and glutamate pyruvate transaminase GPT (ALT) test kits were purchased from Span diagnostics Ltd., Surat, India. Rabbit anti-mouse IgG linked to alkaline phosphatase was procured from Bangalore Genei Pvt. Ltd (Bangalore, India).
Animals and experimental design
Thirty-two healthy 8-week-old male Swiss albino mice weighing approximately 25–30 g were housed in stainless steel cages individually in a controlled temperature room (25 ± 2°C) and a 12-h light/12-h dark cycle. The animals were provided with standard mouse diet (NMC Oil Mills Ltd, Pune, India) and randomly divided into four groups as given below. All regulations of the Animal Ethics Committee of the University were strictly followed during the experiment to protect the welfare of the animals.
- Group I ::
-
Nonexposed animals (Control)
- Group II ::
-
15 mg NaF/L treated through drinking water for 30 days
- Group III ::
-
150 mg NaF/L treated through drinking water for 30 days
- Group IV ::
-
15 mg NaF/L treated through drinking water for 90 days
Measurement of body weight and water consumption
The body weight of all the animals was recorded initially and also during the course of the treatment. Rate of water consumption and gain in body weight were recorded for each individual mouse at certain time intervals during the experiment.
Determination of organo-somatic index (OSI)
The weight of the mice was recorded before killing under light ether anesthesia. Liver and kidney were dissected out carefully, blotted free of blood and fresh weight was recorded. OSI was calculated and compared with the control mice.
Liver function tests
Estimation of serum glutamate oxaloacetate transaminase (SGOT) (aspartate transaminase; AST) and serum glutamate pyruvate transaminase (SGPT) (alanine transaminase; ALT).
Serum GOT (AST) and GPT (ALT) levels were determined following the manufacturer’s protocol.
Determination of reduced glutathione (GSH)
The reduced glutathione of the liver and kidney was measured following the method of Beutler et al. (1964). In brief, liver and kidney tissues were dissected very quickly and blanched in ice-cold isotonic saline. A 10% homogenate was prepared from each tissue with ice-cold saline-EDTA at 4°C. One milliliter of freshly prepared 20% ice-cold trichloroacetic acid (TCA) was added to equal volume of homogenate and the mixture was vortexed and allowed to stand for 10 min in 4°C. The mixture was then centrifuged at 5,000 rpm for 5 min. The clear supernatant was used as the reduced glutathione (GSH) sample from which 1 ml of supernatant was taken, mixed with 3 ml of 0.3 M di-sodium hydrogen phosphate buffer and 1 ml of 5,5′-dithiobis-2-nitro benzoic acid (DTNB) solution. After 5 min, the optical density of the samples was measured at 412 nm. The GSH content of the sample was expressed as μM GSH/mg protein.
Assay of glutathione-s-transferase (GST)
GST Activity was assessed in the liver and kidney cytosolic fractions as described by Habig et al. (1974) using 1-chloro-2,4-dinitrobenzene (CDNB) (1 mM final concentration) as substrate in the presence of excess GSH (5 mM). The rate of CDNB conjugation was estimated by direct spectrophotometry at 340 nm for 3 min. The result was expressed as μM GS-CDNB formed/min/mg protein.
Assay of thiobarbituric acid reactive substances (TBARS) level in tissues
Lipid peroxidation products namely malondialdehyde (MDA) was estimated in liver and kidney microsomes assuming high PUFA content of microsomal membranes. The level of lipid peroxidation as measured by TBARS was determined according to the method of Buege and Aust (1978). Briefly, 1 ml of microsomal sample was mixed with 2 ml of TBA-TCA-HCl mixture thoroughly and heated for 15 min in a boiling water bath. After cooling, the flocculent precipitate was removed by centrifugation at 1,000 g for 10 min. The absorbance of the supernatant was determined at 535 nm. The MDA concentration of the sample was calculated using an extinction coefficient of 1.56 ×105/mol/cm and expressed in terms of nM MDA/mg protein.
Histopathological studies
Portions of liver and kidney tissues of all animals were fixed in Bouin’s fluid, dehydrated through graded alcohol, embedded in paraffin and routine microtomy was carried out to obtain 6-μm-thick tissue sections. Sections were stained by routine hematoxylin-eosin (HE) technique and viewed under light microscope.
Estimation of heat shock protein 70 (Hsp 70)
Sample preparation
The homogenates (10% w/v) of liver and kidney tissues were prepared in 50 mM phosphate buffer (pH 7.5), and samples were centrifuged at 10,000 g for 20 min. The cytosolic supernatant was collected very carefully, and the protein content of the sample was measured following the method of Lowry et al. (1951).
Methods for western blotting
The samples were run through a 10% SDS–PAGE at constant voltage and blotted on a polyvinylidene fluoride membrane following the method as described by Agarwal et al. (2009). Anti-Hsp 70 monoclonal antibody raised in rabbit was used as primary antibody, and goat anti-rabbit IgG linked to alkaline phosphatase was used as secondary antibody. The membrane was washed thoroughly and then incubated in the presence of the substrate (BCIP/NBT) until a colored band developed at the 70-kDa position.
Statistical analysis
The data were analyzed by Fisher’s t test using the Sigma plot 8.0 statistical package. Difference between control and experimental group(s) with a value of P < 0.05 was considered significant.
Results
Mortality and clinical observations
All mice were observed daily for any clinical signs of toxicity. There was no death in control and treatment groups, and no clinical symptoms were found to appear in any of the treatment groups.
Change in body weight and water consumption rate
There was no significant difference in water consumption rate as well as change in body weight (weight gain) recorded between the control and treatment groups (data not shown).
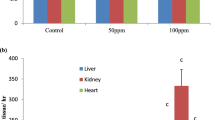
Organo-somatic index (OSI)
No significant difference in the OSI of liver (Fig. 1a) and kidney (Fig. 1b) of any treatment group with the control group was observed.
Liver function tests
An overall increase was noted in the serum GOT (AST) and GPT (ALT) levels noted in all groups (Fig. 2). The GPT level increased significantly in all the treatment groups whereas GOT level increased significantly in group III (65%) and VI (73%).
GSH-GST response and TBARS production
GSH level of liver and kidney decreased in all the treatment groups recording a significant decrease (32%) in the liver of group II mice (Fig. 3). GST activity increased significantly in the liver of all the treatment groups (Fig. 4a) whereas in kidney, it increased only in the Gr-II mice (Fig. 4b). The lipid peroxidation product i.e., MDA production increased gradually in the liver of all the treatment groups and significant increase was found in Gr-IV mice (exposed to the lower dose of F for 90 days) (Fig. 4c). In kidney, the TBARS level increased in all the treatment groups and it increased significantly in the Gr-II mice exposed to lower dose of F for 30 days)(Fig. 4d).
Histopathological studies
Histopathology of liver
Histopathological studies demonstrated an extensive alteration in liver and kidney histoarchitecture. The control group of liver appeared normal and healthy (Fig. 5a). In the liver of group II mice, extensive vacuolar degeneration (Fig. 5b) in the cytoplasm was found. In group III mice, intoxication in the liver was severe showing extensive vacuolization in cytoplasm and loss of integrity in the epithelium lining of central vein (Fig. 5c). In group IV mice treated with NaF 15 mg/L for 90 days, hepatocellular hypertrophy, cytoplasmic vacuolization and hepatic sinusoidal dilation were extensive (Fig. 5d). The central vein was enlarged and surrounded by deep-blue erythrocytes peripherally. The lining of the central vein showed distortion of the parenchymatous cells.
Changes in liver histology. Microphotographs of liver sections (6 μm) stained with hematoxylin and eosin (HE). The original magnification: ×100. a Normal histological appearance of liver tissue of control mice. Central vein (CV). b Group II: Vacuolar degenerations (thick arrows) and disruption of epithelium lining (thin arrows) of the central vein. c Group III: Extensive vacuolization in cytoplasm (thick arrows), loss of integrity in epithelium lining the central vein (moderately thick arrows) and hepatocellular degeneration (thin arrows). d Group IV: Sinusoidal dilation (thin arrows), enlarged central vein (ECV) surrounded peripherally by deep-blue erythrocytes (thick arrows)
Histopathology of kidney
In group I control mice, kidney histoarchitecture was found to be normal but in treated groups, severe alteration of renal histological structures was noticed (Figs. 6 and 7). Atrophy of glomeruli was common to all treated groups. Blood-filled spaces (Figs. 6c, d and 7b) and varying degree of degeneration of tubular epithelium were noticed in Gr-II and Gr-IV mice (Figs. 6d and 7c).
Changes in kidney histology. Microphotograph of kidney sections (6 μm) stained with hematoxylin and eosin (HE). a Normal histological appearance of kidney tissue of control mice. The original magnification ×100. b Control kidney with normal sized glomerulus. The original magnification: ×400. c Group II: Atrophied glomerulus (thin arrow) and blood-filled spaces (thick arrows). The original magnification: ×100. d Group II: Atrophied and necrotic glomerulus (thin and long arrow) with varying degree of degeneration of tubular epithelium (thick arrow). The original magnification: ×400
Changes in kidney histology. Microphotograph of kidney sections (6 μm) stained with hematoxylin and eosin (HE). a Group III: Atrophied glomeruli (arrows). The original magnification: ×400 b Group IV: Blood-filled spaces (thick arrows), vacuolization (moderately thick arrows), atrophied glomeruli (thin arrows). The original magnification: ×100. c Group IV: Disintegration of renal tubular epithelium and vacuolization (arrows) in medullary ray region. The original magnification: ×1,000
Heat shock protein 70 profile
Elevated expression of Hsp 70 against the control was found in all the treatment groups of kidney; 15 mg NaF/L treatment (i.e. group II and group IV) had higher expression than group III of kidney (Fig. 8). In contrast to kidney, liver Hsp 70 reduced gradually dose and time dependently from the control. The decrease was most in the Gr-IV mice, exposed to lower dose of fluoride (NaF 15 mg/L) for 90 days.
Discussion
The main physiological entry route of F in a chronic exposure to human is through drinking water. Therefore, in the present study F was administered in mice through the same route. It was reported that F administration decreased the body weight gain in a dose- and time-dependent manner in rats (Shanthakumari et al. 2004). Darmani et al. (2001) and Dunipace et al. (1989) did not find any significant effect in water consumption rate but Darmani et al. (2001) found a significant increase in relative ovary weights at high F dose given through drinking water. In our study, we did not find any significant change in the rate of water consumption, in body weight gain or in the OSI of liver and kidney.
Liver is the main organ responsible for metabolism and detoxication. It indirectly affects hematopoiesis by destructing the normal and abnormal blood cells (Gale et al. 1978). Furthermore, as liver is involved in the metabolism of toxic compounds produced during systemic transformations and exogenous toxins entering into the organisms from the environment, it was assumed that NaF exposure would induce both pathomorphological and metabolic changes in liver (Dabrowaska et al. 2006). When toxic blood levels of F prevailed, there was selective damage to the tubular structures of the kidney by passage of the glomerular filtrate and consequent lesions produced in the kidney (Ogilvie 1953).
The common means of detecting liver damage is determination of two pathophysiological enzymes, serum glutamate pyruvate transaminase (SGPT) and serum glutamate oxalate transaminase (SGOT) in the serum. Guo et al. (2003) and Shanthakumari et al. (2004) reported significant increase in serum SGOT and SGPT levels in rats in a dose-dependent manner following NaF treatment (50, 100,150 mg/L for 3 months and 25 ppm/day for 8 and 16 weeks). Trivedi et al. (2008) also reported a significant dose-dependent increase in serum SGOT and SGPT levels in mice after oral administration of NaF (6 and 12 mg/kg body weight/day) for 30 days. Our findings also revealed significant increase in SGPT and SGOT activity in all the treatment groups when exposed to both high and low doses of NaF for 30 and 90 days, respectively.
There are many conflicting reports regarding F-induced toxicity in liver and kidney. Bosworth and McCay (1962) found no significant effect in kidney of rats after administration of as much as 10 ppm of NaF in their drinking water daily over the entire life span. DeCamargo and Merzel (1980) also did not find any detectable change with respect to weight gain, morphology of liver and kidney of rats receiving 1, 10 and 100 mg NaF/kg in drinking water for 180 days. Machalinska et al. (2002) did not detect any lesion in the kidney and liver of Balb C mice following tail vein injections of NaF. However, in rabbits, a relatively high concentration of NaF injected subcutaneously for a prolonged period (15 weeks) led to detectable necrotic and degenerative changes in these organs (Shashi and Thapar 2000, 2002). Our findings corroborate earlier observation as observed zonal necrosis and pycnosis of hepatocyte nuclei and disintegration of the arrangement of hepatic cords in fluoride-treated rats (Chinoy et al. 1991), recorded degenerative lesions in the liver of rats with only 1 ppm F in their drinking water (Mello et al. 1963), hepatocellular adenoma, hepatic foci of clear cells and basophilic alteration and sinusoidal dilation in liver when rats and mice were exposed up to 2,500 ppm vinyl fluoride (Bogdanffy et al. 1995), hypertrophy and hyperplasia in the renal tubules of rats treated with 1, 5 and 100 ppm of F for 500 days through drinking water (Ramseyer et al. 1957), shrunken kidney structure, atrophy of glomeruli, degeneration of tubular cells and dilation of convoluted tubules in mice (Kour and Singh 1980).
The liver and kidney toxicity could be mediated by reactive oxygen species generated during F-induced oxidative stress. Jeji et al. (1985), Saralakumari and Rao (1991) reported a close association between chronic F toxicity and increased oxidative stress in humans. There are reports on remarkable rise in malondialdehyde (MDA) and subsequent elevation in the activity of antioxidant enzymes in the liver (Guo et al. 2003), significant elevation of thiobarbituric acid reactive substances (TBARS) and hydroperoxides and decrease in the activities of SOD, GSH, glutathione peroxidase (GPx) and catalase (CAT) in the liver and kidney of experimental rats (Shanthakumari et al. 2004). Mittal and Flora (2006, 2007) reported a marked increase in TBARS level in mice liver and kidney and inhibition of antioxidative enzyme superoxide dismutase (SOD) but no changes in reduced glutathione (GSH) and oxidized glutathione (GSSG) level were observed by them. Chouhan and Flora (2008) found maximum decline in GSH/GSSG ratio in lowest dose and thereafter, less pronounced depletion with the higher concentration of F exposure resulted. The decrease in GSH level in liver and kidney was also evident in our study, which was significant in mice exposed to 15 mg NaF/L for 30 days.
GST is considered to contribute to the biotransformation of xenobiotics conjugating to these compounds which are often electrophilic and somewhat lipophilic in nature, with reduced glutathione to facilitate dissolution in the aqueous cellular and extracellular media, and then are removed from the body. This activity detoxifies endogenous compounds such as peroxidized lipids (Leaver and George 1998) as well as breakdown of different xenobiotics. In our present investigation, we found increased level of TBARS in all the treatment groups, which increased significantly in the liver of Gr- IV mice and in kidney of the Gr-II mice. The GST activity also showed high increase in the same treatment group suggesting an up-regulation of GST to minimize F-induced stress. GST activity significantly increased in the liver of the Gr-IV mice, and the TBARS production was also high in the same group. In Gr-II and IV mice, significant increase in the TBARS in the liver and kidney suggests a high oxidative burden within the body. This may be correlated with our earlier report on significant increase in chromosomal aberrations of bone marrow cells in mice exposed to lower doses of F in comparison with higher dose (Podder et al. 2008a, b).
Hsps are expressed in numerous tissues of several animal species, in response to a harmful stress situation or adverse life conditions. To the best of our knowledge, this is the first report on the expression of Hsp 70 in F-treated mice in vivo. F treatment resulted in increased Hsp 70 expressions in kidney of all the treatment groups whereas in liver its expression was down regulated. The chronic F exposure (NaF 15 mg/L for 90 days) reduced the expression level of Hsp 70 to a minimum in the liver where the TBARS showed a few fold increases compared to the control. On the contrary, kidney showed lower TBARS level with the increase in Hsp expression in the chronic F-exposed groups. These patterns of Hsp 70 expression suggest that F exposure can exhibit tissue-specific response of Hsp 70, which could be correlated with its organ-specific toxicity.
Conclusion
The present study clearly indicates that F induces hepatotoxicity and nephrotoxicity in mice evidenced by oxidative stress, histopathological changes in the liver and kidney with concomitant effects on normal hepatic function. Fluoride is a potent inducer of Hsp 70 in kidney, but it is negatively regulated in liver of mouse. These tissue-specific alterations in heat shock protein synthesis may provide useful biochemical fingerprints or toxicological signature to characterize F toxicity in human.
References
Agarwal S, Roy S, Ray A, Mazumder S, Bhattacharya S (2009) Arsenic trioxide and lead acetate induce apoptosis in adult rat hepatic stem cells. Cell Biol Toxicol 25:403–413
Alarcón-Herrera MT, Martín-Domínguez IR, Trejo-Vázquez R, Rodriguez-Dozal S (2001) Well water fluoride, dental fluorosis, and bone fractures in the Guadiana Valley of Mexico. Fluoride 34:139–149
Beutler E, Duron O, Kelly BM (1964) Improved method for the determination of blood glutathione. J Lab Clin Med 61:812–818
Bogdanffy MS, Makovee GT, Frame SR (1995) Inhalation oncogenicity bioassay in rats and mice with vinyl fluoride. Fundam App Toxicol 26:223–228
Bosworth EB, McCay CM (1962) Pathologic studies of rat kidneys: absence of effects ascribed to fluoride following long-term ingestion of drinking water containing fluoride. J Dent Res 41:940–960
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. In: Fleisher S, Packer L (eds) Methods in Enzymology. Academic Press, New York, pp 302–310
Carlson CH, Armstrong WD, Singer L (1960) Distribution and excretion of radio fluoride in the human. Proc Soc Exp Biol Med 104:235–239
Chinoy NJ, Joseph R, Sequeira E, Narayana MV (1991) Effects of sodium fluoride on the muscle and liver of albino rats. Ind J Environ Biol 1:129–134
Chouhan S, Flora SJS (2008) Effects of fluoride on the tissue oxidative stress and apoptosis in rats: biochemical assays supported by IR spectroscopy data. Toxicology 254:61–67
Dabrowaska E, Letko R, Balunowska M (2006) Effect of sodium fluoride on the morphological picture of the rat liver exposed to NaF in drinking water. Adv Med Sci 51:91–95
Darmani H, Ai-Hiyasat AS, Elbetieha AM (2001) Effects of sodium fluoride in drinking water on fertility in female mice. Fluoride 34:242–249
DeCamargo AM, Merzel J (1980) Histological and histochemical appearance of livers and kidneys of rats after long-term treatment with different concentrations of sodium fluoride in drinking water. Acta Anat 108:288–294
Dunipace AJ, Zhang W, Noblitt TW, Li Y, Stookey GK (1989) Genotoxic evaluation of chronic fluoride exposure: micronucleus and sperm morphology studies. J Dent Res 68:1525–1528
Gale RP, Spakes RS, Golde DW (1978) Bone marrow origin of hepatic macrophages (kupffer cells) in humans. Science 201:937–938
Guo X, Sun G, Sun Y (2003) Oxidative stress from fluoride-induced hepatotoxicity in rats. Fluoride 36:25–29
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S- transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Jacyszyn K, Marut A (1986) Fluoride in blood and urine in humans administered fluoride and exposed to fluoride-polluted air. Fluoride 19:26–32
Jeji J, Sharma R, Jolly SS, Pamnani S (1985) Implication of glutathione in endemic fluorosis. Fluoride 18:117–119
Kotoky P, Barooah PK, Baruah MK, Goswami A, Borah GC, Gogoi HM, Ahmed F, Gogoi A, Paul AB (2008) Fluoride and endemic fluorosis in the Karbianglong district, Assam, India. Fluoride 41:72–75
Kour K, Singh J (1980) Histological findings in kidneys of mice following sodium fluoride administration. Fluoride 13:163–167
Kour K, Koul ML, Koul RL (1981) Histological changes in liver following sodium fluoride ingestion. Fluoride 14:119–123
Leaver MJ, George SG (1998) A piscine glutathione S-transferase which efficiently conjugates the end-products of lipid peroxidation. Mar Environ Res 46:71–74
Liu K, Wang G, Ma L, Jang P, Xiao B, Zhang C (1999) Adverse effects of combined arsenic and fluoride on liver and kidney in rats. Fluoride 32:11–247
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Measurement of protein with the folin phenol reagent. J Biol Chem. 193:265–275
Machalinska A, Wiszniewska B, Tarasiuk J, Machalinski B (2002) Morphological effects of sodium fluoride on hematopoietic organs in mice. Fluoride 35:231–238
Machalinski B, Baskiewicz-Masiuk M, Sadowska B, Machalinska A, Marchlewicz M, Wiszniewska B, Stecewicz I (2003) The influence of sodium fluoride and sodium hexafluorosilicate on human leukemic cell lines. Fluoride 36:231–240
Mello CF, Barberio JC, Campos MAF (1963) Histological analysis of the influence of calcium ion and the action of fluoride ion in the albino rat kidney and liver. Revista Associacao Paulista Cirurgia Dent 17:35–41
Mittal M, Flora SJS (2006) Effects of individual and combined exposure to sodium arsenate and sodium fluoride on tissue oxidative stress, arsenic and fluoride levels in male mice. Chem Biol Int 162:128–139
Mittal M, Flora SJS (2007) Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug Chem Toxicol 30:263–281
Ogilvie AL (1953) Histologic findings in the kidney, liver, pancreas, adrenal, and thyroid glands of the rat following sodium fluoride administration. J Dent Res 32:386–397
Podder S, Chattopadhyay A, Bhattacharya S (2008a) In vivo suppression by fluoride of chromosome aberrations induced by mitomycin-c in mouse bone marrow cells. Fluoride 41:40–43
Podder S, Chattopadhyay A, Bhattacharya S, Ray MR (2008b) Differential in vivo genotoxic effect of lower and higher concentrations of fluoride in mouse bone marrow cells. Fluoride 41:301–307
Ramseyer WF, Smith CAH, McCay CM (1957) Effect of sodium fluoride administration on body changes in old rats. J Gerontol 12:14–19
Saralakumari D, Rao RP (1991) Red cell membrane alterations in human chronic fluoride toxicity. Biochem Int 23:639–648
Shanthakumari D, Srinivasalu S, Subramanian S (2004) Effects of fluoride intoxication on lipidperoxidation and antioxidant status in experimental rats. Toxicology 204:219–228
Shashi A, Thapar SP (2000) Histopathology of fluoride-induced hepatotoxicity in rabbits. Fluoride 34:34–42
Shashi A, Singh JP, Thapar SP (2002) Toxic effects of fluoride on rabbit kidney. Fluoride 35:38–50
Shulman JD, Wells LM (1997) Acute fluoride toxicity from ingesting home-use dental products in children, birth to 6 years of age. J Public Health Dent 57:150–158
Susheela AK, Bhatnagar M (2002) Reversal of fluoride induced cell injury through elimination of fluoride and consumption of diet rich in essential nutrients and antioxidants. Mol Cell Biochem 234(235):335–340
Susheela AK, Das KT (1988) Chronic fluoride toxicity: a scanning electron microscopic study of Duodenal Mucosa. Clin Toxicol 26:467–476
Trivedi MH, Verma RJ, Chinoy NJ (2008) Amelioration by black tea of sodium fluoride-induced effects on DNA, RNA, and protein contents of liver and kidney, and serum transaminase activities in swiss albino mice. Fluoride 41:61–66
Whitford GM (1983) Fluorides: metabolism, mechanisms of action and safety. Dent Hyg 57(5):16–29
Zhan X, Wang M, Xu Z, Li J (2006) Toxic effects of fluoride on kidney function and histological structure in young pigs. Fluoride 39:22–26
Zhang JH, Liang C, Ma JJ, Niu RY, Wang JD (2006) Effects of sodium fluoride and sulfur dioxide on sperm motility and serum testosterone in male rats. Fluoride 39:126–131
Acknowledgments
Authors acknowledge Professor. S.K. Maitra for his help in analyzing the histological changes. Authors are grateful to Inter University Accelerator Centre (IUAC), New Delhi for the Project Fund (UFUP-40316) to AC and University Grants Commission (UGC), New Delhi for the Centre for Advanced Studies (CAS) grant to the Department of Zoology. SP is grateful for a CAS Project Fellowship, and SA is grateful to CSIR for the award of Senior Research Fellowship.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chattopadhyay, A., Podder, S., Agarwal, S. et al. Fluoride-induced histopathology and synthesis of stress protein in liver and kidney of mice. Arch Toxicol 85, 327–335 (2011). https://doi.org/10.1007/s00204-010-0588-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-010-0588-7