Abstract
Selenium is an oligoelement with essential biological functions. Diet is the most important selenium source, and intake of this element depends on its concentration in food and amount of food consumed. Among the essential human micronutrients, selenium is peculiar due to its beneficial physiological activity and toxicity. It may have anticarcinogenic effects at low concentrations, whereas at concentrations higher than those necessary for nutrition, it can be genotoxic and carcinogenic. Because of that, selenium is probably the most widely investigated of all the oligonutrients. In the last decades, there has been increasing interest in several nutritional Se compounds because of their environmental, biological, and toxicological properties, particularly for their cancer- and disease-preventing activities. This article gives an overview of the results of in vitro studies on mutagenicity, genotoxicity, cytotoxicity, and DNA repair conducted within the last decades with different organic and inorganic selenium compounds. Results from these studies provide a better knowledge on the selenium activity and help to elucidate the reasons underlying its duality in order to regulate its correct use in nutrition and clinic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium
Selenium (Se) is an essential trace element for humans, animals, and some bacteria. It is important for many cellular processes, because it is a component of several selenoproteins with essential biological functions (Letavayová et al. 2008a). There are at least 25 human selenoproteins and 24 in the mouse, each characterized by the incorporation of selenium into the primary sequence as the amino acid selenocysteine (SeCys; Kryukov et al. 2003; Foster et al. 2006). Some of these selenoproteins are selenoenzymes, such as thioredoxin reductase and glutathione peroxidase, which represent fundamental antioxidative systems for the maintenance of cellular redox homeostasis (Rayman 2000). Thus, Se functions in the body as an antioxidant, in thyroid hormone metabolism, redox reactions, reproduction, and immune function (Rayman 2000; Combs et al. 2009).
Nevertheless, Se is also toxic, and chronic exposure in humans or animals results in selenosis (Goldhaber 2003). Other related toxic effects are a disruption of endocrine function, synthesis of thyroid hormones and growth hormones, and an insulin-like growth factor metabolism (Navarro-Alarcon and Cabrera-Vique 2008). The mechanism of Se toxicity has not been clarified but mostly attributed to its ability to induce oxidative stress both in vitro and in vivo (Kitahara et al. 1993; Yan and Spallholz 1993).
Diet is the most important Se source, and intake of this essential element depends on its concentration in food and amount of food consumed (Navarro-Alarcon et al. 2005). Se bioavailability varies according to the Se source and nutritional status of the subject, being significantly higher for organic Se forms (Navarro-Alarcon and Cabrera-Vique 2008). Se contents in several foods are gathered in Table 1. Se content in food is influenced by geographical location, seasonal changes, protein content, and food processing. As a result, Se levels in foods can vary manyfold not only between countries but also between regions in a country. A food may have more than tenfold difference in Se content, depending on where it was produced (Reilly 2006).
Se intake is mainly in the form of organic compounds ingested in grains, meat, yeast, and vegetables (Cao et al. 2004). The US Food and Nutrition Board (1980) considered to be the Estimated Safe and Adequate Daily Intake for Se of 50–200 μg, being 55 μg/day the Recommended Dietary Allowance (RDA) for Se for both men and women. Nevertheless, per capita intakes of Se can vary widely between countries. A major reason for this is the difference in food consumption patterns and, especially, in the types of staple foods consumed (Reilly 2006). The no observed adverse effect level (NOAEL) of dietary Se was estimated to be 1,540–1,600 μg/day (Whanger 2004). At these doses, Se has the potential to induce toxic side effects such as induction of DNA damage (Reid et al. 2004; Wycherly et al. 2004).
The major chemical forms of Se are organic, as selenomethionine (SeMet), SeCys, and metylselenocysteine (MeSeCys), and inorganic as selenite and selenate (Letavayová et al. 2006). Studies on the short-term effects of Se showed that inorganic Se (selenate, selenite) is more toxic and less bioavailable than organic forms (Letavayová et al. 2008a), and one potential reason for differences in genotoxicity observed among selenocompounds is their distinct metabolism (Suzuki et al. 2006a, b). However, Se toxicity depends not only on the Se compound and dose but also on the method of administration, animal species, exposure time, idiosyncrasy, physiological status, and interaction with other metals, nutrients, etc. (Burk and Levander 2002).
In the last decade, there has been increasing interest in several nutritional Se compounds because of their environmental, biological, and toxicological properties, particularly for their cancer- and disease-preventing activities. Among the essential trace mineral nutrients, Se is unique due to its catalytic activity and toxicity (Letavayová et al. 2008a). Hamilton (2004) reported the existence of three Se levels of biological activity: (1) trace concentrations are required for normal growth and development; (2) moderate concentrations can be stored, and homeostatic functions maintained; and (3) elevated concentrations can result in toxic effects. As a general rule, Se may have anticarcinogenic effects at low concentrations, whereas at concentrations higher than those necessary for nutrition, it can be genotoxic and carcinogenic (Bronzetti et al. 2001), and its toxic level is relatively close to that required for normal health (FAO 2001). Nevertheless, the mechanisms of action of Se compounds, either via a prooxidant pathway, as seen in cytotoxicity and apoptosis, or via an antioxidant pathway, as proposed in cancer chemoprevention, are still unclear but intriguing (Shen et al. 2001; Hurst et al. 2008).
Selenium controversy
In the last two decades, there has been much progress in our knowledge and understanding of the biological roles of Se and its importance in human nutrition (Navarro-Alarcon and Cabrera-Vique 2008). Different chemical forms of this element produced genotoxic effects in a great variety of in vitro and in vivo studies (Ammar and Couri 1981; Biswas et al. 2000; Cemeli et al. 2006); nevertheless, Se genotoxicity still generates controversy, and IARC (1987) concluded that there were not sufficient data to consider Se as carcinogen for humans. On the other hand, several organic and inorganic Se compounds have been reported to be effective chemopreventive agents against multiple models of animal tumorigenesis (Letavayová et al. 2008a). However, despite its antioxidant properties and requirement for human and animal nutrition, the appropriate form of Se for supplementation continues to be debated, as well as the optimal concentrations of Se that provide protection against genetic damage with the least toxicity (Santos and Takahashi 2008).
Results of the two great trials performed with Se to date are really representative of this controversy. A randomized controlled trial, the Nutritional Prevention of Cancer study (Clark et al. 1996), demonstrated substantial reduction in the risk of several cancers, most notably cancer of the prostate (Duffield-Lillico et al. 2002), among subjects supplemented with Se in the form of selenized yeast. These clinical data, supported by other epidemiological and preclinical data, led to intense interest in the potential of the Se as a non-toxic mean of preventing prostate and other cancers. On this basis, the largest cancer prevention trial ever performed (Selenium and Vitamin E Cancer Prevention Trial, SELECT) was designed to test the hypothesis that daily use of Se (as SeMet) or vitamin E, alone or in combination, could prevent prostate cancer in more than 32,000 men (Klein et al. 2000). However, against all predictions, SELECT recently reported that neither Se nor vitamin E had any beneficial effect on major health outcomes (Lippman et al. 2009).
Given this considerable controversy generated by the results of these and many other Se studies, a more detailed characterization of the effects of this element is required to define the conditions in which they appear and to be able to establish proper standards of Se use. This is especially imperative nowadays, since many people consume Se supplements. On the other hand, there is no international consensus on how to evaluate the potential risk of genotoxic carcinogens in food; moreover, oligoelements present at low levels in food, as Se, frequently give rise to difficulties to evaluate the potential risk of genotoxicity (O’Brien et al. 2006). In this regard, in vitro studies have become increasingly important in the last decades, since they can complement and provide more specific information than those performed in vivo.
Evaluation of selenium activity: in vitro vs. in vivo tests
As the human being is continuously exposed to several chemical, physical, and biological agents, there is a need to evaluate diverse types of biological alterations in order to thoroughly assess the genotoxic/mutagenic potential of a substance, and this requires the use of a battery of in vivo and in vitro assays (Maurici et al. 2005). Data obtained from animal experiments yield information pertaining to the dose for lethal or sublethal toxicity which corresponds to many different general toxic mechanisms and effects. The information derived from in vivo studies is essential for determining the potential toxicity of a chemical to humans and other life forms (Barile 2008). The in vitro systems must try to imitate the real organism conditions, but many times, in vitro metabolic activation systems cannot mimic entirely the mammalian in vivo conditions. The test, therefore, does not provide direct information on the mutagenic and carcinogenic potency of a substance in mammals.
Thus, the use of animals in mutagenicity testing is primarily required when it is necessary to investigate whether mutagenic activity detected in vitro is reproduced in vivo. However, except in those cases in which high, or moderate and prolonged human exposure is expected (e.g., many human medicines), there is no justification for the routine use of animals for mutagenicity tests when there is no evidence for activity at in vitro assays (COM 2000). Moreover, there are compounds for which standard in vivo tests do not provide additional useful information. This includes compounds for which data from studies on toxicokinetics or pharmacokinetics indicate that they are not systemically absorbed and therefore are not available for the target tissues in standard in vivo genotoxicity tests. In all these cases, it may be appropriate to base the evaluation only on in vitro testing (FDA 2007).
For that reason, although current in vitro methods are not considered sufficient to serve as full animal replacements at this time, different institutes are currently developing in vitro tests able to predict compound effects in vivo. Standardized and validated in vitro methods have replaced or reduced some human and animal studies (Carfi et al. 2007). In case of Se, for example, in vitro bioaccessibility methods of simulated digestion are an alternative to in vivo bioavailability procedures for calculating the percentage of an element which is transformed into absorbable forms in the digestive tract. In vitro bioaccessibility analytical procedures are often useful, because they are simple, rapid, inexpensive, and allow individual experimental variables to be easily controlled (Cabrera et al. 1996).
In general terms, the in vivo and in vitro tests are equally necessary, since both provide relevant information on the characterization of the action mechanisms of an agent. However, there are certain situations where it is preferable to use one kind of test and not another. In vivo tests are especially important to evaluate the toxicokinetic effects or the metabolism alterations in the organism, for example. Nevertheless, in vitro methods are significantly faster and less expensive than in vivo assays; moreover, animal studies require a high number of individuals and raise important ethical concern (Carfi et al. 2007). The advantages of in vitro studies include the rapid assessment of large numbers of chemicals, the suggestion of a mechanism for carcinogenicity or mutagenicity, the reduction, replacement, and refinement of animal testing, and a contribution to human and animal risk assessments that correlates as well as the predictive ability of animal toxicology testing (Barile 2008).
Next sections of this review describe the results of the in vitro studies carried out in the last years to evaluate and characterize Se molecular activity.
Toxic effects of selenium
Several in vivo, in vitro, and epidemiological studies describe adverse effects of Se. It was found that Se can induce DNA damage (Biswas et al. 2000; Machado Mda et al. 2009), produce oxidative stress (Wycherly et al. 2004), and increase lipid peroxidation (Colado-Megía et al. 2004), generate neurotoxicity in mice (Ammar and Couri 1981), provide no protection against adverse actions induced by other compounds as arsenic (Hasgekar et al. 2006) or sodium metavanadate (Zwolak and Zaporowska 2009), be ineffective at preventing basal cell carcinoma, and increase, in a non-significant way, the incidence of squamous cell carcinoma and total non-melanoma skin cancer in people supplemented with Se (Duffield-Lillico et al. 2003). However, many of these effects depend on the Se level and chemical form, and results of in vitro studies could help to delimitate those conditions in which toxicity becomes evident.
Selenium mutagenicity and genotoxicity
An effective strategy in mutagenicity and genotoxicity assessment uses tests that produce reproducible and biologically relevant data based upon three stages (Barile 2008). Stage 1 uses bacterial gene mutation assays, stage 2 assays are cytogenetic tests that monitor clastogenicity and aneugenicity, and stage 3 assays record the induction of gene mutations in cultured mammalian cells.
Bacterial mutagenesis assays are the most widely used short-term tests for screening for potential mutagens and carcinogens. They are highly sensitive for genotoxic agents, technically easy, fast, and inexpensive (Barile 2008). Several studies aimed at evaluating the genotoxicity of Se by means of bacterial mutagenesis assays are collected in the literature. Inorganic forms of Se, such as selenites, do not give any indication of being mutagenic in the Salmonella/microsome assay despite of producing positive results in the Bacillus subtilis rec-assay (Nakamuro et al. 1976; Lofroth and Ames 1978). However, in other studies, selenate and selenite were shown to be weakly mutagenic, giving rise to base-pair substitution (Noda et al. 1979), and high concentrations of selenite induced mutagenicity in the S. typhimurium strain TA104 (Kramer and Ames 1988).
Besides a few Se studies using aquatic invertebrates (Tran et al. 2007), fish cell lines (Al-Sabti 1994) or plants (Yi and Si 2007), the mainly eukaryotic non-mammalian cell system used to test mutagenicity and genotoxicity is the yeast. Sodium selenite mutagenicity and genotoxicity was early detected in different strains of Saccharomyces cerevisiae (Rosin 1981; Anjaria and Madhavanath 1989). Rosa et al. (2004) combined the use of the Salmonella/microsome assay and the yeast S. cerevisiae to test for putative mutagenicity, genotoxicity, and recombinogenicity of diphenyl diselenide and to determine whether DNA damage produced is repairable. They showed that this Se compound is a weak mutagen which probably generates DNA strand breaks through both an intercalating action and a prooxidant effect. In a more recent study, the effects of Se (sodium selenite and SeMet) at the genetic level were analyzed by means of a S. cerevisiae-based assay (Seitomer et al. 2008). They determined which genes are involved in responding to high environmental Se using a collection of viable haploid null allele strains representing the major stress pathways. Results suggested that both selenite and SeMet are likely inducing DNA damage by generating reactive species. Letavayová et al. (2008a) characterized three different nutritionally available Se compounds (sodium selenite, SeMet and MeSeCys) for their toxicity and mutagenicity as well as potential detrimental effects on DNA using the budding yeast S. cerevisiae. Only sodium selenite manifested significant toxic effect in yeast, and this effect was accompanied by a promutagenic activity observed only in the stationary phase of growth. The data also suggested that, in inducing oxidative DNA damage, sodium selenite may generate double-strand breaks in replicating yeast cells (Letavayová et al. 2008a).
Despite its usefulness, it is not always possible to extrapolate the results of bacterial or yeast assays to the mammalian system. In such case, or in order to complete these results, mammalian cell systems should be employed. Machado Mda et al. (2009), for example, observed that DFDD (3′,3-ditrifluoromethyldiphenyl diselenide) is not mutagenic for bacteria or yeast; however, it may induce weak genotoxic effects in V79 cells. Se mutagenicity and genotoxicity has been tested in a great variety of in vitro assays with mammalian cell systems. Results of all of them emphasize the importance of the chemical form (Nakamuro et al. 1976; Sirianni and Huang 1983; Smith et al. 2004) and level (Biswas et al. 2000; Weitberg et al. 1985; Abul-Hassan et al. 2004) in the Se effects.
Nakamuro et al. (1976) tested five Se compounds for their ability to induce chromosome aberrations (CA) in cultured human leukocytes. They all showed chromosome breaking activity, but it was significantly higher for the compounds with four-valent than with six-valent Se, the efficiency being in the decreasing order selenious acid > sodium selenite > Se dioxide ≫ selenic acid > sodium selenate. These results seem to be represented in other subsequent Se studies. Inorganic Se compounds, as sodium selenite, sodium selenate, and sodium selenide, were reported to increase CA rates in different cell lines. Selenite induced CA in human fibroblasts (Lo et al. 1978) and lymphocytes (Biswas et al. 2000; Abul-Hassan et al. 2004; Whiting et al. 1980; Khalil 1989), and DNA damage induced by sodium selenate (Biswas et al. 2000; Whiting et al. 1980) and sodium selenide (Whiting et al. 1980) was also found in human cells. Furthermore, organic forms of Se, as SeMet (Khalil 1989) and other synthetic organo-Se compounds (Kalhil and Maslat 1990), have shown their ability to induce CA in human lymphocytes.
The different capabilities of Se compounds (sodium selenide, Se dioxide, Se(0), sodium selenate, and sodium selenite) to induce sister chromatid exchanges (SCE) were clearly demonstrated in an early study of Ray and Altenburg (1980). The SCE-inducing abilities in decreasing order of their effectiveness were Se(0) > Se dioxide > sodium selenide > sodium selenite > sodium selenate. Increases in SCE rates induced by Se, mainly as sodium selenite, were also found in other in vitro studies (Sirianni and Huang 1983; Ray et al. 1978; Morimoto et al. 1982; Ray and Altenburg 1982).
Other studies using micronucleus (MN) test also reported genotoxic effects of several Se compounds in different cell lines. Treatment with diphenyl diselenide, for example, induced an increase in the number of MN in V79 Chinese hamster cells, showing mutagenic risk by this molecule at high concentrations (Rosa et al. 2007a). Selenous acid increased MN formation in mouse bone marrow cells (Itoh and Shimada 1996), in human lymphocytes, and in TK6 lymphoblastoid cell line (Cemeli et al. 2006); sodium selenate and sodium selenite also showed genotoxicity in TK6 cells (Cemeli et al. 2006). Nevertheless, some studies have shown that Se does not produce considerable increase in MN frequency (Berces et al. 1993). Moreover, the work of Ebert et al. (2006) on bone marrow stromal cells with low antioxidative capacity concluded that selenite supplementation of cultures appears to be an important countermeasure to restore their antioxidative capacity and to reduce cell damage in the context of tissue engineering and transplantation procedures.
Prooxidant responses of Se compounds have also been reported. DNA damage induced by sodium selenate, sodium selenite, and selenous acid on their own was detected with the single cell gel electrophoresis (comet) assay in human lymphocytes (Cemeli et al. 2003). Results obtained with this test also showed that selenite induced oxidative stress and apoptosis, and these effects were significantly attenuated by superoxide dismutase, catalase and deferoxamine (Shen et al. 1999). Prooxidant activity exhibited by organoselenium compounds when used in relatively high concentrations was suggested to be linked to genotoxicity observed in human leukocytes by the comet assay (Santos et al. 2009). In this study, the organoselenium amino acid derivatives were more genotoxic than the aromatic derivatives. Methylseleninic acid induced apoptosis without induction of reactive oxygen species (ROS) into two prostate cancer cell lines, whereas selenite generated strand breaks in DNA of LNCaP cells and induced apoptosis by producing superoxide to activate p53 (Li et al. 2007). At high doses, diphenyl diselenide also generated DNA strand breaks, as detected using the comet assay (Rosa et al. 2007a). Lu et al. (1995, 1996) also observed by means of filter elution analyses that sodium selenite and sodium selenide induce single and double DNA strand breaks in a mouse mammary epithelial cell line, whereas MeSeCys and Se-garlic extract only induce single-strand breaks and in lesser degree in the same cells. However, no significant genotoxic effect was found for selenite, selenate, SeMet, or Se-MeSeCys in C6 rat glial cells (Yeh et al. 2006), for SeMet in human lymphocytes (Laffon et al. 2009) and human fibroblasts (Seo et al. 2002), and for ebselen in HepG2 (Yang et al. 1999) and V79 cells (Miorelli et al. 2008).
Selenium cytotoxicity: effects on cell cycle and apoptosis
The effect of Se alone or in combination with other compounds on the growth and proliferation of different mammalian cells has been investigated mainly by means of flow cytometry techniques. In an early study, Se, as sodium selenite, was shown to decrease the growth of fibroblasts and hepatoma cells in a dose-dependent manner, and this inhibition was reversible upon removal of Se from the growth medium (LeBoeuf et al. 1985).
Later, it was reported that selenite inhibited cell growth by G2/M arrest in a mammary tumor cell line (Lu et al. 1995), in human esophageal cancer cells when combined with zinc (Xiao et al. 2008), and in lymphoblastic leukemia MT-4 cells (Philchenkov et al. 2007); however, it promoted cell proliferation at high concentrations (Xiao et al. 2008). An increase in the S-phase fraction in the presence of Se was found in a human maxillary cancer cell line (Yamamoto et al. 1996). The effect of Se–garlic extract and Se-MeSeCys on cell morphology, cell growth, and cell cycle progression was also studied in mammary epithelial cells, both agents inducing growth inhibition by G1-phase cell cycle arrest (Lu et al. 1996). SeMet also induced G2/M arrest in certain prostate and colon cancer cell lines (Goel et al. 2006; Zhao and Brooks 2007), methylseleninic acid caused G0/G1 arrest in prostate cancer cells (Zhao et al. 2004), and ebselen interfered with both the proton-translocating function and the ATPase activity of the plasma membrane H+-ATPase, inhibiting yeast growth in a concentration- and time-dependent manner (Chan et al. 2007). Another recent work investigated the variability of the effects on cell viability, redox modulation, and disruption of subcellular compartments by different selenocompounds (SeMet, methylseleninic acid, and selenazolidines) in several human lung cell lines (Poerschke et al. 2008). Results of this study demonstrated that all selenocompounds behave different, and that the chemical form of the organic selenocompound is a major determinant in the expected cellular response.
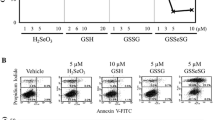
Results from apoptosis studies have shown that several selenocompounds (mainly sodium selenite but also SeMet, Se dioxide, and methylseleninic acid) induce cell death in different mammalian cell lines: human prostate cancer cells (Xiang et al. 2009), lymphoblastic leukemia MT-4 cells (Philchenkov et al. 2007), HepG2 cells (Zou et al. 2007), colon cancer cell lines (Goel et al. 2006), lymphoma cell lines and primary lymphoma cultures (Last et al. 2006), leukemia cell lines (Wang et al. 2004), human pulmonary adenocarcinoma cells (Chen et al. 2003), and brain tumor cell lines (Rooprai et al. 2007). The methylated Se compounds, such as methylselenocyanate or MeSeCys, also induced cell injury and death by apoptosis in a mouse leukemia cell line (Wilson et al. 1992). The precise mechanisms of apoptosis induced by the Se compounds are not well understood (Philchenkov et al. 2007); however, it is believed that ROS may play a crucial role in Se-decreased cell viability and Se-induced apoptosis (Zou et al. 2007).
Shen et al. (2001) designed a study to investigate the interaction effects of selenite and SeMet plus vitamin C, trolox (a water-soluble vitamin E), and copper sulfate, on cell viability and induction of 8-hydroxy-2′-deoxyguanosine (8-OHdG) adduct formation in DNA of primary normal human keratinocytes (NHK). The data showed that selenite, but not SeMet, induced oxidative DNA damage as 8-OHdG adducts, but coincubation with vitamin C or copper sulfate protected NHK cells against that selenite-induced cytotoxicity. However, synergistic effects were observed between selenite and trolox resulting in enhanced cytotoxicity. On the other hand, no effects on cell viability were observed when cells were treated with SeMet plus vitamin C, trolox, or copper sulfate. Previous findings had already shown that high doses of selenite, acting as a prooxidant, induced cytotoxicity and DNA adducts in mouse skin cells, whereas SeMet did not (Stewart et al. 1999). Furthermore, other studies reported that selenite and its metabolites at high doses resulted in cytotoxicity, DNA fragmentation (Garberg et al. 1988; Wilson et al. 1992), and cellular apoptosis (Stewart et al. 1997; Davis et al. 1998).
Zhong and Oberley (2001) employed several methodologies (western blot, structural evaluation of mitochondria, cell growth analysis…) to investigate the effects of Se, as sodium selenite, in the LNCaP human prostate cell line. The data enabled the authors to conclude that the in vitro biological consequences of selenite exposure were different between acute and long-term exposure. In acute exposure, selenite caused cell death, mainly apoptosis attributable to oxidative stress; in chronic long-term exposure, selenite caused only minimal cell death but inhibited cell growth by modifying gene expression and cell cycle progression.
Morris et al. (2006) assayed the BrdU incorporation into DNA of primary epithelial prostate and LNCaP cells treated with SeMet or Se(0) to determine DNA synthesis. The results of the study demonstrated that both chemical Se forms can induce delay in DNA synthesis in a dose-dependent manner in both cell lines. Li et al. (2007) treated two human prostate cancer cell lines with selenite and methylseleninic acid, and results obtained showed that these Se forms induce ROS formation and apoptosis in both cell lines. In another work, the effects of methylseleninic acid on gene expression were evaluated by means of western blot and oligonucleotide array analysis in human prostate cancer cells (Dong et al. 2003). Data showed that Se alters the expression of different important genes inducing an increase in p21WAF1 and p19INK4d protein synthesis and a down-regulation of CDK1, CDK2, and cyclin A. This agrees with previous studies which reported that Se can upregulate or downregulate certain genes (El-Bayoumy and Sinha 2005).
Effect of selenium on DNA repair and synthesis
A role for Se in DNA repair was first noticed when Se treatment was shown to enhance host cell reactivation of a UV-damaged reporter plasmid template by enhancing DNA repair protein complexes (Seo et al. 2002). Enhancement of DNA repair could be a mechanism of chemoprevention, and only very few compounds have been yet shown to act by this mechanism (Collins et al. 2003). Furthermore, Zhang et al. (2008) inferred that Se only enhances DNA repair of normal tissues as a consequence of the selective modulation of Se on Nrf2 in tumor and normal tissues (Kim et al. 2007).
Yeasts are very useful and powerful model systems for elucidating many DNA repair phenomena and pathways highly relevant to areas of investigation in human biology (Letavayová et al. 2008a). Human genetic defects associated with DNA repair can often be addressed directly in yeast because of evolutionary conservation of genes and systems (Resnick and Cox 2000). In a recent study, Letavayová et al. (2008b) used Saccharomyces to test DNA repair processes and concluded that the Rad52 protein is indispensable for repairing sodium selenite-induced double-strand breaks, suggesting a fundamental role of homologous recombination in this repair process and providing the first evidence that this pathway may have a fundamental role in the repair of sodium selenite-induced toxic DNA lesions.
On the other hand, the unscheduled DNA synthesis (UDS) test is commonly used to in vitro assay the influence of different chemical and physical agents on DNA synthesis and repair processes of mammalian cells (Barile 2008). UDS studies were conducted to determine the effects of Se on cell proliferation and the stages of the cell cycle affected by this element (LeBoeuf et al. 1985). Despite of the fact that many of the in vitro studies concluded that Se (mainly as selenite form) induces an inhibition of DNA synthesis (reviewed by Frenkel and Falvey 1988), Se has also been suggested to be a DNA repair promoter (Russell et al. 1980). Whiting et al. (1980) studied the induction of UDS in cultured human cells by different inorganic and organic Se compounds. They found that inorganic compounds (sodium selenate, sodium selenite, and sodium selenide) induced low levels of UDS in absence of glutathione, but high levels of UDS were found in the presence of this peptide. Nevertheless, no UDS was detected in cells treated with organic compounds (selenocystamine or selenomethione), with or without added glutathione, and only selenocystine induced a low level of UDS, being also enhanced by glutathione. In one recent study, different mammalian cells lines (rat gut epithelial cells, primary mouse bone marrow cells, and human squamous cell carcinoma of the head and neck cells) were treated with SeMet and with a variety of DNA-damaging agents, and then UDS was determined. Data showed that SeMet pretreatment caused a DNA repair response, which protected from subsequent challenge with DNA-damaging agents (Fischer et al. 2007).
The comet assay is another test usually employed in DNA repair studies because of its sensitivity for the measurement of radiation- or chemically induced DNA damage and repair in viable cells (McKelvey-Martin et al. 1993). In this regard, Seo et al. (2002) confirmed by means of this assay that SeMet induces DNA repair in normal human fibroblasts in vitro after a challenge with UV-radiation, and Laffon et al. (2009) reported that bleomycin-induced DNA damage in human lymphocytes was repaired better in the presence of SeMet.
DNA synthesis was also evaluated in vitro by measuring incorporation of 3H-thymidine into rat lens following systemic delivery of a cataractogenic dose of selenite. UDS was found to be ~10% of the total DNA formed, but there was a 30 and 70% increase in this putative DNA repair in the lenses from selenite-treated animals at 6 and 24 h after the injection, respectively; 3H-thymidine incorporation into DNA remained elevated compared to controls through 96 h (Huang et al. 1990). The effect of Se (as Se dioxide) on the accuracy of DNA synthesis in vitro was also analyzed by means of the fidelity assay. Se did not alter fidelity under normal conditions of magnesium activation, nor affected the mutagenicity of manganese (Tkeshelashvili et al. 1980). However, several Se-derived compounds (dimethylselenone, diphenylselenone, sodium selenite, and MeSeCys) reversed the proangiogenesis effect of arsenic, which is initiated at the endothelial cell plasma membrane by activation of the ERK1/2 signal transduction pathway (Mousa et al. 2007).
Verma et al. (2004) demonstrated that gastric adenocarcinoma SNU-1 cells responded to SeMet with a biphasic proliferative curve: enhanced incorporation of 3H-thymidine into DNA within a very narrow range of SeMet concentrations, followed by decreased 3H-thymidine uptake at higher levels. This biphasic effect of Se on cell growth was also observed in another previous in vitro study (Medina and Oborn 1984): some Se concentrations stimulated cell growth, whereas others were cytotoxic, and the inhibition of cell growth by Se was reversed when these doses were removed from the growth medium. The increased cell growth was reflected by an increased cell number, increased uptake of 3H-thymidine into DNA, increased DNA labeling index, and increased rate of DNA synthesis. The differential effects of Se were manifested by 48 h after the addition of Se to the cell culture medium.
As general conclusions from results of different assays to study the influence of Se on DNA synthesis, it seems that it depends mainly on the cell line employed (Webber et al. 1985; Vadgama et al. 2000), the chemical Se form (Whiting et al. 1980; Frenkel 1985; Bansal and Sood 1999), and the Se concentrations assayed (Medina and Oborn 1984; Morrison et al. 1988; Nano et al. 1989; Verma et al. 2004).
Selenium antigenotoxicity and protective effect
Several studies described important beneficial properties of Se as antioxidant agent (Roussyn et al. 1996; Hassan et al. 2009; Machado Mda et al. 2009), as protector element against UV light (Rafferty et al. 2003), lead (Aykin-Burns and Ercal 2006), mercury (Lemire et al. 2006; Kaur et al. 2009; Peterson et al. 2009), and cadmium (Frisk et al. 2002), as reducer of progression of HIV infection (Hurwitz et al. 2007), as enhancer of immune system (Kiremidjian-Schumacher et al. 1994), and as anticarcinogenic agent against different types of cancer (Clark et al. 1996; Reid et al. 2002; Cai et al. 2006).
As happened with Se genotoxicity, mutagenicity, and cytotoxicity, the results of most of Se in vitro studies indicate that the antigenotoxic properties of Se compounds are highly dependent upon the conditions under which they are evaluated (Cemeli et al. 2006), and that the protection offered by Se compounds against damage induced in genetic material is time and dose dependent (An et al. 1988). A clear example of this is the study by Weitberg et al. (1985) who showed that sodium selenite had variable effects on the number of SCE induced by stimulated human phagocytes in mammalian cells depending on the concentration used. Low concentrations of sodium selenite protected target cells; however, intermediate concentrations had no effect on oxidant-induced SCE formation, and high concentrations increased the number of exchanges.
Using the Ames test, it was reported that sodium selenite was effective in the reduction of the mutagenicity induced by a variety of mutagens (Martin et al. 1981). Furthermore, co-incubation of sodium selenite and N-methyl-N-nitrosourea (MNU) or N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) with bacterial cells (S. typhimurium) yielded an evident inhibition of the mutagenicity induced by these alkylating agents (Balansky 1992). It was also observed that this Se compound reduced only very slightly the genotoxic effect of nitrofurans (Gajewska et al. 1990), and that pretreatment of cells with a non-lethal dose of selenite induced the synthesis of proteins which protected the cells from killing by H2O2 or high doses of selenite (Kramer and Ames 1988). The genotoxic effects of three Se compounds (sodium selenate, sodium selenite, and selenous acid) were tested in the Ames test and also investigated for their interaction with potassium dichromate (Cemeli et al. 2003). None of them showed significant effect in the Ames test without metabolic activation, and moreover, sodium selenate showed antigenotoxic properties against potassium dichromate.
The possible antigenotoxic effects of Se were also investigated in yeasts by several authors using different Se compounds as ebselen (Chan et al. 2007; Miorelli et al. 2008) or SeMet (Longo et al. 1995; Bronzetti et al. 2001). The antioxidant, mutagenic, and antimutagenic effects of ebselen were evaluated in S. cerevisiae strains proficient and deficient in antioxidant defences. Ebselen showed strong activity against H2O2-induced oxidative damage in the antimutagenic assay using N123 strain and in the antioxidative assay using strains lacking antioxidant defences (Miorelli et al. 2008). In order to examine the antimutagenic effects of sodium selenite and SeMet, S. cerevisiae was treated with H2O2 (Bronzetti et al. 2001). D7 strain of S. cerevisiae was used, because it constitutes a rapid and inexpensive genetic model to investigate the toxic and mutagenic effect of various compounds. The antimutagenic effect was evident for both sodium selenite and SeMet, according to data previously described in the literature (Longo et al. 1995).
Many in vitro studies have proven that adequate levels of Se can reduce the CA induced by different mutagenic compounds. Se (as sodium selenite) was found to protect cells against sodium arsenite by reducing the frequency of gaps and chromatid breaks induced by this compound (Sweins 1983; Beckman and Nordenson 1986). Also, Se-enriched green tea was both able to prevent the CA induced by mytomicin C in mouse spermatocytes and to enhance glutathione peroxidase and superoxide dismutase activity in blood serum and liver (Li et al. 2009). Another study concluded that sodium selenite under specific conditions reduces the percentage of cells with N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced CA (An et al. 1988), but this protective effect is clearly time and dose dependent, resulting in toxic effects at high concentrations. Se (sodium selenite and SeMet) also protected mammalian cells against lead acetate- and sodium sulfite- ( Beckman and Nordenson 1986), carbon tetrachloride- (Siviková et al. 2001) and doxorubicin-induced damage (Santos and Takahashi 2008).
Se was also found to reduce the SCE levels induced by different compounds. Sodium selenite, for example, significantly reduced SCE frequencies induced by fluorescent light in human fibroblasts (Parshad et al. 1980), and also by arsenic (Hu et al. 1996), and by carbon tetrachloride (Siviková et al. 2001) in peripheral lymphocytes. Moreover, some studies demonstrated that sodium selenite can antagonize the ability of other compounds to cause DNA damage leading to the formation of SCE. This was the case of two mercury derivatives (Morimoto et al. 1982), and methyl methanesulfonate or N-hydroxy-2-acetylaminofluorene (Ray and Altenburg 1978), which cause an increase in SCE, but simultaneous addition of sodium selenite to the cultures resulted in SCE frequencies below the sum of the SCE frequencies produced by the individual compounds.
Several works designed to evaluate the antigenotoxic properties of different Se compounds by means of MN test are collected in the literature. Diphenyl diselenide at low concentrations showed antimutagenic properties against H2O2, methyl methanesulphonate, and UVC radiation in lung fibroblast cells (Rosa et al. 2007b); supplementation of human MCF-7 breast carcinoma cells or mouse fibroblasts with low levels of sodium selenite protected these cells from ultraviolet-induced chromosome damage (Baliga et al. 2007); selenous acid and sodium selenate reduced the DNA damage induced by potassium dichromate in human lymphocytes and TK6 cells, respectively (Cemeli et al. 2006); sodium selenite decreased the MN rate induced by MNNG in children’s foreskin fibroblasts (An et al. 1988); V79 cells showed diminished cadmium-induced MN frequency when treated with sodium selenite (Hurná et al. 1997), and its protective effect was also demonstrated in ovine peripheral lymphocytes cultured with carbon tetrachloride (Siviková et al. 2001).
The literature agrees with the protective effect of Se evaluated with the comet assay against a variety of chemical or physical toxic agents. In vitro investigations with this assay found that Se (sodium selenite and ebselen) prevented DNA damage from H2O2 in murine lymphoma cells (Bouzyk et al. 1997), in HepG2 cells (Yang et al. 1999), in Chinese hamster V79 cells (Miorelli et al. 2008), and in mouse hepatoma Hepa 1c1c7 cells (Keck and Findley 2006). Sodium selenite also inhibited the DNA damage caused by cadmium chloride in rat hepatic cells (Yu and Chen 2004). Sodium selenate avoided DNA strand breaks mediated by UVA radiation in human skin fibroblasts (Emonet-Piccardi et al. 1998) and by quenched potassium dichromate in human lymphocytes (Cemeli et al. 2003). Sodium selenite and SeMet protected keratinocytes against UV-induced oxidative damage (Rafferty et al. 2003), as well as SeMet protected against genotoxicity induced by doxorubicin in human lymphocytes (Santos and Takahashi 2008). SeMet was also found to protect against bleomycin-induced DNA damage on human lymphocytes (Laffon et al. 2009). Finally, low concentrations of diphenyl diselenide showed antimutagenic properties in V79 cells treated with H2O2, methyl methanesulphonate, and UVC radiation, probably due to the antioxidant properties of diphenyl diselenide (Rosa et al. 2007a, b).
Regarding to the cell growth, the effect of two Se compounds and methyl mercury was also studied in cell cultures (Alexander et al. 1979). Selenite at low concentration and seleno-di-N-acetyl-glycine in 1,000-fold higher concentrations offered considerable protection against the growth inhibiting effect and the stimulation of glucose and lactate uptake caused by methyl mercury in rat Morris hepatoma cells. However, no protective effect of Se was observed in other cell types as human lymphocytes and human embryonic fibroblasts. The data obtained suggested that Se compounds exert their protective effect through cell-specific processes rather than by a direct chemical reaction between selenite and methyl mercury. In another study, Hurst et al. (2008) exposed two human prostate cell lines to nutritionally relevant doses of MeSeCys and selenite, ranging from deficient to the equivalent of Se supplementation in humans. Several Se-responsive genes were identified by means of two microarray platforms, many of which have been ascribed to cancer cell growth and progression. The study revealed that MeSeCys can alter the expression of several types of collagen and thus potentially modulate the extracellular matrix and stroma, which may at least partially explain the anticancer activity of MeSeCys.
Concluding remarks
Se is one of the oligoelements most studied because of its particular properties. Like some other trace elements, Se is bimodal in nature whereby its beneficial properties occur in a limited range of daily intake below which it cannot perform its essential functions, and above which it is toxic (Alaejos et al. 2000). This nutritional range between essentiality and toxicity in Se is fairly narrow in comparison with the other essential trace elements (Letavayová et al. 2008a), and it could explain, among other causes, the enormous variability in the results of Se studies. As a consequence of these properties, Se can be included in the class of “Janus compounds”, having two “faces” on the same head (Miorelli et al. 2008). In general, at low concentrations, Se compounds are antimutagenic and anticarcinogenic, whereas at high concentrations, they are mutagenic, toxic, and possibly carcinogenic (Letavayová et al. 2008a).
When the effects of different selenocompounds were evaluated by means of the different in vitro assays, results obtained varied highly showing a great controversy. As general conclusions, Se resulted in no mutagenic or weakly mutagenic effects in bacterial assays (Lofroth and Ames 1978; Noda et al. 1979; Morimoto et al. 1982), but mutagenicity and genotoxicity of this element, mainly as sodium selenite, was observed in numerous studies with yeasts (Rosin 1981; Anjaria and Madhavanath 1989; Letavayová et al. 2008a). On the other hand, antigenotoxic properties of Se against a great variety of mutagenic agents were also detected in both cell systems (Martin et al. 1981; Longo et al. 1995; Bronzetti et al. 2001). This agrees with the results of different in vitro studies performed in mammalian cell systems. Data showed that Se induces CA (Nakamuro et al. 1976; Biswas et al. 2000) and SCE (Ray and Altenburg 1980, 1982; Sirianni and Huang 1983), inhibits DNA synthesis (Frenkel and Falvey 1988) and cell growth (Lu et al. 1995; Goel et al. 2006; Philchenkov et al. 2007), and promotes apoptosis (Chen et al. 2003; Last et al. 2006; Xiang et al. 2009). But also antigenotoxic and antimutagenic properties of adequate doses of Se against many chemical and physical agents have been described (Parshad et al. 1980; Sweins 1983; Beckman and Nordenson 1986; An et al. 1988; Hu et al. 1996; Bouzyk et al. 1997; Siviková et al. 2001; Cemeli et al. 2006; Rosa et al. 2007b; Santos and Takahashi 2008).
Nevertheless, all these results are not constant in the literature and vary enormously even when the same in vitro tests are employed. Many factors contribute to this great variety of results, mainly its chemical form (Nakamuro et al. 1976; Whiting et al. 1980; Sirianni and Huang 1983) and the concentration used (Weitberg et al. 1985; Biswas et al. 2000; Verma et al. 2004), but also the exposure time (Ray and Altenburg 1978; An et al. 1988), the treatment conditions (Cemeli et al. 2006; Ray et al. 1978), the cell type or the target tissue (Webber et al. 1985; Vadgama et al. 2000), and other previous factors as method of administration, animal species, physiological status, interaction with other compounds, etc. (Burk and Levander 2002). So, although it is common to speak of Se in the universal term of the element, just Se, the dose and form of the Se species actually determine its biological activity, be it the dietary essential nutrient, the cancer-preventing agent, or the toxicant (Letavayová et al. 2008a).
Se is an important element with beneficial properties as nutrient, and its dietary deficiency is linked to some diseases, e.g., Keshan disease and Kashin-Beck disease (Thomson 2004). Moreover, solid evidence based on epidemiological studies conducted in the last 50 years shows an inverse relationship between Se intake and cancer incidence (Alaejos et al. 2000; Surai 2006). For these reasons, today many people consume Se supplements on a regular basis to increase their intake and improve their nutritional status. They do this in the belief either that Se levels in the diet are inadequate or that the additional intake will provide protection against a variety of health problems. Much of current interest in Se as a supplement was triggered by the report by Clark et al. (1996) (Nutritional Prevention of Cancer study). The use of dietary supplements is considerable in many countries and appears to be increasing. These products are tested in a battery of genotoxicity assays (Griffiths and Matulka 2006), as those described in this paper, before being commercially available, normally in tablet form, in quantities up to 200 μg, and sometimes more, per tablet (Reilly 2006).
Despite the recent results of SELECT (Lippman et al. 2009), supplemental Se has been shown to have cancer-protective effects in a variety of experimental settings and clinical studies (reviewed by Whanger 2004) and to reduce the incidence and mortality of total cancer (Clark et al. 1996), prostate cancer (Duffield-Lillico et al. 2002), liver cancer (Yu et al. 1997), and stomach cancer (Blot et al. 1993) in human interventional trials. In general, the anticarcinogenic effect of Se against leukemia and cancers of the colon, rectum, pancreas, breast, ovaries, prostate, bladder, lung, and skin seems clear at least under some conditions (reviewed by Sunde 2000) and is closely related to its role in selenoproteins-reducing oxidative stress, to its ability to enhance the immune response or, more likely, to its ability to produce antitumorigenic metabolites (e.g., methylselenol or its precursors) that can perturb tumor-cell metabolism, inhibit angiogenesis and induce apoptosis in cancer cells (Rayman 2000; Whanger 2004). The source of the Se supplement (SeMet) in SELECT and the relatively high initial levels of Se in the enrolled men have been suggested to contribute to the negative results obtained in this trial (Hatfield and Gladyshev 2009).
But in spite of the extensive literature describing the antimutagenic and anticarcinogenic effects of Se compounds, little is known on their mode of action (Miorelli et al. 2008), although the anticancer activity of Se seems to be also dose dependent and species specific (Hurst et al. 2008). The bulk of our knowledge on the mechanisms of cancer prevention by Se is based on animal data and from studies conducted in in vitro systems (El-Bayoumy and Sinha 2005), and the modulation of certain in vitro markers may also be of value in predicting the effectiveness of novel forms of Se for cancer prevention. Thus, there is a plausible correlation between the relevance of these in vitro markers and the consequence of in vivo cancer protection. Whether these markers apply only to the biology of Se chemoprevention or could be extended to other classes of anticancer agents remains to be investigated (Lu et al. 1996).
In short, nowadays, besides the beneficial properties that Se has as nutrient and the fact that it seems to be effective in cancer prevention, the genotoxic effects of Se are currently being demonstrated in present studies. In this sense, the enormous variety of in vitro assays are allowing to describe, characterize, and delimit these effects in order to provide important information on the correct use of Se supplements in human health and chemoprevention. These assays show several advantages, as allowing controlling the features of the exposure and employing human cell lines that can provide a more real view of its effects on the human organism, what make them a perfect complement to in vivo assays when these can be used or an appropriate substitute when not.
References
Abul-Hassan KS, Lehnert BE, Guant L, Walmsley R (2004) Abnormal DNA repair in selenium-treated human cells. Mut Res 565:45–51
Alaejos MS, Diaz Romero FJ, Diaz Romero C (2000) Selenium and cancer: some nutritional aspects. Nutrition 16:376–383
Alexander J, Hostmark AT, Forre O, von Kraemer Bryn M (1979) The influence of selenium on methylmercury toxicity in rat hepatoma cells, human embryonic fibroblasts and human lymphocytes in culture. Act Pharmacol Toxicol 45:379–396
Al-Sabti K (1994) Micronuclei induced by selenium, mercury, methylmercury and their mixtures in binucleated blocked fish erythrocyte cells. Mut Res 320:157–163
Ammar EM, Couri D (1981) Acute toxicity of sodium selenite and selenomethionine in mice after ICV or IV administration. Neurotoxicology 2:383–386
Amodio-Cochieri R, Arnese A, Roncioni A, Silvestri G (1995) Evaluation of the selenium content of the traditional Italian diet. Int J Food Sci Nutr 46:149–154
An J, Chen QG, Gao FZ, Zheng E (1988) Effect of Na2SeO3 on the damages of genetic materials induced by MNNG in children′s foreskin fibroblasts in vitro. Zhonghua Zhong Liu Za Zhi 10:180–183
Anjaria KB, Madhavanath U (1989) Genotoxicity of selenite in diploid yeast. Mut Res 204:605–614
Aykin-Burns N, Ercal N (2006) Effects of selenocystine on lead-exposed Chinese hamster ovary (CHO) and PC-12 cells. Toxicol Appl Pharmacol 4:136–143
Balansky R (1992) Effects of sodium selenite and caffeine on mutagenesis induced by N-methyl-N-nitrosourea, N-methyl-N’-nitro-N-nitrosoguanidine and aflatoxin B1 in S. typhimurium. Mutat Res 269:307–317
Baliga MS, Wang H, Zhuo P, Schwartz JL, Diamond AM (2007) Selenium and GPx-1 overexpression protect mammalian cells against UV-induced DNA damage. Biol Trace Elem Res 115:227–242
Bansal MP, Sood S (1999) Influence of sodium selenite and selenomethionine on DNA/RNA synthesis and BaP binding to spleen lymphocytes in culture. Biol Trace Elem Res 70:21–28
Barile FA (2008) Principles of toxicology testing. CRC Press, Boca Raton
Beckman L, Nordenson I (1986) Interaction between some common genotoxic agents. Hum Hered 36:397–401
Berces J, Otos M, Szirmai S, Crane-Uruena C, Köteles GJ (1993) Using the micronucleus assay to detect genotoxic effects of metal ions. Environ Health Perspect 101:11–13
Biswas S, Talukder G, Sharma A (2000) Chromosome damage induced by selenium salt in human peripheral lymphocytes. Toxicol In Vitro 14:405–408
Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY, Yu Y, Liu BQ, Tangrea J, Sun YH, Liu F, Fraumeni JF, Zhang YH, Li B (1993) Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. JNCI 85:1483–1492
Bouzyk E, Iwanenko T, Jarocewicz N, Kruszewski M, Sochanowicz B, Szumiel I (1997) Antioxidant defense system in differentially hydrogen peroxide sensitive L5178Y sublines. Free Radic Biol Med 22:697–704
Bronzetti G, Cini M, Andreoli E, Caltavuturo L, Panunzio M, Croce CD (2001) Protective effects of vitamins and selenium compounds in yeast. Mut Res 496:105–115
Burk RF, Levander OA (2002) In: Shils M, Shike M, Ross AC, Caballero B, Cousins RJ (eds) Modern nutrition in health and disease. Lippincott Williams and Wilkins, Philadelphia, pp 312–325
Cabrera C, Lorenzo ML, De Mena C, López MC (1996) Chromium, copper, iron, manganese, selenium and zinc levels in dairy products: in vitro study of absorbable fractions. Int J Food Sci Nutr 47:331–339
Cai L, Mu LN, Lu H, Lu QY, You NC, Yu SZ, Le AD, Zhao J, Zhou XF, Marshall J, Heber D, Zhang ZF (2006) Dietary selenium intake and genetic polymorphisms of the GSTP1 and p53 genes on the risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 15:294–300
Cao S, Durrani FA, Rustum YM (2004) Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res 10:2561–2569
Carfi M, Gennari A, Maleaba I, Corsini E, Pallardy M, Pieters R, Van Loveren H, Vohr HW, Hartung T, Grimaldo L (2007) In vitro tests to evaluate immunotoxicity: a preliminary study. Toxicology 229:11–22
Cemeli E, Carder J, Anderson D, Guillamet E, Morillas MJ, Creus A, Marcos R (2003) Antigenotoxic properties of selenium compounds on potassium dichromate and hydrogen peroxide. Teratog Carcinog Mutag 23:53–67
Cemeli E, Marcos R, Anderson D (2006) Genotoxic and antigenotoxic properties of selenium compounds in the in vitro micronucleus assay with human whole blood lymphocytes and TK6 lymphoblastoid cells. The ScientificWorld J 6:1202–1210
Chan G, Hardej D, Santoro M, Lau-Cam C, Billack B (2007) Evaluation of the antimicrobial activity of ebselen: role of the yeast plasma membrane H+ -ATPase. J Biochem Mol Toxicol 21:52–264
Chen WX, Cao XZ, Zhu RZ (2003) Effect of selenium dioxide on proliferation, apoptosis, and telomerase activity of human lung cancer cell line in vitro. Ai Zheng 22:927–931
Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL Jr, Park HK, Sanders BB Jr, Smith CL, Taylor JR (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. J Am Med Assoc 276:1957–1963
Colado-Megía MI, Sánchez-Sánchez V, Camarero-Jiménez J, O’Shea-Gaya E (2004) Effect of dietary selenium on MDMA (“ecstasy”)-induced neurotoxicity in brain mouse (in Spanish). Mapfre Medicina 15:53–62
Collins AR, Harrington V, Drew J, Melvin R (2003) Nutritional modulation of DNA repair in a human intervention study. Carcinogenesis 24:511–515
COM (2000) Guidance on a strategy for testing of chemicals for mutagenicity. London: committee on mutagenicity of chemicals in food, consumer products and the environment
Combs GF Jr (1988) Selenium in foods. Adv Food Nutr Res 32:85–113
Combs GF Jr, Midthune DN, Patterson KY, Canfield WK, Hill AD, Levander OA, Taylor PR, Moler JE, Patterson BH (2009) Effects of selenomethionine supplementation on selenium status and thyroid hormone concentrations in healthy adults. Am J Clin Nutr 89:1808–1814
Davis RL, Spallholz JE, Pence BC (1998) Inhibition of selenite-induced cytotoxicity and apoptosis in human colonic carcinoma (HT29) cells by copper. Nutr Cancer 32:181–189
Dong Y, Zhang H, Hawthorn L, Ganther HE, Ip C (2003) Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Cancer Res 63:52–59
Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC (2002) Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev 11:630–639
Duffield-Lillico AJ, Slate EH, Reid ME, Turnbull BW, Wilkins PA, Combs GF Jr, Park HK, Gross EG, Graham GF, Stratton MS, Marshall JR, Clark LC (2003) Selenium supplementation and secondary prevention of nonmelanoma skin cancer in randomized trial. J Nat Cancer Inst 95:1477–1481
Ebert R, Ulmer M, Zeck S, Meissner-Wiegl J, Schneider D, Stopper H, Schupp N, Kassem M, Jakob F (2006) Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells 24:1226–1235
El-Bayoumy K, Sinha R (2005) Molecular chemoprevention by selenium: A genomic approach. Mut Res 591:224–236
Emonet-Piccardi N, Richard MJ, Ravanat JL, Signorini N, Cadet J, Beani JC (1998) Protective effects of antioxidants against UVA-induced DNA damage in human skin fibroblasts in culture. Free Radic Res 29:307–313
FAO/WHO (2001) Human vitamin and mineral requirements. Report of a joint FAO/WHO expert consultation.: Food and nutrition division, Rome, FAO, pp 235–256
FDA (2007) Guidance for industry and other stakeholders. Toxicological principles for the safety assessment of food ingredients. Redbook 2000, U.S. Food and Drug Administration, 2000, updated. Available at: http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodIngredientsandPackaging/Redbook/default.htm
Fischer JL, Mihelc EM, Pollok KE, Smith ML (2007) Chemotherapeutic selectivity conferred by selenium: a role for p53-dependent DNA repair. Mol Cancer Ther 6:355–361
Food and Nutrition Board (1980) Recommended Dietary Allowances, 9th ed. National Academy of Sciences. Washington, DC, pp. 162–164
Foster CB, Aswath K, Chanock SJ, McKay HF, Peters U (2006) Polymorphism analysis of six selenoprotein genes: support for a selective sweep at the glutathione peroxidase 1 locus (3p21) in Asian populations. BMC genetics 7:56
Frenkel GD (1985) Effects of sodium selenite and selenate on DNA and RNA synthesis in vitro. Toxicol Lett 25:219–223
Frenkel GD, Falvey D (1988) Evidence for the involvement of sulfhydryl compounds in the inhibition of cellular DNA synthesis by selenite. Mol Pharmacol 34:573–577
Frisk P, Yaqob A, Lindh U (2002) Indications of selenium protection against cadmium toxicity in cultured K-562 cells. Sci Total Environ 296:189–197
Gajewska J, Szczypka M, Tudek B, Szymczyk T (1990) Studies on the effect of ascorbic acid and selenium on the genotoxicity of nitrofurans: nitrofurazone and furazolidone. Mut Res 232:191–197
Garberg P, Stahl A, Warholm M, Hogberg J (1988) Studies of the role of DNA fragmentation in selenium toxicity. Biochem Pharmacol 37:3401–3406
Goel A, Fuerst F, Hotchkiss E, Boland CR (2006) Selenomethionine induces p53 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cancer Biol Ther 5:529–535
Goldhaber SB (2003) Trace element risk assessment: essentiality vs. toxicity. Regul Toxicol Pharmacol 38:232–242
Griffiths JC, Matulka RA (2006) Genotoxicity studies on Sel-Plex®, a standardized, registered high-selenium yeast. Int J Toxicol 25:477–485
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326:1–31
Hasgekar N, Beck JP, Dunkelberg H, Hirsch-Ernst KI, Gebel TW (2006) Influence of antimonite, selenite, and mercury on the toxicity of arsenic in primary rat hepatocytes. Biol Trace Elem Res 111:167–183
Hassan W, Ibrahim M, Nogueira C, Ahmed M, Rocha J (2009) Effects of acidosis and Fe(II) on lipid peroxidation in phospholipid extract: Comparative effect of diphenyl diselenide and ebselen. Environ Toxicol Pharmacol 28:152–154
Hatfield DL, Gladyshev VN (2009) The outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol Interv 9:18–21
Hu G, Liu X, Liu J (1996) Protective effects of sodium selenite and selenomethionine on genotoxicity to human peripheral lymphocytes induced by arsenic. Zhonghya Yu Fang Yi Xue Za Zhi 30:26–29
Huang LL, Hess JL, Bunce GE (1990) DNA damage, repair, and replication in selenite-induced cataract in rat lens. Curr Eye Res 9:1041–1050
Hurná E, Siklenka P, Hurná S (1997) Effect of selenium on cadmium genotoxicity investigated by micronucleus assay. Veterinární Medicína (Praha) 42:339–342
Hurst R, Elliott RM, Goldson AJ, Fairweather-Tait SJ (2008) Se-methylselenocysteine alters collagen gene and protein expression in human prostate cells. Cancer Lett 269:117–126
Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, Greeson JM, Baum MK, Shor-Posner G, Skyler JS, Schneiderman N (2007) Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation. A randomized controlled trial. Arch Inter Med 167:148–154
IARC (1987) IARC monographs on the evaluation of the carcinogenic risks to humans. Overall evaluations of carcinogenicity: an updating of IARC mongraphs volumes 1 to 42. Supplement 7. International Agency for Research on Cancer, Lyon
Itoh S, Shimada H (1996) Micronucleus induction by chromium and selenium, and suppression by metallothionein inducer. Mut Res 367:233–236
Kalhil AM, Maslat AO (1990) Chromosome aberrations, sister-chromatid exchanges and cell-cycle kinetics in human peripheral blood lymphocytes exposed to organoselenium in vitro. Mut Res 232:227–232
Kaur P, Evje L, Aschner M, Syversen T (2009) The in vitro effects of selenomethionine on methylmercury-induced neurotoxicity. Toxicol In Vitro 23:378–385
Keck AS, Findley JW (2006) Aqueous extracts of selenium-fertilized broccoli increase selenoprotein activity and inhibit DNA single strand breaks, but decrease the activity of quinone reductase in Hepa1c1c7 cells. Food Chem Toxicol 44:695–703
Khalil AM (1989) The induction of chromosome aberrations in human purified peripheral blood lymphocytes following in vitro exposure to selenium. Mut Res 224:503–506
Kim Y-J, Baek S-H, Bogner PN, Ip C, Rustum YM, Fakih MG, Ramnath N, Park YM (2007) Targeting the Nrf2-Prx1 pathway with selenium to enhance the efficacy and selectivity of cancer therapy. J Cancer Molecules 3:37–43
Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G (1994) Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biol Trace Elem Res 41:115–127
Kitahara J, Seko Y, Imura N (1993) Possible involvement of active oxygen species in selenite toxicity in isolated rat hepatocytes. Arch Toxicol 67:497–501
Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, Coltman C (2000) SELECT: the selenium and vitamin E cancer prevention trial: rationale and design. Prostate Cancer Prostatic Dis 3:145–151
Kramer GF, Ames BN (1988) Isolation and characterization of a selenium metabolism mutant of Salmonella typhimurium. J Bacteriol 170:736–743
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443
Laffon B, Valdiglesias V, Pásaro E, Méndez J (2009) The organic selenium compound selenomethionine modulates bleomycin-induced DNA damage and repair. Biol Trace Elem Res. doi:10.1007/s12011-009-8407-9
Last K, Maharaj L, Perry J, Strauss S, Fitzgibbon J, Lister TA, Joel S (2006) The activity of methylated and non-methylated selenium species in lymphoma cell clines and primary tumours. Ann Oncol 7:773–779
LeBoeuf RA, Laishes BA, Hoekstra WG (1985) Effects of selenium on cell proliferation in rat liver and mammalian cells as indicated by cytokinetic and biochemical analysis. Cancer Res 45:5496–5504
Lemire M, Mergler D, Fillion M, Passos CJ, Guimaraes JR, Davidson R, Lucotte M (2006) Elevated blood selenium levels in the Brazilian Amazon. Sci Total Environ 366:101–111
Letavayová L, Vlcková V, Brozmanová J (2006) Selenium: from cancer prevention to DNA damage. Toxicology 227:1–14
Letavayová L, Vlasakova D, Spallholz JE, Brozmanova J, Chovanec M (2008a) Toxicity and mutagenicity of selenium compounds in Saccharomyces cerevisiae. Mut Res 638:1–10
Letavayová L, Vlasáková D, Vlcková V, Brozmanová J, Chovanec M (2008b) Rad52 has a role in the repair of sodium selenite-induced DNA damage in Saccharomyces cerevisiae. Mut Res 652:198–203
Li GX, Hu H, Jiang C, Schuster T, Lü J (2007) Differential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cells. Int J Cancer 120:2034–2043
Li F, Xu J, Zhou J, Zhao L, Sheng J, Sun G, Hu Q (2009) Inhibition of mitomycin C-induced chromosomal aberrations by micrometer powder of selenium-enriched green tea in mice spermatocytes. Mut Res 675:11–16
Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL Jr, Baker LH, Coltman CA Jr (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT). J Am Med Assoc 301:39–51
Lo LW, Koropatnik J, Stich HF (1978) The mutagenicity and cytotoxicity of selenite, “activated” selenite, and selenate for normal and DNA repair-deficient human fibroblasts. Mut Res 49:305–312
Lofroth G, Ames BN (1978) Mutagenicity of inorganic compounds in Salmonella typhimurium: arsenic, chromium, and selenium. Mut Res 53:65–66
Longo VD, Gralla EB, Valentine JS (1995) Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. J Biol Chem 271:12275–12280
Lu J, Jiang C, Kaeck M, Ganther H, Vikit SV, Ip C, Thompson H (1995) Dissociation of the genotoxic and growth inhibitory effects of selenium. Biochem Pharmacol 50:213–219
Lu J, Pei H, Ip C, Lisk DJ, Ganther H, Thompson HJ (1996) Effect of an aqueous extract of selenium-enriched garlic on in vitro markers and in vivo efficacy in cancer prevention. Carcinogenesis 17:1903–1907
Machado Mda S, Villela IV, Moura DJ, Rosa RM, Salvador M, Lopes NP, Braga AL, Roesler R, Saffi J, Henriques JA (2009) 3’3-ditrifluoromethyldiphenyl diselenide: a new organoselenium compound with interesting antigenotoxic and antimutagenic activities. Mut Res 673:133–140
Marro N (1996) The 1994 Australian market basket survey. Australian government publishing service, Camberra
Martin SE, Adams GH, Schillaci M, Milner JA (1981) Antimutagenic effect of selenium on acridine orange and 7, 12-dimethylbenzo [α]anthracene in the Ames Salmonella/microsomal system. Mut Res 82:41–46
Maurici D, Aardema M, Corvi R, Cléber M, Krul C, Laurent C, Loprieno N, Pasanen M, Pfuhler S, Phillips B, Sabbioni E, Sanner T, Vanparys P (2005) Genotoxicity and mutagenicity. ATLA 33:117–130
McKelvey-Martin VJ, Green MHL, Schmezer P, Pool-Zobel BL, De Méo MP, Collins A (1993) The single cell gel electrophoresis assay (comet assay): a European review. Mut Res 288:47–63
McNaughton SA, Marks GC (2002) Selenium content of Australian foods: a review of literature values. J Food Comp Analysis 15:169–182
Medina D, Oborn CJ (1984) Selenium inhibition of DNA synthesis in mouse mammary epithelial cell line YN-4. Cancer Res 44:4361–4365
Miorelli ST, Rosa RM, Moura DJ, Rocha JC, Carneiro Lobo LA, Pêgas Henriques JA, Saffi J (2008) Antioxidant and anti-mutagenic effects of ebselen in yeast and in cultures mammalian V79 cells. Mutagenesis 23:93–99
Morimoto K, Iijima S, Koizumi A (1982) Selenite prevents the induction of sister-chromatid exchanges by methyl mercury and mercuric chloride in human whole-blood cultures. Mut Res 102:183–192
Morris JDH, Pramanik R, Zhang X, Carey A-M, Ragavan N, Martin FL, Muir GH (2006) Selenium- or quercetin-induced retardation of DNA synthesis in primary prostate cells occurs in the presence of a concomitant reduction in androgen-receptor activity. Cancer Lett 239:111–122
Morrison DG, Dishart MK, Medina D (1988) Intracellular 58-kd selenoprotein levels correlate with inhibition of DNA synthesis in mammary epithelial cells. Carcinogenesis 9:1801–1810
Mousa SA, O’Connor L, Rossman TG, Block E (2007) Pro-angiogenesis action of arsenic and its reversal by selenium-derived compounds. Carcinogenesis 28:962–967
Murphy J, Cashman KD (2001) Selenium content of a range of Irish foods. Food Chem 74:493–498
Nakamuro K, Yoshikawa Y, Sayato Y, Kurata H, Tonomura M, Tonomura A (1976) Studies on selenium-related compounds, V. Cytogenetic effect and reactivity with DNA. Mut Res 40:177–184
Nano JL, Czerucka D, Menguy F, Rampal P (1989) Effect of selenium on the growth of three human colon cancer cell lines. Biol Trace Elem Res 20:31–43
Navarro-Alarcon M, Cabrera-Vique C (2008) Selenium in food and the human body: a review. Sci Total Environ 400:115–141
Navarro-Alarcon M, Gil Hernández F, Gil Hernández A (2005) In: Gil A (ed) Tratado de nutrición: bases Fisiológicas y Bioquímicas de la Nutrición. Acción Médica, Madrid, pp 997–1036
Noda M, Takano T, Sakurai H (1979) Mutagenic activity of selenium compounds. Mut Res 66:175–179
O’Brien J, Renwick AG, Constable A, Dybing E, Müller DJ, Schlatter J, Slob W, Tueting W, Van Benthem J, Williams GM, Wolfreys A (2006) Approaches to the risk assessment of genotoxic carcinogens in food: a critical appraisal. Food Chem Toxicol 44:1613–1635
Panigati M, Falciola L, Mussini P, Beretta G, Fancino RM (2007) Determination of selenium in Italian rices by differential pulse cathodic stripping voltammetry. Food Chem 105:1091–1098
Pappa EC, Pappas AC, Surai PF (2006) Selenium content in selected foods from the Greek marked and estimation of the daily intake. Sci Total Environ 372:100–108
Parshad R, Taylor WG, Sanford KK, Camalier RF, Gantt R, Tarone RE (1980) Fluorescent light-induced chromosome damage in human IMR-90 fibroblasts. Role of hydrogen peroxide and related free radicals. Mut Res 73:115–124
Peterson SA, Ralston NV, Peck DV, Van Sickle J, Robertson JD, Spate VL, Morris JS (2009) How might selenium moderate the toxic effects of mercury in stream fish of the western U.S.? Environ Sci Technol 43:3919–3925
Philchenkov A, Zavelevich M, Khranovskaya N, Surai P (2007) Comparative analysis of apoptosis inductions by selenium compounds in human lymphoblastic leukaemia MT-4 cells. Exp Oncol 29:257–261
Poerschke RL, Franklin MR, Moos PJ (2008) Modulation of redox status in human lung cell lines by organoselenocompounds: selenazolidines, selenomethionine, and methylseleninic acid. Toxicol In Vitro 22:1761–1767
Rafferty TS, Green MHL, Lowe JE, Arlett C, Hunter JAA, Beckett GJ, McKenzie RC (2003) Effects of selenium compounds on induction of DNA damage by broadband ultraviolet radiation in human keratinocytes. Br J Dermatol 148:1001–1009
Ray JH, Altenburg IC (1978) Sister-chromatid exchange induction by sodium selenite: Dependence on the presence of red blood cells or red blood cell lysate. Mut Res 54:343–354
Ray JH, Altenburg IC (1980) Dependence of the sister-chromatid exchange-inducing abilities of inorganic selenium compounds on the valence state of selenium. Mut Res 78:261–266
Ray JH, Altenburg IC (1982) Sister-chromatid exchange induction by sodium selenite. Plasma protein-bound selenium is not the active SCE-inducing metabolite of Na2SeO3. Mut Res 102:285–296
Ray JH, Altenburg IC, Jacobs MM (1978) Effects of sodium selenite and methyl methanesulphonate or N-hydroxy-2-acetylaminofluorence co-exposure on sister chromatid exchange production in human blood cultuResearch. Mut Res 57:359–368
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Reid ME, Duffield-Lillico AJ, Garland L, Turnbull BW, Clark LC, Marshall JR (2002) Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev 11:1285–1291
Reid ME, Stratton MS, Lillico AJ, Fakih M, Natarajan R, Clark LC, Marshall JR (2004) A report of high-dose selenium supplementation: response and toxicities. J Trace Elem Med Biol 18:69–74
Reilly C (2006) Selenium in food and health. Springer, New York
Resnick MA, Cox BS (2000) Yeast and honorary mammal. Mut Res 451:1–11
Rooprai HK, Kyriazis I, Nuttall RK, Edwards DR, Zicha D, Aubyn D, Davies D, Gullan R, Pilkington GJ (2007) Inhibition of invasion and induction of apoptosis by selenium in human malignant brain tumour cells in vitro. Int J Oncol 30:1263–1271
Rosa RM, Sulzbacher K, Picada JN, Roesler R, Saffi J, Brendel M, Pêgas Henriques JA (2004) Genotoxicity of diphenyl diselenide in bacteria and yeast. Mut Res 563:107–115
Rosa RM, Moura DJ, Romano e Silva AC, Saffi J, Pêgas Henriques JA (2007a) Antioxidant activity of diphenyl diselenide prevents the genotoxicity of several mutagens in Chinese hamster V79 cells. Mut Res 63:44–54
Rosa RM, Picada JN, Saffi J, Pêgas Henriques JA (2007b) Cytotoxic, genotoxic, and mutagenic effects of diphenyl diselenide in Chinese hamster lung fibroblasts. Mut Res 8:87–98
Rosin MP (1981) Inhibition of spontaneous mutagenesis in yeast cultures by selenite, selenate and selenite. Cancer Lett 13:7–14
Roussyn I, Briviba K, Masumoto H, Sies H (1996) Selenium-containing compounds protect DNA from single-strand breaks caused by peroxynitrite. Arch Biochem Biophys 330:216–218
Russell GR, Nader CJ, Partick EJ (1980) Induction of DNA repair by some selenium compounds. Cancer Lett 10:75–81
Santos RA, Takahashi CS (2008) Anticlastogenic and antigenotoxic effects of selenomethionine on doxorubicin-induced damage in vitro in human lymphocytes. Food Chem Toxicol 46:671–677
Santos DB, Schiar VPP, Ribeiro MCP, Schwab RS, Meinerz DF, Allebrandt J, Rocha JB, Nogueira CW, Aschner M, Barbosa NB (2009) Genotoxicity of organoselenium compounds in human leukocytes in vitro. Mut Res 676:21–26
Seitomer E, Balar B, He D, Copeland PR, Kinzy TG (2008) Analysis of Saccharomyces cerevisiae null allele strains identifies a larger role for DNA damage versus oxidative stress pathways in growth inhibition by selenium. Mol Nutr Food Res 52:1305–1315
Seo YR, Christopher S, Smith ML (2002) Selenomethionine induction of DNA repair response in human fibroblasts. Oncogene 21:3663–3669
Shen HM, Yang CF, Ong CN (1999) Sodium selenite-induced oxidative stress and apoptosis in human hepatoma HepG2 cells. Int J Cancer 81:820–828
Shen CL, Song W, Pence BC (2001) Interactions of selenium compounds with other antioxidants in DNA damage and apoptosis in human normal keratinocytes. Cancer Epidemiol Biomarkers Prev 10:385–390
Sirianni SR, Huang CC (1983) Induction of sister chromatid exchange by various selenium compounds in Chinese hamster cells in the presence and absence of S9 mixture. Cancer Lett 18:109–116
Sirichakwal PP, Puwastein P, Polngam J, Kongkachuichai R (2005) Selenium content of Thai foods. J Food Comp Analysis 18:7–59
Siviková K, Piesová E, Dianovský J (2001) The protection of vitamin E and selenium against carbon tetrachloride-induced genotoxicity in ovine peripheral blood lymphocytes. Mut Res 494:135–142
Smith ML, Lancia JK, Mercer TI, Ip C (2004) Selenium compounds regulate p53 by common and distinctive mechanisms. Anticancer Res 24:1401–1408
Stewart MS, Davis RL, Walsh LP, Pence BC (1997) Induction of differentiation and apoptosis by sodium selenite in human colonic carcinoma cells (HT29). Cancer Lett 117:35–40
Stewart MS, Spallholz JE, Neldner KH, Pence BC (1999) Selenium compounds have disparate abilities to impose oxidative stress and induce apoptosis. Free Radic Biol Med 26:42–48
Sunde RA (2000) Biochemical and physiological aspects of human nutrition. W.B. Saunders Company, New York, pp 782–809
Surai PF (2006) Selenium in nutrition and health. Nottingham University Press, Nottingham, pp 643–808
Suzuki KT, Doi C, Suzuki N (2006a) Metabolism of 76Se-methylselenocysteine compared with that of 77Se-selenomethionine and 82Se-selenite. Toxicol Appl Pharmacol 217:185–195
Suzuki KT, Kurasaki K, Ogawa S, Suzuki N (2006b) Metabolic transformation of methylseleninic acid through key selenium intermediate selenide. Toxicol Appl Pharmacol 215:189–197
Sweins A (1983) Protective effect of selenium against arsenic-induced chromosomal damage in cultured human lymphocytes. Hereditas 98:249–252
Thomson CD (2004) Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clinical Nutr 58:391–402
Tkeshelashvili LK, Shearman CW, Zakour RA, Koplitz RM, Loeb LA (1980) Effects of arsenic, selenium, and chromium on the fidelity of DNA synthesis. Cancer Res 40:2455–2460
Tran D, Moody AJ, Fisher AS, Foulkers ME, Jha AN (2007) Protective effects of selenium on mercury-induced DNA damage in mussel haemocytes. Aquatic Toxicol 84:11–18
Vadgama JV, Wu Y, Shen D, Hsia S, Block J (2000) Effect of selenium in combination with Adriamycin or Taxol on several different cancer cells. Anticancer Res 20:1391–1414
Verma A, Atten MJ, Atter BM, Holian O (2004) Selenomethionine stimulates MAPK (ERK) phosphorylation, protein oxidation, and DNA synthesis in gastric cancer cells. Nutr Cancer 49:184–190
Wang XH, Wei YM, Bai H, Ou JF, Lu JH, Zheng RL (2004) Apoptosis and regulation of expressions of apoptosis-related gene Bcl-2 and p53 induced by selenium dioxide in three leukaemia cell lines. Di Yi Jun Yi Da Xue Xue Bao 24:1160–1163
Webber MM, Perez-Ripoll EA, James GT (1985) Inhibitory effects of selenium on the growth of DU-145 human prostate carcinoma cells in vitro. Biochem Biophys Res Commun 130:603–609
Weitberg AB, Weitzman SA, Clark EP, Stossel TP (1985) Effects of antioxidants on oxidant-induced sister chromatid exchange formation. J Clin Invest 75:1835–1841
Whanger PD (2004) Selenium and its relationship to cancer: an update. Br J Nutr 91:11–28
Whiting RF, Wei L, Stich HF (1980) Unscheduled DNA synthesis and chromosome aberrations induced by inorganic and organic selenium compounds in the presence of glutathione. Mut Res 78:159–169
Wilson AC, Thompson HJ, Schedin PJ, Gibson NW, Ganther HE (1992) Effect of methylated forms of selenium on cell viability and the induction of DNA strand breakage. Biochem Pharmacol 43:1137–1141
Wycherly BJ, Moak MA, Christensen MJ (2004) High dietary intake of sodium selenite induces oxidative DNA damage in rat liver. Nutr Cancer 48:78–83
Xiang N, Zhao R, Zhong W (2009) Sodium selenite induces apoptosis by generation of superoxide via the mitochondrial-dependent pathway in human prostate cancer cells. Cancer Chemother Pharmacol 63:351–362
Xiao HJ, Huang CY, Wang HY, Li M (2008) Effect of selenium and zinc on the proliferation of human esophageal cancer Eca109 cell line in vitro. Nan Fang Yi Ke Da Xue Xue Bao 28:2117–2120
Yamamoto Y, Inoue I, Takasaki T, Takahashi H (1996) Inhibitory effects of selenium, vitamin A and butylated hydroxytoluene on growth of human maxillary cancer cells in vitro. Auris Nasus Larynx 23:91–97
Yan L, Spallholz JE (1993) Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem Pharmacol 45:429–437
Yang CF, Shen HM, Ong CN (1999) Protective effect of ebselen against hydrogen peroxide-induced cytotoxicity and DNA damage in HepG2 cells. Biochem Pharmacol 57:273–279
Yeh JY, Ou BR, Liang YC, Burchfield J, Butler JA, Forsberg NE, Whanger PD (2006) Mechanism for proliferation inhibition by various selenium compounds and selenium-enriched broccoli extract in rat glial cells. Biometals 19:611–621
Yi H, Si L (2007) Vicia root-micronucleus and sister chromatid exchange assays on the genotoxicity of selenium compounds. Mut Res 630:92–96
Yu RA, Chen XM (2004) Effects of selenium on rat hepatocellular DNA damage induced by cadmium in vitro. Zhonghua Yu Fang Yi Xue Za Zhi 30:29–32
Yu SY, Zhu YJ, Li WG (1997) Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol Trace Elem Res 56:117–124
Zhang J, Peng D, Lu H, Liu Q (2008) Attenuating the toxicity of cisplatin by using selenosulfate with reduced risk of selenium toxicity as compared with selenite. Toxicol Appl Pharmacol 226:251–259
Zhao H, Brooks JD (2007) Selenomethionine induced transcriptional programs in human prostate cancer cells. J Urol 77:43–750
Zhao H, Whitfield ML, Xu T, Botstein D, Brooks JD (2004) Diverse effects of methylselenic acid on the transcriptional program of human prostate cancer cells. Mol Biol Cell 15:506–519
Zhong W, Oberley T (2001) Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cell line. Cancer Res 61:7071–7078
Zou Y, Yang J, Liu X, Yuan J (2007) Relationship between reactive oxygen species and apoptosis in HepG2 cells induced by sodium selenite. Wei Sheng Yan Jiu 36:272–274
Zwolak I, Zaporowska H (2009) Preliminary studies on the effect of zinc and selenium on vanadium-induced cytotoxicity in vitro. Acta Biol Hung 60:55–56
Acknowledgments
This work was funded by a grant from the Xunta de Galicia (INCITE08PXIB106155PR). V. Valdiglesias was supported by a fellowship from the University of A Coruña.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valdiglesias, V., Pásaro, E., Méndez, J. et al. In vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: a review. Arch Toxicol 84, 337–351 (2010). https://doi.org/10.1007/s00204-009-0505-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-009-0505-0