Abstract
Histological and histochemical alterations in the liver and kidneys of the frog Rana ridibunda, which was exposed to 200 ppm aqueous solutions of cadmium for 4, 10 and 30 days, respectively were investigated. In both the liver and kidneys, essential changes appeared after 10 days’ exposure, the maximum changes being apparent after 30 days of exposure. In the liver, what was very characteristic was an increase in the area occupied by Kupffer cells, with the area in the animals exposed to cadmium for 30 days being the largest observed. What was also apparent was karyomegaly, polyploidy and infiltration. In addition with regard the kidneys, Hyaline Globules (HG) and apoptotic bodies occurred at a higher rate. At 30 days’ exposure, most of the above changes were enhanced. In comparison with 10 days’ exposure, fibrosis around the blood vessels and between hepatocytes, as well as Proliferating Cell Nuclear Antigen (PCNA) reactivity and apoptotic bodies increased lightly in the liver. In the kidneys, the most pronounced changes were the increase in numbers of apoptotic bodies, PCNA reactivity and hyaline globules. It was concluded that the variability in positive reactions for various proteins in the hyaline globules may be an indication that these (and possibly other) proteins are synthesized by tumor cells, but, most probably, that they may represent liver damage, progressive nephropathy, or progressive glomerulonephropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a non-essential heavy metal, which is of major environmental and public health concerns (WHO 1992). The effects of Cd on early life stages of amphibia have been well-documented (Nebeker et al. 1994). Larval teratogenicity and developmental abnormalities (Calevro et al. 1998) as well as growth retardation (Loumbourdis et al. 1999) have been reported.
The Cd has an extremely long biological half-life (about 30 years) in both human and experimental animals’ bodies and accumulates in tissues, and in particular in the kidneys (Friberg et al. 1974; Vogiatzis and Loumbourdis 1998). Thus, Cd acts as a hepatotoxin, producing hepatic dysfunction, which is characterized by increased excretion of the plasma alanine aminotransferase (GPT). The Cd has also been reported as a possible cause of various histopathological lesions in the liver, such as periportal liver cell necrosis and bile duct hyperplasia in rats (Mitsumori et al. 1998), cell death and regeneration (Tanimoto et al. 1993), dilatation of sinusoids, and an increase in the number of Kupffer cells (Meiss et al. 1982) as well as Kupffer cell enlargement (Sudo et al. 1996; Swiergosz et al. 1998).
The earliest sign of kidney damage is an increase in proteinuria as a result of damage to the Proximal Convoluted Tubules (PCT), characterized by the urinary excretion of a number of low-molecular-weight proteins including β2-microglobulin, retinol-binding protein (RBP) and N-acetylglucosaminidase (NAG). The tubular proteinuria may be followed by other signs of kidney dysfunction, such as glomelural proteinuria (urinary excretion of high molecular weight proteins, e.g. albumin), increased urinary excretion of glucose, amino acids, calcium, phosphorus and uric acid.
The portion of kidney affected by Cd is that of the PCT (Hamada et al. 1994; Shibutani et al. 2000). According to Herak-Kramberger and Sabolic (2001), nephrotoxic heavy metals (such as Cd) directly damage the integrity of proximal tubule cell plasma membrane, causing thus shortening and loss of microvilli and to basolateral invaginations in heavy metals-treated experimental animals in vivo.
Various hypotheses have been proposed to explain the pathogenesis of Cd nephrotoxicity. The hypothesis most commonly accepted is that Cd is taken up by the liver, where it binds to metallothionein (MT–Cd). The MT–Cd complex is released from the liver into plasma and is then filtered through the glomeruli and taken up by the proximal tubules of the kidneys. The Cd taken up by the proximal tubular cells damages these cells. A different hypothesis has been proposed by Nomiyama and Nomiyama (1998). According to this hypothesis, the Cd, which is not bound to metallothionein, can induce free radicals, which may, in turn, depress hepatic functions. Hepatic Cd–thionein is then released into the bloodstream. Plasma Cd–thionein passes through the glomeruli and reaches the proximal tubular lumen. There, Cd–thionein may split by an unknown mechanism into Cd ions and holo-thionein on or the brush border membrane of the proximal tubular cells. Free radicals, which are initiated by Cd ion, may injure the brush border membranes.
More recently, proposed cellular biomarkers of Cd exposure in aquatic organisms are programmed cell death (apoptosis) (Berntssen et al. 2001) and cell proliferation. Apoptosis has been suggested as a sensitive and early indicator of chronic chemical stress, loss of cellular function and structure, and organism death (Sweet et al. 1999). Under toxic exposure, apoptosis is suggested to remove critically damaged cells followed by compensatory cell regeneration to maintain tissue structure and function (Habeebu et al. 1998).
Measurements of cell proliferation in normal or neoplastic cell populations can be informative indicators both of tissue regeneration and of the carcinogenic nature of many drugs or chemicals. Immunohistochemical markers of cell proliferation such as Proliferating Cell Nuclear Antigen (PCNA) have recently been used successfully (Hadjolov and Bitsch 1997; Shibutani et al. 2000; Berntssen et al. 2001).
Hyaline globules (HG) have been identified in numerous types of malignant and benign tumors as well as in non-neoplastic tissue (Papadimitriou et al. 2000; De Rijk et al. 2003; Yokoo et al. 2003). They are cytoplasmic, either intracellular or extracellular, and are usually stained with acidic dyes, such as eosin, PAS and acid fuchsine. They are immunohistochemically positive for alpha-antitrypsin, albumin and ferritin, and are thus connected to erythrophagocytosis, kappa and lambda light immunoglobulin chains, IgG and many others (Papadimitriou et al. 2000). Ultrastructurally, the HG consist of large osmiophilic inclusions containing membranes or nuclear debris at various stages of digestion, consistent with secondary lysosomes or phagosomes (Papadimitriou et al. 2000).
In a series of experiments, adult frogs Rana ridibunda were exposed to 200 ppm of Cd aqueous solution (as CdCl2) in order to study Cd tissue distribution and hepatic metallothionein and glutathione levels, as well as changes in the hepatic metabolism as a result of exposure to Cd (Vogiatzis and Loumbourdis 1998, 1999a). This paper is part of that project and its aim is to study the impact of exposure to Cd on various hepatic and renal histological lesions.
Materials and methods
Experiments were conducted during the animals’ active period in the summer of 1997. Adult female R.ridibunda were purchased from a local dealer who collected them from a relatively unpolluted area of Northern Greece.
The experimental conditions have been described elsewhere (Vogiatzis and Loumbourdis, 1998, 1999b). Twelve frogs were placed in a plastic aquarium (120×65×60 cm) in an aqueous solution of 200 mg/L Cd, while another four frogs, served as the control group, were kept in clean tap water. Frogs were sacrificed at the end of the 4th (N=4 animals, 10th(N=4 animals) and 30th (N=4 animals) days’ of Cd exposure.
Part of the liver and kidney from control and exposed frogs was fixed in 10% neutral buffered formalin, dehydrated, cleaned, embedded in paraffin and cut at 7 μm thickness. For general histopathological study the tissue was stained using Hematoxylin–Eosin (H–E) stain. For histochemical detection of fibrosis, Sirius Red (Juncueira et al. 1979) was used and for detection of hemosiderin Perl’s acid ferrocyanide (Pearse 1985) stain was used. From each section of the liver stained by Perl’s method, five areas were arbitrarily selected under 200 times magnification, and then digitized and stored as a file in jpeg format for later analysis. To identify any PAS positive material in the tissues, PAS stain (Pearse 1985) was employed. To avoid differences in staining intensity, all the sections to be stained with the same method were stained simultaneously.
Apoptotic bodies (AB) were detected by fluorescence microscopy, using a Zeiss Axioskop microscope equipped with an epifluorescence unit (BP 450–490, FT 510, LP 520). This detection of AB by fluorescence microscopy is a novel technique (Stinchcombe et al. 1995) based on the observation that AB show strong eosin fluorescence in H–E stained sections. Because of this, identification of AB was confirmed by using transmitted light microscope, and by switching between fluorescent and light microscope.
To estimate the rate of cell regeneration, PCNA immunostaining of liver and kidney sections was performed, using a mouse monoclonar primary antibody and immunoreactivity was detected by the ImmunoCruz staining system. Formalin-fixed, paraffin-embedded tissue sections were prepared from both the control and exposed animals. The whole procedure was performed in strict accordance with the instructions, which accompanied the kit.
In the liver sections, which were stained using Perl’s method, five randomly selected fields were digitized and stored as jpeg files. An electronic imaging program (Sigma Scan Pro 5.0) was used to assist in area measurement by projecting the electronic image of the above mentioned files with the area stained blue representing the iron stored as hemosiderin and/or ferritin.
The values of the studied parameters were compared by analysis of variance (ANOVA) and Dunnet’s comparison test was used to compare the means. Differences were deemed statistically significant at P < 0.05. Statistical analysis was carried out with SPSS program Version 10.0 for windows.
Results
Liver from the Cd-treated groups did not exhibit any macroscopic alterations in structure. Similarly, light microscopic studies did not reveal any significant changes in structure in the control and 4 days exposed frogs.
After 10 days’ exposure, marked alterations in the liver were detected. The area occupied by Kupffer cells was significantly larger than that of controls and 4 days exposed frogs (Table 1). The karyomegaly and polyploidy was also noteworthy (Fig. 1). Diffuse necrotic cells and solitary or small foci of infiltrates, mainly lymphocytes, monocytes and neutrophils were evident. Fibrosis around blood vessels and between hepatocytes was lightly prominent.
At 30 days exposure, most histological lesions were enhanced. Thus, the foci of infiltrates had increased in number. The AB and PCNA positive foci were evident. There were no signs of increased fibrosis. The area occupied by Kupffer cells was the largest observed in all the sample studied (Table 1).
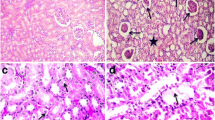
The changes observed in kidneys followed the same pattern more or less. Thus after 4 days’ exposure no changes were observed. After 10 days’ exposure, prominent changes such as karyomegaly and polyploidy (Fig. 2), infiltration and vacuolization had taken also place. For the first time, HG, 1–15 μm in diameter made their appearance. Most of them were strongly eosinophilic in routine H–E staining. They were located within the cytoplasm of PCT cells or, less frequently, in the lumen of PCT (Fig. 3).
After 30 days’ exposure, tubular degeneration was enhanced, and many PCT had lost their shape. In one animal, some PCT had degenerated completely, their cells were lost and an amorphous mass filled the entire lumen (Fig. 4). The HG observed after 10 days exposure increased in number and in some of them nuclear fragments were evident (Fig. 5), suggesting a similarity with the AB. Perl stain revealed a more complex situation. Some HG were completely Perl positive, suggesting the presence of iron in the whole globule. Some other were Perl negative and a third category was partly Perl positive. Most of the HG, especially those smaller in diameter, were strongly PAS positive, while some others were lightly stained and the rest of them were completely unstained (Fig. 6). A few globules were moderately stained with Sirius red, suggesting the presence of collagenous proteins. The PCNA immunoreactive cells were preferentially detected in the PCTs undergoing degeneration (Fig. 7). Scattered AB were also seen, most of them enclosed in epithelial cells, and some others lying in the lumen of PCT cells. Two types of AB were detected, namely those with DNA fragments, and those without fragments. The fluorescence of the second type was stronger and some AB are detectable only with fluorescence microscopy (Figs. 8a, b).
a, b Section of frog kidney stained with H–E, showing apoptotic bodies (arrows) and two hyaline globules (arrowheads) visualized by transmitted light microscope (a) and fluorescent microscope (b). Note that the less fluorescent apoptotic bodies in b contain DNA fragments and the hyaline globules do not fluoresce at all. Scale bar 25 μm
Discussion
The present study was conducted to elucidate the relationship between levels of Cd exposure and sensitivity, which induces hepatic and renal toxicity during a 30-day period.
The liver is well known as the primary organ for Cd accumulation and for its gradual redistribution to other organs, mainly the kidneys (Vogiatzis and Loumbourdis 1998). In the liver, Cd is stored mainly as a metallothionein complex (Cd–MT), since the concentration of this low molecular weight protein seems to increase accordingly with increasing concentrations of Cd (Vogiatzis and Loumbourdis 1998). However, other proteins such as glutathione (GSH) may be involved (Vogiatzis and Loumbourdis 1998).
The first signs of liver toxicity appear after 10 days exposure. First of all, an increase in the concentration of melanomacrophage (MMC) was detectable (Loumbourdis and Vogiatzis 2002), as a result of exposure to Cd. It was found that this concentration was dose-dependent.
In this study, an increase in the area occupied by Kupffer cells was detected in Perl stained material. It is well known that Perl’s method stains ferrous and ferric iron. Iron (Fe) is mainly stored in hemoglobin (60–70%) and to a great extent (25–27%) in cells of the reticuloendothelial system, like Kupffer cells (Stryer 1988). An increase in the area, which is stained blue using Perl’s method is an indication of an increase in the Fe content of Kupffer cells. It is well known that Cd causes rupturing of small blood vessels (Rani and Ramamurthi 1989; Taylor 1996), resulting in the discharge of erythrocytes to the surrounding tissues. The Fe inside the Kupffer cells is stored in the form of ferritin and hemosiderin. With time, ferritin is engulfed by lysosomes and is catabolized to hemosiderin, a nonspecific mixture of partially degraded protein, lipid and Fe. Ferritin is the only other well-characterized system for the intracellular storage and sequestration of metals, apart from metallothionein and glutathione (Roesijadi and Robinson 1994), which are both elevated in the liver of R.ridibunda after exposure to Cd (Vogiatzis and Loumbourdis 1998).
In the light microscopic examination the increase in the number and size of Kupffer cells in the rats injected with Cd subcutaneously was particularly remarkable (Meis et al. 1982).
In the last years, the negative role of Kupffer cells in liver damage has been documented. Thus, Sauer et al. (1997) found that inflammatory cells, which include resident macrophages (Kupffer cells), neutrophils and monocytes, participate in the pathogenesis of tissue injury by releasing cytotoxic mediators such as cytokines, superoxide anion, nitric oxide and proteases. Sauer et al. (1997) also found that CdCI2 significantly enhances the activity of Kupffer cells. Thus, it seems likely that agents, which stimulate Kupffer cell activity potentiate the hepatotoxicity of Cd. However, because all these mediators are non-specific, their release in excess quantities or in an uncontrolled manner, can also destroy normal tissues. This represents what Laskin and Laskin (2001) termed the “dark side” of macrophages.
A new finding of the present study was the visualization of globules, 1–15 μm in diameter, lying in the lumen and into the cytoplasm of PCT cells, which most probably resemble the hyaline droplets described by other authors (De Rijk et al. 2003; Yokoo et al. 2003). The diverse stainability of HG using histochemical and immunohistochemical methods have led to explanatory theories such as that of erythrophagocytosis, disturbed secretion of glycoproteins in areas of tumor cell injury (Scroggs et al. 1989) or derivation exclusively from basement membrane substances (Pierce et al. 1970). The multiplicity of positive immunohistochemical reactions for various proteins was considered to be an indication that these diverse proteins are synthesized by the tumor cells in some cases (Wolfe and Palmer 1981). However, intracytoplasmic HG can also appear in cases of chronic progressive nephropathy (CPN) and progressive glomerulonephropathy (De Rijk et al. 2003).
The differential staining of HG with PAS, Perl’s, Eosin and Sirius red, may also indicate the different proteins enclosed in the HG. Thus, they may be glycoproteins and/or albumin (PAS positive), ferritin and/or hemosiderin (Perl positive) resulting from red blood cell phagocytosis, and collagen. Thus, the possibility of CPN and glomerulonephropathy could not be ruled out. The continuous loss of hepatic proteins observed in the very same animals (Vogiatzis and Loumbourdis 1998) may be a strong indication of some kind of nephropathy.
According to Papadimitriou et al. (2000), the HG reflect stages of cell injury, which in most instances relate to apoptotic cell death. They are specifically associated with the cytoplasmic blebbing and condensation typically seen in this form of cell death. These phenomena are accompanied by plasma protein influx and the formation of distinct intracellular fragments.
Analysis for PCNA has the potential to identify cells in different phases of the cell cycle. In the present study, the increase in PCNA is reported, particularly in the frogs exposed for 30 days, when degeneration of PCT begins. Similarly, Shibutani et al. (2000) observed PCNA positive cells, preferentially in the PCT undergoing degeneration. Therefore, the degree of Cd-induced tubular injury could be predicted by counting cells undergoing mitotic division.
In general, increased proliferative activity in cells resulting from toxicity cell death and regeneration due to toxicants, has been reported to be closely associated with tumor development (Yoshida et al. 1999). The persistent enhancement of cell proliferation as a result of damage caused by toxicants might be crucial for the carcinogenesis. This appears to be the mechanism of action of non-genotoxic carcinogenesis (Yoshida et al. 1999). Thus, measurements of cell proliferation in normal or neoplastic cell populations can be informative indicators of tissue regeneration and of the carcinogenic nature of many drugs or chemicals.
Although eosin is usually not regarded as a fluorochrome, the advantage of using a fluorescence microscope (FM) in the detection of eosinophilic structures has recently been thoroughly described (Stinchcombe et al. 1995). Eosinophilic cellular components, which are poorly visible under bright field illumination, are more precisely recognizable due to the high sensitivity of eosin fluorescence. Thus, large numbers of small AB show a high level of fluorescence in H&E staining sections and are therefore much more rapidly recognized, compared to transmission light microscope. In this way, quantitative determination of AB is more accurate compared to bright field microscopy. Functionally, apoptosis is a normal and strategic event with an important role in the development process, as, for instance, in the frog tadpoles development, but also in the removal of damaged cells to protect the organism from carcinogenesis (Biagioli et al. 2001).
Declaration
The experiments performed in this study all comply with the current laws of Greece.
References
Berntssen MHG, Aspholm O, Hyllard K, Wendelaar-Bonga SE, Lundedye A-K (2001) Tissue metallothionein, apoptosis and cell proliferation responses in Atlantic salmon (Salmo salar) parr fed elevated dietary cadmium. Comp Biochem Physiol C 128:299–310
Biagioli M, Watjen W, Beyersmann D, Zoncu R, Cappellini C, Ragghianti M, Cremisi F, Bucci S (2001) Cadmium-induced apoptosis in murine fibroblasts is supported by Bcl-2. Arch Toxicol 75:313–320
Calevro F, Campani S, Ragghianti M, Bucci S, Mancino G (1998) Tests of toxicity and teratogenicity in biphasic vertebrates treated with heavy metals (Cr3+, Al3+, Cd2+). Chemosphere 37:651–659
De Rijk EPCT, Ravesloot WT, Wijnands Y, Van Esch E (2003) A fast histochemical staining method to identify Hyaline droplets in the rat kidney. Toxicol Pathol 31:462–464
Friberg L, Piscator M, Nordberg G, Kjellstrom T (1974) Cadmium in the environment, 2nd edn. CRC Press, Cleveland, OH
Habeebu SSM, Liu J, Klaasen CD (1998) Cadmium-induced apoptosis in mouse liver. Toxicol Appl Pharmacol 149:203–209
Hadjolov N, Bitch A (1997) Early effects in chemical-induced rat liver carcinogenesis: an immunohistochemical study following exposure to 0.04% AAF. Apoptosis 2:91–100
Hamada T, Tanimoto A, Iwai S, Fujiwara H, Sasaguri Y (1994) Cytopathological changes induced by cadmium-exposure in canine proximal tubular cells: a cytochemical and ultrastructural study. Nephron 68:104–111
Herak-Kramberger CM, Sabolic I (2001) The integrity of renal cortical brush-border and basolateral membrane vesicles is damaged in vitro by nephrotoxic heavy metals. Toxicology 156:139–147
Juncueira LCU, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11:447–455
Laskin DL, Laskin JD (2001) Role of macrophages and inflammatory mediators in chemically induced toxicity. Toxicology 160:111–118
Loumbourdis NS, Vogiatzis AK (2002) Impact of cadmium on liver pigmentary system of the frog Rana ridibunda. Ecotox Environ Saf 53:52–58
Loumbourdis NS, Kyriakopoulou-Sklavounou P, Zachariadis G (1999) Effects of cadmium exposure on bioaccumulation and larval growth in the frog Rana ridibunda. Environ Pollut 104:429–433
Meiss R, Robenek H, Rassat J, Themann H, Reichert B (1982) Ultrastructural alterations in the hepatic parenchyma of the rat following acute cadmium intoxication. Arch Environ Contam Toxicol 11:283–289
Mitsumori K, Shibutani M, Sato S, Onodera H, Nakagawa J, Hayashi Y, Ando M (1998) Relationship between the development of hepato-renal toxicity and cadmium accumulation in rats given minimum to large amounts of cadmium chloride in the long-term: preliminary study. Arch Toxicol 72:545–552
Nebeker AV, Schuytema GS, Ott SL (1994) Effects of cadmium on limb regeneration in the northwestern salamander Ambystoma gracile. Arch Environ Contam Toxicol 27:318–322
Nomiyama K, Nomiyama H (1998) Cadmium-induced renal dysfunction: new mechanism, treatment and prevention. J Trace Elem Exp Med 11:275–288
Papadimitriou JC, Drachenberg CB, Brenner DS, Newkirk C, Trump BF, Silverberg SG (2000) “Thanatosomes”: a unifying morphogenetic concept for tumor hyaline globules related to apoptosis. Hum Pathol 31:1455–1465
Pearse AGE (1985) Histochemistry, theoretical and applied, vol II. Churchill Livingstone, London
Pierce GB, Bullock WK, Huntington RW Jr (1970) Yolk sac tumors of the testis. Cancer 25:644–658
Rani UA, Ramamurthi R (1989) Histopathological alterations in the liver of freshwater teleost Tilapia mossambica in response to cadmium toxicity. Ecotoxicol Environ Saf 17:221–226
Roesijadi G, Robinson WE (1994) Metal regulation in aquatic animals: mechanisms of uptake, accumulation and release. In: Malins DC, Ostrander GK (eds) Molecular biology and biochemical approach to aquatic toxicology. Lewis, Boca Raton FL, pp 387–420
Sauer J-M, Waalkes MP, Hooser SB, Kuester RK, McQueen CA, Sipes IG (1997) Suppression of Kupffer cell function prevents cadmium induced hepatocellular necrosis in the male Sprague–Dawley rat. Toxicology 121:155–164
Scroggs MW, Roggli VL, Fraire AE (1989) Eosinophilic intracytoplasmic globules in pulmonary adenocarcinomas: a histochemical, immunohistochemical, and ultrastructural study of six cases. Hum Pathol 20:845–849
Shibutani M, Mitsumori K, Niho N, Satoh S, Hiratsuka H, Satoh M, Sumiyoshi M, Nishijima M, Katsuki Y, Suzuki J, Nakagawa J, Ando M (2000) Assessment of renal toxicity by analysis of regeneration of tubular epithelium in rats given low-dose cadmium chloride or cadmium-polluted rice for 22 months. Arch Toxicol 74:571–577
Stinchcombe S, Buchmann A, Bock KW, Schwarz M (1995) Inhibition of apoptosis during 2,3,7,8-tetrachlodibenzo- p-dioxin-mediated tumour promotion in rat liver. Carcinogenesis 16:1271–1275
Stryer L (1988) Biochemistry, 3rd edn. WH Freeman, New York
Sudo J, Hayashi T, Kimura S, Kakuno K, Terui J, Takashima K, Soyama M (1996) Mechanism of nephrotoxicity induced by repeated administration of cadmium chloride in rats. J Toxicol Environ Health 48:333–348
Sweet LI, Passinoreader DR, Meier PG, Omnan GM (1999) Xenobiotic-induced apoptosis: significance and potential application as a general biomarker of response. Biomarkers 4:237–253
Swiergosz R, Zakrzewska M, Sawicka-Kapusta K, Bacia K, Janowska I (1998) Accumulation of cadmium in and its effect on Bank Vole tissues after chronic exposure. Ecotox Environ Saf 41:130–136
Tanimoto A, Hamada T, Koide O (1993) Cell death and regeneration of renal proximal tubular cells in rats with subchronic cadmium intoxication. Toxicol Pathol 21:341–352
Taylor AE (1996) Cardiovacular effects of environmental chemicals. Otolaryngol Head Neck Surg 114:209–211
Vogiatzis AK, Loumbourdis NS (1998) Cadmium accumulation in liver and kidneys and hepatic metallothionein and glutathione levels in Rana ridibunda after exposure to CdCI2. Arch Environm Contam Toxicol 34:64–68
Vogiatzis AK, Loumbourdis NS (1999a) A study of glycogen, lactate, total fats, protein, and glucose concentration in the liver of the frog Rana ridibunda, after exposure to cadmium for 30 days. Environ Pollut 104:335–340
Vogiatzis AK, Loumbourdis NS (1999b) Exposure of Rana ridibunda to lead I. Study of lead accumulation in various tissues and hepatic δ-Aminolevulinic Acid Dehydratase activity. J Appl Toxicol 19:25–29
Wolfe HJ, Palmer PE (1981) Alpha-1-antitrypsin: its immunohistochemical localization and significance in diagnostic pathology. In: DeLellis RA (ed) Diagnostic immunohistochemistry. Masson Publishing USA, New York, NY, pp 227–238
World Health Organization (WHO) (1992) Environmental health criteria 134:cadmium. WHO, Geneva
Yokoo H, Arai H, Isoda K, Arai M, Sasaki A, Hirato J, Nakazato Y (2003) Characterization of eosinophilic hyaline droplets in schwannoma. Acta Neuropathol 105:170–176
Yoshida M, Miyajima K, Shiraki K, Ando J, Kudoh K, Nakae D, Takahashi M, Maekawa A (1999) Hepatotoxicity and consequently increased cell proliferation are associated with flumequine hepatocarcinogenesis in mice. Cancer Lett 141:99–107
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loumbourdis, N.S. Hepatotoxic and nephrotoxic effects of Cadmium in the frog Rana ridibunda. Arch Toxicol 79, 434–440 (2005). https://doi.org/10.1007/s00204-005-0652-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-005-0652-x