Abstract
Micronucleus (MN) and other nuclear abnormalities have been measured in the hemocytes of mussels Perna perna to verify whether feeding mussels with different concentrations of Prorocentrum lima results in accumulation of levels of okadaic acid (OA) capable of inducing genotoxic effects at the chromosome level, as evidenced by micronuclei and nuclear abnormalities. Four groups of 12 mussels housed individually in aquaria containing filtered seawater were fed with different concentrations of P. lima. Another group collected directly from the production area served as outdoor control. A significantly higher frequency of MN and nuclear lesions was observed in hemocytes from the groups fed P. lima.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prorocentrum lima (Ehrenberg) (Dodge 1975) is a toxic benthic, epiphytic, photosynthetic dinoflagellate usually found attached to or associated with macrophytes, floating detritus, debris, or other substrates and, less commonly, found in plankton (Steindinger 1983). P. lima is widely distributed in both temperate and tropical regions. It is an important primary producer of okadaic acid (OA) (Faust 1991), which has been associated with diarrheic shellfish poisoning (DSP) episodes in different regions of the world (Murakami et al. 1982; Morton et al. 1999). Murakami et al. (1982) identified one of the lipid-soluble toxins in P. lima as OA, one of the causative toxins responsible for DSP.
OA was first isolated from the black sponge Halinchondria okadaii (Tachibana et al. 1981) and later from the dinoflagellate P. lima (Murakami et al. 1982). OA is attracting substantial interest because it is a tumor promoter which accumulates in the digestive organs of filter-feeding shellfish and is the cause of DSP in humans, therefore posing an environmental and public health problem (Sueoka and Fujiki 1997). The most prominent human symptoms are gastrointestinal, including diarrhea, nausea, vomiting, and abdominal pain (Yasumoto et al. 1978).
Some authors have verified the cytotoxic, neurotoxic, genotoxic, and tumorigenic effects of OA by use of different tests (Fessard et al. 1996; Matias and Creppy 1998; Matias et al. 1999; Carvalho Pinto-Silva et al. 2003). These authors tried to elucidate the possible interaction of OA with the genetic material of mammals and its mechanism of action in tumor promotion.
One method currently used as an alternative and simple means of assessing chromosome damage in vivo is the micronucleus (MN) assay. This type of test has been recommended for environmental biomonitoring studies, principally because of its capacity to evaluate structural and numeric alterations in chromosomes at certain stages in the life cycle of cells (Carrano and Natarajan 1988). MN are small DNA-containing bodies that appear near the cell nucleus during interphase and originate from acentric chromosome fragments or whole chromosomes, lagging behind during cell division (Fenech 2000).
The MN assay in hemocytes from mussels has proved an excellent indicator of cumulative chromosomal damage, even during short exposure or contamination (Wrisberg et al. 1992). Considering that mussel cells filter large quantities of seawater every day, the micronuclei assay performed in these cells is a suitable test for monitoring the extent of genotoxic action of marine toxins.
The main purpose of this study was to verify whether feeding mussels with different concentrations of P. lima, results in accumulation of OA capable of inducing genotoxic effects at the chromosome level as evidenced by MN formation and nucleoplasmic bridges (NL) in mussel hemocytes of Perna perna, a species native to the south coast of Brazil.
Material and methods
Culture of P. lima clones
Clones of P. lima strain PL2V were obtained from Vigo (Spain). Liquid culture growth was promoted in 1 L of K medium (Keller and Guillard 1985) under a 12:12 light/dark cycle (4×20 W cool-white fluorescent lamps), at 22±1°C, with constant stirring.
Chemicals
Ethanol, methanol, acetic acid, and basic fuchsin were purchased from Merck (Germany). Ultra-pure water was prepared by use of a Milli-Q plus water-purification system (Millipore, Bedford, MA, USA). Fast green was purchased from Sigma–Aldrich (Brazil).
Mussels
Mussels (Perna perna; 7–8 cm shell length) were collected in 2002 from Experimental Mussel-Farming Station on Ratones Island (located in the North Bay of Santa Catarina Island-Brazil 27°28′30′′S and 48°33′40′′W) that belongs to the Marine Mollusks Farming Laboratory of the Federal University of Santa Catarina, Brazil.
The mussels were transferred to the Environmental Toxicology Laboratory (UFSC) where the experiments were carried out. Animals were placed in aquaria of aerated filtered seawater at 24°C and acclimatized to laboratory conditions for 7 days.
Contamination of mussels with toxins by feeding with P. lima
The experiments were carried out with four groups of 12 mussels housed individually in 1-L aquaria containing filtered seawater. To assess the possible induction of MN by the stress produced by the laboratory, one group (12 mussels) was collected directly from the open-air site of production and served as outdoor control.
The first group was used as a laboratory control and mussels were fed with approximately 6,000 cells per animal of non-toxic Chaetoceros sp., twice a day for 2 days. The second group was fed with 200 cells per animal of P. lima, twice a day for 2 days. Under the same experimental conditions, another group received 1,000 cells of P. lima and finally, the last group was fed 10,000 cells of P. lima per animal. Hemocytes were extracted at 24 and 48 h.
Determination of micronuclei frequency
Hemolymph was withdrawn from the posterior adductor muscle into a sterile 5-mL hypodermic syringe containing 1 mL of a solution of methanol and acetic acid (1:1 v/v). The preparations were then fixed in the syringe for 7 min, after which two slides per animal were prepared for microscopic analysis. The slides were dried at room temperature, fixed in methanol for 10 min, and stained using the Feulgen/Fast-Green method (Beçak and Paulete 1976).
From each animal, 1,000 cells (500 per slide whenever possible) were scored under 1000× magnification to determine the average of MN and NL. Coded and randomized slides were scored using blind review by a single observer.
Extraction of OA from the mussels
Extraction was carried out according to the procedure described by Lee et al. (1987). The hepatopancreases were homogenized with 80% methanol in water for 2 min at room temperature. The homogenate was centrifuged at 1,000g for 10 min and the supernatant was transferred to a test tube to be extracted with petroleum ether. To the lower phase was added 1 mL water and 1 mL chloroform. The mixture was shaken for 20 s on a vortex mixer and centrifuged at 3,000g for 15 min. The lower phase was transferred to a graduated test tube and the upper phase was re-extracted with chloroform.
HPLC system
An HP 1050 chromatograph equipped with HP 1046 A spectrofluorimeter was used. Analysis of the toxin extracted from the hepatopancreases was performed at room temperature on an ultrasep C18 column (250×4 mm) using acetonitrile–methanol–water, 8:1:1 (v/v) as mobile phase. The flow rate was maintained at 0.5 mL min−1. The excitation and emission wavelengths were 365 and 412 nm, respectively.
Statistical analysis
Results of cytogenetics for MN and NL frequencies found in the five groups are shown as mean±SD. The Kruskal–Wallis test was employed to compare MN and NL frequencies between the groups. The 0.05 level of probability was used as significance criterion.
Results
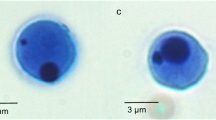
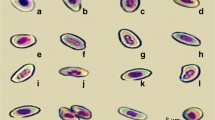
In the cells studied two main types of nuclear abnormality were found, MN and NL. MN were non-refractory particles only, the same color as the nucleus of the cells and with a round or ovoid shape (Fig. 1a). NL were considered only in binucleated cells with NL (Fig. 1b) and in binucleated cells with extruded nuclear buds that appeared like MN with a nucleoplasmic connection to the main nucleus (Fig. 1c) (Fenech et al. 2003).
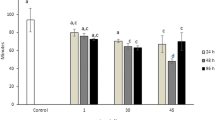
Mean±SD MN were counted for groups of animals fed with different concentrations of P. lima, for different periods of exposure, and for the animals of the control groups. A significant increase in MN formation occurred in animals fed with 200 and 1,000 cells of P. lima. However, it was observed that in these groups, the OA concentration was low. On the other hand, in animals fed with 10,000 cells of P. lima, decreased incidence of MN was observed. The results showed that the higher the concentration of OA the lower the formation of MN (Fig. 2).
Figure 3 shows the average number of NL in groups of animals fed with different concentrations of P. lima, for different periods of exposure, and in animals of the control groups. The results indicated that only in the groups which were fed with 10,000 cells of P. lima was the number of NL substantially increased in comparison with the controls.
Discussion
Although, intensively studied in various mammalian systems, the cytotoxicity and impact of marine toxins in mollusks, the main vectors for DSP, has been given little attention. Although some mussels are exposed to marine toxins for prolonged periods every year, no apparent increase in mortality within populations of mussels has been observed. Thus, it seems likely that organisms which are naturally exposed to marine toxins have some protective mechanisms against its harmful effects (Svensson and Forlin 1998). In seawater the number of P. lima is never zero, whatever the season. Thus mussels are continuously exposed to cytotoxic and genotoxic shellfish poisons.
Our results indicated that, in the conditions tested, only in the groups of animals fed with 200 and 1,000 cells of P. lima was there a significantly increase in the frequency of MN. Because the average number of MN in these groups was the highest compared with controls, it can be concluded that the OA present in the cells of P. lima is genotoxic even at low concentrations. Botana et al. (1996) have shown cytotoxicity under similar conditions.
Svensson and Forlin (1998) demonstrated that mussels fed P. lima accumulated OA at concentrations corresponding to those prevailing in Swedish mussel populations during blooms of toxic dinoflagellates. Several authors, using other types of test, have observed the genotoxic action of OA. Matias and Creppy (1998) showed that OA induced hypermethylation of DNA in Vero cells, the ratio of 5-methylcytosine in nuclei increased from 3.8 to 7.8%, indicating possible interference of OA with the expression, replication, and regulation of DNA.
Carvalho Pinto-Silva et al. (2003) showed that pure OA induces a significant increase and higher frequency of MN in mussels Perna perna.
The highest average number of MN was found after exposure for 24 h, suggesting a rapid effect of OA. According to Matias and Creppy (1998), the rapid establishment of the genotoxic effect of OA could be because of rapid metabolic transformation of OA into a very active compound during the first hour of exposure, suggesting a mechanism of action in which the DNA becomes the ultimate target of OA, and possibly its derivatives, directly or indirectly. The fact that genotoxicity is higher after 24 h may also be explained by reduction of the concentration of the toxin after transformation of OA into a less active metabolite during the course of incubation. Such a hypothesis is reinforced by the data of Tohda et al. (1993), who demonstrated that OA significantly increased levels of sister-chromatid exchanges (SCE) and increased the number of mitotic cells characterized by marked chromosome condensation; they also showed that OA induced chromosome fragmentation in human lymphoblastoid cells and fragmented nuclei in Chinese hamster cells.
The decrease in the average number of MN in the mussels fed with 10,000 cells of P. lima and the increase in the average number of NL may be because of cell mortality caused by excessive exposure to the genotoxic and cytotoxic agent and/or to a chronic effect, although the length of time the mussel is able to tolerate this stress is not known. It is believed, however, that these results are because of the death of hemocytes, once OA interferes with various cell functions and inhibits the synthesis of proteins. This is apparent from the study of Matias et al. (1999) who demonstrated that in a mammalian system, in vitro (Vero cells), pure OA at a concentration of 15 ng mL−1 caused cell death. Traore et al. (2001) also demonstrated that OA induces apoptosis in Caco2 cells.
Fenech et al. (2003), on the other hand, demonstrated that the NL observed in Fig. 1b and Fig. 1c occurred because of elimination of amplified DNA and possibly elimination of DNA-repair complexes. The authors suggest that the nuclear buds originate from the dicentric chromosomes and the whole centromeres are pulled apart to opposite poles of the cell at anaphase.
The influence of the stress caused by experimental conditions was observed in the results and in the average increase of MN in the laboratory control group.
In conclusion, MN and other nuclear buds occur in mussels fed with P. lima. After 48 h exposure an increase in the frequency of MN effects was observed in the laboratory control group of mussels, probably because of stress.
References
Beçak W, Paulete J (1976) Técnicas de Citologia e Histologia, vol I, Livro Técnicas Científicas (ed) SA Rio de Janeiro, RJ, 305 pp
Botana LM, Rodrigues-Vieytes M, Alfonso A, Louzão CM (1996) Phycotoxins: paralytic shellfish poisoning and diarrhetic shellfish poisoning. In: Nollet LML (ed) Handbook of food analysis, vol. 2. Marcel Dekker, New York
Carrano AV, Natarajan AT (1988) Considerations for populations monitoring using cytogenetic techniques. Mutat Res 204:379–406
Carvalho Pinto-Silva CR, Ferreira JF, Costa RHR, Belli Filho P, Creppy EE, Matias WG (2003) Micronucleus induction in mussels exposed to okadaic acid. Toxicon 41:93–97
Dodge JD (1975) Revision of the Taxonomy within the genus Prorocentrum. Bot J Linn Soc 71:102–105
Faust M (1991) Morphology of ciguatera-causing Prorocentrum lima (Pyrrophyta) from widely differing sites. J Phycol 24:642–648
Fenech M (2000) The in vitro micronucleus technique. Mutat Res 455:81–95
Fenech M, Chang WP, Kirsh-Volders M, Holland N, Bonassi S, Zeiger E (2003) HUMN project: detailed description on the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 534:65–75
Fessard V, Grosse Y, Pfohl-Leszkowics A, Puiseux-Dao S (1996) Okadaic acid treatment induces DNA adduct formation in BHK21 C13 fibroblasts, HESV keratinocytes. Mutat Res 361:133–141
Keller MD, Guillard RRL (1985) Media for the Culture of oceanic ultraphytoplankton. J Phycol 23:633–638
Lee JS, Yanagi T, Kenma R, Yasumoto T (1987) Fluorimetric determination of diarrhetic shellfish toxins by high-performance liquid chromatography. Agric Biol Chim 51:877–881
Matias W G, Creppy EE (1998) 5-Methyldeoxycytosine as a biological marker of ADN damage induced by okadaic acid in Vero cells. Environ Toxicol Water Qual. 13:83–88
Matias WG, Traore A, Bonini M, Sanni A, Creppy EE (1999) Oxygen reactive radicals production in cell culture by okadaic acid and their implication in protein synthesis inhibition. Human Exp Toxicol 18:634–639
Morton DL, Leighfield TA, Haynes BL, Van Dolah FM, Gowan J (1999) Evidence of diarrhetic shellfish poisoning along the Coast of Maine. J Shellfish Res 18:681–686
Murakami Y, Oshima Y, Yasumoto T (1982) Identification of okadaic acid as a toxic component of a marine dinoflagellate Prorocentrum lima. Bull Jpn Soc Sci Fish 48:69–72
Steindinger KA (1983) A reevaluation of toxic Dinoflagellate biology and ecology. Physiol Res 2:147–188
Sueoka E, Fujiki HJ (1997) Carcinogenesis of okadaic acid class tumor promoters derived from marine natural products. Cancer Res Clin Oncol 123:413–419
Svensson S, Forlin L (1998) Intracellular effects of okadaic acid in the blue mussel Mytilus edulis and rainbow trout Oncorhynchus mykiss. Marine Environ Res 46:449–452
Tachibana K, Scheur PJ, Tsukitani Y, Kikuchi H, Engen DV, Clardy J, Gopichand Y (1981) Okadaic acid, a cytotoxic polyether from two marine sponges of the genus Halichondria. J Am Chem Soc 103:2469–2471
Tohda H, Nagao M, Sugimura T, Oikawa A (1993) Okadaic acid. A protein phosphatase inhibitor induces sister-chromatid exchanges depending on the presence of bromodeoxyuridine. Mutat Res 289:275–280
Traoré A, Baudrimont I, Ambaliou S, Dano SD, Creppy EE (2001) DNA breaks and cell cycle arrest induced by okadaic acid in Caco-2 cells, a human colonic epithelial cell line. Arch Toxicol 75:110–117
Wrisberg MN, Bulbo C, Spliid H (1992) Induction of micronuclei in hemocytes of mussels and statistical analysis. Ecotoxicol Environ Saf 23:191–205
Yasumoto T, Oshima Y, Yamaguchi M (1978) Occurrence of a new type of shellfish poisoning in the Tohoku district. Nippon Suisan Gakkai Shi 44:1249–1275
Acknowledgements
The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)-Brazil, supported this work. The authors are grateful to the French National Academy of Medicine for the prize awarded to Prof. E.E. Creppy 2001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pinto-Silva, C.C., Creppy, E.E. & Matias, W.G. Micronucleus test in mussels Perna perna fed with the toxic dinoflagellate Prorocentrum lima. Arch Toxicol 79, 422–426 (2005). https://doi.org/10.1007/s00204-004-0645-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-004-0645-1