Abstract
The release of pollutants is increasing along the coast of Chile, and the use of biomarkers in biomonitoring programs is essential to assess the early biological effects of marine contamination. The Micronucleus (MN) test was carried out using hemocytes of the mussel Perumytilus purpuratus from two sites in northern-central Chile (La Pampilla and Totoralillo). Nuclear abnormalities were assessed, and high frequencies of micronucleus (10.7–14.4‰) and other abnormalities were found (51.9–76.6‰). These values tended to be higher in La Pampilla, possibly due to the large presence of pollution sources in that site. However, considerably high values were observed in both sites. P. purpuratus is a suitable bioindicator and further monitoring along the Chilean coast using this species should be developed using the MN test and/or other biomarkers to comprehend the effects of human activities on the coastal environment of Chile.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Marine pollution is an increasing problem for the global marine environments (Devier et al. 2005). It causes growing concerns due to its negative impacts on marine ecosystems that threaten the capacity of the oceans to maintain the ecosystem functions and provide goods and services, now and in the future (Halpern et al. 2012). Common pollutants in marine environments include metals, hydrocarbons, pesticides, polychlorinated biphenyls, and plastic debris, among other substances which are mainly originated from human land-based activities (Devier et al. 2005; Vikas and Dwarakish 2015). Such xenobiotics can generate a range of effects on marine organisms, including embryotoxicity, cytotoxicity, immunotoxicity, genotoxicity, metabolic disturbance, endocrine disruption, and long-term damages that could persist for several generations (Jha et al. 2000; Ohe et al. 2004).
The assessment of environmental risks due to pollution is receiving increasing importance, as established in the United Nations Sustainable Development Goals (SDGs), specifically in the SDG #14 “Life Below Water—Conserve and sustainably use the oceans, seas and marine resources for sustainable development” (UN 2019). Approaches used to assess pollution include chemical analyses, toxicity tests, community structure description, and biomarkers, among others (Chapman and Anderson 2005). Fish and invertebrates are suitable biomonitors of marine pollution (USEPA 1993), including bivalves which have been widely used worldwide to assess contaminants’ bioaccumulation and effects (Burns and Smith 1981). Due to their sessile and filter-feeding habits, wide distribution, abundance in environment, commercial and ecological importance, and easy field collection, they represent suitable indicators of the environmental quality (Kehrig et al. 2006; Silva et al. 2006; Boening 1999). The use of marine bivalves in biomonitoring frequently involves quantification of contaminants in soft-tissues and measuring adverse effects through biomarkers. Biomarkers can be defined as biochemical, physiological, and/or histological measurements that indicate biochemical or cellular alterations in living organisms as response to toxicants (Van Der Oost et al. 2003). From the organismal and sub-organismal responses, biomarkers allow extrapolating and warning about ecosystem changes (Depledge 1998; Thain et al. 2008).
Genotoxicity biomarkers consist of tools that measure DNA damage caused by direct or indirect action of chemical substances (Depledge 1998) and have been widely used for monitoring marine pollution, either alone or as part of integrated assessments (e.g. Dailianis et al. 2003; Pereira et al. 2011; Brooks et al. 2012). DNA damages might be caused by DNA adducts, or directly by the pollutants, which may act as an electrophile and/or produce reactive oxygen species (ROS), which cause DNA oxidation (Lee and Steinert 2003). The micronucleus (MN) test is one of the most used genotoxicity biomarkers (Hayashi 2016). Micronuclei are fragments of chromosomes that are left aside in some stage of the cellular division, mainly in the mitosis (Ku 2017). They are usually caused by aneugenic and clastogenic compounds (Schmidt 1975) and can be easily observed in optical microscopy (Brunetti et al. 1988). Mussels have been broadly used in ecotoxicological and biomonitoring studies involving MN and genotoxicity (e.g. Izquierdo et al. 2003; Siu et al. 2004; Barsiene et al. 2008, 2010; Touahri et al. 2016; Gaete et al. 2019).
Marine pollution is a concern along the Chilean coast, as high concentrations of different pollutants have been found in coastal waters, sediments, and/or marine organisms, such as metals (Valdés et al. 2009) and plastic debris (e.g. Thiel et al. 2003). Besides, increasing amounts of wastes coming from urban, port, mining, and industrial activities tend to increase the levels of pollutants in the environment to the point of harming marine ecosystems and the marine resources extracted for human consumption (De Gregori et al. 1996). In spite of the local importance and the global concerns regarding this issue, the monitoring of the environmental quality of Chilean coastal ecosystems is insufficient. The monitoring of the marine pollutants in Chile was carried out by the Coastal Environment Observation Program (POAL—the acronym to “Programa de Observación del Ambiente Litoral”) until 2017. Annual concentrations of several pollutants in water and sediments were measured in this program. Also, this program included the quantification of pollutants in soft tissues of the mussel Perumytilus purpuratus but did not involve the evaluation of adverse effects. Worse, the program seems to be discontinued and new analysis have not been carried out since 2018.
Perumytilus purpuratus is a small mussel (maximum length 3 cm) that inhabits the exposed rocky intertidal reefs along the entire Chilean coast (18° S to 54° S) (Zagal and Hermosilla 2007). Due to its sensitivity to contaminants and status of non-commercial species, this mussel is the target species of the P.O.A.L and has been used successfully as bioindicator of pollution, including metals (De Gregori et al. 1994, 1996; Salamanca et al. 2004) and polycyclic aromatic hydrocarbons (Mendoza et al. 2005; Palma-Fleming et al. 2008). This species has been used in some biomarker studies involving exposure to copper (Cu) in laboratory conditions. Riveros et al. (2002) studied lysosomal stability and other cellular responses, while Riveros et al. (2003) used copper metallothionein-like proteins as biomarker for Cu. More recently, MN rates were measured in P. purpuratus exposed to Cu (Gaete et al. 2019). This work evaluated the genotoxicity in individuals of P. purpuratus collected at two sites presenting different sources of contaminants to determine whether individuals from the more impacted site exhibited larger signs of stress. The aim of the present study was to evaluate the utility of the MN test for the monitoring of environmental pollution on the Chilean coast.
Materials and Methods
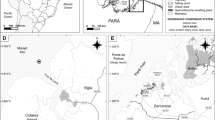
Individuals of P. purpuratus (25–35 mm) were collected from two sites located on northern-central Chile: La Pampilla, Coquimbo (29° 57′ 18.4′′ S–71° 21′ 41.8′′ W), and Totoralillo (30° 04′ 51.5′′ S–71° 22′ 18.9′′ W). La Pampilla is a site influenced by the release of urban, port and fishing wastes, while Totoralillo (30° 04′ 51.5′′ S–71° 22′ 18.9′′ W) is to a small town and watering place. These sites were chosen because of the presence of populations of P. purpuratus, and differences on the existing pollution sources. The first survey was done between May 30th, 2017 (Totoralillo) and June 8th, 2017 (La Pampilla), while the second one was conducted 3 months later (August 29th and 30th). May–June survey was carried out after heavy rainstorms that occurred between May 10th and 13th, 2017. This event affected both sampling sites, but collections in La Pampilla were delayed due to unsuitable conditions which prevailed until May 30th. After the sampling, individuals were immediately carried to the laboratory of the Universidad Católica del Norte for haemolymph extraction.
To develop the MN test, haemolymph was extracted from the posterior adductor muscle of the mussel (Fig. S1) of 13 individuals from each study site. This was done using a 1 mL thin-tipped syringe containing 0.2 mL of a fixing solution of methanol-acetic acid (Carnoy) (3:1 v/v). Haemolymph was left in the syringe for 10 min. Then, three or four droplets were placed on labelled glass slides and fixed in methanol for 5 min. Slides were left drying over ambient temperature and rinsed with distilled water; three slides per individual were prepared. The slides were stained for 30 s with Giemsa (4%–5%) in phosphate buffer (pH = 6.8), and then, rinsed again. Finally, the preparations were sealed permanently with a cover slide, using the mounting medium Entellan (Merck, see simplified protocol scheme in Fig. S2). The extraction of haemolymph and slides preparation was made based on the protocol described by Bolognesi and Fenech (2012) with some modifications. Details of these modifications and protocol are described in the supplementary material (Table S1).
1000 hemocytes per mussel were counted using a light microscope. Hemocytes were separated in normal agranular cells and cells with nuclear abnormalities. This was done considering three different groups (Bolognesi and Fenech 2012): (1) micronucleus, that include all nuclear structures in the cytoplasm less than 1/3 of the diameter of the main nucleus, which were not in contact with the principal nucleus and that were of the same colour and structure than the main nucleus (Rocha and Rocha 2016), (2) binucleated cells, that are hemocytes with two nucleus totally or partially separated, and (3) nuclear buds, which are cells with structures similar to a micronuclei but that are in contact to the principal nucleus (Fig. 1). In the second sampling (August), the shell lengths were additionally measured to relate the size of individuals to the number of nuclear abnormalities present in their hemocytes.
Frequency in a total of 1000 cells (‰) of the three types of nuclear abnormalities and their sum (total nuclear abnormalities) were considered for statistical analyses. Generalised linear models (GLM) with negative binomial distribution (due to overdispersion of the data) were used to compare frequencies of nuclear abnormalities with “sampling” and “site” as fixed factors. Four separated GLM analyses were run to compare frequencies of micronucleus (MN), nuclear buds (NB), binucleated cells (BC) and total nuclear abnormalities (Total NA) from organisms obtained in different sites and sampling dates. The effects of shell size in the different nuclear abnormalities were analysed using linear regressions again separately for each nuclear abnormality. Analyses and figures were prepared using R studio v. 1.3.959 (R Studio Team 2020).
Results and Discussion
For total NA, the highest value was 76.6 ‰ in La Pampilla in May–June sampling, while the lowest value was 51.9‰ in Totoralillo in August (Table S2). In the case of Micronucleus, the lowest frequency of MN was 10.7‰ in Totoralillo on August and the highest value was shared between La Pampilla in August and Totoralillo in May–June (14.4 ‰). MN was the nuclear abnormality that showed lower frequencies (compared to NB and BC), and this have also been observed in other studies using the MN test and other nuclear abnormalities (e.g. Barsiene et al. 2006; Van Ngan et al. 2007). These differences in the number of abnormalities indicate a different kind of damage; the damage can be genotoxic, in the case of MN and NB, and cytotoxic, when BC are counted (Rodilla 1993), also other nuclear abnormalities appeared in higher frequencies than MN. For this reason, the use of the three nuclear abnormalities analysed here could provide a wider view about the cytogenotoxicity of the environment in biomonitoring programs (Barsiene et al. 2006; Fernández et al. 2011). In general, the number of nuclear abnormalities was higher in La Pampilla than Totoralillo, as well as in May–June than August (Fig. 2). This trend is significant for the frequency of NB in August, that was 0.6 times higher in La Pampilla than Totoralillo (p = 0.01). This high frequency of NB and the general trend of nuclear abnormalities to be higher in La Pampilla might be due to the large amount of pollution sources present there (see below). Detailed results of the statistical test can be found in the Table S3.
Frequency of nuclear abnormalities in 1000 agranular hemocytes (Abnormality ‰) of Perumytilus purpuratus from La Pampilla and Totoralillo in May–June and August sampling. Nuclear abnormalities include micronucleus (MN), nuclear buds (NB), binucleated cells (BC) and total abnormalities (Total NA). Box plots show data distribution. Boxes represent data corresponding to the first and third quartiles (n = 7–10, see Table S2). Bold line inside boxes indicates the value of the median of each NA and vertical lines represents the interquartile range. Dots outside the range are outliers
Shell lengths of the mussels ranged between 25.5 and 35 mm, while the average size in Totoralillo was 30.43 ± 3.45 mm, and 28.33 ± 1.9 mm in La Pampilla. There was no relationship between the frequency of NAs and the size of the individuals, thus, the size of mussels did not affect the observed level of genotoxicity. Besides, the use of organisms of a similar range of sizes is recommended to reduce the variation due to individual characteristics (Bolognesi et al. 2004). Therefore, the size range used in this study is recommended for further studies with P. purpuratus.
The observed frequencies of nuclear abnormalities can be considered high when they are compared with results from other studies. Regarding to MN frequency, Touahri et al. (2016) found MN rates of 12.5 ‰ in hemocytes of M. galloprovincialis collected in a contaminated port zone from Algeria, while in the reference clean site, mussels presented MN rates of 3.24 ‰. About total NA, Barsiene et al. (2006) evaluated the total NA in gills of Mytilus spp. from the Gulf of Gdansk which is polluted by polychlorinated biphenyls (PCB) and polycyclic aromatic hydrocarbons (PAHs, Konat and Kowalewska 2001; Lubecki and Kowalewska 2012) and they found rates of ~ 25 ‰. Such numbers are very low compared with average NAs observed in this study.
A possible explanation might be the high pollution in the study sites. In the case of La Pampilla, it receives contamination from different anthropogenic sources. One of the most important sources is the sewage outfall of Punta Tinaja, which consists of a main source of domestic sewage to the adjacent waters. Sediment analysis near the sewage outfall (Coquimbo’s water bodies, Bahía La Herradura) developed by the POAL found concentrations of genotoxic substances, such as Cadmium (Cd), Mercury (Hg), Lead (Pb), PAHs and Cu (DIRECTEMAR 2017). There is no information on the concentrations of metals and PAHs for Totoralillo. In that analysis, Cu concentration was considerably high (145 mg kg −1), when contrasted to the sediment quality guidelines proposed by the NOAA (Buchman 2008). Cu is a well-known genotoxic agent (e.g. Bolognesi et al. 1999). Furthermore, Gaete et al. (2019) observed genotoxicity on P. purpuratus exposed to Cu from concentrations of 1 μg L−1, and also an increased number of MN with time of exposure. Cu concentrations in Chile are naturally high and this metal may occur in waters, sediments, and mussels from the northern coast of Chile, as observed by De Gregori et al. (1996). Cu is also related to several sources of pollution, such as sewage or residues from mining or port activities (Valdés et al. 2015). Furthermore, there are several studies focused on the effects of Cu concentrations in Chilean coastal environments and organisms (e.g. Riveros et al. 2003; Medina et al. 2005; Sáez et al. 2015; Gaete et al. 2019). Other metals found near the sampling site have also increased the frequency of MN in mussels (Bolognesi et al. 1999). PAHs are also known to have genotoxic potential to mussels (Siu et al. 2004). The presence of Cu and other xenobiotics like these might explain the number of NAs observed.
Gaete et al (2019) discussed that P. purpuratus exposed to Cu presented sensitivity similar to other mussels (about MN frequency) and that low MN basal values were observed for this species. Then, higher rates of NA observed in our investigation could be due to pollution, or natural environmental stressors present in the sampling sites. Another factor that might influence the elevated frequencies observed in P. purpuratus was the fact that this species inhabits the intertidal zone of the rocky reef, thus they keep exposed to the air and sun during low tides, and such fact may represent a natural stressor and increase the number of MN (Brunetti et al. 1992). This temporal anoxic condition combined with the contaminants present in the environment might cause stronger genotoxic effects.
High frequencies of NA may also have been influenced by the heavy and uncommon thunderstorms occurred in the zone (Coquimbo) between May 10 and 13, 2017 (2–3 weeks before sampling); in that extreme climatic event, the volume of rain precipitation was almost equivalent to a year’s average for the zone. This possibly caused the introduction of stormwater rainfall and superficial leachates into the coastal waters, carrying sediments and contaminants from urban zones and mining activities. It is known that short acute exposure to contaminants may cause genotoxicity to mussels (Woznicki et al. 2004), and this effect may last up to 5 years (Barsiene et al. 2008, 2012). Also, temporal variations in effluents and organic contents in seawater can generate variations in the concentrations of pollutants in soft tissues of P. purpuratus (Riveros et al. 2003). Therefore, an abrupt increase of contaminants and freshwater input could have affected the organisms, especially in the first sampling survey. This indicates that this test also can be used to evaluate the effects of extreme environmental changes on marine organisms and not only direct effect of pollutants.
Perumytilus purpuratus appears as potential organism to be used in wider monitoring programs and risk assessments, using MN test and/or other biomarkers capable to show the effects caused by xenobiotics or changes in the environment, such as those generated by the climate change. Previous studies conducted in Chile also showed that this species can provide useful information on the environmental contamination (Riveros et al. 2002, 2003). Also, as this organism is not commercially exploited, the populations tend to be stable, abundant, and available to provide a suitable number of test-organisms. The use of the MN and other abnormalities on P. purpuratus was considered effective and simple to evidence environmental genotoxicity. This test also showed the utility to assess the effect of extreme climatic events, which will increase in intensity and frequency in the future (Ummenhofer and Meehl 2017).
References
Barsiene J, Schiedek D, Rybakovas A, Syvokiene J, Kopecka J, Forlin L (2006) Cytogenetic and cytotoxic effects in gill cells of the blue mussel Mytilus spp. From different zones of Baltic Sea. Mar Pollut Bull 53:469–478. https://doi.org/10.1016/j.marpolbul.2005.11.015
Barsiene J, Rybakovas A, Förlin L, Syvokiene J (2008) Environmental genotoxicity studies in mussels and fish from the Göteborg Area of the North Sea. Acta Zool Litu 18:240–246. https://doi.org/10.2478/v10043-008-0032-x
Barsiene J, Andreikenaite L, Bjornstad A (2010) Induction of micronuclei and other nuclear abnormalities in blue mussels Mytilus edulis after 1 -, 2-, 4-, 8-day treatment with crude oil from the North Sea. Ekologija 56:124–151. https://doi.org/10.2478/v10055-010-0018-4
Barsiene J, Rybakovas A, Garnaga G, Andreikenaite L (2012) Environmental genotoxicity and cytotoxicity studies in mussels before and after an oil spill at the marine oil terminal in the Baltic Sea. Environ Monit Assess 184:2067–2078. https://doi.org/10.1007/s10661-011-2100-0
Boening DW (1999) An evaluation of bivalves as biomonitors of heavy metals pollution in marine waters. Environ Monit Assess 55:459–470. https://doi.org/10.1023/A:1005995217901
Bolognesi C, Fenech M (2012) Mussel micronucleus cytome assay. Nat Protoc 6:1125–1137. https://doi.org/10.1038/nprot.2012.043
Bolognesi C, Landini E, Roggieri P, Fabbri R, Viarengo A (1999) Genotoxicity biomarkers in the assessment of heavy metal effects in mussels: experimental studies. Environ Mol Mutagen 33:287–292. https://doi.org/10.1002/(SICI)1098-2280(1999)33:4%3c287::AID-EM5%3e3.0.CO;2-G
Bolognesi C, Frenzili G, Lasagna C, Perronea E, Roggieria P (2004) Genotoxicity biomarkers in Mytilus galloprovincialis: wild versus caged mussels. Mutat Res 552:152–162. https://doi.org/10.1016/j.mrfmmm.2004.06.012
Brooks S, Harman C, Soto M, Cancio I, Glette T, Marigómez I (2012) Integrated coastal monitoring of a gas processing plant using native and caged mussels. Sci Total Environ 426:375–386. https://doi.org/10.1016/j.scitotenv.2012.03.059
Brunetti R, Majone F, Gola I, Beltrame C (1988) The micronucleus test: examples of application to marine ecology. Mar Ecol Prog Ser 44:65–68. https://doi.org/10.3354/meps044065
Brunetti R, Fumagalli O, Valerio P, Gabriele M (1992) Genotoxic effects of anoxia on Mytilus galloprovincialis. Mar Ecol Prog Ser 83:71–74. https://doi.org/10.3354/meps083071
Buchman M (2008) NOAA screening quick reference tables, NOAA OR&R report 08–1. Office of Response and Restoration Division, National Oceanic and Atmospheric Administration, Seattle, p 34
Burns KA, Smith JL (1981) Biological monitoring of ambient water quality: the case for using bivalves as sentinel organisms for monitoring petroleum pollution in coastal waters. Estuar Coast Shelf Sci 13:433–443. https://doi.org/10.1016/S0302-3524(81)80039-4
Chapman PM, Anderson J (2005) A decision-making framework for sediment contamination. Integr Environ Assess Manage 1:163–173. https://doi.org/10.1897/2005-013r.1
Dailianis S, Domouhtsidou GP, Raftopoulou E, Kaloyianni M, Dimitriadis VK (2003) Evaluation of neutral red retention assay, micronucleus test, acetylcholinesterase activity and signal transduction molecule (cAMP) in tissues of Mytilus galloprovincialis (L.), in pollution monitoring. Mar Environ Res 56:443–470. https://doi.org/10.1016/S0141-1136(03)00005-9
Depledge MH (1998) The ecotoxicological significance of genotoxicity in marine invertebrates. Mutat Res 399:109–122. https://doi.org/10.1016/S0027-5107(97)00270-4
Devier M-H, Augagneur S, Budzinski H, Le Menach K, Mora P, Narbonne J-F, Garrigues P (2005) One-year monitoring survey of organic compounds (PAHs, PCBs, TBT), heavy metals and biomarkers in blue mussels from the Arcachon Bay, France. J Environ Monit 7:224–240. https://doi.org/10.1039/b409577d
De Gregori I, Pinochet H, Delgado D, Gras N, Muñoz L (1994) Heavy metals in bivalve Mussels and their habitats from different sites along the Chilean Coast. Bull Environ Contam Toxicol 52:261–268. https://doi.org/10.1007/BF00198497
De Gregori I, Pinochet H, Gras N, Muñoz L (1996) Variability of cadmium, cooper and zinc levels in molluscs and associated sediments from Chile. Environ Pollut 92:359–368. https://doi.org/10.1016/0269-7491(95)00077-1
DIRECTEMAR (2017) Datos POAL. Directemar, Armada de Chile. https://www.directemar.cl/directemar/site/tax/port/fid_adjunto/taxport_44_145_377_1html. Accessed 13 Aug 2020.
Fernández B, Campillo JA, Martínez-Gómez C, Benedicto J (2011) Micronuclei and other nuclear abnormalities in mussels (Mytilus galloprovincialis) as biomarkers of cyto-genotoxic Pollution in Mediterranean Waters. Environ Mol Mutagen 52:479–491. https://doi.org/10.1002/em.20646
Gaete H, Guerra R, Espinoza P, Fernández D (2019) Lysosomal membrane stability in hemocytes and micronuclei in gills of Perumytilus purpuratus lamarck 1819 (bivalvia: mytilidae) exposed to copper. Bull Environ Contam Toxicol 103:796–801. https://doi.org/10.1007/s00128-019-027
Halpern BS, Longo C, Hardy D, McLeod KL, Samhouri JF, Katona SK, Kleisner K, Lester SE, O’Leary J, Ranelletti M, Rosenberg AA, Scarborough C, Selig ER, Best BD, Brumbaugh DR, Chapin FS, Crowder LB, Daly KL, Doney SC, Elfes C, Fogarty MJ, Gaines SD, Jacobsen KI, Karrer LB, Leslie HM, Neeley E, Pauly D, Polasky S, Ris B, St Martin K, Stone GS, Sumaila UR, Zeller D (2012) An index to assess the health and benefits of the global ocean. Nature 488:615–620. https://doi.org/10.1038/nature11397
Hayashi M (2016) The micronucleus test—most widely used in vivo genotoxicity test. Genes Environ 38:18–24. https://doi.org/10.1186/s41021-016-0044-x
Izquierdo JI, Machado G, Ayllon F, d’Amico VL, Bala LO, Vallarino E, Elias R, García-Vazquez E (2003) Assessing pollution in coastal ecosystems: a preliminary survey using the micronucleus test in the mussel Mytilus edulis. Ecotox Environ Safe 55:24–29. https://doi.org/10.1016/S0147-6513(02)00041-6
Jha A, Hagger J, Hill S, Depledge M (2000) Genotoxic, cytotoxic and developmental effects of tributyltin oxide (TBTO): an integrated approach to the evaluation of the relative sensitivities of two marine species. Mar Environ Res 50:563–573. https://doi.org/10.1016/S0141-1136(00)00112-4
Kehrig HA, Costa M, Moreira I, Malm O (2006) Total and methyl mercury in different species of molluscs from two estuaries in Rio de Janeiro State. J Braz Chem Soc 17:1409–1418. https://doi.org/10.1590/S0103-50532006000700031
Konat J, Kowalewska G (2001) Polychlorinated biphenyls (PCBs) in sediments of the southern Baltic Sea—trends and fate. Sci Total Environ 280:1–15. https://doi.org/10.1016/S0048-9697(01)00785-9
Ku W (2017) A preliminary study on the relationship between micronucleus formation and cell cycle. Gene Sci Eng 1:28–36. https://doi.org/10.18063/gse.v1i1.523
Lee RF, Steinert S (2003) Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutat Res 544:43–64. https://doi.org/10.1016/S1383-5742(03)00017-6
Lubecki L, Kowalewska G (2012) Indices of PAH origin—a case study of the gulf of Gdansk (se Baltic) sediments. Polycycl Aromat Comp 32:335–363. https://doi.org/10.1080/10406638.2011.640734
Medina M, Andrade S, Faugeron S, Lagos N, Mella D, Correa JA (2005) Biodiversity of rocky intertidal benthic communities associated with copper mine tailing discharges in northern Chile. Mar Pollut Bull 50:396–409. https://doi.org/10.1016/j.marpolbul.2004.11.022
Mendoza G, Gutierrez L, Pozo-Gallardo K, Fuentes-Rios D, Montory M, Urrutia R, Barra R (2005) Polychlorinated biphenyls (PCBs) in mussels along the chilean coast. Environ Sci Pollut R 13:67–74. https://doi.org/10.1065/espr2006.01.011
Ohe T, Watanabe T, Wakabayashi K (2004) Mutagens in surface waters: a review. Mutat Res 567:109–149. https://doi.org/10.1016/j.mrrev.2004.08.003
Palma-Fleming H, Cornejo C, González M, Pérez V, González M, Gutierrez E, Sericano JL, Seeger M (2008) Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in coastal environments of Valdivia and Valparaíso, Chile. J Chil Chem Soc 53:1533–1538. https://doi.org/10.4067/S0717-97072008000200020
Pereira CDS, Martín-Díaz ML, Zanette J, Cesar A, Choueri RB, Abessa DMS, Catharino MGM, Vasconcellos MBA, Bainy ACD, Sousa ECPM, Del Valls TA (2011) Integrated biomarker responses as environmental status descriptors of a coastal zone. Ecotox Environ Safe 74:1257–1264. https://doi.org/10.1016/j.ecoenv.2011.02.019
R Studio Team (2020). RStudio: integrated development environment for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/. Accessed 12 Feb 2021
Riveros A, Zuñiga M, Hernández A, Camaño A (2002) Cellular biomarkers in native and transplanted populations of the mussel Perumytilus purpuratus in the intertidal zones of San Jorge Bay, Antofagasta, Chile. Arch Environ Con Toxicol 42:303–312. https://doi.org/10.1007/s00244-001-0031-4
Riveros A, Zuñiga M, Larraín A (2003) Copper metallothionein-like proteins as exposure biomarker in native and transplanted intertidal populations of the mussel Perumytilus purpuratus from San Jorge Bay, Antofagasta, Chile. Bull Environ Contam Toxicol 70:233–241. https://doi.org/10.1007/s00128-002-0182-7
Rocha SMD, Rocha CAMD (2016) Micronucleus test in bivalve mollusks as an important tool for xenobiotic exposure risk assessment. Acta Fish 4:70–79
Rodilla V (1993) Origin and evolution of binucleated cells and binucleated cells with micronuclei in cisplatin-treated CGO cultures. Mutat Res 300:281–291. https://doi.org/10.1016/0165-1218(93)90062-i
Salamanca M, Jara B, Rodríguez T (2004) Niveles de Cu, Pb y Zn en agua y Perumytilus purpuratus en Bahia San Jorge, Norte de Chile. Gayana (Concepc) 68:53–62. https://doi.org/10.4067/S0717-65382004000100005
Sáez CA, Roncarati F, Moenne A, Moody AJ, Brown MT (2015) Copper-induced intra-specific oxidative damage and antioxidant responses in strains of the brown alga Ectocarpus siliculosus with different pollution histories. Aquat Toxicol 159:81–89. https://doi.org/10.1016/j.aquatox.2014.11.019
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15. https://doi.org/10.1016/0165-1161(75)90058-8
Silva CAR, Smith BD, Rainbow PS (2006) Comparative biomonitors of coastal trace metal contamination in tropical South America (N. Brazil). Mar Environ Res 61:439–455. https://doi.org/10.1016/j.marenvres.2006.02.001
Siu WHL, Mak E, Cao J, De Luca-Abbott SB, Richardson BJ, Xu L, Lam PKS (2004) Micronucleus induction in gill cells of green-lipped mussels (Perna viridis) exposed to mixtures of polycyclic aromatic hydrocarbons and chlorinated pesticides. Environ Toxicol Chem 23:1317–1325. https://doi.org/10.1897/03-225
Thain JE, Vethaak AD, Hylland K (2008) Contaminants in marine ecosystems: developing an integrated indicator framework using biological-effect techniques. ICES J Mar Sci 65:1508–1514. https://doi.org/10.1093/icesjms/fsn120
Thiel M, Hinojosa I, Vásquez N, Macaya E (2003) Floating marine debris in coastal waters of the SE-Pacific (Chile). Mar Pollut Bull 46:224–231. https://doi.org/10.1016/S0025-326X(02)00365-X
Touahri HG, Boutiba Z, Benguedda W, Shaposhniko S (2016) Active biomonitoring of mussels Mytilus galloprovincialis with integrated use of micronucleus assay and physiological indices to assess harbor pollution. Mar Pollut Bull 110:52–64. https://doi.org/10.1016/j.marpolbul.2016.06.029
Ummenhofer CC, Meehl GA (2017) Extreme weather and climate events with ecological relevance: a review. Philos Trans R Soc B 372:20160135. https://doi.org/10.1098/rstb.2016.0135
UN (2019) GSDR 2019: global sustainable development report 2019. United Nations, Department of Economic and Social Affairs. New York, USA. https://sustainabledevelopment.un.org/content/documents/24797GSDR_report_2019.pdf. Accessed on 7 July 2020
USEPA (1993) Guidance manual: Bedded sediment bioaccumulation tests. AScI Corp, Newport
Valdés J, Román D, Guiñez M, Rivera L, Morales T, Ávila J, Cortés P (2009) Distribution and temporal variation of trace metal enrichment in surface sediments of San Jorge Bay, Chile. Environ Monit Assess 167:185–197. https://doi.org/10.1007/s10661-009-1041-3
Valdés J, Román D, Guiñez M, Rivera L, Ávila J, Cortés P, Castillo A (2015) Trace metal variability in coastal waters of San Jorge Bay, Antofagasta, Chile: an environmental evaluation and statistical approach to propose local background levels. Mar Pollut Bull 100:544–554
Van Der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental assessment: a review. Environ Toxicol Pharm 13:57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Van Ngan P, Gomes V, Passos MJACR, Ussami KA, Campos DYF, Rocha AJS, Pereira BA (2007) Biomonitoring of the genotoxic potential (micronucleus and erythrocyte nuclear abnormalities assay) of the Admiralty Bay water surrounding the Brazilian Antarctic Research Station ‘“Comandante Ferraz”,’ King George Island. Polar Biol 30:209–217. https://doi.org/10.1007/s00300-006-0174-x
Vikas M, Dwarakish GS (2015) Coastal pollution: a review. Aquat Procedia 4:381–388. https://doi.org/10.1016/j.aqpro.2015.02.051
Woznicki P, Lewandowka R, Brzuzan P, Ziomek E, Bardega R (2004) The level of DNA damage and the frequency of micronuclei in haemolymph of freshwater mussels Anodonta woodiana exposed to Benzo[A]pyrene. Acta Toxicol 12:41–45
Zagal CJ, Hermosilla C (2007) Guía de invertebrados marinos del sur de Chile, 2nd edn. Editorial Fantástico Sur, Puerto Natales, p 263
Acknowledgements
We thank to the volunteers that helped in different stages of this work. We also are grateful to the team of the laboratories of in which this work was carried out: Cytogenetics from Universidad de La Serena, Cytogenetics from Universidad Católica del Norte and NEPEA from UNESP. CAM thanks “Becas Santander Iberoamérica 2015” for the scholarship for academic exchange to UNESP, where this research started. DMSA thanks Brazilian National Council for Scientific and Technological Development (Grant No. 311609/2014-7) (CNPq) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Musrri, C.A., Palma-Rojas, C., von Brand, E. et al. Environmental Genotoxicity Assessment Using Micronucleus (and Nuclear Abnormalities) Test on Intertidal Mussel Perumytilus purpuratus: A Tool for Biomonitoring the Chilean Coast. Bull Environ Contam Toxicol 107, 77–83 (2021). https://doi.org/10.1007/s00128-021-03132-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03132-8