Abstract
This study aimed to investigate the biomass production of Bacillus subtilis in flask and bioreactor conditions. It is necessary to carry the culture from the shake flask to the pH, air, temperature and stirring controlled bioreactor in order to reduce the working time and increase the production efficiency and product quality. In this study, Bacillus was isolated from soil and grown under flask and bioreactor conditions as biocontrol agent against Botrytis cinerea and Fusarium oxysporum. In this process, a pH value of 7.5, 100% O2 saturation, 30% dissolved O2, at the temperature of 37 °C, total flow of 0.1 Lmin−1 and mixing speed of 150 min−1 were preferred for optimal concerning high production yield of B. subtilis in bioreactor. To test whether B. subtilis has antifungal activity on the growth of B. cinerea and F. oxysporum, a dual culture assay in a PDA medium was carried out. Ultimately, high biomass production in a short incubation period by reaching 2.2 µg/mL after 9 h in the bioreactor. It was observed that the bacteria produced in the bioreactor cultivation grew stronger and showed high antifungal activity which resulted 33.33% inhibition percentage against B. cinerea. It was concluded that B. subtilis can be used as a green-fungicide against B. cinerea and F. oxysporum, and bacterial metabolites from B. subtilis could pave the way for the development of next generation green/biopesticides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, approximately 50% of total agricultural fruit production is lost after harvesting due to the phytopathogen fungi (Carmona-Hernandez et al. 2019). Because of their low pH, higher moisture content and nutrient composition, fruit can be decomposed by phytopathogen fungi. Postharvest diseases generally occur due to the fungal species within the genera such as Penicillium, Botrytis, Monilinia, Rhizopus, Alternaria, Aspergillus, Fusarium, Geotrichum, Gloeosporium and Mucor which release mycotoxins that threat human health (Liu et al. 2013). In order to prevent postharvest loss, synthetic fungicides are applied to fruits generally. Yet, usage of synthetic fungicides cause environmental problems and threat to human and animal health. Therefore, their usage is limited because of their carcinogenicity, teratogenicity, high and acute residual toxicity, long degradation period (Tripathi and Dubey 2004). Moreover, deterioration in fruit quality also leads to reduction in economic value (Liu et al. 2019; Bu et al. 2021). Recently, people have become more conscious about consuming organic foods and prefer foods that do not use synthetic preservatives. For this reason, researchers are trying to develop a safer and more environmentally friendly strategy to treat postharvest diseases of fruit (Li et al. 2021).
In recent years, utilizing probiotic bacteria as a biocontrol agent come into prominence to control phytopathogens (Carmona-Hernandez et al. 2019). As a probiotic, Bacillus subtilis is used for the treatment of mild gastrointestinal diseases or as a dietary supplement (Pryor et al. 2007). In addition to that, gram-positive Bacillus spp. spores can be used as biological control agents due to its diverse structure and activity, as well as its ability to produce a number of secondary metabolites with broad-spectrum antimicrobial potential (Prakash and Arora 2021; Chen et al. 2010). B. subtilis produces resistant spores and antifungal lipopeptides such as iturin, surfactin and fengycin (Pryor et al. 2007; Caulier et al. 2019). As a biocontrol agent, the endospore formation of Bacillus gives them superiority in nature as well as long-term storage and enables the conversion of Bacillus-based natural products into commercial products. According to the literature studies, B. subtilis maximum spore yield values are between 1.00 × 109 spores/mL and 7.40 × 109 spores/mL (Luna et al. 2002; Monteiro et al. 2005).
In large-scale production of bacteria, the stirred tank bioreactor is the most preferred bioreactor which provides a controlled environment and high oxygen uptake rate during fermentation. The bioreactor enables to control the temperature, pH and substrate condition which affects the cellular metabolism of the bacteria (Lidén 2002). Also, the metabolic performance of a microbial culture in bioreactor depends largely on the complex interactions between different operating conditions. During the bioprocess, high oxygen uptake is also necessary for the product concentration, yield and volumetric productivity. Since both high cell density and viability are crucial for commercial production of the fermentation process, detailed evaluation is required to carry out appropriate operating conditions (Sarmiento-López et al. 2022; Hathi et al. 2021).
In this study, as an alternative to chemical fungicides, the production of microbial-derived biocontrol agents by utilizing the probiotic B. subtilis, and its application on Botrytis cinerea and Fusarium oxysporum which are the most common fungal species that cause postharvest disease were investigated. It was aimed to put forth the high efficacy of the bioreactor culture against fungal species in comparison with the conventional productions. In the literature, there are studies which Bacillus species were evaluated against various fungal species including B. cinerea and F. oxysporum. However, neither of these studies investigated B. subtilis produced in bioreactor against B. cinerea and F. oxysporum to be used as a biocontrol agent. It was seen that the inhibition rate of the B. subtilis produced in bioreactor was almost 1.5 times higher than the productions in flask or on solid medium owing to the controlled environment and efficient production.
Materials and methods
Materials
Luria–Bertani (LB), Tryptic Soy Broth (TSB), Tryptic Soy Agar (TSA) and Potato Dextrose Agar (PDA) were supplied from Merck (Merck, Darmstadt, Germany). B. cinerea and F. oxysporum were isolated from rotten apples and identified as mentioned by Yilmaz et al. 2016. B. subtilis was isolated from soil and deposited in GenBank under accession number OQ000948.
Soil sampling and preparation
One gram of each soil sample was dispersed in 9 mL of distilled water and the suspension was heated at 80 °C for 15 min. This treatment was carried out to distinguish the Bacillus genus from other heat-sensitive pathogenic species. After that the samples were serially diluted with phosphate-buffered saline (PBS), and 100 μL of the sample was distributed on the TSA plate and incubated at 37 °C for overnight. The plates were then examined and suspect colonies were stained by the Gram stain and spore stain method. Finally, gram-positive, spore-forming, rod-shaped colonies were selected (Amin et al. 2015). Then, the B. subtilis strain was determined by PCR identification among the obtained Bacillus strains.
Identification of B. subtilis isolate
The 16S rRNA gene sequencing was performed with primers AMP_F (5´-GAGAGTTTGATYCTGGCTCAG-3´) and AMP_R (5´-AAGGAGGTGATCCARCCGCA3´) (Baker et al. 2003). PCR reaction mixtures and PCR conditions for 16S rRNA gene sequencing were used as described by Dertli et al. (2016). The amplification products were run on gel electrophoresis and sent to Medsantek (Turkey) for sequencing.
Production of probiotic bacteria
Before the experiments, B. subtilis was maintained at − 20 °C in Luria–Bertani (LB) supplemented with 25% (v/v) glycerin. To obtain a single colony of the pure culture of B. subtilis, probiotic bacteria were grown on TSA Petri dishes at 37 °C for 24 h. After selecting the colony, bacteria were inoculated in 10 mL of TSB liquid medium and incubated at 37 °C for 24 h (Arutchelvan et al. 2006; Vehapi and Özçimen 2021).
Spore production of B. subtilis was firstly carried out in 1 L Erlenmeyer flask containing 500 mL of TSB medium. The flask was inoculated with 1% (v/v) bacteria and incubated at 37 °C for 30 h on a rotary shaker at 100 rpm.
As for the production in bioreactor, batch fermentation was carried out in a 1 L bioreactor (INFORS, Minifors 2). The bioreactor was first sterilized by autoclaving at the temperature of 121 °C and 1.2 atm for 15 min. After sterilization, required values (pH, temperature, mixing speed and air inlet speed) were entered to the control system. For calibration at one point on the oxygen electrodes, the saturation value of the dissolved oxygen in the bioreactor medium was set to 100% saturation. The preculture of the TSB medium was transferred into the bioreactor with an inoculation rate of 1% and 500 mL medium (TSB) was added to the bioreactor with an initial pH of 7.38. The culture was grown with a stirring speed of 100 rpm and at the temperature of 37 °C for 9 h. At the end of the fermentations, ability to form spores in the productions in flask and bioreactor was compared and growth kinetics were determined.
Growth rate measurement
The PG Instruments T-60 UV Spectrophotometer was used for the measurement of optical density (OD) of flask and bioreactor fermentation of B. subtilis. The optical density of the bacteria was measured at 600 nm at regular intervals every hour for 9 h of bioreactor fermentation and 30 h of flask cultivation (Arutchelvan et al. 2006). The specific cell growth rate and doubling time were calculated using Eqs. 1–3.
where t is the time, X is the CFU mL−1 at time t, X0 is the CFU mL−1 at time t0, µ is the specific growth rate (h−1), td is the doubling time (h)
Carbohydrate analysis
The total carbohydrate content in the medium where bacteria were grown was determined by the phenol–sulfuric acid assay. The phenol was prepared to be 80% (w/w), and 0.05 mL of phenol was added to the samples and glucose solutions. Glucose was used as the standard for carbohydrate analysis and was prepared at different concentrations in the tubes to form the standard curve. Analysis was followed by the addition of 5 mL of H2SO4. After 15 min at room temperature, the absorbance of the samples was measured at 490 nm with a UV spectrophotometer (Dubois et al. 1956).
Extraction of volatile compounds
After the production in bioreactor, bacterial cells were separated from the culture broth by centrifugation at 8000 rpm for 10 min at 4 °C. The collected supernatant was acidified with 6 N HCl to pH 2.0 and allowed to settle at 4 °C overnight. A white precipitate containing the biosurfactant was then recovered by centrifugation at 10,000 rpm for 15 min. The precipitate was suspended in a minimal amount of distilled water and adjusted to pH 7.0 using 1 N NaOH. The solution was then lyophilized and stored for GC–MS analysis (Fooladi et al. 2018).
Identification of volatile compounds
The volatile compounds were identified by solid phase microextraction (SPME) coupled with gas chromatography–mass spectrometry (GCMS-QP2010, Shimadzu, Japan) combined with a CTC-Combi-PAL-Autosampler (Bender and Holbein, Zurich, Switzerland) and the column used for chromatographic separation was Restec (Bellefonte, USA) Rtx-5MS fused silica capillary column (30 m × 0.25 mm, 0.25 μm). The vials were hold at 35 °C for 40 min and then a SPME fiber 75 mm, carboxen/polydimethylsiloxane (CAR/PDMS) was exposed to the headspace of the vials while maintaining the sample at 35 °C for 10 minutes. Compounds were then desorbed for 10 min in the injection port of the gas chromatograph at 220 °C for 10 min with the purge valve off (split-less mode). The injector temperature was 250 °C; detector temperature was 220 °C; and carrier gas (He) flow rate was 1 mL min−1. The GC oven temperature program began when the fiber was inserted and was held at 40 °C for 2 min, ramped to 200 °C at 10 °C per min, then ramped from 200 to 250 °C at 15 °C per min and finally held at 250 °C for 5 min (Chaves-López et al. 2015). The volatile compounds quantities were expressed as percentage area.
Antifungal activity
30 μL of bacteria which was cultivated in the bioreactor in TSB medium at 37 °C for 9 h was used for dual culture assay against B. cinerea and F. oxysporum. To produce the inoculum, the fungal pathogen was cultivated on PDA plates for 6 days at 27 °C in an incubator. A zone of inhibition was generated by the inhibition of mycelial growth by the antifungal activity of B. subtilis. The growth inhibition percentage (I %) of treated plates (Tp) compared to the control plates (Cp) is calculated using the following Eq. (5) (Ryu et al. 2014; Saravanakumar et al. 2019; Liu et al. 2019; Vehapi et al. 2021):
Statistical analysis
Data are presented as means with ± standard deviations (n = 3). Analysis of variance was performed using the JMP (release 6.0.0, SAS) package program. The significance ratings between the averages were determined by Student’s t test (p < 0.05 was considered significant).
Results and discussion
Identification of B. subtilis isolate
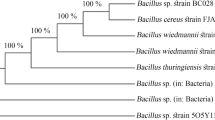
In this study, probiotic bacteria were isolated from soil samples in Turkey and identified as B. subtilis (Table 1). The sequence obtained was aligned with the NCBI database using the BLAST algorithm with a similarity criterion of 100%. The partial 16S sequence of the identified strain was deposited in GenBank under accession number OQ000948.
The isolates were analyzed for cell shape, colony appearance (size, shape, smoothness of the edges and surface, matt and gloss state, etc.) and endospore formation. To determine the morphological features of Gram-positive Bacilli, it was cultured in liquid TSB and solid TSA media and various morphological features such as Gram staining, spore staining, colony type, colony color, and colony size were determined (Table 1).
Production of probiotic bacteria
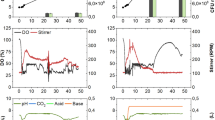
Bioreactor and flask cultivation were compared to study cultures that underwent both growth and sporulation, specifically to study the effects of growth rate on sporulation or changes in growth rate. In addition to that the kinetics of cell growth were investigated under similar conditions (Fig. 1). It was determined that the specific growth rates of the cells cultivated in flask and bioreactor were 0.36 and 0.67 h−1, respectively. Doubling time of the cells grown in flask was almost twice as cells grown in bioreactor. Doubling time of the cells was found as 1.92 and 1.08 h for cells grown in flask and bioreactor, respectively.
According to Fig. 1, in the flask cultivation, B. subtilis reached the stationary phase after 23 h of incubation with an absorbance value of 1.150 and the maximum absorbance value of the B. subtilis was found as 1.33 at the 30th h. On the other hand, the maximum absorbance value of the bioreactor cultivation reached 1.67 after 9 h (Fig. 1). Therefore, it was found that the cultivation in bioreactor resulted in a higher growth in less time in comparison with the growth in flask.
During the production in bioreactor, pH values were also observed automatically and it was found that while the cell concentration increased rapidly, the pH decreased. As the cells grew rapidly within 5 h, the oxygen level decreased. The oxygen saturation, which was 100% at the beginning, decreased to 10%, but since the reactor was initially set not to fall below 30% oxygen saturation, the stirring speed was increased from 150 to 600 rpm to increase the oxygen saturation (Table 2).
In addition to that it was observed that the carbohydrate concentration decreased rapidly in the 3 h and the rate of decrease in the carbohydrate concentration decreased after 4 h. After 7 h, the carbohydrate concentration gradually decreased to 15.43 µg/mL. At the end of the treatment, more than 96.40% of the initial carbohydrate concentration had been consumed (Fig. 2). The change of carbohydrate concentration with the biomass concentration is also presented in Fig. 3.
Similar results were reported in the following literature studies. Yeh et al. studied B. subtilis ATCC 21,332 production in an innovative bioreactor. Time versus cell growth, pH and residual glucose concentration results showed that while cell concentration increased, pH and residual glucose concentration decreased as expected (Yeh et al. 2006). In another study carried out by Posada-Uribe et al., culture conditions were optimized to increase spore production of B. subtilis EA-CB0575. It was reported that biomass concentration (g/L) increased for 10 h and reached to 3.5 g/L. On the other hand, cells consumed 96.9% of reducing sugars (Posada-Uribe et al. 2015). Pandey and Vakil were evaluated the growth of Bacillus coagulans in shake flasks and stirred tank bioreactor. It was found that, while the shake flask fermentation of B. coagulans yielded 8.0 g/L productivity, stirred tank bioreactor fermentation of B. coagulans yielded 18 g/L biomass with 60% spore efficiency (Pandey and Vakil 2016). In studies with bacteria other than Bacillus species, efficient productions were conducted by using bioreactors rather than shaken flasks. Gamboa-Suasnavart et al. investigated the production of Streptomyces lividans under flask and bioreactor conditions to obtain a specified compound from Mycobacterium tuberculosis. It was reported that increase in the biomass concentration in bioreactor was carried out quicker than shaken flasks. Moreover after 30 h, biomass concentration was still continued to increase (Gamboa-Suasnavart et al 2013).
Identification of volatile compounds
Volatile compounds produced by B. subtilis were analyzed by GC–MS. Eighteen compounds were identified (Table 3). The area of major peaks was detected as 26.41%, 26.05%, 7.94%, 6.54%, 5.94%, and 4.78% which corresponds to hexadecane, 2, 6, 10, 14-tetramethyl, heptadecane, heneicosane, octadecane, 1-chloro-, hexadecane and 4-tert-butylcyclohexyl acetate. Minor peaks were detected as 3.72%, 3.21%, 2.23%, 1.95%, 1.71%, 1.67%, 1.26%, 1.24%, 1.13%, 1.05%, 1.03%, and 1.02% which corresponds to dodecane, phenol, 2,4-bis(1,1-dimethylethyl), octadecamethylcyclononasiloxane, hexadecamethylcyclooctasiloxane, triacetin, alpha.-terpinenyl acetate, octadecane, 5-methyl-, eicosane, docosane, eicosane, 2,4-dimethyl, eicosamethylcyclodecasiloxane and decane, 1-iodo-. The hydrophobic components of the hexane extract allow for strong interactions with microbial membranes. As a result, modifications to the microbial membrane increase permeability and allow microbial cells to consume a large amount of the active substance (Chaieb et al. 2011).
It was stated that heptadecane is an antifungal compound (Ponnusamy et al. 2018). Rajaofera et al. (2019) showed that octadecane and docosane produced by B. atrophaeus HAB-5 had antifungal activity against Colletotrichum gloeosporioides (Rajaofera et al. 2019). Prakash and Arora (2021) reported that the phenol, 2,4-bis(1,1-dimethylethyl) had a significant role in biocontrol activity of several phytopathogens (Prakash and Arora 2021). Another metabolite octadecamethylcyclononasiloxane was indicated as an antifungal compound (Isbilen and Volkan 2020). Vanitha et al. (2020) investigated the antimicrobial activity of heneicosane from leaf extract. They found that it has a good inhibitory activity against Aspergillus fumigatus (Vanitha et al. 2020). Triacetin which is one of the minor compounds also has antifungal activity (Quinn and Ziolkowski 2015).
Antifungal effect of B. subtilis
The biocontrol of pathogens using probiotics as antagonists is currently recognized as an important part of integrated management in crops. Among microbial antagonists, Bacillus spp. plays a fundamental role as potential biocontrol agents against pathogens due to their ability to produce numerous antimicrobial compounds such as fengycin, surfactin and iturin (Sarwar et al. 2018). According to the study of Li et al., MALDI-TOF–MS analysis showed a broad m/z peak range, indicating that Bacillus atrophaeus strain B44 produces a complex mixture of iturin, surfactin and fengycin lipopeptides (Li et al. 2021). Particularly B. subtilis produces surfactin, iturin, and fengycin lipopeptides with antifungal activity, and the antifungal activity of these peptides has been attributed to changes in cell membrane permeability (Popov et al. 2021). In the study Mohd Isa et al. carried out, HPLC analysis results of surfactin from isolated B. subtilis were compared to the standard chromatogram of 100 mg/L surfactin standard. 41.6 mg/L, 59.75 mg/L, 26.90 mg/L and 84.08 mg/L surfactin were produced from B-budu, B-cincalok, B-tapai and B-tempeh strain of B. subtilis (Mohd Isa et al. 2020).
Bacillus strains have been shown to have broad-spectrum antifungal activities in various biocontrol studies (Elshaghabee et al. 2017; Liu et al. 2017). In the study by Chitarra et al., it was shown that the antifungal compound produced by B. subtilis YM10-20 strain isolated from corn before harvest inhibited spore germination and growth of Penicillium roqueforti (Chitarra et al. 2003). Kumar et al., investigated the effects of variables such as different media content, pH, incubation period, aeration and temperature on the antifungal activity of B. subtilis MTCC8114 strain isolated from soil. Nutrient broth (NB), sucrose broth (SB), trypticase dextrose broth (TDB) and trypticase soy broth (TSB) were used as the medium and it was determined that the antifungal production in the TSB medium was maximum (Kumar et al. 2009).
To test whether B. subtilis has antifungal activity on the growth of pathogenic fungi, a dual culture assay was carried out. In the presented study, B. subtilis and fungi were incubated in the same petri dish (PDA) medium using dual culture method. For this reason, the antifungal effect was not only due to volatile compounds, but also as a result of microbial competition. As shown in Tables 4, 5, B. subtilis isolate showed antifungal activity against F. oxysporum and B. cinerea. The in vitro effect of B. subtilis on the sixth day of incubation, the mean mycelium diameter of B. cinerea was 60.00 mm. In addition, the mean mycelium diameter of control B. cinerea was observed as 90.00 mm. It was seen that an inhibition rate of B. subtilis against F. oxysporum was 39.16 mm on the sixth day of incubation and the control mycelium had an average diameter of 47.00 mm. The highest antifungal activity was observed with B. subtilis isolate against B. cinerea. The results revealed that B. subtilis plays a vital role in biocontrol efficacy. Mean values of all groups were further analyzed by the comparison test when a significant (p < 0.05) main effect was found (Table 6).
Fungistatic rates showed that B. cinerea and F. oxysporum were successfully inhibited as 33.33% and 16.68% in the dual culture assay at 6th incubation day, respectively. As a result, it can be reported that B. subtilis showed higher inhibition rate against B. cinerea compared to F. oxysporum. In the literature, similar antifungal effects were observed against these fungal pathogens and other pathogens. The investigation of Gajbhiye et al., was based on the isolation of B. subtilis from cotton rhizosphere and it was evaluated as a biological control agent against F. oxysporum and exhibited more than 50% mycelial inhibition in dual culture bioassay (Gajbhiye et al. 2010). In the study of Rong et al., the antifungal activities of the B. safensis B21 against Magnaporthe oryzae were evaluated. B. safensis B21 isolated from Osmanthus fragrans fruit showed high antifungal activity against M. oryzae (Rong et al. 2020). Hussain and Khan described that B. subtilis HussainT-AMU strain significantly inhibited the growth of R. solani by 45 ± 0.30% growth inhibition rate in comparison to control (Hussain and Khan 2020).
It was also seen that the antifungal activity of the B. subtilis produced in bioreactor was higher than the productions in flask owing to the controlled environment and efficient production. On the sixth day, inhibition of B. subtilis grown in flasks on B. cinerea and F. oxysporum was calculated as 26.5% and 10.42%, respectively. This result can be explained with the quality of the spores. It can be said that there were more alive spores in bioreactor culture which grew and affect the fungi more than the culture in flask. The results from Kefi et al. (2015), Mardanova et al. (2016), Khan et al. (2018) and Khedher et al. (2021) who investigated the antifungal effect of various Bacillus species cultivated in flask or solid medium against fungal pathogens including B. cinerea and F. oxysporum showed that the inhibition rate calculated from this study is superior to these results.
Conclusion
In this study, the biomass yield of B. subtilis in shake flask and bioreactor production was compared and an effective approach to the environmentally friendly biocontrol of fungal pathogens by using probiotic bacteria that were grown under bioreactor conditions was suggested. It was found that B. subtilis can inhibit mycelial growth, the germination of Botrytis cinerea and Fusarium oxysporum. The antifungal activity of B. subtilis was attributed with the synthesis of antimicrobial volatile compounds. It was also put forth that the importance of controlled environment for probiotic production to achieve higher productivity and improved antifungal activity. In conclusion, B. subtilis grown in suitable and controlled conditions can be utilized in agriculture as an effective biocontrol agent for agriculturally important plants to prevent postharvest diseases.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Amin M, Rakhisi Z, Zarei AA (2015) Isolation and identification of Bacillus species from soil and evaluation of their antibacterial properties. Avicenna J Clin Microbiol Infect 2(1):1–4
Arutchelvan V, Kanakasabai V, Elangovan R, Nagarajan S, Muralikrishnan V (2006) Kinetics of high strength phenol degradation using Bacillus brevis. J Hazard Mater 129(1–3):216–222
Baker GC, Smith JJ, Cowan DA (2003) Review and re-analysis of domain-specific 16S primers. J Microbiol Methods 55(3):541–555. https://doi.org/10.1016/j.mimet.2003.08.009
Bu S, Munir S, He P, Li Y, Wu Y, Li X, He Y (2021) Bacillus subtilis L1–21 as a biocontrol agent for postharvest gray mold of tomato caused by Botrytis cinerea. Biol Control 157:104568
Carmona-Hernandez S, Reyes-Pérez JJ, Chiquito-Contreras RG, Rincon-Enriquez G, Cerdan-Cabrera CR, Hernandez-Montiel LG (2019) Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: a review. Agron 9(3):121
Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J (2019) Overview of the antimicrobial compounds produced by members of the Bacillus subtilis Group. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00302
Chaieb K, Kouidhi B, Jrah H, Mahdouani K, Bakhrouf A (2011) Antibacterial activity of thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement Altern Med 11(1):1–6
Chaves-López C, Serio A, Gianotti A, Sacchetti G, Ndagijimana M, Ciccarone C, Stellarini A, Corsetti A, Paparella A (2015) Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J Appl Microbiol 119(2):487–499
Chen ZM, Li Q, Liu HM, Yu N, Xie TJ, Yang MY, Chen XD (2010) Greater enhancement of Bacillus subtilis spore yields in submerged cultures by optimization of medium composition through statistical experimental designs. Appl Microbiol Biotechnol 85(5):1353–1360
Chitarra GS, Breeuwer P, Nout MJR, Van Aelst AC, Rombouts FM, Abee T (2003) An antifungal compound produced by Bacillus subtilis YM 10–20 inhibits germination of Penicillium roqueforti conidiospores. J Appl Microbiol 94:159–166
Dertli E, Mercan E, Arıcı M, Yılmaz MT, Sağdıç O (2016) Characterisation of lactic acid bacteria from Turkish sourdough and determination of their exopolysaccharide (EPS) production characteristics. LWT-Food Sci Technol 71:116–124
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Elshaghabee FMF, Rokana N, Gulhane RD, Sharma C, Panwar H (2017) Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol. https://doi.org/10.3389/fmicb.2017.01490
Fooladi T, Abdeshahian P, Moazami N, Soudi MR, Kadier A, Yusoff WMW, Hamid AA (2018) Enhanced biosurfactant production by Bacillus pumilus 2IR in Fed-batch fermentation using 5-L BIOREACTOR. Iran J Sci Technol Trans A Sci 42(3):1111–1123
Gajbhiye A, Rai AR, Meshram SU, Dongre AB (2010) Isolation, evaluation and characterization of Bacillus subtilis from cotton rhizospheric soil with biocontrol activity against Fusarium oxysporum. World J Microbiol Biotechnol 26(7):1187–1194
Gamboa-Suasnavart RA, Marín-Palacio LD, Martínez-Sotelo JA, Espitia C, Servín-González L, Valdez-Cruz NA, Trujillo-Roldán MA (2013) Scale-up from shake flasks to bioreactor, based on power input and Streptomyces lividans morphology, for the production of recombinant APA (45/47 kDa protein) from Mycobacterium tuberculosis. World J Microbiol Biotechnol 29(8):1421–1429
Hathi Z, Mettu S, Priya A, Athukoralalage S, Lam TN, Choudhury NR, Lin CSK (2021) Methodological advances and challenges in probiotic bacteria production: Ongoing strategies and future perspectives. Biochem Eng J 176:108199
Hussain T, Khan AA (2020) Bacillus subtilis HussainT-AMU and its Antifungal activity against Potato Black scurf caused by Rhizoctonia solani on seed tubers. Biocatal Agric Biotechnol 23:101443
Isbilen O, Volkan E (2020) Anticancer activities of Allium sativum L. against MCF-7 and MDA-MB-231 breast cancer cell lines mediated by Caspase-3 and Caspase-9. Cyprus J Med Sci 5(2):305–312
Kefi A, Slimene IB, Karkouch I, Rihouey C, Azaeiz S, Bejaoui M, Limam F (2015) Characterization of endophytic Bacillus strains from tomato plants (Lycopersicon esculentum) displaying antifungal activity against Botrytis cinerea Pers. World J Microbiol Biotechnol 31(12):1967–1976
Khan N, Martínez-Hidalgo P, Ice TA, Maymon M, Humm EA, Nejat N, Hirsch AM (2018) Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front Microbiol 9:2363
Khedher SB, Mejdoub-Trabelsi B, Tounsi S (2021) Biological potential of Bacillus subtilis V26 for the control of Fusarium wilt and tuber dry rot on potato caused by Fusarium species and the promotion of plant growth. Biol Control 152:104444
Kumar A, Saini P, Shrivastava JN (2009) Production of peptide antifungal antibiotic and biocontrol activity of Bacillus subtilis. Indian J Exp Biol 47:57–62
Li X, Zhang M, Qi D, Zhou D, Qi C, Li C, Wang W (2021) Biocontrol ability and mechanism of a broad-spectrum antifungal strain Bacillus safensis sp. QN1NO-4 against strawberry anthracnose caused by Colletotrichum fragariae. Front Microbiol 1–14
Lidén G (2002) Understanding the bioreactor. Bioprocess Biosyst Eng 24(5):273–279
Liu J, Sui Y, Wisniewski M, Droby S, Liu Y (2013) Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int J Food Microbiol 167(2):153–160
Liu H, Wang S, Cai Y, Guo X, Cao Z, Zhang Y, Zhou Y (2017) Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol 60:326–333
Liu L, Zhao XY, Tang QB, Lei CL, Huang QY (2019) The mechanisms of social immunity against fungal infections in eusocial insects. Toxins 11(5):244
Luna CL, Mariano RLR, Souto-Maior AM (2002) Production of a biocontrol agent for crucifers black rot disease. Braz J Chem Eng 19:133–140
Mardanova AM, Hadieva GF, Lutfullin MT, Khilyas IVE, Minnullina LF, Gilyazeva AG, Sharipova MR (2016) Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agric Sci 8(1):1–20
Mohd Isa MH, Shamsudin NH, Al-Shorgani NKN, Alsharjabi FA, Kalil MS (2020) Evaluation of antibacterial potential of biosurfactant produced by surfactin-producing Bacillus isolated from selected Malaysian fermented foods. Food Biotechnol 34(1):1–24
Monteiro SM, Clemente JJ, Henriques AO, Gomes RJ, Carrondo MJ, Cunha AE (2005) A procedure for high-yield spore production by Bacillus subtilis. Biotechnol Prog 21:1026–1031
Pandey KR, Vakil BV (2016) Development of bioprocess for high density cultivation yield the probiotic Bacillus coagulans and its spores. J Biosci Biotechnol 5(2):173–181
Ponnusamy R, Vaikuntavasan P, Subramanian R, Mathiyazhagan K, Palanisamy M, Ayyanar K (2018) Molecular detection of antimicrobial peptide genes and identification of antifungal compounds of Bacillus sp. against Fusarium oxysporum f. sp. cubense causing wilt in banana. Curr J Appl Sci 28(2):1–8
Popov IV, Algburi A, Prazdnova EV, Mazanko MS, Elisashvili V, Bren AB, Chikindas ML (2021) A review of the effects and production of spore-forming probiotics for poultry. Animals 11(7):1941
Posada-Uribe LF, Romero-Tabarez M, Villegas-Escobar V (2015) Effect of medium components and culture conditions in Bacillus subtilis EA-CB0575 spore production. Bioprocess Biosyst Eng 38(10):1879–1888
Prakash J, Arora NK (2021) Novel metabolites from Bacillus safensis and their antifungal property against Alternaria alternata. Anton Leeuw 114:1245–1258
Pryor SW, Gibson DM, Hay AG, Gossett JM, Walker LP (2007) Optimization of spore and antifungal lipopeptide production during the solid-state fermentation of Bacillus subtilis. Appl Biochem Biotechnol 143(1):63–79
Quinn MJ, Ziolkowski D (2015) Wildlife toxicity assessment for triacetin. In: Williams MA, Reddy G, Quinn MJ, Johnson MS (eds) Wildlife toxicity assessments for chemicals of military concern. Elsevie, r, pp 291–299
Rajaofera MJR, Wang Y, Dahar GY, Jin P, Fan L, Xu L, Liu W, Miao W (2019) Volatile organic compounds of Bacillus atrophaeus HAB-5 inhibit the growth of Colletotrichum gloeosporioides. Pestıc Biochem Phys 156:170–176
Rong S, Xu H, Li L, Chen R, Gao X, Xu Z (2020) Antifungal activity of endophytic Bacillus safensis B21 and its potential application as a biopesticide to control rice blast. Pestic Biochem Physiol 162:69–77
Ryu H, Park H, Suh DS, Jung GH, Park K, Lee BD (2014) Biological control of Colletotrichum panacicola on Panax ginseng by Bacillus subtilis HK-CSM-1. J Ginseng Res 38(3):215–219
Saravanakumar D, Thomas A, Banwarie N (2019) Antagonistic potential of lipopeptide producing Bacillus amyloliquefaciens against major vegetable pathogens. Eur J Plant Pathol 154(2):319–335
Sarmiento-López LG, López-Meyer M, Maldonado-Mendoza IE, Quiroz-Figueroa FR, Sepúlveda-Jiménez G, Rodríguez-Monroy M (2022) Production of indole-3-acetic acid by Bacillus circulans E9 in a low-cost medium in a bioreactor. J Biosci Bioeng 134(1):21–28
Sarwar A, Hassan MN, Imran M, Iqbal M, Majeed S, Brader G, Hafeez FY (2018) Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol Res 209:1–13
Tripathi P, Dubey NK (2004) Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol Technol 32(3):235–245
Vanitha V, Vijayakumar S, Nilavukkarasi M, Punitha VN, Vidhya E, Praseetha PK (2020) Heneicosane—a novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Ind Crops Prod 154:112748
Vehapi M, İnan B, Kayacan-Cakmakoglu S, Sagdic O, Özçimen D (2021) Optimization of growth conditions for the production of Bacillus subtilis using central composite design and its antagonism against pathogenic fungi. Probiot Antimicrob Proteins 1–12
Vehapi M, Özçimen D (2021) Antimicrobial and bacteriostatic activity of surfactants against B. subtilis for microbial cleaner formulation. Arch Microbiol 203(6):3389–3397
Yeh MS, Wei YH, Chang JS (2006) Bioreactor design for enhanced carrier-assisted surfactin production with Bacillus subtilis. Process Biochem 41(8):1799–1805
Yilmaz A, Bozkurt F, Cicek PK, Dertli E, Durak MZ, Yilmaz MT (2016) A novel antifungal surface-coating application to limit postharvest decay on coated apples: Molecular, thermal and morphological properties of electrospun zein–nanofiber mats loaded with curcumin. Innov Food Sci Emerging Technol 37:74–83
Acknowledgements
Meyrem Vehapi is supported by TUBİTAK 2244—Industrial Ph.D. Program No: 118C075.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflicts of interest exist.
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vehapi, M., İnan, B., Kayacan-Cakmakoglu, S. et al. Production of Bacillus subtilis soil isolate as biocontrol agent under bioreactor conditions. Arch Microbiol 205, 52 (2023). https://doi.org/10.1007/s00203-022-03381-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03381-z