Abstract
Abnormal cervicovaginal microbiota play an important role in HPV persistence and progression to cervical cancer. The present study aimed at isolating and identifying potential probiotics from vaginal swabs of healthy women and evaluating their activity against vaginal pathogens isolated from cervical cancer patients. Based on probiotic, acid-bile tolerance and antimicrobial properties, 13 lactic acid bacteria (LAB) from the healthy group were identified by MALDI TOF MS (Matrix Assisted Laser Desorption and Ionisation, Time Of Flight Mass Spectrometry). Among these, four strains, Lactobacillus gasseri P36Mops, Limosilactobacillus fermentum P37Mws, Lactobacillus delbrueckii P31Mcs and Enterococcus faecium P26Mcm, exhibited significant antimicrobial activity against 8 vaginal pathogens (Staphylococcus haemolyticus P41Tcs, Escherichia coli P30Tcs, E. coli P79Bcm, Enterococus faecalis P29Mops, E. faecalis P50Tws, E. faecalis P68Tcb, S. haemolyticus P48Bcb and S. haemolyticus P58Bcb) isolated from precancerous and cervical cancer patients. 16S rRNA sequencing of four potential probiotics revealed congruency with the MALDI-TOF MS identification and phylogenetic analysis showed genetic relationship with previously reported LAB strains. The selected LAB showed strain specific hydrophobicity (35.88–56.70%), auto-aggregation (35.26–61.39%) and antibiotic susceptibility. Interestingly, L. gasseri P36Mops was resistant to five standard antibiotics routinely used against urogenital or vaginal infections. LCMS (Liquid Chromatography Mass Spectrometry) analyses of the CFS (cell-free supernatant) of the four potential probiotics revealed the presence of metabolites such as N-(1-deoxy-1-fructosyl)valine, hygroline, acetoxy-2-hydroxy-16-heptadecen-4-one, avocadyne 4-acetate, avocadyne 2-acetate, taraxinic acid glucosyl ester, 6-hydroxypentadecanedioic acid, with reported antimicrobial activity. The overall data suggest the bio-therapeutic potential of the identified vaginal probiotics against cervical cancer-associated pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the second leading cause of cancer death among Indian women (Siegel et al. 2018). Infection with high-risk human papilloma viruses (HPVs) such as HPV16 and 18 is the major cause for developing cervical cancer (Siegel et al. 2018).
Healthy vaginal microbiota are mostly dominated by beneficial microbes such as Lactobacillus and opportunistic pathogens. Changes in the vaginal microbiota could modulate immune responses and favour the growth of pathogens, thereby facilitating development of several diseases (Kalia et al. 2020; Mitra et al. 2020). Depletion of beneficial Lactobacillus could increase infection with diverse aerobic (Staphylococcus spp., Pseudomonas spp sp E. coli, and E. faecalis) and anaerobic (Gardnerella spp., Atopobium spp., Eggerthella spp., Sneathia spp. and Prevotella spp.) bacteria. Such vaginal dysbiosis can lead to either bacterial vaginosis (BV) and/or invasive cervical carcinoma (ICC) (Biswal et al. 2014; Mitra et al. 2020). Recent evidences suggest that the local cervico-vaginal microbiota play a critical role in the development of pre-cancerous cervical intraepithelial neoplasia (CIN), which if left untreated could progress to invasive cervical carcinoma (ICC) (Dai et al. 2021). The exact role of pathogenic bacteria in cervical carcinogenesis is unknown; however, it is anticipated that they produce toxins and carcinogenic metabolites (nitrosamines) that could trigger increased production of proinflammatory cytokines and DNA adducts, which could lead to CIN and ICC (Kyrgiou et al. 2017).
The current standard of care (SOC) drugs for treating vaginal infections or during cervical cancer therapy usually include metronidazole or clindamycin, norfloxacin and cotrimoxazole (Degu et al. 2017; Kietpeerakool et al. 2017; Mulu et al. 2015; Petrina et al. 2017; Thulkar et al. 2012; Turovskiy et al. 2009). The indiscriminate use of antibiotics mostly results into development of resistant micro‐organisms (Ahire et al. 2021), thereby increasing the risk of secondary infections in the cervical cancer patients (Gao et al. 2020). Use of antibiotics may also be associated with vaginal pain, metrorrhagia, nausea, vomiting, diarrhoea and renal failure (Mubarak and Kazi 2014). To overcome such issues, probiotics are being used as adjuncts during treatment of cervical cancer-associated bacterial infections (Mejía-Caballero et al. 2021) or BV (Happel et al. 2020; Mejía-Caballero et al. 2021).
In the present study, we have isolated and identified vaginal Lactobacillus and non-Lactobacillus (Enterococcus) species from healthy women. The isolates were evaluated for probiotic potential and antibacterial activity against vaginal pathogens isolated from cervical cancer patients.
Materials and method
Subject selection and sampling
The study was approved by Institutional Ethics Committees of Bharati Vidyapeeth (Deemed to be) University Medical College (BVMC) (Ref: BVDU/MC/57), and B. J. Government Medical College and Sassoon General Hospitals (BJGMCSGH), Pune (Ref No. BJGMC/IEC/Pharmac/ND-Dept 0,119,007-007). Vaginal swabs from healthy (n =45 ); low-grade squamous intraepithelial lesion, LSIL (n = 1); high-grade squamous intraepithelial lesion, HSIL (n = 1) and invasive cervical carcinoma ICC (n = 6) patients (aged between 18 and 55 years) were collected by the clinicians at the respective clinical sites. All the patients provided informed written consent. The patients were screened for eligibility criteria based upon Pap test, vaginal cytology or colposcopy findings and were categorized under healthy, LSIL, HSIL and ICC groups. Post-collection, the samples were immediately placed on ice and taken to IRSHA for further analysis within 2 h of the collection.
Culture and reagents
The clinical strain, Pseudomonas aeruginosa MCC 2081, was procured from National Centre for Microbial Resource (NCMR), NCCS, Pune. All the media components such as de Man, Rogosa and Sharpe (MRS), Tryptone Soya (TSA) or Brain Heart Infusion (BHI), L-cysteine, Bile, Sheep blood agar plates were purchased from HiMedia Laboratories, Mumbai, India. Anaero Gas Pack, antibiotics discs and sterile cotton swabs were procured from HiMedia Laboratories, Mumbai, India. Pepsin and pancreatin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Plasticware was purchased from Tarsons Products Private Limited, India. Ethanol (99% v/v) was procured from Changshu Hongsheng Fine Chemical Co. Ltd. (China).
Isolation and cultivation of microorganisms
For isolation of microbiota, aliquots from the collected swabs were plated on de Man, Rogosa and Sharpe (MRS), Tryptone Soya (TSA) or Brain Heart Infusion (BHI) media. Based on the morphology and biochemical tests, Lactobacillus and non-Lactobacillus colonies were separated and purified. All the Lactobacillus strains grown on MRS media were supplemented with 0.05% L-cysteine at 37 °C for 48 h in anaerobic jars, supplemented with Anaero Gas Pack (HiMedia, Mumbai). Non-Lactobacillus strains were grown either on TSA or BHI media and incubated at 37 °C for 24 h. The pure colonies were stored in 20% glycerol at – 80 °C until further use.
Hemolysis assay
All the isolates were determined for hemolytic activity by streaking the strains on sheep blood agar plates (HiMedia, Mumbai) and incubated at 37 °C for 24–48 h. The hemolytic activity was detected by observing either a clear zone of hydrolysis around the colonies (beta hemolysis), or a greenish zone (alpha hemolysis), or no zone (gamma hemolysis).
Acid and bile tolerance
The tolerance assay was done as reported earlier (Azat et al. 2016; Ehrmann 2002). Briefly, the isolates were grown, harvested and the pellet was washed and resuspended in 1X PBS (pH 3) with a final concentration of 8–9 logCFU/ml or PBS (pH 7.4) containing 0.3% bile, followed by incubation at 37 °C for 3 h. Appropriate dilutions from each sample were spread plated on the respective agar plates and incubated anaerobically using Gas-Pack system at 37 °C for 24–48 h. The tolerance was evaluated by total viable count method (log CFU/ml) after 3 h of incubation. The survival rate (%) was calculated as per the following equation:
where N0 and N1 are the total viable counts of the selected strains before and after treatment, respectively.
MALDI-TOF MS analysis
The identification of isolates by MALDI-TOF MS was carried out at National Centre for Microbial Resources (NCMR), National Centre for Cell Sciences (NCCS), Pune. Sample preparation for MALDI-TOF MS analysis was performed as described earlier (Kurli et al. 2018). Briefly, a smear of actively grown bacterial single colonies was made as a thin film directly onto the spot on a MALDI target plate. The bacterial smear was overlaid with 1 µl of alphacyano-4-hydroxycinnamic acid (HCCA) solution and allowed to dry at room temperature. The samples were loaded onto the MALDI-TOF MS instrument (AUTOFLEX speed, Bruker Daltonics, GmbH, Germany) and MALDI Biotyper software 3.1 (Bruker Daltonik GmbH, Germany) to identify the isolates and visualize the mass spectra. The strain showing ≥ 1.7 log value with the strain in the database was confirmed as the member of that genus, and strains showing ≥ 2.0 log values were confirmed to be the member of that species.
Preparation of cell-free supernatant (CFS)
The LAB strains were grown for 24–48 h and centrifuged at 3000 g for 15 min. The CFS was collected and filter-sterilized with 0.22 µm syringe filter. The resultant CFS was used for antimicrobial assays, and for LCMS, lyophilized powder was stored until further use.
Antibacterial activity
The antibacterial activity of CFS of the selected isolates was determined by agar well diffusion method (Reuben et al. 2019). Briefly, cultures of overnight grown indicator pathogens were adjusted to OD600nm of 0.5 McFarland. 100 µL adjusted pathogen culture was aseptically spread on the nutrient agar plates. Wells of 8 mm diameter were cut out with sterile cork borer, and 150 µl CFS was loaded. The plates were kept at 4 °C for 2 h for diffusion and incubated further at 37 °C for 24 h. After incubation, the diameter (mm) of the zone of inhibition (ZOI) around the well was measured. Pseudomonas aeruginosa MCC 2081 was used as the standard clinical pathogen. The pathogens (with beta and alpha hemolysis) used in this study were isolated from cervical cancer patients and identified by MALDI TOF MS method. Antibacterial activity of the eight standard antibiotics [carbenicillin (100 μg), cefoxitin (30 μg), clindamycin (2 μg), chloramphenicol (30 μg), erythromycin (15 μg), metronidazole (5 μg), penicillin G (10 unit) and tetracycline (30 μg)] was also evaluated against pathogens by disc diffusion method (Bayer et al. 1966).
Liquid chromatography mass spectroscopy (LCMS)
LCMS analysis of the lyophilized CFS of the selected potential probiotics was carried out at Center for Applications of Mass Spectrometry (CAMS), Venture Centre, Pune. Samples were analyzed by non‑targeted LCMS QTOF using Agilent 1290 HPLC system as described previously (Aphale et al. 2018). Briefly, 8 µl of sample was injected onto an Agilent 1290 HPLC system having Zorbax Eclipse Plus C18 column (2.1 mm × 50 mm, 1.8 µm particle sizes). The mobile phases consisted of (A) water and (B) acetonitrile (LCMS grade, J. T. Baker) with flow rate of 0.3 ml/min and 95:5 acetonitrile/water. Both mobile phases were modified with 0.1% (v/v) formic acid for MS analysis in positive mode and with 5 mm ammonium acetate for analysis in negative mode. The chromatographic conditions included first 18 min run of B from 95 to 5% gradient, applied from 18 to 30 min, followed by 3 min isocratically at 100%. MS analysis was performed on an Agilent 6530 Quadrupole time‑of‑flight spectrometer fitted with an electrospray ionization source in both positive and negative mode. Data were analyzed by using Mass Hunter Qualitative Analysis Software Package (Agilent Technologies) and online database Metlin. Compound lists were screened against online mass databases; METLIN Metabolomics Database and MassBank Database.
16S rRNA gene sequencing and phylogenetic analysis
The identification of isolates was carried out at the sequencing facility of NCMR, Pune. The genomic DNA was isolated by the standard phenol/chloroform extraction method (Sambrook et al.1989), followed by PCR amplification of the 16S rRNA gene using universal primers 16F27 [5'-CCA GAG TTT GAT CMT GGC TCA G-3'] and 16R1492 [5'-TAC GGY TAC CTT GTT ACG ACT T-3']. The amplified 16S rRNA PCR product was purified by PEG-NaCl precipitation and directly sequenced on ABI® 3730XL automated DNA sequencer (Applied Biosystems, Inc., Foster City, CA) as per the manufacturer’s instructions. Assembly was carried out using Lasergene package, followed by identification using the EzBioCloud database (Yoon et al. 2017). The phylogenic trees were constructed using the neighbor-joining method (Tamura et al. 2013) with MEGA6 software.
Tolerance to simulated gastrointestinal conditions
The ability of Lactobacilli to tolerate the simulated gastric conditions was determined as described previously (Pino et al. 2019; Pithva et al. 2014). Briefly, simulated gastric juice (SGJ) consisting of 0.3% pepsin in NaCl solution was adjusted to pH 2.5 by adding 1 M HCl. On the other hand, simulated intestinal fluid (SIF) comprising of 0.1% (w/v) pancreatin and 0.3% (w/v) bile in NaCl solution was adjusted to pH 8 by adding 1 M NaOH. Both the solutions were prepared immediately before use and sterilized using 0.22 µm filter. The potential probiotics were grown for 48 h, centrifuged for 3000 × g for 15 min. The pellet was washed with saline, resuspended in gastric fluid and incubated at 37 °C for 3 h. After incubation, the cells were pelleted down by centrifugation, washed with 1X PBS and resuspended in SIF, followed by incubation at 37 °C for 3 h. The cells were washed with 1X PBS, serially diluted and plated on MRS agar for determining viability by total viable count method. The survival rate (%) was calculated as described for the acid and bile tolerance test.
Hydrophobicity and auto-aggregation properties
Hydrophobicity (H) (Kang et al. 2018) and auto‐aggregation (A) (Juárez Tomás et al. 2005) of the selected potential probiotic strains were determined as described earlier. Overnight grown bacterial suspensions in MRS broth were harvested by centrifugation at 3000 g for 15 min at room temperature. The cells were washed twice with 1X PBS and adjusted to an optical density (OD) of 0.5 ± 0.1 (A0) at 600 nm. To determine the hydrophobicity of the cell surface, xylene was used as a solvent. It was added to each bacterial suspension in the ratio of 1:1, and the mixtures were vortexed for 1 min and incubated for 5 h at 37 °C. After phase stabilization and separation, the aqueous phase was removed, followed by measurement of absorbance (At) at 600 nm. Hydrophobicity percentage was calculated from the formula, H(%) = A0 − At/A0 × 100, where A0 and At are the optical densities before and after extraction with xylene, respectively. For the auto-aggregation assay, each bacterial suspension (initial OD600nm = 0.5 ± 0.1) was vortexed for 10 s and incubated at 37 °C for 5 h without agitation. The absorbance (At) was measured at 600 nm in microplate reader (Epoch, BioTek Instruments, Inc., USA). The percentage of auto-aggregation was calculated from the formula A(%) = A0 − At/A0 × 100, where A0 is the OD at initial time (0 h) and At is the OD at final time (5 h) of the assay.
Biofilm formation
For biofilm formation (Terraf et al. 2012), the potential probiotic strains were inoculated in MRS media at 37 °C for 24 h. Around 200 μl of the grown bacterial culture (OD 0.5) was added into the each well of 96-well plate and incubated at 37 °C for 72 h. The biofilm formed in the wells was washed twice with 200 μl 1X PBS and dried for 30 min at 37 °C, followed by incubation with 200 μl of 0.1% (w/v) crystal violet for 30 min at room temperature (RT). The well was washed twice with 200 μl distilled water, dried for 10 min at RT. The residual crystal violet was dissolved in 200 μl solution containing 95% ethanol and 0.1% acetic acid in water, followed by measurement of the absorbance at 570 nm in multiplate reader.
Antibiotic susceptibility
Susceptibility of the potential probiotics to antibiotics was determined by the agar diffusion method by using two different antibiotics disks (Combi II and Combi III, HiMedia, Mumbai). A total of 16 antibiotics were tested that included carbencillin (100 μg), cefoxitin (30 μg), clindamycin (2 μg), chloramphenicol (30 μg), erythromycin (15 μg), metronidazole (5 μg), penicillin G (10 unit), tetracycline (30 μg), ampicillin (10 μg), norfloxacin (10 μg), nitrofurantoin (300 μg), nalidixic acid (30 μg), gentamicin (10 μg), cotrimoxazole (25 μg), cefalotin (30 μg), and cefotaxime (30 μg).
Statistical analysis
The data have been presented as mean ± standard deviation (SD) and was analyzed for statistical significance by one-way and two-way analysis of variance (ANOVA) by using Tukey's multiple comparisons test. Statistical significance level was defined at p value < 0.05. All the statistical analyses were performed by using Graph Pad Prism version 9 (GraphPad, San Diego, USA).
Accession number
The nucleotide sequences of 16S rRNA of the four strains were deposited at the GenBank database under the following accession numbers: Lactobacillus delbrueckii P31Mcs (OM049479), Lactobacillus gasseri P36Mops (OM049480), Limosilactobacillus fermentum P37Mws (OM049481) and Enterococcus faecium P15Mcm (OM049482).
Results
Isolation and characterization of vaginal microbiota
From the vaginal swabs of 45 healthy women, 111 microbiota were isolated and characterized for their microscopic structure, Gram staining and catalase activity (Supplementary Table S1). From 40 healthy vaginal swabs, most of the isolates showed Lactobacillus-related morphology (rods, Gram positive and catalase negative) (88.89%). Other 5 vaginal swabs showed presence of cocci and non-Lactobacilli. All the isolates were further evaluated for hemolysis. 79 isolates revealed non-hemolytic nature (Supplementary Table S1), out of which four were omitted that showed morphological features similar to yeast and Gram negative cocci. From the vaginal swabs of the patients, 29 microbiota [four isolates from LSIL (n = 1), five from HSIL (n = 1) and twenty from ICC (n = 6)] (Supplementary Table S2), were isolated. Among these, 17.24% isolates showed Lactobacillus-related morphology, rest were cocci with either catalase negative or positive activity, and few were Gram-negative rods. Out of 29 isolates, 13 exhibited beta hemolysis and 5 showed alpha hemolysis (Supplementary Table S2), while the remaining 11 showed gamma hemolysis. Out of 18 isolates, five with beta hemolysis from LSIL (1), HSIL (1), ICC (3) and three with alpha hemolysis from ICC group were randomly selected as indicator pathogens for the antibacterial assay.
Non-hemolytic microbiota exhibited acid and bile tolerance
The non-hemolytic strains (75) from healthy controls were evaluated for acid bile tolerance. Around 90.66% strains showed tolerance to acidic pH (Supplementary Table S3). After incubation with bile (0.3%), the viability of 40 isolates was reduced by approximately more than 2-log with survival rates less than 75% (Supplementary Table S3). Another 22 isolates were totally inhibited in the presence of bile. Interestingly, only thirteen isolates showed tolerance to both acid and bile with less than 2-log reductions and were taken further for antibacterial studies.
MALDI-TOF MS identification of the isolates
All the thirteen isolates showing acid and bile tolerance were further identified by MALDI-TOF MS (Table 1). For each LAB strain, the score value was in the range of 1.7–2. Among the 13 identified LAB from healthy group, Lactobacillus gasseri (53.85%) was found to be dominant, followed by Limosilactobacillus fermentum (15.38%), Enterococcus faecium (15.38%), Lactobacillus reuteri (7.69%) and Lactobacillus delbrueckii (7.69%). Out of 18 pathogenic isolates, 8 strains were identified by MALDI-TOF MS (score value above 2.0) (Supplementary Table S4), S. haemolyticus P41Tcs and E. coli P79Bcm were isolated from the swabs of LSIL and HSIL patients, respectively. E. coli P30Tcs, E. faecalis P29Mops, E. faecalis P50Tws, E. faecalis P68Tcb, S. haemolyticus P48Bcb and S. haemolyticus P58Bcb were isolated from the swabs of 6 cervical cancer patients. Among these, S. haemolyticus (37.5%) and E. faecalis (37.5%) were found to be the dominant strains, followed by E. coli (25%). The identified pathogenic strains were used as indicators for further antimicrobial studies.
CFS of selected strains from healthy group exhibited antimicrobial activity
The cell-free supernatant (CFS) of the acid-bile tolerant isolates (13) from healthy individuals was evaluated for antimicrobial activity against standard (commercially obtained) pathogen, P. aeruginosa 2081 and eight isolated pathogenic strains from cervical cancer patients (Table 2). Among the thirteen isolates, L. fermentum P37Mws exhibited significantly high (p < 0.05) antimicrobial activity against four pathogenic strains, P. aeruginosa, E. coli P79Bcm, S. haemolyticus P48Bcb and P58Bcb. L. gasseri P36Mops also showed antibacterial activity (p < 0.05) against four pathogens, E. coli P30Tcs and P79Bcm; S. haemolyticus P41Tcs and P58Bcb. L. delbrueckii P31Mcs showed significant (p < 0.05) zone of inhibition against (three pathogens) E. coli P30Tcs, S. haemolyticus P48Bcb and 58Bcb. Interestingly, E. faecium P15Mcm showed significantly higher zone of inhibition (> 18 mm) against three pathogenic strains of E. faecalis P29Mops, P50Tws and P68Tcb. Thus, overall data showed that four strains (L. fermentum P37Mws, L. delbrueckii P31Mcs, L. gasseri P36Mops and E. faecium P15Mcm) exhibited probiotic characteristics and antibacterial activity against more than two pathogens. The vaginal pathogens were also tested for their susceptibility to eight standard antibiotics that included carbencillin, cefoxitin, clindamycin, chloramphenicol, erythromycin, metronidazole, penicillin G and tetracycline. Tetracycline exhibited antibacterial activity against all the eight vaginal pathogens, whereas metronidazole did not show any activity against the pathogens (Supplementary Table S5). On the other hand, carbencillin displayed antibacterial activity only against the strains of E. faecalis. Rest of the antibiotics displayed varied spectrum of antibacterial activity against the pathogens.
CFS showed presence of metabolites with reported antimicrobial activity
LCMS analysis (non-targeted) of the CFS from the potential probiotics was carried out to identify the potential antibacterial compounds secreted by the respective strains. Around 119 compounds from L. gasseri, 95 from L. fermentum, 106 from L. delbrueckii P31Mcs and 117 from E. faecium P15Mcm were identified. The major metabolites with antibacterial activity are shown in Table 3, and their respective chromatograms are included in Supplementary Fig. S1. The antibacterial metabolites from CFSs of L. gasseri P36Mops included N-(1-deoxy-1-fructosyl)valine, homoarecoline, 2-Isopropyl-1,4-benzenediol, 1-Acetoxy-2-hydroxy-16-heptadecen-4-one, avocadyne 4-acetate, avocadyne 2-acetate, grandidentatin, taraxinic acid glucosyl ester and nigellicine. The metabolites detected from L. fermentum P37Mws included methylarmepavine, (-)-hygroline, avocadyne 4-acetate, avocadyne 2-acetate and 1-acetoxy-2-hydroxy-16-heptadecen-4-one. The CFS of L. delbrueckii P31Mcs showed the presence of metabolites such as ( +)-O-methylarmepavine, 6-methylquinoline, quinaldine, 1-acetoxy-2-hydroxy-16-heptadecen-4-one, 3-O-sulfogalactosylceramide. The metabolites such as nepetalactam, hordenine, cuminaldehyde, 4-phenyl-3-buten-2-ol, 6-hydroxypentadecanedioic acid, hygroline, taraxinic acid glucosyl ester, avermectin A2b aglycone were detected from E. faecium P15Mcm. Thus, the CFS of the potential probiotic strains showed presence of antimicrobial metabolites, which could be responsible for their observed antibacterial activity.
16 s rRNA sequencing and phylogenetic analysis
The potential probiotic strains were subjected to molecular identification and phylogenetic analysis by the Sanger sequencing method and EzBioCloud database, respectively (Supplementary Table S6). A comparative 16S rRNA gene-based phylogenetic analysis of the four vaginal strains, P36Mops, P37Mws, P31Mcs and P15Mcm, revealed their closest similarity (ranging from 100 to 99%) to the sequences of the type strains, L. gasseri ATCC 33323 (Azcarate-Peril et al. 2008), L. fermentum CECT562 (Zheng et al. 2020), L. delbrueckii DSM 20072 (Schoch et al. 2020) and E. lactis BT159 (Morandi et al. 2012), respectively, from GenBank database (Supplementary Fig. S2-S5).
Probiotics survived the simulated gastric juice (SGJ) and intestinal fluid (SIF) conditions
All the selected strains displayed > 80% survival in 0.3% pepsin (Table 4), representing SGJ. L. fermentum P37Mws exhibited significant (p < 0.0001) survival (96.3%) with viable count of 8.86 ± 0.09 log CFU/ml, followed by E. faecium P15Mcm (94.16%). On the other hand, L. gasseri P36Mops and L. delbrueckii P31Mcs showed survival up to 85.94 to 84.86%, respectively. All the four potential probiotics showed 60.09–76.30% survival up to 3 h in the presence of pancreatin enzyme. The viable count for L. fermentum P37Mws and L. gasseri P36Mops did not differ significantly (p > 0.05) under SIF environment. The viable count was decreased to approximately 6 logs CFU/ml from the initial count (approx. 8–9 logs CFU/ml) in all, except in L. delbrueckii P31Mcs that showed viable count of 5.24 log CFU/ml. Thus, the probiotic strains survived the gastrointestinal (GI) transit, which is a prerequisite for colonization to the host epithelial cells for providing health benefits.
Probiotics exhibited properties of hydrophobicity, auto-aggregation and biofilm formation
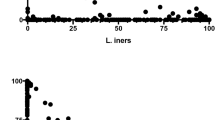
All the potential probiotics, after 5 h of incubation with the xylene, showed moderate hydrophobicity (35.88–56.70%). L. gasseri P36Mops showed highest (56.70% ± 1.63) hydrophobicity (Table 5), followed by L. delbrueckii P31Mcs (55.92% ± 2.41), E. faecium P15Mcm (39.69% ± 2.83) and L. fermentum P37Mws (35.88% ± 1.97). There was no significant difference (P > 0.05) in the hydrophobicity between L. gasseri P36Mops and L. delbrueckii P31Mcs. The tested probiotics showed highest auto-aggregation with E. faecium P15Mcm (61.39% ± 1.49; p < 0.0001), followed by L. fermentum P37Mws (52.20% ± 0.93; p < 0.0001), L. delbrueckii P31Mcs (43.12% ± 2.27; p < 0.0001) and L. gasseri P36Mops (35.26% ± 2.03; p < 0.0001) (Table 5). All the four potential probiotic strains showed higher optical density (ODT) at 570 nm that was 4 times greater (4ODc < ODT; p < 0.0001), than the OD of control media (ODc = 0.321 ± 0.04). Based on biofilm production, the strains were divided into non- (ODT ≤ ODc); weak (ODc < ODT ≤ 4ODc); and strong (4ODc < ODT) producers, where ODc is OD of control media (Terraf et al. 2012). It was noted that L. fermentum P37Mws produced more biofilm, (3.25 ± 0.15; p < 0.0001), which was followed by E. faecium P15Mcm (2.57 ± 0.43; p < 0.0001), L. delbrueckii P31Mcs (2.40 ± 0.41; p < 0.0001) and L. gasseri P36Mops (1.41 ± 0.35; p < 0.0001) (Fig. 1). Thus, the selected strains exhibited probiotic properties of hydrophobicity, auto aggregation and biofilm production.
Probiotics exhibited property of biofilm formation. The figure shows biofilm property of the potential probiotics. The data have been presented as mean ± SD of three independent experiments, each performed in triplicates. ****p < 0.0001 indicates statistically significant differences compared to the media control
Probiotics exhibited resistance toward standard antibiotics
The potential probiotics were evaluated for their resistance toward 16 different antibiotics (carbencillin, cefoxitin, clindamycin, chloramphenicol, erythromycin, metronidazole, penicillin G, tetracycline, ampicillin, norfloxacin, nitrofurantoin, nalidixic acid, gentamicin, cotrimoxazole, cefalotin and cefotaxime), mostly prescribed for bacterial vaginosis or urogenital infections. L. gasseri exhibited resistance toward five antibiotics that included nalidixic acid, norfloxacin, nitrofurantoin, metronidazole and cotrimoxazole (Supplementary Table S7). L. fermentum P37Mws showed resistance to four antibiotics namely, cefoxitin, metronidazole, nalidixic acid and cotrimoxazole. E. faecium P15Mcm exhibited resistance to four antibiotics (carbencillin, metronidazole, nalidixic acid and cotrimoxazole) and L. delbrueckii showed resistance toward two antibiotics (nalidixic acid and cotrimoxazole).
Discussion
In the present study, the microbiota isolated from the vaginal swabs of the healthy women was shown to exhibit probiotic properties. The microbiota also showed antimicrobial activity against pathogenic bacteria, isolated from the vaginal swabs of the precancerous (LSIL, HSIL) and ICC patients. Depletion of the beneficial vaginal Lactobacilli, with increased load of pathogenic bacteria, is one of the high-risk factors for cervical cancer progression (Adebamowo et al. 2017; Mitra et al. 2020). The vaginal microbiota of cervical cancer patients predominantly consist of different anaerobic (Ureaplasma parvum, Atopobium vaginae, Megasphaera, Prevotella, Gardnerella, Sneathia sanguinegens, Fusobacteria, Fastidiosipila and Dialister) and aerobic (S. epidermidis, E. faecalis, E. coli) pathogens (Adebamowo et al. 2017; Caselli et al. 2020; Kalia et al. 2020; Mitra et al. 2020). These microbiota produce toxins and carcinogenic metabolites (nitrosamines) that trigger DNA oxidation and production of pro-inflammatory cytokines, which together aid in the progression of cervical cancer (Hatta et al. 2021; Mitra et al. 2020). Thus, a balanced vaginal microbiome would not only prevent vulvo-vaginal infections but would also regulate cancer development (Godoy-Vitorino et al. 2018; Chen et al. 2019).
The present data showed that out of 75 non-hemolytic isolates from the healthy group, only 13 strains survived the gastro-intestinal transit (GIT) with more than 75% survival, a prerequisite for the development of oral probiotics. For oral delivery, probiotics should (a) be non-pathogenic, (b) tolerate the unfavourable conditions (acidic pH and bile acid concentrations) of the gastrointestinal tract, and (c) reach the intestine in a viable state (Dinçer and Kıvanç 2021; Oh et al. 2018). Orally ingested probiotics have been reported to repopulate in the vagina by passing through the intestine, reaching the rectum, and ascending to the vagina (Reid et al. 2001; Morelli et al. 2004; Cribby et al. 2008; Bohbot and Cardot 2012). The tolerance of other vaginal probiotics such as L. rhamnosus (Pino et al. 2019; Pithva et al. 2014), L. helveticus and L. salivarius (Pino et al. 2019); L. gasseri and L. plantarum (Bouridane et al. 2016); and L. fermentum (Brandt et al. 2020) to simulated GIT conditions has been reported earlier. The acid-bile tolerance for L. gasseri (Oh et al. 2018) and L. fermentum (Archer and Halami 2015) with survival above 70–80% has been reported. Thus, the thirteen strains from healthy individuals exhibited probiotic properties by surviving the GIT.
Initially, MALDI-TOF MS analysis was used to identify the thirteen stains, among which L. gasseri was found to be the dominant strain, followed by L. fermentum, E. faecium, L. reuteri and L. delbrueckii. The species level identification of the LAB strains was further confirmed by 16S rRNA identification, with scores in the range of 1.7–2.0. The isolates from LSIL, HSIL and ICC vaginal swabs were either cocci or Gram negative rods and only few had Lactobacilli-like morphology. Among these isolates, three strains of E. faecalis exhibited alpha hemolytic activity whereas three strains of S. haemolyticus and two strains of E. coli exhibited beta hemolytic activity, thereby confirming their pathogenic nature. Alpha haemolytic activity by E. faecalis, isolated from patients with intestinal infection, has been reported before (Bello Gonzalez et al. 2017). Beta hemolysis has been reported for several clinical strains of E. coli (Navidinia et al. 2012; Navidinia 2014; Toval et al. 2014) and S. haemolyticus (Pinheiro et al. 2015), isolated from patients with urinary tract and blood infections, respectively.
The CFSs of all the thirteen strains inhibited the growth of the standard pathogen, P. aeruginosa MCC 2081. CFS of four isolates, L. fermentum P37Mws, L. gasseri P36Mops, L. delbrueckii P31Mcs and E. faecium P15Mcm exhibited varying degrees of antibacterial activity against pathogens isolated from the vaginal swabs of LSIL, HSIL and ICC patients. The vaginal probiotic L. fermentum, isolated from healthy Algerian women, was reported to exhibit antibacterial activity against the vaginal pathogens E. coli, Staphylococcus spp., Enterococcus spp., and Candida spp. (Ouarabi et al. 2019). CFS from Lactobacillus strain VLb3 showed antibacterial activity against the vaginal pathogen G. vaginalis ATCC14018 (Andreeva et al. 2016). Bacteriocin extracted from the vaginal probiotic Lactobacillus showed activity against the cervicovaginal pathogens Salmonella, Gardnerella, Chlamydia, Trichomonas and Neisseria, isolated from the patients (Dasari et al. 2014). The healthy vaginal microbiota is mostly dominated by Lactobacillus species and opportunistic pathogens (Kyrgiou et al. 2017). However, Lactobacillus dominant communities protect the host against genital infections through the production of antimicrobial compounds and short chain fatty acids (SCFA), which acidify the local microenvironment by keeping vaginal pH below 4.5 (Kyrgiou et al. 2017). Depletion of beneficial Lactobacillus increase the vaginal pH, thereby increasing its susceptibility to infection with diverse aerobic (Staphylococcus spp, Pseudomonas spp. E. coli, and E. faecalis) and anaerobic (Gardnerella spp., Atopobium spp., Eggerthella spp., Sneathia spp. and Prevotella spp.) bacteria. Such dysbiosis can modulate the immune responses and lead to pathogenesis of several diseases, including cervical cancer.
The CFS from L. gasseri P36Mops was found to be rich in antimicrobial compounds such as N-(1-deoxy-1-fructosyl)valine (amino acid and derivative) (Fuochi et al. 2019); alkaloids, homoarecoline (Machová et al. 2021) and hygroline (Cretton et al. 2021); 2-isopropyl-1,4-benzenediol (hydroquinone) (Jurica et al. 2017); long chain fatty alcohols, 1-acetoxy-2-hydroxy-16-heptadecen-4-one, avocadyne 4-acetate and avocadyne 2-acetate (Rodríguez-Sánchez et al. 2019); grandidentatin (cinnamate ester) (Tyśkiewicz et al. 2019); taraxinic acid glucosyl ester (sesquiterpene lactone) (Cartagena et al. 2008); and alkaloid, nigellicine (Mohammed et al. 2019). L. fermentum P37Mws CFS showed presence of methylarmepavine (benzylisoquinolines) with reported anti-Leishmanial and antibacterial activities (Do Nascimento et al. 2015); and antimicrobial compounds such as 6-hydroxypentadecanedioic acid (long-chain fatty acid) (Rocchetti et al. 2020), hygroline (Jurica et al. 2017), avocadyne 4-acetate, avocadyne 2-acetate and 1-acetoxy-2-hydroxy-16-heptadecen-4-one (Rodríguez-Sánchez et al. 2019). CFS from L. delbrueckii P31Mcs showed the presence of antimicrobial metabolites such as ( +)-O-methylarmepavine (Do Nascimento et al. 2015), 1-acetoxy-2-hydroxy-16-heptadecen-4-one, avocadyne 4-acetate, avocadyne 2-acetate (Rodríguez-Sánchez et al. 2019); and quinoline derivatives, 6-methylquinoline and quinaldine (Bawa et al. 2009; Jeon et al. 2009). E. faecium P15Mcm CFS had antimicrobial compounds such as alkaloids, ephedrine (Tulgar et al. 2018) and hordenine (Zhou et al. 2018); nepetalactam (tetrahydropyridine) (Aridoss et al. 2008); cuminaldehyde (benzaldehyde) (Wongkattiya et al. 2019); anethole (phenylpropanoid) (Esfandyari-Manesh et al. 2013); estragole (olefinic compound) (Song et al. 2016); 6-hydroxypentadecanedioic acid (Rocchetti et al. 2020); hygroline (Jurica et al. 2017); taraxinic acid glucosyl ester (Cartagena et al. 2008; Tyśkiewicz et al. 2019); oxane and tertiary allylic alcohol derivatives, avermectin A2b aglycone; and avermectin B2a aglycone (El-Saber Batiha et al. 2020). L. fermentum TcUESC01 having valine and benzeneacetic acid as metabolites, was reported to show antimicrobial activity against Streptococcus mutans UA159 (de Souza Rodrigues et al. 2020). Different strains of L. fermentum and L. gasseri, isolated from human (oral and vaginal) samples, showed antibacterial activity against P. aeruignosa (Fuochi 2016) and Legionella pneumophila. The metabolite hydroquinone has reported antibacterial activity against E. faecalis (Jurica et al. 2017). Different species of Lactobacillus producing metabolites such as 2,4-hexadienoic acid and hydroxypentadecanedioic acid, showed antimicrobial activity against Candida vini (Lipinska-Zubrycka et al. 2020). L. rhamnosus and L. salivarius, producing valine, acetate, ethanol, 2–3-butanediol, uridine, 3 hydroxyphenylacetate have shown antibacterial activity against L. pneumophila (Fuochi et al. 2019). L. plantarum, producing organic acids such as 1,2-benzenedicarboxylic, palmitic, oleic, pentadecanoic acid, inhibited the growth of E. coli (Kanjan and Hongpattarakere 2016). Lactiplantibacillus plantarum producing different metabolites showed antibacterial activity against S. aureus (Ray Mohapatra et al. 2022). The antibacterial metabolite, taraxinic acid, was present in all the four potential probiotics. Others such as 1-acetoxy-2-hydroxy-16-heptadecen-4-one, avocadyne 4-acetate and avocadyne 2-acetate were common in L. gasseri P36Mops, L. fermentum P37Mws and L. delbrueckii P31Mcs. Hygroline was present in L. gasseri P36Mops, L. fermentum P37Mws and E. faecium P15Mcm. The antibacterial metabolite, hydroxypentadecanedioic acid was present in CFS of L. fermentum P37Mws and E. faecium P15Mcm. These common metabolites belong to the class of sesquiterpene lactones, alkaloids, long chain fatty acids or alcohols. Since all the four potential probiotic strains belong to the LAB group, they can have few common metabolites, suggesting that these metabolites could be used as potential biomarkers for predicting the risk for development of cervico-vaginal infections and cervical cancer.
The selected potential probiotic strains showed moderate hydrophobicity (35.88–56.70%) toward xylene (apolar solvent), with L. delbrueckii P31Mcs (56.70%) showing highest hydrophobicity. Cell surface hydrophobicity and auto-aggregation properties correlate with adhesion of the probiotics to the epithelial cells for a longer time, a prerequisite for preventing colonization of pathogens at the epithelial surface (Krausova et al. 2019). Hydrophobicity of any probiotic is measured by evaluating their affinity to hydrocarbon solvent by microbial adhesion to the hydrocarbons (MATH). Hydrophobicity can be divided into low (< 33%), moderate (33–66%), or high (> 66%) (Fonseca et al. 2021). Bacteria with higher hydrophobicity can bind efficiently to the epithelial cells, thereby preventing the colonization of the pathogens (Krausova et al. 2019). Interestingly, E. faecium P15Mcm exhibited strong auto-aggregation and others showed moderate auto-aggregation. Different vaginal L. fermentum species have shown 60–80% auto-aggregation, whereas vaginal L. gasseri UBLG36 has shown 32.98% auto-aggregation (Ahire et al. 2021). The potential probiotic strains produced strong biofilm on the plastic surface of the 96-well microplate with higher production by L. fermentum P37Mws. Biofilm formation prevents the colonization of pathogenic bacteria and thus is an important property of the probiotic strains (Salas-Jara et al. 2016). Biofilm producing probiotics such as L. rhamnosus and L. reuteri have been successfully used in adjuvant treatment of bacterial vaginosis (Ventolini 2015). Biofilm formation has been reported from two strains of L. plantarum, LSC3 and LSC22 (Gheziel et al. 2019), L. delbrueckii HY5 and three strains of L. fermentum (RGM3, RCM11 and RCM13) (Aziz et al. 2019).
L. gasseri P36Mops, L. fermentum P37Mws and E. faecium P15Mcm showed resistance to most of the antibiotics such as norfloxacin, nitrofurantoin, metronidazole, nalidixic acid and cotrimoxazole. These antimicrobials are generally used for the treatment of BV (Bradshaw and Sobel 2016; Larsson et al. 2011; Schwebke and Desmond 2007) and urinary tract infections (Anger et al. 2019). Most of the vaginal Lactobacillus sp. have shown resistance toward norfloxacin, nitrofurantoin, nalidixic acid (Fonseca et al. 2021); co-trimoxazole (Salas-Jara et al. 2016) and metronidazole (Mastromarino et al. 2002; Pithva et al. 2014). Resistance to antibiotics is considered to be important for restoration of vaginal microbiota (Wiik et al. 2019). Decrease in Lactobacilli load favours the growth of HPV and microorganisms in bacterial vaginosis (Happel et al. 2020). A significant correlation has been reported between BV and cervical cancer (Muñoz et al. 2006). Thus, the antibiotic resistant probiotic strains, L. gasseri P36Mops, L. fermentum P37Mws, L. delbrueckii P31Mcs and E. faecium P15Mcm could be used to restore the normal cervico-vaginal microbiota during the vaginal infections or during cervical cancer.
Probiotics are live microorganisms, which confer health benefit to the host and commonly include Lactobacillus, Bifidobacterium, Saccharomyces, Streptococcus, Enterococcus, Escherichia, and Bacillus (FAO/WHO 2001; Han and Ren 2021). They work through different mechanisms by (1) production of organic acids (lactic acid and short chain fatty acids) that maintain vaginal pH below 4.5, and antimicrobial agents such as hydrogen peroxide, bacteriocins and peptides (Tachedjian et al. 2017); (2) increasing the mucosal viscosity in the vagina (Di Cerbo 2016); (3) stimulation of the immune system (Di Cerbo 2016); and (4) formation of biofilms at the epithelial layer, thus inhibiting colonization of pathogens (Di Cerbo 2016; Mitra et al. 2020). Long-term treatment with probiotics has been reported to reduce the recurrence of vaginal infections and help in the clearance of PAP-smear abnormalities and HPV in cervical cancer patients (Palma et al. 2018; Verhoeven et al. 2013). Probiotic supplementation in cervical cancer patients has been reported to reduce the radiotherapy associated side effects such as diarrhoea, vaginal dryness, itching and risk of vaginal infections (Linn et al. 2019). The identified potential probiotics could be used both in the prevention of cervical cancer risk in women as well as for the treatment of vaginal infections, in general or during cervical cancer. Moreover, the common metabolites present in the LAB from the healthy group could be used to predict the risk for development of cervico-vaginal infections or cervical cancer.
Conclusion
The present study indicated that healthy vaginal ecosystem is an excellent source of Lactobacillus and Enteroccocus spp. with promising probiotic characteristics. These probiotics hold a great potential in managing the vaginal infections and cervical cancer-associated bacterial infections. However, extensive studies are warranted to identify the most beneficial and safe probiotic strains for maintaining the overall vaginal health.
Data availability
The data generated in this study have been included in the article and in the Supplementary information files.
References
Adebamowo SN, Ma B, Zella D, Famooto A, Ravel J, Adebamowo C, ACCME Research Group (2017) Mycoplasma hominis and Mycoplasma genitalium in the vaginal microbiota and persistent high-risk human papillomavirus infection. Front Public Health. 5:140. https://doi.org/10.3389/fpubh.2017.00140
Ahire JJ, Sahoo S, Kashikar MS, Heerekar A, Lakshmi SG, Madempudi RS (2021) In vitro assessment of Lactobacillus crispatus UBLCp01, Lactobacillus gasseri UBLG36, and Lactobacillus johnsonii UBLJ01 as a potential vaginal probiotic candidate. Probiot Antimicrob Proteins 20:1–2. https://doi.org/10.1007/s12602-021-09838-9
Andreeva P, Shterev A, Danova S (2016) Antimicrobial activity of vaginal lactobacilli against Gardnerella vaginalis and pathogens. Int J Adv Res Biol Sci 3:200–207. SOI: http://s-o-i.org/1.15/ijarbs-2016-3-5-29
Anger J, Lee U, Ackerman AL, Chou R, Chughtai B, Clemens JQ, Hickling D et al (2019) Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol 202:282–289. https://doi.org/10.1097/JU.0000000000000296
Aphale S, Pandita S, Raina P, Mishra JN, Kaul-Ghanekar R (2018) Phytochemical standardization of panchavalkala: An ayurvedic formulation and evaluation of its anticancer activity in cervical cancer cell lines. Phcog Mag 14:554–560. https://doi.org/10.4103/pm.pm_252_18
Archer AC, Halami PM (2015) Probiotic attributes of Lactobacillus fermentum isolated from human feces and dairy products. Appl Microbiol Biotechnol 99:8113–8123. https://doi.org/10.1080/14712598.2021.1828858
Aridoss G, Amirthaganesan S, Kumar NA, Kim JT, Lim KT, Kabilan S, Jeong YT (2008) A facile synthesis, antibacterial, and antitubercular studies of some piperidin-4-one and tetrahydropyridine derivatives. Bioorg Med Chem Lett 18:6542–6548. https://doi.org/10.1016/j.bmcl.2008.10.045
Azat R, Liu Y, Li W, Kayir A, Lin DB, Zhou WW, Zheng XD (2016) Probiotic properties of lactic acid bacteria isolated from traditionally fermented Xinjiang cheese. J Zhejiang Univ Sci B 17:597–609. https://doi.org/10.1631/jzus.B1500250
Azcarate-Peril MA, Altermann E, Goh YJ, Tallon R, Sanozky-Dawes RB, Pfeiler EA et al (2008) Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Appl Environ Microbiol 74:4610–4625. https://doi.org/10.1128/AEM.00054-08
Aziz K, Tariq M, Zaidi A (2019) Biofilm development in L. fermentum under shear flow & sequential GIT digestion. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnz064
Bawa S, Kumar S, Drabu S, Kumar R (2009) Synthesis and antimicrobial activity of 2-chloro-6-methylquinoline hydrazone derivatives. J Pharm Bioall Sci 1(1):27. https://doi.org/10.4103/0975-7406.62683
Bayer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 45:493–496. https://doi.org/10.1093/ajcp/45.4_ts.493
Bello Gonzalez TdJ, Pham P, Top J, Willems RJL, van Schaik W, van Passel MWJ, Smidt H (2017) Characterization of Enterococcus isolates colonizing the intestinal tract of intensive care unit patients receiving selective digestive decontamination. Front Microbiol 8:1596. https://doi.org/10.3389/fmicb.2017.01596
Biswal BM, Singh KK, Ismail MB, Jalal MI, Safruddin EI (2014) Current concept of bacterial vaginosis in cervical cancer. J Clin Gynecol Obstet 3:1–7. https://doi.org/10.14740/jcgo175w
Bohbot JM, Cardot JM (2012) Vaginal impact of the oral administration of total freeze-dried culture of LCR 35 in healthy women. Infect Dis Obstet Gynecol 2012:503648. https://doi.org/10.1155/2012/503648
Bouridane H, Sifour M, Idoui T, Annick L, Thonard P (2016) Technological and probiotic traits of the Lactobacilli isolated from vaginal tract of the healthy women for probiotic use. Iran J Biotechnol 14:192–201. https://doi.org/10.15171/ijb.1432
Bradshaw CS, Sobel JD (2016). Current treatment of bacterial vaginosis limitations and need for innovation. J Infect Dis 214(suppl_1):S14–S20. https://doi.org/10.1093/infdis/jiw159
Brandt K, Nethery MA, O’Flaherty S, Barrangou R (2020) Genomic characterization of Lactobacillus fermentum DSM 20052. BMC Genom 21:328. https://doi.org/10.1186/s12864-020-6740-8
Cartagena E, Montanaro S, Bardón A (2008) Improvement of the antibacterial activity of sesquiterpene lactones. Rev Latinoam Química 36:43–50
Caselli E, D’Accolti M, Santi E, Soffritti I, Conzadori S, Mazzacane S, Greco P et al (2020) Vaginal microbiota and cytokine microenvironment in HPV clearance/persistence in women surgically treated for cervical intraepithelial neoplasia: an observational prospective study. Front Cell Infect Microbiol 10:540900. https://doi.org/10.3389/fcimb.2020.540900
Chen Y, Hong Z, Wang W, Gu L, Gao H, Qiu L, Di W (2019) Association between the vaginal microbiome and high-risk human papillomavirus infection in pregnant Chinese women. BMC Infect Dis 19:677. https://doi.org/10.1186/s12879-019-4279-6
Cretton S, Genta-Jouve G, Kaiser M, Mäser P, Muñoz O, Bürgi T, Cuendet M et al (2021) Hygroline derivatives from Schizanthus tricolor and their anti-trypanosomatid and antiplasmodial activities. Phytochem 192:112957. https://doi.org/10.1016/j.phytochem.2021.112957
Cribby S, Taylor M, Reid G (2008) Vaginal microbiota and the use of probiotics. Interdiscip Perspect Infect Dis 2008:256490. https://doi.org/10.1155/2008/256490
Dai W, Du H, Li S, Wu R (2021) Cervicovaginal microbiome factors in clearance of Human Papillomavirus infection. Front Oncol 2021:2983. https://doi.org/10.3389/fonc.2021.722639
Dasari S, Shouri RN, Wudayagiri R, Valluru L (2014) Antimicrobial activity of Lactobacillus against microbial flora of cervicovaginal infections. Asian Pac J Trop Dis 4:18–24. https://doi.org/10.1016/S2222-1808(14)60307-8
de Souza Rodrigues JZ, Passos MR, de Macêdo Neres NS, Almeida RS, Pita LS, Santos IA, Silveira PH et al (2020) Antimicrobial activity of Lactobacillus fermentum TcUESC01 against Streptococcus mutans UA159. Microb Pathog 142:104063. https://doi.org/10.1016/j.micpath.2020.104063
Degu A, Njogu P, Weru I, Karimi P (2017) Assessment of drug therapy problems among patients with cervical cancer at Kenyatta National Hospital, Kenya. Gynecol Oncol Res Prac 4:1–5. https://doi.org/10.1186/s40661-017-0054-9
Di Cerbo A, Palmieri B, Aponte M, Morales-Medina JC, Iannitti T (2016) Mechanisms and therapeutic effectiveness of lactobacilli. J Clin Pathol 69:187–203. https://doi.org/10.1136/jclinpath-2015-202976
Dinçer E, Kıvanç M (2021) In vitro evaluation of probiotic potential of Enterococcus faecium strains isolated from Turkish pastırma. Arch Microbiol 203:2831–2841. https://doi.org/10.1007/s00203-021-02273-y
Do Nascimento RF, De Sales IR, de Oliveira FR, Barbosa-Filho JM, Sobral MV, Tavares JF, Diniz MD et al (2015) Activity of alkaloids on peptic ulcer: what’s new? Molecules 20:929–950. https://doi.org/10.3390/molecules20010929
Ehrmann MA, Kurzak P, Bauer J, Vogel RF (2002) Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J Appl Microbiol 92:966–975. https://doi.org/10.1046/j.1365-2672.2002.01608.x
El-Saber Batiha G, Alqahtani A, Ilesanmi OB, Saati AA, El-Mleeh A, Hetta HF, Magdy Beshbishy A. (2020) Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects. Pharmaceuticals 13(8):196.https://doi.org/10.3390/ph13080196
Esfandyari-Manesh M, Ghaedi Z, Asemi M, Khanavi M, Manayi A, Jamalifar H, Atyabi F et al (2013) Study of antimicrobial activity of anethole and carvone loaded PLGA nanoparticles. J Pharm Res 7:290–295.https://doi.org/10.1016/j.jopr.2013.04.019
FAO/WHO (2001) Report of a Joint FAO/WHO expert consultation on evaluation of health nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. World Health Organization and Food and Agriculture Organization of the United Nations, London, Ontario, Canada
Fonseca HC, de Sousa MD, Ramos CL, Dias DR, Schwan RF (2021) Probiotic properties of lactobacilli and their ability to inhibit the adhesion of enteropathogenic bacteria to Caco-2 and HT-29 cells. Probiot Antimicrob Proteins 13:102–112. https://doi.org/10.1007/s12602-020-09659-2
Fuochi V, Coniglio MA, Laghi L, Rescifina A, Caruso M, Stivala A, Furneri PM (2019) Metabolic characterization of supernatants produced by Lactobacillus spp. with in vitro anti-Legionella activity. Front. Microbiol. 10:1403. https://doi.org/10.3389/fmicb.2019.01403
Fuochi V (2016) Amensalistic activity of Lactobacillus spp., isolated from human samples. Catania: Università degli Studi di Catania
Gao Y, Shang Q, Li W, Guo W, Stojadinovic A, Mannion C, Man YG et al (2020) Antibiotics for cancer treatment: A double-edged sword. J Cancer 11:5135–5149. https://doi.org/10.7150/jca.47470
Gheziel C, Russo P, Arena MP, Spano G, Ouzari HI, Kheroua O, Saidi D (2019) Evaluating the probiotic potential of Lactobacillus plantarum strains from Algerian infant feces: towards the design of probiotic starter cultures tailored for developing countries. Probiot Antimicrob Proteins 11:113–123. https://doi.org/10.1007/s12602-018-9396-9
Godoy-Vitorino F, Romaguera J, Zhao C, Vargas-Robles D, Ortiz-Morales G, Vázquez-Sánchez F, Sanchez-Vázquez M et al (2018) Cervicovaginal fungi and bacteria associated with cervical intraepithelial neoplasia and high-risk human papillomavirus infections in a hispanic population. Front Microbiol 9:2533. https://doi.org/10.3389/fmicb.2018.02533
Han Y, Ren QL (2021) Does probiotics work for bacterial vaginosis and vulvovaginal candidiasis? Current Opin Pharmacol 61:83–90. https://doi.org/10.1016/j.coph.2021.09.004
Happel AU, Kullin B, Gamieldien H, Wentzel N, Zauchenberger CZ, Jaspan HB, Dabee S et al (2020) Exploring potential of vaginal Lactobacillus isolates from South African women for enhancing treatment for bacterial vaginosis. PLoS Pathog 16:e1008559. https://doi.org/10.1371/journal.ppat.1008559
Hatta MN, Mohamad Hanif EA, Chin SF, Neoh HM (2021) Pathogens and Carcinogenesis: A Review. Biology 10:533. https://doi.org/10.3390/biology10060533
Jeon JH, Lee CH, Lee HS (2009) Antimicrobial activities of 2-methyl-8-hydroxyquinoline and its derivatives against human intestinal bacteria. J Korean Soc Appl Biol Chem 52:202–205. https://doi.org/10.3839/jksabc.2009.037
Juárez Tomás MS, Wiese B, Nader-Macías ME (2005) Effects of culture conditions on the growth and auto-aggregation ability of vaginal Lactobacillus johnsonii CRL 1294. J Appl Microbiol 99:1383–1391. https://doi.org/10.1111/j.1365-2672.2005.02726.x
Jurica K, Gobin I, Kremer D, Čepo DV, Grubešić RJ, Karačonji IB, Kosalec I (2017) Arbutin and its metabolite hydroquinone as the main factors in the antimicrobial effect of strawberry tree (Arbutus unedo L.) leaves. J Herb Med 8:17–23. https://doi.org/10.1016/j.hermed.2017.03.006
Kalia N, Singh J, Kaur M (2020) Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann Clin Microbiol Antimicrob 19:1–19. https://doi.org/10.1186/s12941-020-0347-4
Kang CH, Han SH, Kim Y, Paek NS, So JS (2018) In vitro probiotic properties of Lactobacillus salivarius MG242 isolated from human vagina. Probiot Antimicrob Proteins 10:343–349. https://doi.org/10.1007/s12602-017-9323-5
Kanjan P, Hongpattarakere T (2016) Antibacterial metabolites secreted under glucose-limited environment of the mimicked proximal colon model by lactobacilli abundant in infant feces. Appl Microbiol Biotechnol 100:7651–7664. https://doi.org/10.1007/s00253-016-7606-5
Kietpeerakool C, Chumworathayi B, Thinkhamrop J, Ussahgij B, Lumbiganon P (2017) Antibiotics for infection prevention after excision of the cervical transformation zone. Cochrane Database Syst Rev 1:1–30. https://doi.org/10.1002/14651858.CD009957.pub2
Krausova G, Hyrslova I, Hynstova I (2019) In vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation 5:1–11. https://doi.org/10.3390/fermentation5040100
Kurli R, Chaudhari D, Pansare AN, Khairnar M, Shouche YS, Rahi P (2018) Cultivable microbial diversity associated with cellular phones. Front Microbiol 9:1229. https://doi.org/10.3389/fmicb.2018.01229
Kyrgiou M, Mitra A, Moscicki AB (2017) Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res 179:168–182. https://doi.org/10.1016/j.trsl.2016.07.004
Larsson PG, Brandsborg E, Forsum U, Pendharkar S, Andersen KK, Nasic S, Hammarström L et al (2011) Extended antimicrobial treatment of bacterial vaginosis combined with human lactobacilli to find the best treatment and minimize the risk of relapses. BMC Infect Dis 11:1–4. https://doi.org/10.1186/1471-2334-11-223
Linn YH, Thu KK, Win NH (2019) Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiot Antimicrob Proteins 11(2):638–647. https://doi.org/10.1007/s12602-018-9408-9
Lipinska-Zubrycka L, Klewicki R, Sojka M, Bonikowski R, Milczarek A, Klewicka E (2020) Anticandidal activity of Lactobacillus spp. in the presence of galactosyl polyols. Microbiol. Res 240:126540. https://doi.org/10.1016/j.micres.2020.126540
Machová M, Bajer T, Šilha D, Ventura K, Bajerová P (2021) Volatiles composition and antimicrobial activities of areca nut extracts obtained by simultaneous distillation–extraction and headspace solid-phase microextraction. Molecules 26:7422–7433. https://doi.org/10.3390/molecules26247422
Mastromarino P, Brigidi P, Macchia S, Maggi L, Pirovano F, Trinchieri V, Conte U et al (2002) Characterization and selection of vaginal Lactobacillus strains for the preparation of vaginal tablets. J Appl Microbiol 93:884–893. https://doi.org/10.1046/j.1365-2672.2002.01759.x
Mejía-Caballero A, Salas-Villagrán VA, Jiménez-Serna A, Farrés A (2021) Challenges in the production and use of probiotics as therapeuticals in cancer treatment or prevention. J Ind Microbiol Biotechnol. https://doi.org/10.1093/jimb/kuab052
Mitra A, MacIntyre DA, Ntritsos G, Smith A, Tsilidis KK, Marchesi JR, Bennett PR et al (2020) The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat Commun 11:1–3. https://doi.org/10.1038/s41467-020-15856-y
Mohammed SJ, Amin HH, Aziz SB, Sha AM, Hassan S, Abdul Aziz JM, Rahman HS (2019) Structural characterization, antimicrobial activity, and in vitro cytotoxicity effect of black seed oil. Evid Based Complement Alternat Med 2019:1–9. https://doi.org/10.1155/2019/6515671
Morandi S, Cremonesi P, Povolo M, Brasca M (2012) Enterococcus lactis sp. nov., from Italian raw milk cheeses. Int J Syst Evol Microbiol 62:1992–1996. https://doi.org/10.1099/ijs.0.030825-0
Morelli L, Zonenenschain D, Del Piano M, Cognein P (2004) Utilization of the intestinal tract as a delivery system for urogenital probiotics. J Clin Gastroenterol 38:S107–S110. https://doi.org/10.1097/01.mcg.0000128938.32835.98
Mubarak M, Kazi JI (2014) Acute renal failure in a young female with vaginal bleeding with partial recovery. J Nephropathol 3:45–47. https://doi.org/10.12860/jnp.2014.10
Mulu W, Yimer M, Zenebe Y, Abera B (2015) Common causes of vaginal infections and antibiotic susceptibility of aerobic bacterial isolates in women of reproductive age attending at Felegehiwot Referral Hospital, Ethiopia: a cross sectional study. BMC Womens Health 15:1–9. https://doi.org/10.1186/s12905-015-0197-y
Muñoz N, Castellsagué X, de González AB, Gissmann L (2006) HPV in the etiology of human cancer. Vaccine 24:S1. https://doi.org/10.1016/j.vaccine.2006.05.115
Navidinia M, Karimi A, Rahbar M, Fallah F, Ahsani RR, Malekan MA, Jahromi MH et al (2012) Study prevalence of verotoxigenic E. coli isolated from urinary tract infections (UTIs) in an Iranian children hospital. Open Microbiol J 6:1–4. https://doi.org/10.2174/1874285801206010001
Navidinia M, Peerayeh SN, Fallah F, Bakhshi B, Sajadinia RS (2014) Phylogenetic grouping and pathotypic comparison of urine and fecal Escherichia coli isolates from children with urinary tract infection. Brazilian J Microbiol 45:509–514. https://doi.org/10.1590/s1517-83822014000200019
Oh NS, Joung JY, Lee JY, Kim Y (2018) Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 14(13):e0192021. https://doi.org/10.1371/journal.pone.0192021
Ouarabi L, Chait YA, Seddik HA, Drider D, Bendali F (2019) Newly isolated lactobacilli strains from Algerian human vaginal microbiota: Lactobacillus fermentum strains relevant probiotic’s candidates. Probiot Antimicrob Proteins 11:43–54. https://doi.org/10.1007/s12602-017-9360-0
Palma E, Recine N, Domenici L, Giorgini M, Pierangeli A, Panici PB (2018) Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: A promising solution against HPV-infection. BMC Infect Dis 18:13. https://doi.org/10.1186/s12879-017-2938-z
Petrina MA, Cosentino LA, Rabe LK, Hillier SL (2017) Susceptibility of bacterial vaginosis (BV)-associated bacteria to secnidazole compared to metronidazole, tinidazole and clindamycin. Anaerobe 47:115–119. https://doi.org/10.1016/j.anaerobe.2017.05.005
Pinheiro L, Brito CI, De Oliveira A, Martins PY, Pereira VC, Da Cunha MD (2015) Staphylococcus epidermidis and Staphylococcus haemolyticus: molecular detection of cytotoxin and enterotoxin genes. Toxins 7(9):3688–3699. https://doi.org/10.3390/toxins7093688
Pino A, Bartolo E, Caggia C, Cianci A, Randazzo CL (2019) Detection of vaginal lactobacilli as probiotic candidates. Sci Rep 9:3355. https://doi.org/10.1038/s41598-019-40304-3
Pithva S, Shekh S, Dave J, Vyas BR (2014) Probiotic attributes of autochthonous Lactobacillus rhamnosus strains of human origin. Appl Biochem Biotech 173:259–277. https://doi.org/10.1007/s12010-014-0839-9
Ray Mohapatra A, Harikrishnan A, Lakshmanan D, Jeevaratnam K (2022) Targeting Staphylococcus aureus and its biofilms with novel antibacterial compounds produced by Lactiplantibacillus plantarum SJ33. Arch Microbiol 204:1–5. https://doi.org/10.1007/s00203-021-02630-x
Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B (2001) Oral probiotics can resolve urogenital infections. FEMS Immunol Medical Microbiol 30:49–52. https://doi.org/10.1111/j.1574-695X.2001.tb01549.x
Reuben RC, Roy PC, Sarkar SL, Alam RU, Jahid IK (2019) Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol 19:1–20. https://doi.org/10.1186/s12866-019-1626-0
Rocchetti G, Bernardo L, Pateiro M, Barba FJ, Munekata PE, Trevisan M, Lorenzo JM et al (2020) Impact of a pitanga leaf extract to prevent lipid oxidation processes during shelf life of packaged pork burgers: An untargeted metabolomic approach. Foods 9:1668. https://doi.org/10.3390/foods9111668
Rodríguez-Sánchez DG, Pacheco A, Villarreal-Lara R, Ramos-González MR, Ramos-Parra PA, Granados-Principal S, Díaz de la Garza RI et al (2019) Chemical profile and safety assessment of a food-grade acetogenin-enriched antimicrobial extract from avocado seed. Molecules 24:2354. https://doi.org/10.3390/molecules24132354
Salas-Jara MJ, Ilabaca A, Vega M, García A (2016) Biofilm forming Lactobacillus: new challenges for the development of probiotics. Microorganisms 4:1–14. https://doi.org/10.3390/microorganisms4030035
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R et al (2020) NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database. https://doi.org/10.1093/database/baaa062
Schwebke JR, Desmond R (2007) A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am J Obstet Gynecol 196:517–516. https://doi.org/10.1016/j.ajog.2007.02.048
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
Song YR, Choi MS, Choi GW, Park IK, Oh CS (2016) Antibacterial activity of cinnamaldehyde and estragole extracted from plant essential oils against Pseudomonas syringae pv. actinidiae causing bacterial canker disease in kiwifruit. Plant Pathol J 32:363–370. https://doi.org/10.5423/PPJ.NT.01.2016.0006
Tachedjian G, Aldunate M, Bradshaw CS, Cone RA (2017) The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol 168:782–792. https://doi.org/10.1016/j.resmic.2017.04.001
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Terraf ML, Juárez Tomás MS, Nader-Macías ME, Silva C (2012) Screening of biofilm formation by beneficial vaginal lactobacilli and influence of culture media components. J Appl Microbiol 113:1517–1529. https://doi.org/10.1111/j.1365-2672.2012.05429.x
Thulkar J, Kriplani A, Agarwal N (2012) A comparative study of oral single dose of metronidazole, tinidazole, secnidazole and ornidazole in bacterial vaginosis. Indian J Pharmacol 44:243–245. https://doi.org/10.4103/0253-7613.93859
Toval F, Köhler CD, Vogel U, Wagenlehner F, Mellmann A, Fruth A, Schmidt MA et al (2014) Characterization of Escherichia coli isolates from hospital inpatients or outpatients with urinary tract infection. J Clin Microbiol 52:407–418. https://doi.org/10.1128/JCM.02069-13
Tulgar S, Alasehir EA, Selvi O (2018) The antimicrobial activity of ephedrine and admixture of ephedrine and propofol: an in vitro study. Braz J Anesthesiol 68(1):69–74. https://doi.org/10.1016/j.bjan.2017.08.001
Turovskiy Y, Ludescher RD, Aroutcheva AA, Faro S, Chikindas ML (2009) Lactocin 160, a bacteriocin produced by vaginal Lactobacillus rhamnosus, targets cytoplasmic membranes of the vaginal pathogen, Gardnerella vaginalis. Probiot Antimicrob Proteins 1:67–74. https://doi.org/10.1007/s12602-008-9003-6
Tyśkiewicz K, Konkol M, Kowalski R, Rój E, Warmiński K, Krzyżaniak M, Gil Ł et al (2019) Characterization of bioactive compounds in the biomass of black locust, poplar and willow. Trees 33:1235–1263. https://doi.org/10.1007/s00468-019-01837-2
Ventolini G (2015) Vaginal Lactobacillus: biofilm formation in vivo–clinical implications. Int J Womens Health 7:243–247. https://doi.org/10.2147/IJWH.S77956
Verhoeven V, Renard N, Makar A, Royen PV, Bogers J-P, Lardon F et al (2013) Probiotics enhance the clearance of human papillomavirus-related cervical lesions: A prospective controlled pilot study. Eur J Cancer Prev 22:46–51. https://doi.org/10.1097/CEJ.0b013e328355ed23
Wiik J, Sengpiel V, Kyrgiou M, Nilsson S, Mitra A, Tanbo T, Jonassen CM (2019) Cervical microbiota in women with cervical intra-epithelial neoplasia, prior to and after local excisional treatment, a Norwegian Cohort study. BMC Womens Health 19:1–9. https://doi.org/10.1186/s12905-019-0727-0
Wongkattiya N, Sanguansermsri P, Fraser IH, Sanguansermsri D (2019) Antibacterial activity of cuminaldehyde on food-borne pathogens, the bioactive component of essential oil from Cuminum cyminum L. collected in Thailand. J Complem Integr Med 16:20180195. https://doi.org/10.1515/jcim-2018-0195
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. https://doi.org/10.1099/ijsem.0.001755
Zheng J, Wittouck S, Salvetti E, Franz CM, Harris H, Mattarelli P et al (2020) A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 70:2782–2858. https://doi.org/10.1099/ijsem.0.004107
Zhou JW, Luo HZ, Jiang H, Jian TK, Chen ZQ, Jia AQ (2018) Hordenine: a novel quorum sensing inhibitor and antibiofilm agent against Pseudomonas aeruginosa. J Agric Food Chem 66:1620–1628. https://doi.org/10.1021/acs.jafc.7b05035
Acknowledgements
The authors would like to thank Director, IRSHA, Dr. A. C. Mishra for constant support and encouragement.
Funding
This study was funded by the Department of Science and Technology Women Scientist Scheme-A (DST WOS-A), Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical standards
The study was conducted according to the guidelines of the Helsinki Declaration, and all the procedures involving human patients were approved by the Institutional Ethics Committee (IEC)s of Bharati Vidyapeeth (Deemed to be) University Medical College (Ref: BVDU/MC/57) and B. J. Government Medical College Sassoon General Hospitals (Ref No. BJGMC/IEC/Pharmac/ND-Dept 0119007–007], Pune.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamble, A., Naik, S., Talathi, M. et al. Cervicovaginal microbiota isolated from healthy women exhibit probiotic properties and antimicrobial activity against pathogens isolated from cervical cancer patients. Arch Microbiol 204, 491 (2022). https://doi.org/10.1007/s00203-022-03103-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03103-5