Abstract
In this study, a successfully rapid, simple approach was applied for biosynthesis of silver nanoparticles AgNPs using for the first time the mixed leaves extract of Olea europaea subsp. europaea var. sylvestris and Pistacia lentiscus from natural association aimed to enhance their antimicrobial potential. The plant extract acts both as reducing and capping agents. When the aqueous extract was added to AgNO3 solution, the color was changed from pale to yellow to brown indicating the reduction of Ag ions and synthesis of silver nanoparticles (AgNPs) without any solvent or hazardous reagents. The green synthesized AgNPs were characterized by UV–Vis spectrophotometer, FTIR spectrum and the X-ray crystallography. The AgNPs showed superior antioxidant activity measured by DPPH, Ferric Antioxidant Reducing Power (FRAP) as well as the total antioxidant activity methods. Moreover, the analysis of phytochemical constituents including flavonoids, tannins, alkaloids and total polyphenols contents mentioned the most richness of the silver nanoparticles compared to plant extract. The new synthesized AgNPs demonstrated the bactericidal and fungicidal effects against all the tested bacterial and fungal strains and found to limit the spore germination of filamentous fungi. AgNPs also gave an anti-biofilm activity and synergistic effect with the conventional antibiotic’s drugs. Here we firstly describe the silver nanoparticles effect on virulence factors of Candida species by reduction of enzymes like proteinase and phospholipase, inhibition of morphogenesis of Candida albicans cells. This natural product, acquiring these properties, should be promoted to be used in pharmaceutical and medical industries in future.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal nanoparticles are of great importance in various industrial/scientific applications. Principally, the silver nanoparticles hold enormous antimicrobial potential against the drug resistant clinical bacterial and fungal pathogens (Jyoti et al. 2016; Bharti et al. 2021). Various chemical and physical methods were developed to synthesize silver nanoparticles, but the adsorption of toxic chemicals on the nanoparticles could be the adverse consequence on its applications. Hence, there is a crucial need to generate the alternative green non-toxic approach for the synthesis of Ag NPs (Gomathi et al. 2017). Green synthesis of nanoparticles has been proven to be significant in terms of their eco-friendly nature, rapid and consistent production by single step process providing more stability (Mittal et al. 2014). This method is undoubtedly more competent and simpler for the synthesis of Ag NPs than the traditional chemical and physical methods. Silver nanoparticles disrupt the multiple bacterial cellular processes, including the disulfide bond formation, metabolic and ion homeostasis. These changes increase the formation of reactive oxygen species and membrane permeability of gram-negative bacteria particularly. Hence, it contributes to enhance the activity of broad-spectrum antibiotics, restoring the antibiotic susceptibility among the antibiotic resistant strains (Morones Ramirez et al. 2013).
Nowadays, biotechnology industry targets for new natural antimicrobial drugs. Owing to the antibacterial activity of silver nanoparticles via green synthesis, the plant extracts also contain other chemical constituents such as polyphenols, reducing sugars, ascorbic acids etc., which are responsible for the bio-reduction of metal ions, and also act as capping ligands to stabilize the interface between nanoparticles and interacting medium (Ahmad et al. 2016; Gomathi et al. 2017).
Tunisia is a rich source of natural medicinal plant species such as Olea europaea L. (Oleaceae) and Pistacia Lentiscus L. (Anacardiaceae). In addition, the diverse natural associations among the wild olive trees or oleaster, Olea europaea subspecies europaea var. sylvestris, forests with pistachio, Pistacia lentiscus, build a frequently spreading in natural ecosystem. Therefore, this ecosystem reflects the natural association. The wild olive trees are native in Tunisia, scientifically evidenced by nuclear and cytoplasmic molecular markers in the previous reports (Camps-Fabrer 1997, 1953; Hannachi et al. 2008, 2010). Pistacia lentiscus L. is evergreen shrub widespread in Mediterranean forests (Gardeli et al. 2018).
This study described the enhancement of the antimicrobial and antioxidant activities of AgNPs synthesized by using mixed aqueous extract from oleaster (Olea europaea subsp. europaea var. sylvestris) and pistachio (Pistacia lentiscus) leaves.
In this context, we mainly focused on the use of oleaster and pistachio leaves as natural source of biomolecules and surface reducers of nanoparticles based on their richness on secondary metabolites as phenolic compounds. We have observed the antibacterial, antifungal and their synergistic activities of the synthesized silver nanoparticles help contribute in the reduction of yeast virulence factors, biofilm and spore formation.
Materials and methods
Preparation of the extract
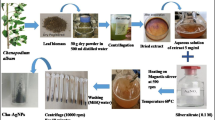
Oleaster (Olea europaea subsp. europaea var. sylvestris) and Pistachio (Pistacia lentiscus) leaves were freshly collected form natural association from Tunisia northern forest. The collected leaves were surface cleaned with running tap water to remove soil and other contaminated organic contents, followed by double distilled water and were air dried at room temperature. A simple approach was applied based on the described method of Dridi et al. 2022. Briefly, 20 g of the leaves (10 g from each specie) were cut into small pieces and then boiled with 100 mL distilled water for 20 min. The obtained extract after filtration was stored at 4 °C for the further use including the analysis of its major chemical constituents.
Synthesis of nanoparticles
Aqueous solution (5 mM) of silver nitrate was prepared. The plant extract (10 mL) was added drop by drop to 20 mL of silver nitrate solution (5 mM). The reaction mixture of AgNO3 and leaf extract was stirred for 2 min. The color changed from yellow to reddish brown color indicating the formation of silver nanoparticles. Then, the AgNPs obtained was purified by repeated centrifugation at 10,000 rpm for 10 min. The pellet was collected and dried (Dridi et al. 2022).
Characterization of synthesized silver nanoparticles
The reduction of pure silver ions was confirmed by measuring the UV–vis spectrum of the reaction mixture against distilled water as a blank. The Spectrum analysis was done using a 2802 UV/Vis spectrophotometer (UNICO) in the 250–700 nm region (Vidhu et al. 2011). The Fourier Transform-Infrared Spectroscopy (FTIR) spectrum was recorded in the range 400–4000 cm−1 on a Varian FTIR 640 spectrophotometer with KBr pellets. The X-ray powder diffraction (XRD) measurements was performed on a D8 ADVANCE BRUKER diffractometer using Cu-Kα radiations and equipped with Lynxeye accelerator (Ghiuta et al. 2018; Luo et al. 2018).
Antioxidant activities
DPPH radical scavenging activity
The DPPH (2,2-Diphenyl-1-picryl-hydrazyl) free radical scavenging activity was estimated by colorimetric method. One mL of sample was added to 2 mL of 1,1-diphenyl-2-picrylhydrazyl methanolic solution (1:2). The mixture was incubated for 30 min in dark after shaking. The presence of an antioxidant donator of hydrogen reduced the DPPH. Radical on 2,2-diphényl-1-picrylhydrazine (DPPH-H) reflected by the color change. The absorbance was measured at 517 nm. The scavenging activity was expressed as percentage of inhibition (PI) following this formula: PI = ((Ac – As)/As) × 100 (Chan et al. 2007) where Ac is the absorbance of the control and As is the absorbance of the sample.
Total antioxidant activity (TAA)
The mixture reaction containing 0.2 mL of sulfuric acid, sodium phosphate (H2SO4, 0.6 M NaHPO4, H2O 28 mL) and ammonium heptamolybdate ((NH4)6Mo7O240.4H2O4mM) at acidic pH was prepared. Then, 0.3 mL of sample was added. The reaction was placed in boiled water at 95 °C for 90 min. The reduction of Mo was accompanied by green color. The absorbance was measured at 695 nm. The total antioxidant activity was expressed as mg of gallic acid equivalent per g of dry matter (DM) (mg GAE/g DM) (Pisoschi and Negulescu 2011).
Ferric reducing antioxidant power (FRAP)
The mixture reaction containing 100 µL of the sample solution (silver nanoparticles or plant extract) and 3 mL of FRAP reagent were incubated at room temperature in dark for 10 min. The FRAP method relies on the reduction by the antioxidants of the complex ferric ion-TPTZ (2,4,6-tri(2-pyridyl)1,3,5-triazine) accompanied by the apparition of blue color. The absorbance was measured at 593 nm (Analytik Jena) spectrophotometer. The FRAP value was expressed as mg Ascorbic Acid Equivalent Antioxidant Capacity (AEAC) per g DM (Athavale et al.2012).
Phytochemical characterization of EA and AgNPs
Total polyphenols content
Total polyphenols content in the sample solution was estimated using the method of Folin–Ciocalteu method as described by Singleton and Rossi (1965) and the results were expressed as mg of gallic acid equivalent (GAE) per mL (mg GAE/mL of extract) based on a gallic acid calibration curve.
Total flavonoids content
The flavonoids content was estimated by using aluminum chloride colorimetric technique at 765 nm. Results were expressed as mg quercetin equivalents per mL of extract based on a quercetin calibration curve (Harris and Ray 1935).
Tannins content
The tannins content was determined by the vanillin method in acid medium. Tannic acid was served as a standard and tannin content was expressed as mg tannic acid equivalent per mL of extract. The absorbance was measured at 760 nm (Rodríguez-Romero et al. 2021; Schanderi 1970).
Total alkaloids content
Total alkaloids content was estimated after extraction with glacial acetic acid and ethanol and precipitated with Draggendroff’s reagent. The residue treated with sodium sulfite and thiourea solution. Atropine standard solution was prepared (Sigma chemical, USA) and optical density was measured at 435 nm (Sreevidya and Mehrotra 2003).
Antibacterial and antifungal potentialities of the silver nanoparticles
Well diffusion for antimicrobial activity detection: the bacterial and fungal clinical strains obtained from a Tunisian Clinical laboratory, were used in this study. The gram-negative bacteria taken as Klebsiella pneumoniae, Escherichia coli, and Enterobacter cloacae, while Staphylococcus aureus and Micrococcus luteus were the gram positives used. Fungi species are belonging to Candida albicans, Candida parapsilosis, Penicillium spp, and Aspergillus spp. Before use, the AgNPs was diluted in distilled water and adjusted to obtain the optimized concentration. The cell suspension (0.1 mL) adjusted to 107 CFU/mL for bacteria and 105spores /mL for fungi were transferred separately into the surface of agar plates and 40 μL of the tested AgNPs were aseptically pipetted into wells (6 mm). The plates were incubated at 37 °C. The observation of inhibition zone around the wells indicates the antimicrobial activity and the diameter of inhibition zone was measured in mm. Ceftazidime CAZ30 was used as positive control for gram negative bacteria and Vancomycin for gram positive bacteria, Amphotericin B and Fluconazole 25 were used as fungicide standards. All tests were performed in triplicate (Essghaier et al. 2014).
Minimum inhibitory concentration (MIC) determination
The MIC was determined by broth dilution method by conducting broth culture of pathogen strains in the presence of different concentrations of silver nanoparticles. The incubation was performed in Eppendorf tube containing 1 mL of Nutrient broth (NB) and 10 CFU/mL of the pathogen strains. The negative control tube contained the NB without AgNPs. High rotational speed of 200 rpm was maintained to avoid the aggregation of the nanoparticles. The absorption was measured at 600 nm, to depict bacterial and fungal growth, no increase in absorbance indicates the MIC (Bharti et al. 2021).
Minimal bactericidal concentration (MBC) and minimal fungicidal concentration (MFC) determinations
The MBC and MFC were determined by transferring an aliquot of 10 µL from the tube corresponding to MIC values on the surface of the appropriate agar plates and the inoculated plates were incubated at 37 °C for 24 h. The lowest concentration of AgNPs at which no visible colony was observed on the surface of the agar plate was reported as the MBC or MFC according to the modified method (Ruparelia et al. 2008). All assays were performed in quadruplicate.
Anti-biofilm activity
The probability effect on the biofilm formation by the bacterial species (Klebsiella pneumoniae, Escherichia coli, Enterobacter cloacae; Staphylococcus aureus; Micrococcus luteus) have been tested based on the method of Kim et al. (2017). The method of Gulati et al. (2018) already applied for Candida species (Candida albicans, Candida parapsilosis).
Spores germination inhibition
To evaluate the effect of the silver nanoparticles on spore germination of fungi species (Penicillium sp. and Aspergillus niger), the mixed reaction consisting of v/v of the AgNPs and the conidial suspensions (105spores/mL) mixed in Eppendorf tube containing 1 mL of 5% glucose as previously described (Sarangi et al. 2010).
Evaluation of AgNPs on the virulence factors of Candida species
Phospholipase detection
The phospholipase activity (Pz) was determined based on the method of Samaranayake et al. (2005). Briefly, the Egg yolk agar medium was used and a volume of 10 µL of yeast suspension adjusted at 107 CFU/mL, was deposit into the wells. Then the plates were incubated for 48 h at 37 °C. The diameter of colonies and the diameter of zone opacity were measured and the phospholipase activity (Pz) was calculated as follow:
Proteolytic activity
The bovine serum albumin medium was used, and the wells punched on the surface were inoculated by 10 µl of Candida suspension (at 107 CFU/mL), after incubation of 48 h at 37 °C, based on the modified method of Staib as detailed by Mohan Da and Ballal (2008). The Proteinase activity (Pz) was calculated as detailed above in phospholipase activity assay.
Morphogenesis change
The effect of the addition of AgNPs on Candida albicans morphogenesis, was examined by direct microscopic observation of 100µL of Candida albicans suspension from 1 mL culture in Sabouraud’s broth for 48 h at 37 °C in the presence of AgNPs (10µL of 250 µg/mL). The coloration with cotton blue was compared to control tube culture without the addition of AgNPs (Zhu et al.2006; Dridi et al. 2022).
Synergistic antibiotic effect of AgNPs with conventional antibiotics
In order to enhance the antibacterial effect of the synthesized AgNPs, we have tested their synergistic effect with conventional antibiotics by using the method of agar diffusion (Danielli et al. 2018).
Statistical analysis
All values are expressed as mean ± SEM. Comparisons between groups were performed using the generalized linear model (GLM) of the SAS statistical program The multiple comparison of means was performed by using Student Newman-Keuls SNK tests at a threshold of 5% (means with same letters are not significantly different).
Results
UV–Vis spectroscopy study
The formation of AgNPs can be visualized by the color change of the mixture which is turned from pale yellow to brown color (Fig. 1A). This color change in the reaction mixture strongly indicates the reduction of Ag+ ions to Ag0. UV–Vis spectroscopic analysis elucidates a single absorbance peak at 422 nm (Fig. 1B) indicating the formation of AgNPs.
FT-IR analysis
FT-IR analysis was performed to identify the bond linkages and functional groups of the active components in the obtained AgNPs based on the peaks and the values in the IR region. All the observed intense bands were compared with standard values to identify the functional groups. As shown in Fig. 2, the intense band of AgNPs at 3226 cm−1 denotes the presence of C-H of aromatic compounds as demonstrated by Zia et al. (2016). The absorption peak at 1633 cm−1 correspond to the C = C bending of alkynes (Dyah et al. 2017). Further, the peak emerged at 1322 cm−1 corresponds to O–H bending vibrations. Finally, the band at 1012 cm−1 can be assigned C-O stretching vibration indicated the presence of phenols and aliphatic amines (Mahiuddin et al. 2020) (Fig. 2A).
Structural studies
The crystalline nature of nanoparticles was confirmed by X-ray crystallography. The XRD pattern of the synthesized AgNPs is illustrated in Fig. 2B. The observed 2θ values at 37.01, 45.38, 64.43 and 78.62° corresponding to the (111), (200), (220) and (311) reflections, respectively, which indicated that spherical silver nanoparticles are crystalline in nature with face-centered cubic structure (fcc) (Ibrahim 2015; Dyah et al. 2017). The crystallite size was calculated using the Scherer’s formula that depends on the peak position and FWHM of the dominant reflection. Scherer’s equation is d = k λ / β cos θ where, d is the average crystallite size of the nanoparticles, k is the geometric factor equal to 0.9, λ is the wavelength of X-ray radiation source equal to 1.54 Å,, β is the angular FWHM (full-width at half maximum) of the XRD peak at the diffraction angle θ (Rajkumar et al. 2015). The calculated average crystallite of the AgNPs is 23 nm.
Antioxidant activities
Based on the obtained results, mixed leaves extract showed high total antioxidant activity (Fig. 3B), this may be due to their richness by various phytoconstituents from both studied plant species. The antioxidant activities using DPPH free radical scavenging, the total antioxidant activity and the Ferric Antioxidant Reducing Power mehtods showed that the AgNPs preserve an important amount of antioxidant activity (Fig. 3) which may be due to the stability of the antioxidant activity in nanomaterials.
Phytochemical characterization of plant extract AE and AgNPs
Results showed that the synthesized nanoparticles were richer in tannins, total polyphenols and flavonoids compared to the mixed leaves aqueous extracts (Table 1). The observed data can explain the enhancement of the antimicrobial properties of the AgNPs compared to the plant extract one.
Antibacterial and anti-candida screening
The synthesized silver nanoparticles (NP) showed an antibacterial activity against all tested gram positive and gram-negative clinical bacterial strains, unlike the aqueous extract which was unable to limit the growth of used bacteria strains (Fig. 4A). These results indicated that the increase of zone inhibition expressed in mm given by the silver nanoparticles compared to AgNO3 and the development of sensitivity/susceptibility of Escherichia coli strain which was resistant to Ag NO3 (Fig. 4A).
A Antibacterial activity of silver nanoparticles (AgNPs) against clinical bacteria strains compared to the antibiotic standard (Vancomycin and Ceftazidime), AgNO3 and Olea europaea var. Sylvestris and Pistacia lentiscus leaves aqueous extract. B Silver nanoparticles effects, on Candida species compared to fungicides (Fluconazole 25 and amphotericin B) and plant extracts. Values expressed diameters in mm. Bars represent Standard errors
The present results mentioned that the synthesized AgNPs (NP) gave more inhibitory activity against Candida species compared to AgNO3. The aqueous plant extract was unable to limit any Candida species. Our synthesized nanoparticles were more effective than the fungicide amphotericin B with 19.66 and 17.33 mm compared to 19 mm and 10 mm against Candida albicans and Candida parapsilosis, respectively by the AgNPs and Amphotericin B (Fig. 4B). The MIC values ranged from 31 to 500 µg/mL related to the tested pathogens strains. Based on calculated value of MBC/MIC ≤ 4, AgNPs showed bactericidal and fungicidal effects against all tested pathogens strains. In the present work, we have investigated the silver nanoparticles effect on filamentous fungi genus Penicillium sp. and Aspergillus niger, and the finding showed that only the synthesized silver nanoparticles can affect mycelium growth compared to the plant extract or AgNO3. Superior inhibitory activity was given against spores germination of Penicillium with 98.4%.
Anti-biofilm activity
The investigation of the synthesized AgNPs effect on biofilm formation elucidated that AgNPs used at the corresponding MIC value were qualified to limit biofilm formation of all tested gram positive and gram negative bacteria with value ranging from 32.74 to 83%, but they were more efficient on biofilm of Candida albicans strains with 87% and Candida parapsilosis with 63% (Table 2).
Silver nanoparticles on virulence factor of Candida strains
To illustrate the ability of the silver nanoparticles on factor virulence of yeast species, we reported the effect of AgNPs on hydrolytic enzymes production and yeast morphogenesis. As a result in Table 3, mentioned that, the enzymes hydrolase proteinase and phospholipase were higly reduced in Candida species growth additioned with silver nanoparticles. Morover, Fig. 5, illustrated that in vitro co-culture model of Candida strains, confirmed the superior inhibitory effect of the AgNPs used at MIC of 250 µg/mL, on Candida albicans morphogenesis compared to untreated Candida albicans culture, the observation indicate the absence of any morphogenesis key virulence change for biofilm resistance such as: germ tube, chlamydospore and filamentous hyphae, as well as the alteration of the scare Blastospore in the presence of AgNPs. For both Candida species, the results indicate that the addition of AgNPs in co-cultural model, affect hardly the number of Blastospore observed and the cell multiplication phenomena.
Antibiotic synergistic effect of AgNPs
According to the observed results, the synthesized nanoparticles booster the effect of the tested conventional antibiotic currently used or make bacterial strains to become susceptible against the regularly used antibiotics. For example, both bacteria strains were resistant to Nalidixic acid (NA) but they were sensitive when it was associated with AgNPs, and Tobramycin (TM) against Micrococcus luteus (Fig. 6).
Discussion
It is well known that the green synthesis of nanoparticle materials has attracted the attention of many studies due to their advancement over the chemical methods, as it is a single step process, cost effective and emerged as ecofriendly environment (Mittal et al. 2014). This report demonstrated for the first time the biosynthesis of AgNPs using aqueous extract from the equal mixture of Olea europaea subsp. europaea var. sylvestris and Pistacia lentiscus leaves. Here we proved that firstly, two Olea europaea subsp. europaea var. sylvestris and Pistacia lentiscus natural associated species can be used as natural sources to synthesize silver nanoparticles by enhancing their antimicrobial potential compared to other AgNPs synthesized from individually plant as reported previously in literature. For example as reported by the P lentiscus leaf extracts which possess moderate antimicrobial activities and scare works focused on the biosynthesis of silver nanomaterial from this specie (El-Chaghaby and Ahmad 2011).
Secondly, here the AgNPs synthesis were completely free from any chemical solvents and hazardous reagents similar to the green synthesis described previously by DeMatteis et al. (2019), based on the use of two cultivars (Leccino and Carolea) of Olea europaea growing in the same pedoclimatic conditions for green biosynthesis of silver nanoparticles with new properties as antibacterial potential against, and were able to induce toxicity in breast cancer cell lines (MCF-7 and Hela cells). In adition, here the mixed leaves extract enhance the biosynthesis of AgNPs, on one hand, the size of silver nanoparticles of 23 nm, were smaller than those obtained only by Pistachio leaves extract as reported by Ghadir et al. (2011) which showed that the AgNPs from Pistachia ethanol extract ranged from 24 to 26 nm. On the other hand, the mixed leaves from both plant species enhance the antibacterial potential as compared to those obtained by silver nanoparticles from Pistachio which not exceed 13 mm of zone inhibition agaisnt Escherchia coli and Staphylococcus aureus (Ghadir et al. 2011).
Currently used antibiotics and Ag NPs are shown to be effective against all the emerging drug resistant and biofilm producing microbes, by demonstrating the multiple antagonism mechanism as compared to the conventional antibiotics. In this work, we revealed a significant antibacterial and antifungal efficacy of AgNPs against all tested clinical strains. These findings may be attributed to its richness with secondary metabolites from its natural sources, such as polyphenol rich compounds are known to display antioxidant, antimicrobial and anti-inflammatory activities. In literature the anti-inflammatory and antibacterial effect against gram positive bacteria is due to the Olive component Oleuropein (Qabahe et al. 2018). It has been reported that the antioxidant activity was correlated with major phenolic compounds in olive leaves and with luteolin, naringin, epicatechin in Pistachio leaves (Sonmezdag et al. 2016; Qabahe et al. 2018).
The examination of the functional groups indicated the presence of the phenols, organics acids and aliphatic amines in the silver nanoparticles. Several literature, mentioned that the phenolic compounds owing the redox potential which make them as reducing agents and hydrogen donors (Irshad et al. 2012 and Benzie et al. 1999).
Furthermore, here the enhancement of AgNPs from the mixed leaves extract observed by the superior antibacterial activity by exhibiting maximum antibacterial effect against both gram negative and gram positive bacteria.
In addition, the results mentioned that the susceptibility of Gram positive and Gram negative bacteria to biosynthesized AgNPs was found to vary from the studies of others, related to the pathogen strains tested (Nagajyothi and Lee 2011) and the concentration of the inoculum or solvent used (Mosconi et al. 2020). For example, here the zone inhibition given by the AgNPs against Staphylococcus aureus and Escherichia coli were 18.66 mm and15.33 mm respectively, these diameters were superior to those obtained by the AgNPs from Pistachia lentiscus with 13 mm and 13 mm, respectively, against the same bacteria strains, these data could be due to the maximum richness of the metabolites from the mixed plant leaves (Nagajyothi and Lee 2011). Few works aimed the antifungal potential of AgNPs, here we successfully describe the high antifungal potential of our AgNPs against Candida species and Penicillium. The observed results on filamentous fungi confirmed that the silver nanoparticles enhanced the antifungal behavior against mycelium and spores (Renzi et al. 2020). Numerous works, described the antifungal potential of Olive plant against filamentous fungi like Rhizopus, Fusarium and Alternaria as reported by Korukluoglu et al. (2008). These funding mentioned that the antifungal behavior of the biosynthesized silver nanoparticles from mixed extracts could be due to the olive composition, which was able to affect the spores and the mycelium fungi growth. In literature, the major active components in Olive leaf are known to be Oleuropein and its derivatives as owing superior antifungal potential against fungi (Farag et al. 2003). It is essential to note that, the new AgNPs exhibited novel bactericidal and fungicidal potential which may be highly relevant in infections caused by filamentous fungi and MDR bacteria strains. Based on the method previously used by Okou et al. (2008), showing that values of MBC/MIC report inferior to 4 reflects the bactericidal or fungicidal effects (MFC/MIC ≤ 4) of the tested compound and if the values exceed 4 it was considered as bacteriostatic or fungistatical agent.
Consequently, the broad spectrum killing caused by AgNPs, have encouraged their use as antimicrobial drugs including multidrug strains MDR (Mlalila et al. 2017; Bharti et al. 2021).
Numerous studies have shown that nanoparticles generally improved the pharmaceutical characteristics of antifungals, as lower toxicity and enhancing antifungal potential, and the possibility of prolonged action (Renzi et al. 2020). The reported data show that the presence of AgNPs in the Candida growth can limit the virulence factor as enzyme production and biofilm formation.
In literature, several mechanisms have been reported for antimicrobial activity of AgNPs such as disruption of the bacterial cell membrane, interference in the respiratory electron transport chain formation of reactive oxygen species (ROS) (Bharti et al. 2015; Mai and Hilt 2017). Nanoparticles exhibited new or improved properties depending upon their size and morphology. The enzyme hydrolases are considered as the virulence factor in Candida spp help formation of biofilm on the surfaces and also support invading the host cell. The present work has been carried out for the very first time highlighting the effects of silver nanoparticles from mixed leaves from oleaster and Pistacia on Candida key virulence factors by means of enzyme production (proteinase, phospholipase) and morphogenesis reduction. Our results illustrated that the addition of silver nanoparticles can reduce the enzyme production and the germ tube and filamentous hyphae. Recently scare works, reported the reduction of enzyme and biofilm by Candida albicans by the addition of green silver nanoparticles e.g. Lara et al. (2015). However, the exact mechanism of action on biofilm by silver nanoparticles is not known. In addition, the inhibition of yeast morphogenesis; like germ tube and filamentous hyphae lead to the suppression of biofilm formation in Candida strains (Eladly and Shabana 2018; Jalal et al. 2019).
Furthermore, a combination of conventional antifungals with natural compounds can also minimize the toxicity of these drugs by reducing the dose request. Therefore, the focus of this study was to explore the new AgNPs by enhancing the antibacterial potential of conventional antibiotic drugs tested on clinical strains. The association of silver nanoparticles with conventional antibiotics is used to act synergistically on bacteria and turn them into antibiotics sensitive strains, rather than being resistant using the conventional antibiotics alone. After demonstrating the simple method of synthesis, the antioxidant potential of AgNPs by testing DPPH radical scavenging, Ferric Antioxidant Reducing Power (FRAP) as well as the total antioxidant activity was determined. Thus the combination of the antioxidant effects and the antibacterial and antifungal activities encourage the use of the green biosynthesized AgNPs in pharmaceutical field.
Conclusion
In conclusion, to our knowledge, this is the first study evaluating the antioxidant and antimicrobial effects of silver nanoparticles biosynthesized from Olea europaea subsp. europaea var. sylvestris and Pistacia lentiscus leaves. The synthesized AgNPs is rich in secondary metabolites and has an antioxidant activity. The reported AgNPs exhibited markedly bactericidal and fungicidal effects against clinical pathogen strains. The synergistic interaction with the conventional antibiotic as well as the effect on bacteria biofilm and the spores of filamentous encouraged their formulation in pharmaceutical and medical purposes.
Availability of data and material
Not applicable.
References
Ahmad S, Mudasir AS, Swami BL (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radia Res Appl Sci 9:1–7. https://doi.org/10.1016/j.jrras.2015.06.006
Athavale A, Jirankalgikar N, Nariya P, Des S (2012) Evaluation of in-vitro antioxidant activity of panchagavya: a traditional ayurvedic preparation. Int J Pharmaceut Sci Res 3(8):2543–2549
Bharti S, Agnihotri S, Mukherji S, Mukherji S (2015) Effectiveness of immobilized silver nanoparticles in inactivation of pathogenic bacteria. J Environ Res Dev 9:849–856
Bharti S, Mukherji S, Mukherji S (2021) Enhanced antibacterial activity of decahedral silver nanoparticles. J Nanopart Res 23(2):36. https://doi.org/10.1007/s11051-020-05106-z
Camps-Fabrer H Olive tree (1997) part one, In the Olive tree and oil In Roman Africa. General government of Algeria. Direction of Interior and Fine Arts In: World Olive Encyclopedia, C.O.I. (Eds.), 30–33
Camps-Fabrer H (1953) part one, The culture of Olive tree in north Africa, Evolution and history. In Olive tree and oil In Roman Africa. General government of Algeria, Antiquities Service. Imp. Off., Alger, pp 1–93
Chan EWC, Lim YY, Omar M (2007) Antioxidant and antibacterial activity of leaves of Etlingera Species (Zingiberaceae) in Peninsular Malaysia. Food Chem 104(4):1586–1593. https://doi.org/10.1016/j.foodchem.2007.03.023
Da Mohan V, Ballal M (2008) Proteinase and phospholipase activity as virulence factors in Candida species isolated from blood. Rev Iberoam Micol 25(4):208–2010. https://doi.org/10.1016/s1130-1406(08)70050-0
Danielli LJ, Pippi B, Duarte JA, Maciel AJ, Lopes W, Machado MM et al (2018) Antifungal mechanism of action of Schinus lentiscifolius Marchand essential oil and its synergistic effect in vitro with terbinafine and ciclopirox against dermatophytes. J Pharm Pharmacol 70(9):1216–1227. https://doi.org/10.1111/jphp.12949
DeMatteis V, Rizzelio L, Ingrosso C, Liatsi-Douvitsa E, De Giorgi ML, De Matteis G, Rinaldi R (2019) Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. Nanomaterials 9(11):1544. https://doi.org/10.3390/nano9111544
Dridi R, Essghaier B, Hannachi H, Benkheder G, Chaffei C, Zid MF (2021) Biosynthesized silver nanoparticles using Anagallis monelli: Evaluation of antioxidant activity, antibacterial and antifungal effects. J Mol Struct 1251(5):132076. https://doi.org/10.1016/j.molstruc.2021.132076
Dyah W, Rengga P, Yufitasari A, Adi W (2017) Synthesis of silver nanoparticles from silver nitrate solution using green tea extract (Camelia sinensis) as Bioreductor. Jurnal Bahan Alam Turbarukan 6 (1): 32–38. https://doi.org/10.15294/jbat.v6i1.6628
Eladly A, Shabana I (2018) Antimicrobial activity of green silver nanoparticles against fluconazole-resistant candida albicans in animal model. Egypt J Bot 58 (1):119–132 https://doi.org/10.21608/EJBO.2018.1292.1110
El-Chaghaby GA, Ahmad AF (2011) Biosynthesis of silver nanoparticles using Pistacia lentiscus leaves extract and investigation of their antimicrobial effect. Orient J Chem 27(3):929–936
Essghaier B, Naouar A, Abdelhak J, Zid MF (2014) Synthesis, crystal structure and potential antimicrobial activities of di (4-sulfamoyl-phenyl-ammonium) sulphate. Microbiol Res 169:504–510. https://doi.org/10.1016/j.micres/2013/11/005
Farag RS, ElBaroty GC, Basuny AM (2003) Safety evaluation of olive phenolic compounds as natural antioxidants. Int J Food Sci Nutr 54:159–174. https://doi.org/10.1080/0963748031000136306
Gardeli C, Vassiliki P, Athanasios M, Kibouris T, Komaitis M (2018) Essential oil composition of Pistacia lentiscus L. Myrtus communis L.: evaluation of antioxidant capacity of methanolic extracts. Food Chem 107:1120–1130. https://doi.org/10.1016/j.foodchem.2007.09.036
Ghadir A, Elchaghaby AF (2011) Biosyhtheiss of Silver Nanoparticels using Pistahcia lentiscus leaves extract and investigation of their antimicrobial effect. Orient J Chem 27(3):929–936
Ghiuta I, Cristea D, Croitoru C, Kost J, Wenkert R, Vyrides I, Anayiotos A, Munteanu D (2018) Characterization and antimicrobial activity of silver nanoparticles, biosynthesized using Bacillus species. Appl Surf Sci 438:66–73. https://doi.org/10.1016/j.apsusc.2017.09.163
Gomathi M, Rajkumar PV, Prakasam A, Ravichandran K (2017) Green synthesis of silver nanoparticles using Datura stramonium leaf extract and assessment of their antibacterial activity. Res Effic Technol 3(3):280–284. https://doi.org/10.1016/j.reffit.2016.12.005
Gulati M, Lohse MB, Ennis CL, Gonzalez CL, Perry AM, Bapat P, Arevalo AV, Rodriguez DL, Nobile CJ (2018) In vitro culturing and screening of Candida albicans Biofilms. Curr Protoc Microbiol 50(1):e60. https://doi.org/10.1002/cpmc.60
Hannachi H, Breton C, Msallem M, Ben El Hadj S, El Gazzah M, Bervillé A (2008) Differences between local and introduced olive cultivars as revealed by morphology of drupes, oil composition and SSR polymorphisms: a case study in Tunisia. Scientia Hortic 116:280–290. https://doi.org/10.1016/j.scienta.2008.01.004
Hannachi H, Breton C, Msallem M, Ben El Hadj S, El Gazzah M, Bervillé A (2010) Genetic relationships between cultivated and Wild Olive Trees (Olea Europaea L. Var. Europaea and Var. Sylvestris) based on nuclear and chloroplast SSR markers. Natl Resour J Scirp 1:95–103. https://doi.org/10.4236/nr.2010.12010
Harris LJ (1935) Ray SN (1935) Determination of plasma Ascorbic acid by 2, 6-dichorphenol indophenols titration. Lancet 1(71):462
Ibrahim HMM (2015) Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci 8(3):265–275. https://doi.org/10.1016/j.jrras.2015.01.007
Jalal M, Aazam Ansari M, Ali SG, Alzohairy MM, Khan HM, Almatroudi A, Siddiqui MI (2019) Anticandidal activity of biosynthesized silver nanoparticles: effect on growth, cell morphology, and key virulence attributes of Candida species. Int J Nanomed 14:4667–4467. https://doi.org/10.2147/IJN.S210449
Jyoti K, Baunthiyal M, Singh A (2016) Characterization of silver nanoparticles synthesized using Urtica dioica Linn leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sci 9(3):217–227. https://doi.org/10.1016/j.jrras.2015.10.002
Kim MK, Zhao A, Wang A, Brown ZZ, Muir TW, Stone HA, Bassler BL (2017) Surface attached molecules control Staphylococcus aureus quorum sensing and biofilm development. Nat Microbiol 2:17080. https://doi.org/10.1038/nmicrobiol.2017.80
Korukluoglu M, Sahan Y, Yigit A (2008) Antifungal properties of olive leaf extracts and their phenolic compounds. J Food Safety 28(1):76–87. https://doi.org/10.1111/j.1745-4565.2007.00096.x
Lara HH, Romero-Urbina DG, Pierce C, Lopez-Ribot JL, Josefina M (2015) Arellano-Jiménez and M Jose-Yacaman Effect of silver nanoparticles on Candida albicans biofilms: an ultrastructural study. J Nanobiotechnol 13:91. https://doi.org/10.1186/s12951-015-0147-8
Luo G, Su W, Li H, Xiong J, Wang W, Yang W, Du J (2018) Antibacterial activity and catalytic activity of biosynthesised silver nanoparticles by flavonoids from petals of Lilium casa blanca. Micro Nano Lett 13(6):824–828. https://doi.org/10.1049/mnl.2018.0055
Mahiuddin Md, Saha P, Ochiai B (2020) Green synthesis and catalytic activity of silver nanoparticles based on piper chaba stem extracts. Nanomaterials 10(9):1777. https://doi.org/10.3390/nano10091777
Mai T, Hilt JZ (2017) Mangetic nanoparticles; reactive oxygen species generation and potential therapeutic applications. J Nanopart Res 19:253. https://doi.org/10.1007/s11051-017-3943-2
Mittal J, Batra A, Singh A, Sharma MM (2014) Phytofabrication of nanoparticles through plant as nanofactories. Adv Natl Sci 5(4):043002. https://doi.org/10.1088/2043-6262/5/4/043002
Mlalila NG, Swai HS, Hilonga A, Kadam DM (2017) Antimicrobial of silver nanoparticles in surface plasmon resonance bands against Escherchia coli. Nanotechnol Sci Appl 10:1–9. https://doi.org/10.2147/NSA.S123681
Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ (2013) Silver enhances antibiotic activity against gram-negative bacteria. Sci Trans Med 5:181–190. https://doi.org/10.1126/scitranslmed.3006276
Mosconi N, Monti L, Giulidori C, Williams PAM, Raimondi M, Bellú S, Rizzotto M (2020) Antifungal, phyto, cyto,genotoxic and lipophilic properties of three complexes of sulfadimethoxine (HSDM) with Ag(I). Synthesis and characterization of [Ag3SDM(SCN)2 ]·H2O and [Ag2(SDM)ophenanthroline]·H2OPolyhedron https://doi.org/10.1016/j.poly.2020.114965
Nagajyothi PC, Lee KD (2011) Synthesis of plant-mediated silver nanoparticles using Dioscorea batatas rhizome extract and evaluation of their antimicrobial activities. J Nanomater 211:9. https://doi.org/10.1155/2011/573429
Okou OC, Yapo SE, Kporou KE, Baibo GL, Monthaut S, Djaman AJ (2018) Evaluation de l’activité antibactérienne des extraits de feuilles de Solanum torvum Swartz (Solanaceae) sur la croissance in vitro de 3 souches d’entérobactéries. J Appl Biosci 122:12287–12295
Pisoschi AM, Negulescu GP (2011) Methods for total antioxidant activity determination: a review. Biochem Analyt Biochem 1(1):1–9. https://doi.org/10.4172/2161-1009.1000106
Qabahe K, AlRimawi F, Qasem A, Naser AS (2018) Oleuropein is responsible for the major anti-inflammatory effects of Olive leaf extract. J Med Food 21(3):302–305. https://doi.org/10.1089/jmf.2017.0070
Rajkumar PV, Ravichandran K, Baneto M, Ravidhas C, Sakthivel B, Dineshbabu N (2015) Enhancement of optical electrical properties of SILAR deposited ZnO thin films through fluorine doping and vacuum annealing for photovoltaic applications. Mater Sci Semicond Process 35:189–196. https://doi.org/10.1016/j.mssp.2015.03.010
Renzi DF, de Almeida CL, Miranda EH, Mainardes RM, Abraham WR, Grigoletto DF, Khalil NM (2020) Nanoparticles as a tool for broadening antifungal activities. Curr Med Chem 27:1–30. https://doi.org/10.2174/0929867327666200330143338
Rodríguez-Romero M, Godoy-Cancho B, Calha IM, Passarinho JA, Moreira AC (2021) Allelopathic effects of three herb species on phytophthora cinnamomi, a pathogen causing severe oak decline in mediterranean wood pastures. Forests 12(3):285. https://doi.org/10.3390/f12030285
Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4(3):707–716. https://doi.org/10.1016/j.actbio.2007.11.006
Samaranayake YH, Dassanayake RS, Jayatilake JS, Cheung BK, Yau JY, Yeung KS et al (2005) Phospholipase B enzyme expression is not associated with other virulence attributes in Candida albicans isolates from patients with human immunodeficiency virus infection. J Med Microbiol 54:583–593
Sarangi N, Athukorala P, Dilantha Fernando WG, Rashid KY, Kievit TD (2010) The role of volatile and non volatile antibiotics produced by Pseudomonas chlororaphis strain PA23 in its root colonization and control of Sclerotinia sclerotiorum. Biocontrol Sci Tech 20(8):875–890
Schanderi SH (1970) Method in food analysis, 1st edn. Elsevier, Academic Press, New York
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Sonmezdag AS, Kelebek H, Selli S (2016) Characterization and comparative evaluation of volatile, phenolic and antioxidant properties of pistachio (Pistacia vera L.) hull. J Essent Oil Res. https://doi.org/10.1080/10412905.2016.1216899
Sreevidya N, Mehrotra S (2003) Spectrophotometric Method for the estimation of Alkaloids Precipitable with Dragendroff‘s reagent in plant materials. J AOAC Int 86(6):1124–1127. https://doi.org/10.1093/jaoac/86.6.1124
Vidhu VK, Aromal SA, Philip D (2011) Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochim Acta Mol Biomol Spectrosc 83(1):392–397. https://doi.org/10.1016/j.saa.2011.08.051
Zhu J, Luther PW, Leng Q, Mixson AJ (2006) Synthetic histidine-rich peptides inhibit Candida species and other fungi in vitro: role of endocytosis and treatment implications. Antimicrob Agents Chemother 50:2797–2805. https://doi.org/10.1128/AAC.00411-06
Zia F, Ghafoor N, Iqbal M, Mehboob S (2016) Green synthesis and characterization of silver nanoparticles using Cydonia oblong seed extract. Appl Nanosci 6:1023–1029. https://doi.org/10.1007/s13204-016-0517-z
Acknowledgements
This work was supported by funds from the authority of the Ministry of Higher Education and Scientific Research of Tunisia.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Essghaier Badiaa: Microbial Methodology, Data curation, writing original draft, Rihab Dridi, Hannachi Hedia, Ben khedher Ghada: methodology, validation, Med Faouzi Zid, Chaffei Chiraz: Supervision.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Essghaier, B., Ben Khedher, G., Hannachi, H. et al. Green synthesis of silver nanoparticles using mixed leaves aqueous extract of wild olive and pistachio: characterization, antioxidant, antimicrobial and effect on virulence factors of Candida. Arch Microbiol 204, 203 (2022). https://doi.org/10.1007/s00203-022-02810-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02810-3