Abstract
The green routs for the synthesis of silver nanoparticles (AgNPs) and its multiple applications have attracted many researchers, because silver nanoparticles synthesized by the green method, in addition to being environmentally friendly, are effective in targeting specific tissues and pathogenic microorganisms. The fruit of the Prickly Pear plant has a wide range of secondary metabolites with high regenerative power that can be used for the biosynthesis of AgNPs. Therefore; in this study, green synthesis of nanoparticles was performed using Cactus fruit extract and its antioxidant and antibacterial properties were examined. Antimicrobial activity of extracts and AgNPs against standard strains of gram-positive bacteria such as Staphylococcus aureus (PTCC 16538), Enterococcus faecalis (ATCC 15753), Streptococcus mutans (ATCC 35668), Streptococcus mitis (ATCC 6249), Klebsiella pneumoniae (PTCC 700603), Staphylococcus epidermidis (ATCC 12228) as well as gram-negative Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853) were determined by micro-broth dilution method. SEM, UV–Vis, EDAX and XRD techniques confirm successful biosynthesis of silver nanoparticles and average particle size was around 40–65 nm. Silver nanoparticles acted as an inhibitor of DPPH radicals and showed desirable antioxidant properties. MIC and MBC values of experimental pathogens were recorded in the range of 2.34–18.75 µg/mL and 2.34–37.5 µg/mL, respectively. The results showed appropriate antibacterial and antioxidant activity of biosynthesized silver nanoparticles. Therefore, the synthesized silver nanoparticles can be used as natural resources to produce antioxidant and antimicrobial supplements in the pharmaceutical industry.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology has unprecedented uses in industries such as biomedical, clinical, agriculture, food, environment, electronics, power, textiles, aviation, and aerospace [1] Nanomaterials science deals with the structure and utility of nano-sized particles across disciplines [2]. In general, nanotechnology deals with particles whose at least one dimension is in the range of 1–100 nm [3]. In recent years, nanoparticles have been considered due to their properties such as high surface-to-volume ratio, very small size, and physical and chemical properties compared to their bulk structure [4]. Nanoparticles are divided into several categories of composite such as core–shell structures, insulating and semiconductor nanoparticles, metal and ceramic including metal oxides nanoparticles [5, 6]. Among these, metal nanoparticles have been used extensively in medicine and pharmaceutical applications [7, 8]. Silver nanoparticles (Ag-NPs) have been reported to possess efficient anti-microbial, anti-oxidant, anti-fungal, anti-inflammatory, anti-viral, and anti-angiogenesis properties [9]. Silver nanoparticles (Ag-NPs) have a large surface area, which leads to biochemical reactivity and significant catalytic activity compared to larger particles with similar chemical properties [10]. Monovalent silver compounds have been used as antibacterial agents for many years. However, studies have shown that silver nanoparticles also have these properties [11]. Ag nanoparticles were observed to penetrate bacterial cells. They produced free oxygen that bound to cell structural components, resulting in bactericidal effects [9, 12]. These properties make nanoparticles more toxic to fungi, bacteria and viruses than bulk metallic silver [13,14,15].
One of the important areas of nano-science is related to the design of experimental methods for the synthesis of nanoparticles with different chemical composition, size, shape and properties [16, 17].There are several methods for the synthesis of silver nanoparticles, including sol–gel method, chemical vapor deposition, laser ablation technique, hydrothermal and combustion synthesis [10, 18]. The application of chemical methods is limited due to the high cost and retention of some toxic reagents on nanoparticles in medicine, and also potential damage to the environment [19, 20]. Due to increased environmental awareness, natural substances have gained attention as potential antibacterial agents [21]. Producing environmentally friendly nanoparticles using synthesis methods without using toxic and dangerous chemicals is becoming increasingly important in today’s world. Nanoparticles can be created using a variety of chemical methods and materials, but many researchers are turning to environmentally friendly processes that use enzymes, microscopic organisms, and plant extracts to synthesize nanomaterials. Recently, the synthesis of nanometals using plant extracts has gained attention due to its low environmental risk and safety, in addition to the use of genetic engineering and transgenic plants that are resistant to pests. The green route is non-toxic, eco-friendly, clean, inexpensive, and safe [22,23,24]. Biochemical, enzymes, vitamins and polysaccharides in micro and microorganisms such as bacteria [25], fungi [26], algae [27] and plants [28, 29] are generally used in green synthesis of nanoparticles [30,31,32]. Green synthesis of metal ions using plant compounds is usually a one-step method that does not require surfactants and other stabilizing agents [33, 34]. Biologically active substances and compounds in plant extracts such as flavonoids, polyol compounds, heterocyclic molecules and other water-soluble metabolites can be used to reduce the metal ions to nano size materials at room temperature [35, 36]. In this method, the surface of nanoparticles is covered by plant metabolites as well as carbohydrates and proteins which in addition to sustainability, makes them biocompatible [35]. Nature of plant extract, metal salt concentration, pH, temperature, and extract concentration are the factors that affect the production rate and quality of nanoparticles [37]. So far, various plants have been used to synthesis silver nanoparticles, including Jatropha curcas [38], Azadirachtaindica [39], Echinacea purpurea[40], Cestrum nocturnum [15] and Ferula persica [41].
Prickly Pear is a subfamily plant of Opuntiaspp and belongs to the Cactus family. This plant is widely found in Mexico, Asia, Latin America, South Africa and the Mediterranean. Mexico is a major producer of Opuntia species and accounts for more than 45% of world cultivation [42,43,44]. Cactus pear fruit, commonly called Prickly Pear fruit, due to the content of betalin and betanin pigments are in purple color and due to the content of indicaxanthin pigments finding in orange as well as white colors [45]. The results of various studies have shown that European and Asian Cactus fruits are a source of nutrients and antioxidants that reduce oxidative stress in patients significantly. It also has benefits such as protective effects on the cardiovascular system, anti-cancer, liver and nerve protection [46,47,48,49]. Intense antibacterial effects are another feature of Prickly Pear [50, 51].
Therefore, in the present study, the Cactus fruit extract was used to synthesize of silver nanoparticles as well as antibacterial and antioxidant properties of synthesized silver nanoparticles, were investigated.
2 Materials and methods
2.1 Materials
In the present study, silver nitrate (AgNO3) was purchased from the Merck Company in order to nanoparticles synthesis. To prepare the plant extract, the fruit of the Cactus plant was collected in spring around the city of Birjand (the capital of South Khorasan Province—Iran) and methanol with a purity of 99.9% (Germany Merck) was used. Also 2,2-Diphenyl-1-picrylhydrazyl (DPPH) were prepared from Sigma Company to test the antioxidant properties of nanoparticles. For antibacterial experiment, Mueller- Hinton Agar and Mueller–Hinton Broth made by Merck, and to adjust the pH, sodium hydroxide solution were used.
2.2 Preparation of Cactus fruit extract
A rotary apparatus was used for methanolic extraction of the prickly pear plant extract. For this purpose, the collected Cactus fruit was dried at room temperature and in the dark condition after washing with double distilled water. After that, 30 g of dried fruit powder was exposed to 400 mL of methanol for a specified period time. Then, the samples were filtered using Whatman paper (No.42) and a rotary device was used for extraction [52].
2.3 Synthesis of silver nanoparticles
The advantages of plant materials use for the biosynthesis of nanoparticles is the perception of adsorb mechanism of metal ions by plants and understanding the possible mechanism of metal nanoparticle formation in plants. To prepare nanoparticles by green synthesis method, AgNO3 was used as a source of silver. For this purpose, 10 mL of prickly pear extract (5 g/L) was combined with 10 mL of silver nitrate solution (5 mM). The solution was stirred at room temperature for 20 min at 200 rpm. The final color change of the solution from pink to brown indicated the synthesis of silver nanoparticles. The absorption spectra were obtained using a double Beam Spectrophotometer in the range of 300 to 500 nm. In order to optimize the silver concentration, 5–30 mM of silver nitrate with 10 mL of Cactus fruit extract was shaken in specific pH and temperature for about 20 min. To evaluate the optimal pH, 10 mL of silver nitrate solution from the previous stage was mixed with 10 mL of Cactus fruit extract and examined at pH range equal 7–14 for 20 min at room temperature. In the next step, to determine the optimal time, 10 mL of Cactus extract with optimized silver nitrate solution was placed on the shaker in fixed temperature and pH for 5–60 min. Finally, the optimal solution was centrifuged (Centurion Scientific, K280R, England) at 6000 rpm to obtain nanoparticle sediment. In order to get pure nanoparticles, the precipitate was washed with distilled water and methanol. Then the obtained nanoparticles were dried in an oven at 60 °C for 24 h [53].
2.4 Characterization
Several advanced methods were used to determine the surface morphology and properties of the synthesized silver nanoparticles. The nanoparticle absorption spectra were taken by UV–Vis spectrophotometer (UV–Vis T80+, PG Instrument Ltd, England). Scanning electron microscopy (SEM)(FE-SEM; TESCAN BRNO-Mira3) was used to analyze and characterize the distribution of nanoparticle constituents. Changes in the crystal structure of nanoparticles was investigated by X-ray diffraction (XRD)(Philips X’pert pro, The Netherlands). Energy-dispersive X-ray spectroscopy (EDAX) spectroscopy was used to analyze and describe the distribution of nanoparticle constituent elements.
2.5 Determination of antibacterial properties
Standard strains bacteria (ATCC) were prepared from the Microbiology Reference Laboratory and frozen. Antimicrobial activity of extract and nanoparticles against gram-positive bacteria such as Staphylococcus aureus (PTCC 16538), Enterococcus faecalis (ATCC 15753), Streptococcus mutans (ATCC 35668), Streptococcus mitis (ATCC 6249), Klebsiella pneumonia (PTCC 700603), and Staphylococcus epidermidis (ATCC 12228) as well as gram-negative Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853) was determined by micro-broth dilution method.
The minimum inhibition concentration (MIC) was determined in 96-well plate. For this purpose, 100 µL of each bacterium with a concentration of 0.5 McFarland was dissolved under sterile conditions in Mueller–Hinton broth culture medium and incubated at 37 °C for 2 h. Briefly, 100 µL of the Mueller–Hinton broth culture medium was poured into 96-well microplate. Then 100 µL of the extraction solution containing silver nanoparticles was added to the first well of each row and diluted from the second well to the third and in the same way it was diluted to well number 6. In the next step, 100 µL was poured out of the end well and the concentration of nanoparticles in each well will be half of the previous. Finally, 100 µL of diluted microbial suspension with 106 CFU/mL was added to all wells. This procedure was performed for all of the mentioned bacteria. Then, the microplates containing Streptococcus bacteria were incubated in the presence of CO2 and the other microplates without CO2 for 24 h at 37 °C. The intensity of turbidity, which indicates the growth of bacteria, was examined visually. According to the definition, the concentration of the last well (the thinnest) without turbidity was considered as MIC. The extract solution alone was used as a control.
To determined Minimum Bactericidal Concentration (MBC), all turbidity-free wells were cultured on Mueller–Hinton agar medium separately. Solid culture medium containing Streptococcus bacteria was incubated in the presence of CO2 and other plates without the presence at 37 °C. After 2 h, the lowest concentration of the extract containing silver nanoparticles in which the bacterium did not grow was reported as MBC (Fig. 1) [53]. Experiments were performed in 3 rounds and the average results were reported.
2.6 Determination of antioxidant properties
Some plants have the ability to prepare silver nanoparticles with antioxidant properties in the green synthesis method. The potential of these plants for bioremediation of Ag+ to Ag° has been identified via spectroscopic methods by reducing the adsorption in 517 nm. Evaluation of DPPH free radical inhibition is one of the most common methods for appraised antioxidant power of various compounds due to its simplicity and high sensitivity. The basis of this method is the reduction of DPPH free radical by antioxidants in the absence of other free radicals in the environment [54,55,56]. For this purpose, different concentrations of silver nanoparticles and extract (1–2-5 mg/mL) were prepared in deionized water. After, 50 µL of the sample was added to 1 mL of stock solution of DPPH and ethanol (Fig. 2). The reaction mixture was stirred and its adsorption was determined after 15 min at 517 nm using a UV–Vis spectrophotometer. Ethanol was used as a blank sample in all assays. Free radical scavenging activity was calculated as DPPH reduction percentage as follows.
3 Results and discussion
Silver nanoparticles (AgNPs) were synthesized using Prickly Pear fruit as reducing agent. The conversion in color of the solution from pink to dark brown shortly after the addition of the extract to 5 mM silver nitrate (AgNO3) due to the bioremediation of silver ions (Ag+) indicated the successful synthesis of silver nanoparticles. Lack of temperature limit and high reaction rate for silver nanoparticles formation can be considered as an advantage of this method. The color change increased over time due to further regeneration of Ag+. In order to confirm the synthesis of silver nanoparticles, ultraviolet spectra were recorded at 300–500 nm. According to result, the maximum absorption peak in the 420 nm wavelength range indicates the presence of silver nanoparticles in the reaction solution. Since the optimization of the important factors in green synthesis can affect the morphology and other properties of nanoparticles, several effective parameters were investigated and optimized in this study.
3.1 Experiments of reaction parameters
3.1.1 Influence of silver nitrate concentration
In order to investigate the effect of silver nitrate concentration on nanoparticles formation, extract solutions in different concentrations of silver nitrate were prepared. Concentrations of 5, 10, 20, and 30 mM silver nitrate with 10 mL extract at constant temperature and pH were placed on the shaker. In order to ensure the formation of nanoparticles after 20 min, the absorption spectra of the solutions were recorded at 300–500 nm using a spectrophotometer. According to reports, the presence of Surface plasmon resonance characteristic (SPR) in the range of 420–440 nm indicates the formation of silver nanoparticles [57]. As shown in Fig. 3, nanoparticle formation rate in concentration of 5 mM solution of silver nitrate is more than other concentrations. Therefore, it was concluded that increasing the concentration of metal ions above the threshold leads to a decrease in nanoparticle synthesis. Therefore, in subsequent experiments, this concentration was considered optimal.
3.1.2 Influence of pH
In order to investigate effect of pH on the silver nanoparticles formation, solutions of Cactus fruit extract were prepared in the alkaline pH range. For this purpose, 10 mL of silver nitrate solution was combined with the optimal concentration of 5 mM with 10 mL of the extract. Then, the pH of the solution was raised to 7, 10, 12, and 14 by adding 2 N sodium hydroxide solution. To ensure the synthesis of nanoparticles after a specified time and at ambient temperature, the visible spectrum was taken in the range of 300–500 nm. As shown in Fig. 4, by changing the pH from neutral to alkaline, a significant increase was observed in the adsorption band intensity, so that the maximum absorption of the final product is at pH equal 14. Therefore, it can be stated that the presence of NaOH facilitates the deposition of silver nanoparticles and thus increases the formation of nanoparticles [58]. Birla et al. Reported similar results from this study, according to which silver nanoparticles are dispersed and stable at alkaline pH. Also, the synthesis of nanoparticles is decrease by reducing the pH at the acidic surface due to the accumulation and density of the particles [59].
3.1.3 Influence of contact time
Reaction time is another important factor in nanoparticle synthesis. This factor is necessary for complete the process of reducing silver salt by the extract and converting into silver nanoparticles. According to reports, in a short time the possibility of converting all Ag+ ions is reduced, while a long time does not much effect on this process [60]. Therefore, optimizing the reaction time for the speed of synthesis as well as the complete synthesis of nanoparticles has particular importance. For this purpose, silver nitrate with the optimal concentration was combined in proportion to the extract and was shaken for 5, 20, 10, 40, 30, and 60 min at optimum temperature and pH. Finally, the absorption spectrum for all samples was taken with a two-beam spectrophotometer in the range of 300 to 500 nm. According to the results (Fig. 5), time of 40 min was sufficient to convert all Ag+ ions to silver nanoparticles and more time did not have a significant effect on synthesis. It should be noted that at times higher than the optimum time, the size of nanoparticles increases and due to compaction become mass. The results of the research of Shameli et al. are similar to the results of the present study, which with increasing time, the synthesis of nanoparticles decreased [61].
3.2 Characterization of the synthesized AgNPs
The reduction of Ag ions was confirmed by ultraviolet–visible spectroscopy (UV–Vis), X-ray diffraction (XRD), X-ray energy diffraction (EDAX), and scanning electron microscopy (SEM).
3.2.1 XRD pattern
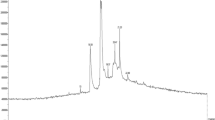
X-ray diffraction pattern (XRD) provides information on the crystal structure and approximate size of nanoparticles [62]. For this purpose, a device with a copper anode lamp source Cu Ka at a wavelength of 1.5406 A˚ was used. Data were taken in the area of 2θ and in the range of 10–80°. According to Fig. 6, the presence of peaks for the Miller Indices (111), (200), (220) and (311), which are related to the absorption peaks at angles of 38.1°, 44.8°, 64.5° and 77.4°; respectively, is fully compliant with the standard silver X-ray diffraction pattern. Also, the synthesized nanoparticles have a face centered cubic crystal structure with a size of about 40–65 nm. The results of the XRD pattern that presented at Fig. 6 confirm the presence of silver crystals in the Cactus plant extract [59, 63].
3.2.2 EDS analysis
X-ray energy diffraction spectroscopy (EDX or EDS) is an analytical method used to analyze the structural or chemical properties of a sample. This method relies on examining the interaction between an X-ray excitation source and a sample. Each of the peaks shown in this diagram is assigned to a specific atom. Intense peaks mean higher element concentrations in the sample [64, 65]. In this study, EDAX spectrum was used to represent the initial composition of silver nanoparticles.The spectrum presented in Fig. 7 identifies the constituent elements of nanoparticles. In the obtained spectral analysis, the strong signal observed in 3 Ke Vis related to the adsorption of silver nanoparticles [66], and the quantity of carbon elements in the amounts of 10.19%, oxygen 21.52% and silver 68.29% has been recorded. According to the results, the percentage of silver metal was significant compared to other chemical elements and these elements act as organic coating agents on the surface of silver nanoparticles.
3.2.3 SEM images
Scanning electron microscopy (SEM) was used to examine the morphology, mean diameter and surface details of the nanoparticles. Figure 8 shows the SEM images with a magnification of 500 nm of synthesized silver nanoparticles. As can be seen, the nanoparticles are mostly single and spherical in shape, with an average diameter in the range of less than 100 nm. They are also accumulation in some areas.
3.3 Antibacterial activity
Although the antimicrobial activity of nanoparticles alone or in combination with standard antibiotics against a wide range of microorganisms including gram-negative and gram-positive bacteria and fungi has been demonstrated, there is still insufficient information on the exact mechanism of action. However, extensive research has been performed to elucidate how they work, and so far three distinct mechanisms have been proposed, including (1) cell wall and membrane damage, (2) intracellular penetration and damage, and (3) oxidative stress [65, 67]. Also, according to studies, the biological type of these nanoparticles has a higher antimicrobial activity than its chemical type [37, 57, 68]. Several studies have shown that the antibacterial ability of nanoparticles is affected by their shape, size, surface charge, concentration and colloidal state [34, 69]. In addition, parameters such as the nature of the plant extract, pH and reaction time affect its size, shape and morphology [70]. However, the specific response of each bacterium depends on its metabolic characteristics.
The action mechanism of silver is related to its interaction with thiol group compounds in the respiratory enzymes of bacterial cells. The high affinity of silver for sulfur, phosphorus, protein, enzymes and DNA in the bacterial cell membrane can be the main cause of its antimicrobial properties. Silver nanoparticles react with sulfur-containing proteins inside or outside the cell membrane, which affects cell survival [65, 67, 71, 72].
Antimicrobial properties of synthesized silver nanoparticles and Cactus fruit extract against clinically pathogenic microorganisms (E. coli, S. aureus, S. mutans, Klebsiella, S. mitis, Pseudomonas, E. faecalis and S. epidermidis) by microbroth Dilution method through the determination of MIC and MBC is shown in Table 1. According to the results, the lowest MIC and MBC values which occurred for Streptococcus mitis was 2.34 µg/mL. In other bacteria, growth inhibition was less and also the same. In general, silver nanoparticles showed a significant antimicrobial effect on the tested samples, so that they prevented the growth of microorganisms with very low concentrations. The antibacterial effect of the extract itself was also measured and the results showed that this extract has no antibacterial effect at the concentrations measured. It can also be stated that the antimicrobial activity of nanoparticles against the mentioned gram-positive bacteria is higher compared to the gram-negative type. Similar results have been reported in research by Joanna [73] and Azarbani [65]. In another study, Madakka et al. recorded higher antimicrobial activity of silver nanoparticles against mycogenic S. aureus than E. coli [74], While some researchers have reported the inverse results with the present study.
3.4 Antioxidant activity
The antioxidant activity of the samples was measured using the adsorb ability of 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) radicals. The potency of silver nanoparticles and Cactus fruit extract with different concentrations in inhibiting DPPH free radicals is shown in Table 2. The results showed that all samples had a high ability to inhibit free radicals, and silver nanoparticles and extracts at a concentration of 5 mg/mL trapped 51.8 and 33.1 percentage of the available free radicals, respectively. It can be stated that with decreasing concentration, the antioxidant power is also degraded and silver nanoparticles have a higher antioxidant effect than the extract alone. This antioxidant property can be related to the effect of various compounds, including the synergistic effect of betalaine and flavonoids in the composition of Cactus fruit [44]. The study of antioxidant properties using Artemisia annua and Sidaacuta leaves is consistent with the present study. Accordingly, the antioxidant properties of the samples depend on their concentration and the antioxidant activity improves with increasing concentration. Also, silver nanoparticles showed excellent antioxidant activity compared to the standard antioxidant ascorbic acid [55].
4 Conclusion
The results of this study showed that Cactus fruit extract has a high potential in the production of silver nanoparticles. The synthesis of nanoparticles in the present method is cost-effective and the process is completed in a very short time. SEM, UV–Vis, EDAX and XRD techniques were used to ensure the synthesis of silver nanoparticles, which confirms its successful biosynthesis. According to the results, silver nanoparticles acted as an inhibitor of DPPH radicals and showed desired antioxidant properties. Also, high antimicrobial activity of biosynthesized nanoparticles from Cactus fruit extract was proven. In general, the results indicated the appropriate antibacterial and antioxidant activity of the biosynthesized nanoparticles.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- AgNPs:
-
Silver nanoparticles
- PTCC 16538:
-
Persian Type Culture Collection Staphylococcus aureus
- ATCC 15753:
-
American Type Culture Collection Enterococcus faecalis
- ATCC 35668:
-
Streptococcus mutans
- ATCC 6249:
-
Streptococcus mitis
- PTCC 700603:
-
Klebsiella pneumoniae
- ATCC 12228:
-
Staphylococcus epidermidis
- ATCC 25922:
-
Escherichia coli
- ATCC 27853:
-
Pseudomonas aeruginosa
- AgNO3 :
-
Silver nitrate
- DPPH:
-
Diphenyl-1-picrylhydrazyl
- XRD:
-
X-ray diffraction
- SEM:
-
Scanning electron microscopy
- EDAX:
-
X-ray energy diffraction
References
Yerpude ST et al (2023) Biomedical, clinical and environmental applications of platinum-based nanohybrids: An updated review. Environ Res 231:116148
Bhilkar PR et al (2023) Phyto-derived metal nanoparticles: Prominent tool for biomedical applications. OpenNano 14:100192
Chouke PB et al (2022) Bioinspired metal/metal oxide nanoparticles: A road map to potential applications. Mater Today Adv 16:100314
Khalil MM et al (2014) Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab J Chem 7(6):1131–1139
Dezfuli AAZ, Abu-Elghait M, Salem SS (2023) Recent Insights into Nanotechnology in Colorectal Cancer. Appl Biochem Biotechnol
Esmati M, Allahresani A, Naghizadeh A (2021) Synthesis and characterization of Graphitic Carbon Nitride/Mesoporous Nano-Silica (g-C3N4/KCC-1) nanocomposite as a novel highly efficient and recyclable photocatalyst for degradation of antibiotic in aqueous solution. Res Chem Intermed 47:1447–1469
Jagtap UB, Bapat VA (2013) Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam seed extract and its antibacterial activity. Ind Crops Prod 46:132–137
Banin U, Ben-Shahar Y, Vinokurov K (2014) Hybrid semiconductor–metal nanoparticles: from architecture to function. Chem Mater 26(1):97–110
Ali F et al (2022) Biosynthesis and characterization of silver nanoparticles using strawberry seed extract and evaluation of their antibacterial and antioxidant activities. J Saudi Chem Soc 26(6):101558
Rafique M et al (2017) A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol 45(7):1272–1291
Bedlovičová Z et al (2020) A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 25(14):3191
Ali F et al (2022) State-of-art of silver and gold nanoparticles synthesis routes, characterization and applications: a review. Z Phys Chem 236(3):291–326
Le Ouay B, Stellacci F (2015) Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today 10(3):339–354
Rawani A, Ghosh A, Chandra G (2013) Mosquito larvicidal and antimicrobial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of Solanum nigrum L. (Solanaceae: Solanales). Acta tropica 128(3):613–622
Keshari AK et al (2020) Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J Ayurveda Integr Med 11(1):37–44
Nagappa B, Chandrappa G (2007) Mesoporous nanocrystalline magnesium oxide for environmental remediation. Microporous Mesoporous Mater 106(1–3):212–218
Rai M, Yadav A (2013) Plants as potential synthesiser of precious metal nanoparticles: progress and prospects. IET Nanobiotechnol 7(3):117–124
Hossein Panahi A et al (2020) Survey of sono-activated persulfate process for treatment of real dairy wastewater. Int J Environ Sci Technol 17:93–98
Yugandhar P, Haribabu R, Savithramma N (2015) Synthesis, characterization and antimicrobial properties of green-synthesised silver nanoparticles from stem bark extract of Syzygium alternifolium (Wt.) Walp. 3 Biotech 5(6):1031–1039
Nasrollahzadeh M et al (2016) Green synthesis of the Pd nanoparticles supported on reduced graphene oxide using barberry fruit extract and its application as a recyclable and heterogeneous catalyst for the reduction of nitroarenes. J Colloid Interface Sci 466:360–368
Bukhari A et al (2023) A novel formulation of triethyl orthoformate mediated durable, smart and antibacterial chitosan cross-linked cellulose fabrics. Int J Biol Macromol 253:126813
Hussain S et al (2023) Potential Antifungal and Antimicrobial Effects of Nano Zinc Oxide Particles Obtained from Cymbogobon citratus Leaf Extract Using Green Technology. Pol J Environ Stud 32(5):4065–4072
Salem SS (2022) Bio-fabrication of selenium nanoparticles using baker’s yeast extract and its antimicrobial efficacy on food borne pathogens. Appl Biochem Biotechnol 194(5):1898–1910
Hashem AH, Salem SS (2022) Green and ecofriendly biosynthesis of selenium nanoparticles using Urtica dioica (stinging nettle) leaf extract: Antimicrobial and anticancer activity. Biotechnol J 17(2):2100432
Iravani S (2014) Bacteria in nanoparticle synthesis: current status and future prospects. International scholarly research notices, 2014
Sastry M et al (2003) Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci 162–170
Hulkoti NI, Taranath T (2014) Biosynthesis of nanoparticles using microbes—a review. Colloids Surf B 121:474–483
Makarov V et al (2014)“Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae (aнглoязычнaя вepcия) 6(1 (20))
Ovais M et al (2016) Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine 12(23):3157–3177
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13(10):2638–2650
Korbekandi H, Iravani S, Abbasi S (2009) Production of nanoparticles using organisms. Crit Rev Biotechnol 29(4):279–306
Emmanuel R et al (2015) Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater Sci Eng C 56:374–379
Said A et al (2023) Antibacterial Activity of Green Synthesized Silver Nanoparticles Using Lawsonia inermis Against Common Pathogens from Urinary Tract Infection. Appl Biochem Biotechnol
Abdelghany TM et al (2023) Phytofabrication of zinc oxide nanoparticles with advanced characterization and its antioxidant, anticancer, and antimicrobial activity against pathogenic microorganisms. Biomass Convers Biorefinery 13(1):417–430
Ayinde WB et al (2018) Biosynthesis of ultrasonically modified Ag-MgO nanocomposite and its potential for antimicrobial activity. J Nanotechnol 2018
Mittal AK, Chisti Y, Banerjee UC (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv 31(2):346–356
Heydari R, Rashidipour M (2015) Green synthesis of silver nanoparticles using extract of oak fruit hull (Jaft): synthesis and in vitro cytotoxic effect on MCF-7 cells. International journal of breast cancer, 2015
Bar H et al (2009) Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A 339(1–3):134–139
Ahmed S et al (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci 9(1):1–7
Gecer EN et al (2021) Green synthesis of silver nanoparticles from Echinacea purpurea (L.) Moench with antioxidant profile. Part Sci Technol 1–8
Hashemi Z et al (2021) Green synthesis of silver nanoparticles using Ferula persica extract (Fp-NPs): Characterization, antibacterial, antileishmanial, and in vitro anticancer activities. Mater Today Commun 27:102264
Aparicio-Fernández X et al (2017) Physicochemical characteristics of fruits from wild Opuntia species from two semiarid regions of Jalisco. Mexico Polibotánica 43:219–244
El-Mostafa K et al (2014) Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 19(9):14879–14901
Madrigal-Santillán E et al (2013) Antioxidant and anticlastogenic capacity of prickly pear juice. Nutrients 5(10):4145–4158
Stintzing FC, Schieber A, Carle R (2001) Phytochemical and nutritional significance of cactus pear. Eur Food Res Technol 212(4):396–407
Cerezal P, Duarte G (2004) Sensory influence of chemical additives in peeled cactus pears (Opuntia ficus-indica (L.) Miller) in syrup conserved by combined methods. J Prof Assoc Cactus Dev 6:102–119
Livrea MA, Tesoriere L (2006) Health benefits and bioactive components of the fruits from Opuntia ficus-indica [L.] Mill. J Prof Assoc Cactus Dev 8(1):73–90
Chavez-Santoscoy R, Gutierrez-Uribe J, Serna-Saldívar S (2009) Phenolic composition, antioxidant capacity and in vitro cancer cell cytotoxicity of nine prickly pear (Opuntia spp.) juices. Plant Foods Human Nutr 64(2):146–152
Butera D et al (2002) Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: betanin and indicaxanthin. J Agric Food Chem 50(23):6895–6901
Fiad M et al (2020) Evaluation of antioxidant and antimicrobial properties of opuntia ficus-indica, seeds and peels extracts. Zagazig J Agric Res 47(2):587–596
Palmeri R et al (2020) Antioxidant and antimicrobial properties of semi-processed frozen prickly pear juice as affected by cultivar and harvest time. Foods 9(2):235
Mortazavi-Derazkola S et al (2021) Green Synthesis and Investigation of Antibacterial Activity of Silver Nanoparticles Using Eryngium bungei Boiss Plant Extract. J Polym Environ 29(9):2978–2985
Mortazavi-Derazkola S et al (2021) Green synthesis and characterization of silver nanoparticles using Elaeagnus angustifolia bark extract and study of Its antibacterial effect. J Polym Environ 1–9
Mani A, Lakshmi S, Gopal V (2012) Bio-mimetic synthesis of silver nanoparticles and evaluation of its free radical scavenging activity. Int J Biol Pharm Res 3:4
Johnson A, Obot I, Ukpong U (2014) Green synthesis of silver nanoparticles using Artemisia annua and Sida acuta leaves extract and their antimicrobial, antioxidant and corrosion inhibition potentials. J Mater Environ Sci 5(3):899–906
Lalitha A, Subbaiya R, Ponmurugan P (2013) Green synthesis of silver nanoparticles from leaf extract Azhadirachta indica and to study its anti-bacterial and antioxidant property. Int J Curr Microbiol Appl Sci 2(6):228–235
Carson L et al (2020) Green synthesis of silver nanoparticles with antimicrobial properties using Phyla dulcis plant extract. Foodborne Pathog Dis 17(8):504–511
Yang B et al (2014) In situ green synthesis of silver–graphene oxide nanocomposites by using tryptophan as a reducing and stabilizing agent and their application in SERS. Appl Surf Sci 316:22–27
Birla SS et al (2013) Rapid synthesis of silver nanoparticles from Fusarium oxysporum by optimizing physicocultural conditions. Sci World J 2013
Pourmortazavi SM et al (2015) Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim Acta Part A Mol Biomol Spectrosc 136:1249–1254
Shameli K et al (2012) Investigation of antibacterial properties silver nanoparticles prepared via green method. Chem Cent J 6(1):1–10
Bousalem S et al (2020) Physical and electrochemical investigations on hybrid materials synthesized by polyaniline with various amounts of ZnO nanoparticle. Chem Phys Lett 741:137095
Alharthi FA et al (2020) Facile one-pot green synthesis of Ag–ZnO Nanocomposites using potato peeland their Ag concentration dependent photocatalytic properties. Sci Rep 10(1):1–14
Cao C et al (2020) Molten salt-assisted processing of nanoparticle-reinforced Cu. Mater Sci Eng A 785:139345
Azarbani F, Shiravand S (2020) Green synthesis of silver nanoparticles by Ferulago macrocarpa flowers extract and their antibacterial, antifungal and toxic effects. Green Chem Lett Rev 13(1):41–49
Rautela A, Rani J, Das MD (2019) Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J Anal Sci Technol 10(1):1–10
de JesúsRuíz-Baltazar Á et al (2017) Green synthesis of silver nanoparticles using a Melissa officinalis leaf extract with antibacterial properties. Results Phys 7:2639–2643
Hong X et al (2016) Shape effect on the antibacterial activity of silver nanoparticles synthesized via a microwave-assisted method. Environ Sci Pollut Res 23(5):4489–4497
El-Khawaga AM et al (2023) Green synthesized ZnO nanoparticles by Saccharomyces cerevisiae and their antibacterial activity and photocatalytic degradation. Biomass Convers Biorefinery
Logaranjan K et al (2016) Shape-and size-controlled synthesis of silver nanoparticles using Aloe vera plant extract and their antimicrobial activity. Nanoscale Res Lett 11(1):1–9
Srikar SK et al (2016) Green synthesis of silver nanoparticles: a review. Green Sustain Chem 6(1):34–56
Garibo D et al (2020) Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci Rep 10(1):1–11
Joanna C et al (2018) A nonspecific synergistic effect of biogenic silver nanoparticles and biosurfactant towards environmental bacteria and fungi. Ecotoxicology 27(3):352–359
Madakka M, Jayaraju N, Rajesh N (2018) Mycosynthesis of silver nanoparticles and their characterization. MethodsX 5:20–29
Acknowledgements
We thank the support of Birjand University of Medical Sciences (BUMS) for the success of this article (Code: 5309).
Funding
This research project was funded by Birjand University of medical sciences.
Author information
Authors and Affiliations
Contributions
Ali Naghizadeh was the supervisor of this research project, all of other authors contributed equally in performing this research project.
Corresponding author
Ethics declarations
Ethical approval
This paper was approved on BUMS ethical committee with code IR.BUMS.REC.1400.003.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bidaki, M.Z., Naghizadeh, A., Yousefinia, A. et al. Environmentally friendly synthesis of silver nanoparticles using Prickly Pear extract and their antimicrobial and antioxidant activities. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-023-05259-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05259-6