Abstract
Enteric fever (typhoid and paratyphoid fever) is a public health concern which contributes to mortality and morbidity all around the globe. It is caused mainly due to ingestion of contaminated food and water with a gram negative, rod-shaped, flagellated bacterium known as Salmonella enterica serotype typhi (typhoid fever) or paratyphi (paratyphoid fever). Clinical problems associated with Salmonellosis are mainly bacteraemia, gastroenteritis and enteric fever. The bacteria undergo various mechanisms to escape itself from immune reaction of the host, modulating immune response at the site of infection leading to virulence factor production and anti-microbial resistance. Biofilm is one of the adaptation mechanisms through which Salmonella survives in unfavourable conditions and thus is considered as a major threat to public health. Another property of the bacteria is “Quorum Sensing”, which is a cell–cell communication and most of the pathogenic bacteria use it to coordinate the production of several virulence factors and other behaviours such as swarming and biofilm formation. Earlier, quorum sensing was believed to be just a medium for communication but, later on, its role in virulence has been studied. However, there are negligible information relating to interaction between quorum sensing and biofilm formation and how these events play crucial role in Salmonella pathogenesis. The review is a summary of updated information regarding how Salmonella uses these properties to spread more and survive better, making a challenge for clinicians and public health experts. Therefore, this review would help bring an insight regarding how biofilm formation and quorum sensing are inter-related and their role in pathogenesis and virulence of Salmonella.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salmonella infection, which is a major public health concern, causes morbidity and mortality throughout the world. It is a gram negative, rod-shaped, flagellated bacterium having two species, Salmonella enterica and Salmonella bongori, which are again divided into different sub-species. Salmonella enterica has six subspecies: arizonae, diarizonae, houtenae, salamae, indica, enterica and each subspecies has different serovars which differ by their antigenic specificity. Interestingly most of the human pathogenic Salmonella serovars belong to enterica subspecies that includes S. enterica serovar typhi, paratyphi, typhimurium, enteritidis and choleraesuis. Throughout our article, all these serovars will be mentioned with genus and serovar names such as S. typhi, S. typhimurium, etc., S. typhimurium is a common inhabitant of gastrointestinal track of ruminants and its infection causes gastroenteritis and fever in human and mice, respectively (Chaudhuri et al. 2018). Similarly, S. enteritidis causes gastrointestinal disorders and food poisoning in human. The major source of this infection in human is contaminated chicken eggs. It causes systemic infection in chicks and laying hens with a prolonged shedding in faeces, which also makes chicken an asymptomatic carrier (Mon et al. 2015). However, S. choleraesuis poses highest threat of food borne infections in human. It causes septicaemic disease in human and paratyphoid fever in swine. Its resistance to many antibiotics and association with development of mycotic aneurysm make it a difficult pathogen to treat (Chiu et al. 2004). Another disease caused by Salmonella in human is typhoid fever, for which the responsible pathogen is Salmonella typhi. According to the most updated WHO evaluation, approximately 11–21 million people suffer from typhoid fever and it claims 128,000–161,000 deaths annually across the globe. Poor response to treatment and delayed diagnosis may lead to serious health complications such as gut wall damage, cerebral dysfunction, gastrointestinal haemorrhage and shock (Mukhopadhyay et al. 2019). Chronic infection with S. typhi and longer persistence in gall bladder combined with continuous excretion to environment have made this pathogen a high risk to public health (Xu et al. 2010). Its persistence and colonization on gallstone have been found to be promoted by biofilm production (Di Domenico et al. 2017). Biofilm formation is an important defence attribute of multidrug-resistant bacteria to survive in unfavourable condition. Since decades, it has attracted many researchers due to its associated high risks including virulence factor production and anti-microbial resistance (Young et al. 2002). Biofilm formation is important for Salmonella spreading as it saves the pathogen from physical, chemical and mechanical stress whereas biofilm formation in gall stone is considered as the hall mark of S. typhi carrier (Marin et al. 2009; Scher et al. 2005; Joseph et al. 2001). Salmonella strains other than S. typhi and S. paratyphi are named as non-typhoidal Salmonella which are mainly found in animal reservoirs. Another property of bacteria called “quorum sensing” is also known to affect the virulence of both typhoidal and non-typhoidal strains of Salmonella. Quorum sensing is a method that bacteria adopt to transmit cell–cell signals and coordinate gene expression. Though it was initially thought to simply play role in signalling and cross-talk among bacteria, it is involved in biofilm formation and production of virulent factors in pathogenic microorganisms (Aswathanarayan and Vittal 2018). luxS protein, a central molecule of quorum sensing is essential for expression of virulent genes in S. typhimurium (Choi et al. 2007). Besides, LsrR, a transcription regulator of quorum sensing regulates the invasion of Salmonella into epithelial cells (Choi et al. 2012). However, detailed knowledge on the interaction between quorum sensing and biofilm formation and their solo/combinatorial effect on Salmonella pathogenesis is still scanty. This review is an updated version of most of the important and necessary information regarding how quorum sensing regulates biofilm formation and role of these two events in Salmonella pathogenesis, virulence and how the whole process makes the pathogen more difficult to treat clinically.
Quorum sensing and Biofilm: platforms for bacteria to gather and talk
For their growth, survival and reproduction in changing environments, microbes create different platforms to communicate within cells. This type of communication that happens between cells is called quorum sensing which is directly dependent upon the microbial cell density and induces multiple factors such as production of virulent factors and biofilm formation in microbes (Choi et al. 2012). Though this special kind of microbial communication was first discovered in bacteria, it was subsequently observed in fungi as well (Mehmood et al. 2019).
Another way of communication among microbes is through biofilm formation. Microbes make an association by attaching themselves with each other and also to a surface bound inside a self-made extracellular matrix. Bacteria form biofilms to adapt to unfavourable environmental conditions and interact with their host. When E. coli are in the process of biofilm formation, they convert their single planktonic growth mode to biofilm mode where they switch off their flagella production and in turn production of curli fimbriae and extracellular polymeric substances (EPS) is switched on. CsgD is known as the master regulator of biofilm formation which activates production of curli fimbriae and EPS. It not only regulates the expression of csg operon, but also a number of genes including adrA, required for modulating the cell physiology according to biofilm lifestyle (Simm et al. 2004). Cell motility for planktonic growth is supressed by CsgD which promotes the switch to biofilm formation and preventing CsgD expression abolishes synthesis of curli and cellulose which ultimately affects biofilm formation (Ogasawara et al. 2011; Khambhati et al. 2021). EPS are known to have key role in biofilm formation and development by modulating surface attachment, mechanical stability, hydrophobicity, porosity, water content, etc. (Flemming and Wingender 2002). At the time of surface colonization, bacteria produce EPS which in turn makes the biofilm matrix. The matrix not only provides protection, it also acts as an adsorbent sieve for nutrition and signalling molecules. There exist some genes such as sdiA and adrA that are involved in EPS production, which are also known to be controlled by quorum sensing (Pellock et al. 2002). sdiA plays a role in biofilm formation and adhesion to epithelial cells whereas adrA activates cellulose synthesis which is a major constituent of biofilm matrix especially in Salmonella (Culler et al. 2018; Da Re and Ghigo 2006). In a recent report, both sdiA and adrA genes were found to be present in all S. enteritidis strains isolated from poultry farm environment and human faeces (except one strain) in Poland (Cwiek et al. 2020). The presence of these genes might provide advantage to Salmonella biofilm formation by producing EPS components and establishing quorum sensing communication. S. typhi is one such bacteria that heavily depends upon biofilm formation to continue long persistence inside an existing host and further transmission to a new host (Moshiri et al. 2018). Though, quorum sensing and biofilm formation are two types of communication platforms for bacteria, approximately 80% of infections occur due to quorum sensing-induced biofilm formation (Arciola et al. 2012). It has been reported that, in pathogens including Salmonella, Escherichia coli (E. coli), Campylobacter, Staphylococcus aureus, Listeria monocytogenes and Bacillus cereus, quorum sensing has a key role in the process of biofilm formation (Machado et al. 2019). According to Cui et al. 2020, deletion of Cas3 in a type I-E CRISPR-Cas system of S. enteritidis, upregulated quorum sensing-related genes and downregulated genes related to biofilm formation and virulence (Cui et al. 2020). Interestingly, the CRISPR-Cas system seems to regulate quorum sensing signalling and biofilm formation in many bacteria (Cui et al. 2020). Besides directly regulating genes involved in biofilm formation, quorum sensing induces production of extracellular DNA which interacts with extracellular matrix to form biofilm (Jennings et al. 2015). There are many quorum sensing regulated factors which influence biofilm formation. The ability to form siderophores (for iron availability) by bacteria is thought to be essential for biofilm formation. Pyoverdin is a quorum sensing-controlled siderophore and mutants incapable of producing this iron chelator were found to be unable to produce biofilm aggregates (Da Silva et al. 2017). Another important finding shows that, acyl homoserine lactone-based quorum sensing enhances biofilm formation by S. enteritidis in anaerobic condition, a situation that is relevant to its pathogenesis (De Almeida et al. 2017). Because of the major involvement of quorum sensing in biofilm formation, strategies used to inhibit quorum sensing have also been found to be useful against reducing bacterial biofilm and pathogenicity (Remy et al. 2018). Berberine, an isoquinoline has been found to be an anti-biofilm agent in S. typhimurium based on its quorum sensing inhibiting property (Aswathanarayan and Vittal 2018). However, a lot of further research need to be done to screen more effective anti-quorum sensing compounds to control biofilm formation in pathogenic bacteria.
Quorum sensing and Salmonella pathogenesis
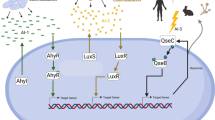
A variety of bacteria phenotypes are regulated by quorum sensing leading to increased pathogenesis and virulence. S. typhimurium is one of the main serovars isolated from human sources and its pathogenicity is contributed by many factors including its flagella (Conceiçao et al. 2015). In S. typhimurium, quorum sensing has been found to increase motility by inducing expression of genes involved in assembly of flagella (Conceiçao et al. 2015). The QseC quorum sensing sensor kinase also has been found to have role in motility and swine colonization of this pathogen. In the same study, deletion of luxS gene also resulted in down regulation of motility related genes, genes present in salmonella pathogenicity island and chemotaxis (Jesudhasan et al. 2010). Isolation of bacteria strains from different environmental conditions and their culture in different settings sometimes become responsible for contrasting results. The quorum sensing mechanism in Salmonella species functions through three types of autoinducers (AI), auto inducer I, II and III (Perrett et al. 2009). Both AI-II and III play important role in regulating pathogenic molecules in Salmonella (Widmer et al. 2007; Moreira et al. 2010). The first and crucial step during infection is adhesion of the pathogen to host cell surface. Effect of quorum sensing on adhesion and biofilm formation has been studied in S. typhi (Prouty et al. 2002), S. enteritidis (Chorianopoulos et al. 2010) and nine other serovars but resulted in ambiguous conclusion (Wang et al. 2013). In addition to its effect on virulence and pathogenesis, quorum sensing in S. typhimurium critically modulates its behaviour during pre-stationary growth phase (Surette and Bassler 1998). The luxS gene is vital for growth of this bacteria and its mutation results in poor growth (Widmer et al. 2012). When it becomes tough for the bacteria to survive under harsh conditions, quorum sensing comes into rescue. Chronic persistence of S. typhi in gall bladder has to deal with bile production as well as generation of reactive oxygen species by producing anti-oxidative enzymes. There is proof that, quorum sensing system of this pathogen keeps its hold on managing the level of these enzymes when the bacteria are under oxidative stress (Walawalkar et al. 2016). Though, Salmonella infection is a risk to human health, Swofford et al. 2015 engineered non-pathogenic Salmonella with integration of a quorum sensing switch that only induces drug expression carried by the bacteria in closely packed colonies present inside the tumor. They have shown that, this quorum sensing engineered Salmonella expressed anti-tumor proteins only inside the tumor and can be used for tumor-specific drug delivery (Swofford et al. 2015). Some of the key roles of quorum sensing in Salmonella virulence are presented in Fig. 1C.
A The graph represents total no. of articles published which reported biofilm producing Salmonella during a period of 2010–2021, B the graph represents total no. of articles published which reported antibiotic resistance property of biofilm producing Salmonella during a period of 2010–2021, C schematic presentation of role of biofilm and quorum sensing in Salmonella virulence
Association of biofilm with Salmonella virulence
In natural condition, most of the bacteria are believed to exist in biofilm forming conditions and research findings showing “approximately 80% bacterial infections are associated with biofilm”, emphasize the importance of this event in bacteria associated diseases (Davies 2003). Some important contributing factors of biofilm to Salmonella virulence are presented in Fig. 1C. The increasing number of reports on biofilm forming Salmonella and their antibiotic resistance during past one decade (Fig. 1A and B, the references of articles presented in these figures are provided in supplementary file, Table 1) provides an alarming picture of its severity if not taken care of appropriately in time. Below is a thorough description of how biofilm formation provides advantages to Salmonella to survive in harsh conditions and promote its virulence, making it a more difficult pathogen clinically.
Antibiotic resistance
Around 60% of nosocomial infections found in humans are thought to be biofilm associated and biofilms increase resistance of bacteria towards host innate defence and antibiotics (Chen and Wen 2011). Of note, acute bacterial infections can be cured with a certain profile of antibiotic treatment whereas the treatment does not show efficacy against an infection caused by biofilm producing bacteria which has a risk of recurrent infection. Sometimes, replacing the initial antibiotic therapy as a treatment regimen helps in resolving these kinds of infections (Song et al. 2013). Biofilm producing bacteria show heterogeneity in replication and metabolism which modulates the biofilm structure and the action of antibiotic, hence making the anti-microbial agent less effective (Dykes et al. 2003). In case of S. typhi, emergence of MDR strains has become a major risk as many easily available antibiotics are no more effective (Wang et al. 2013). The problem becomes more severe in case of gall stone patients, as in most cases, antibiotic treatment does not work effectively because of S. typhi colonization, ultimately leaving gall bladder removal as the only possible therapy (Gunn et al. 2014). Another study, which seconds this finding, proves that, biofilm formation provides protection to S. typhi and S. typhimurium from ciprofloxacin in vitro as well as in a mouse model of chronic carriage (González et al. 2018). Not only biofilm formation induces antibiotic resistance, but also multidrug efflux pump in S. typhimurium plays important role in biofilm formation (Baugh et al. 2012). A study showed that, S. typhimurium biofilms, formed on microplates, exhibited 2000-fold more resistance to ciprofloxacin in comparison to their planktonic cells (Tabak et al. 2019). A significant increase in biofilm and extra polysaccharide production by sub-inhibitory concentration of cefotaxime in clinical Salmonella isolates indicates the threat of uncontrolled use of antibiotics (Majtan et al. 2008). This is a major concern as ciprofloxacin combined with ceftriaxone or cefotaxime are usually administered for treatment of non-typhoidal Salmonellosis (Parry and Threlfall 2008). In another study, 194 S. enterica strains isolated from infected children were tested for their biofilm forming capacity. Surprisingly, 56% of them formed biofilms and simultaneously showed resistance to nine antibiotics with highest resistance against gentamicin and ampicillin (Papavasileiou et al. 2010). Several reports from 2013–2021 have shown an induced antibiotic resistance of different biofilm forming Salmonella species isolated from food sources or clinical samples from different parts of globe (Gong et al. 2013; Tezela et al. 2016; Trampari et al. 2021; Farahani et al. 2018).
Stimulation of Virulence factors and survivability in harsh conditions
Evidences showing that bacterial biofilm may affect the virulence of the pathogen, have led to increased attention of researchers to study diseases from the aspect of their biofilm forming ability. Biofilm formation in Salmonella is considered as one of the virulence weapons which provides survival advantage to the bacteria (Fàbrega and Vila 2013). The extracellular matrix of biofilm contains polysaccharide layers that protects the bacteria from its harsh environment (Jason Chin et al. 2017). Transcriptomic study of S. typhi biofilms revealed that, there is significant increase in expression of genes associated with extracellular matrix and antibiotic resistance which are crucial virulence factors of the pathogen (Jason Chin et al. 2017). Another two important virulence markers involved in S. typhimurium pathogenicity are Salmonella enterotoxin (stn gene, causes mainly gastroenteritis) (Nakano et al. 2012) and invasion gene (invA gene, that helps the pathogen to invade into intestinal epithelium) (Bruno et al. 2009). Expression of these two genes has also been found to be upregulated in biofilm forming S. typhimurium (Xu et al. 2010). Similarly, biofilm formation is also reported to induce expression of type 1 fimbria operon genes in S. typhimurium and these genes are associated with biofilm attachment to abiotic surfaces and colonization on mucosal surface of gut (Escobedo and Gunn 2013). There are some examples of different genes playing key role either in biofilm formation, quorum sensing, antibiotic resistance or virulence of different pathogenic bacteria, presented in Table 1. During their life cycle and pathogenesis, Salmonella has to travel through different types of hosts and different kind of unfavourable environmental conditions including interaction with preservatives, anti-microbial peptides, reactive nitrogen/oxygen species, changing temperature and pH and limited availability of nutrients, etc. The adaptation to changing environment leads to change in its growth pattern, survival and virulence. The polysaccharide matrix of biofilm that covers the biofilm forming Salmonella, protects the bacteria from the outer harsh environmental condition (Jason Chin et al. 2017). A transcriptional analysis of stress response genes of biofilm forming S. typhimurium showed an important role of biofilm in inducing enterotoxin and invasion gene of the pathogen when cultured in varied pH such as pH 5, 6 and 7 (Xu et al. 2010). As acid tolerance response (ATR) is believed to play key role in invasion and colonization making the pathogen more virulent (Abdelwaheb and Ahmed 2009), the increased production of enterotoxin and invasion by S. typhimurium under acidic stress conditions is certainly a matter of worry. Furthermore, stimulating effect of biofilm on the whole stress-response process suggests its involvement in providing survival advantage to this pathogen under unsuitable condition. In a parallel situation, in vitro experiments have shown that both S. typhi and S. typhimurium can produce biofilm on gall stone surface and biofilm provides them protection against high concentration of bile as well as antibiotics (Prouty et al. 2002). In physiological condition, bile acts as a lipid emulsifier, detergent and a potent anti-microbial agent by disrupting cellular homeostasis. As bile containing gall bladder is the main colonizing site of Salmonella, to identify the bile response mechanism, some studies have identified biofilm formation as the major strategy of these pathogens to survive in bile enriched site (Prouty et al. 2002; Crawford et al. 2008). In vivo imaging has shown that biofilm helps the pathogen survive longer outside the host by well-equipped counter mechanisms to tolerate outside extreme conditions (White et al. 2008). However, further research is needed to understand whether S. typhimurium forms biofilm in human gall bladder or not.
Longer persistence inside host and prolonged shedding into environment
From the microbiological point of view, the capability of a pathogen to establish a persistent infection inside host is crucial for its survival as well as transmission which makes the microbe an active reservoir. Salmonella is a common source of zoonotic disease and infectious to both humans and animals. Systemic persistence of S. enterica for a long time inside human body makes the patient asymptomatic carrier in most cases and poses high risk to public health (Dutta et al. 2000). These chronically infected asymptomatic carriers shed bacteria through faecal route and thereby transmit the disease to naive hosts. Faecal-oral route is the common route of spread for Salmonella and humans get infected mostly after getting contact with food contaminated with faeces of infected animal (Mukhopadhyay et al. 2019). There are multiple reports showing a significant number of typhoid patients are asymptomatic, chronic carriers of S. typhi and shed pathogen in their urine and stool for long period of time, sometimes even for life time (Ruby et al. 2012; House et al. 2001). Besides human body, non-typhoidal Salmonella have been found to persist on the surface of raw fruits and vegetable and cause disease outbreaks in the past decade (Beuchat 2002). Interestingly, there are evidences of bacterial biofilm formation on food products and food contact surfaces which may become a potential transmission route of food-borne pathogens (Lee et al. 2020). The profound role of biofilm in attachment and persistence of bacteria on both biotic and abiotic surfaces has led to studies trying to elucidate the mechanism by which biofilm makes the bacteria adhere to the surface for long period. S. typhimurium can form biofilms on glass, polysterene, stainless steel, human epithelial cells, fruits, vegetables and its ability to form biofilm and remain attached on these surfaces is an important strategy of survival (Prouty et al. 2002; Arnold and Bailey 2000; Boddicker et al. 2002; Zogaj et al. 2001; Avila-Novoa et al. 2021). Spread of S. saintpaul, S. rubislaw and S. javiana by paprika, isolation of S. typhimurium, S. ofda, S. tennessee, and S. poona from sesame paste and seed are some examples that have contributed to food born outbreaks (Lehmacher et al. 1995; Brockmann et al. 2004). There was a correlation found between biofilm formation of Salmonella and its persistence in fish meal and feed factories (Vestby et al. 2009). Salmonella is known to produce biofilm on surfaces of plant and plant products and biofilm promotes its persistence by protecting from washing and sanitization (Andino and Hanning 2015). In a study carried out by Hamilton et al. 2009, biofilm of S. typhimurium has been proven as an inducer of bacterial attachment to abiotic surfaces, potential infection reservoir and stimulator of genes involved in tryptophan biosynthesis (trp) and transport. Deletion of trpE gene resulted in decreased attachment of the bacteria showing the importance of its biofilm-mediated induction (Hamilton et al. 2009). A 2–5% of Salmonella-infected patients fail to completely get rid of the bacteria from their body thus becoming chronic carriers. In the situation of chronic infection, Salmonella chooses gall bladder as its niche and invades gall bladder either from liver, small intestine or via biliary tract. Once it is settled on the organ, it starts forming biofilm on the surface leading to many complications including reinfection of intestine, gall stone and transmission to naive host through faecal route (Crawford et al. 2010). A recent finding has very elegantly shown that, Salmonella exists in the form of biofilm in persistently infected host and in absence of biofilm, it kills the host in short span of time by inhibiting innate immune response of the host. In a Caenorhabditis elegans infection model, biofilm formation by Salmonella was found to activate host defence and thereby obtained some growth advantages in vivo. During biofilm formation, downregulation of Salmonella SP-1 virulence genes stimulated the activation of host innate immune pathway and thereby created a state of dormancy for its prolonged survival inside host (Desaia et al. 2019). Another major risk biofilm forming Salmonella in gall bladder causes is gastrointestinal cancer which is still an enigmatic area for the researchers (Koshiol et al. 2016).
Biofilm and gastrointestinal cancer
Though the association of bacteria induced inflammation and cancer progression has been the focus of researchers since many years, the mechanism by which bacteria induce tumorigenesis is poorly understood. Reports have shown that, carriers of S. typhi and S. paratyphi are more susceptible to pancreas, colon, lung and gall bladder cancer (Caygill et al. 1994). According to several epidemiological studies carried out at different places including Ecuador, Bolivia, Chile, Pakistan, Japan, Korea and some parts of India, about 90% of chronically infected S. typhi carriers also have gall stones and this relationship is a major factor for gall bladder cancer development (Gunn et al. 2014; Caygill et al. 1994; Dutta et al. 2000; Nath et al. 2008; Escobedo et al. 2011). Though genetics and life style are two important factors for any kind of malignancy, infection with S. typhi and gall stone remain the most significant cause for gall bladder cancer (Nagaraja and Eslick 2014). In a study carried out in Mexico City, which is a typhoid endemic site, S. typhi carriers were found to have gall stones and biofilm was clearly visible on gall stones. Furthermore, using a S. typhimurium infected mice model, the group has shown presence of biofilm on mice gall stone in addition to human patients and its role in facilitating gall bladder colonization and shedding of the pathogen (Caygill et al. 1994). Despite plenty of evidences showing positive corelation between S. typhi, gall stone and gall bladder cancer, their actual role in the cancer initiation is still a matter of debate. Production of biofilm by S. typhi might be the key player in development of gall bladder cancer, as its stimulatory effect on colonization and chronic persistence of the pathogen in gall bladder, leading to formation of gall stone. Salmonella biofilm embeds the gall stone which becomes a favourable site for bacteria persistence, causes reinfection of intestine, induces transmission through faecal shedding and most importantly chronically making the gall bladder epithelium more vulnerable towards bacteria produced carcinogenic factors (Di Domenico et al. 2017). S. typhi produces many factors of carcinogenic potential such as glucuronidase, nitroso compounds and cytolethal destending toxins (CDT), etc., which might be the contributing factors for gall bladder cancer (Di Domenico et al. 2017). Moreover, biofilm on gall stone provides all kind of advantages to S. typhi (protection from antibiotics, bile and other harsh conditions etc.) to grow and survive better as well as produce carcinogenic factors making a conducive environment to development of gall bladder cancer. Though there is a strong association between S. typhi chronic carriers’ status and presence of gall stone, the putative link between these events and gall bladder cancer has not been explored till date. Molecular characterization of events regulating biofilm formation on gall stone, S. typhi-mediated inflammation and damage of gall bladder and role of CDT toxins that induce damage in DNA and activation of MAPK-AKT is needed to understand the mechanistic ground. However, early detection of S. typhi can be considered as a critical clinical practice to develop preventive measures for gall bladder cancer.
Conclusion
The recent past decade has extensively focussed on generating a wealth of knowledge about how microbes utilize quorum sensing and biofilm as their media of communication and coordination of their diverse functions. This knowledge has further been exploited as a foundation pillar to investigate the role of these events beyond just cross-talk among microbial population. This emerging field of research and its findings are relevant to the interest of this review which aims at understanding how both quorum sensing and biofilm help Salmonella to achieve success in infection establishment in diversified environmental conditions. The current review gathers most important information related to involvement of these two phenomena in Salmonella pathogenesis. Comparatively lesser number of studies on the association of quorum sensing with Salmonella virulence was a major challenge to point out its probable role in the process of disease incidence and progression. Another experimental issue with S. typhi is that, this pathogen is human host restricted. Though S. typhimurium infection in animal models mimics S. typhi infection closely, investigating the exact role of S. typhi biofilm formation in gall stone and gall bladder cancer would have been the ultimate system. Figures presented in this review show the associated risk of biofilm forming Salmonella as their increasing antibiotic resistance could become a public health threat. So, screening for biofilm forming Salmonella during initial period of infection is strongly recommended for better treatment strategy and administration of suitable antibiotics. However, future studies with thorough investigation are required to shed light on biofilm-mediated modulation of host immune system as well as biofilm-induced molecular markers aggravating the pathogenesis. Understanding, whether both quorum sensing and biofilm are directly making the pathogen more virulent or the pathogen is just taking the advantage of the favourable environment made by these events indirectly will also enlighten us regarding developing effective regimens to inhibit their association.
References
Abdelwaheb C, Ahmed L (2009) Acid pre-adaptation enhances virulence of Salmonella enterica serovar Typhimurium dam mutant. Pathol Biol 57(5):358–362. https://doi.org/10.1016/j.patbio.2008.02.020
Al Marjania M-F, Kouhsari E, Ali F-S, Authman S-H (2021) Evaluation of type II toxin-antitoxin systems, antibiotic resistance profiles, and biofilm quorum sensing genes in Acinetobacter baumannii isolates in Iraq. Infect Disord Drug Targets 21(2):180–186. https://doi.org/10.2174/1871526520666200525170318
Andino A, Hanning I (2015) Salmonella enterica: survival, colonization, and virulence differences among serovars. Sci World J. https://doi.org/10.1155/2015/520179
Arciola CR, Campoccia D, Speziale V, Montanaro L, Costerton JW (2012) Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 33(26):5967–5982. https://doi.org/10.1016/j.biomaterials.2012.05.031
Arnold JW, Bailey GW (2000) Surface finishes on stainless steel reduce bacterial attachment and early biofilm formation: scanning electron and atomic force microscopy study. Poult Sci 79(12):1839–1845. https://doi.org/10.1093/ps/79.12.1839
Aswathanarayan JB, Vittal RR (2018) Inhibition of biofilm formation and quorum sensing mediated phenotypes by berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv 8:36133. https://doi.org/10.1039/C8RA06413J
Avila-Novoa MG, Guerrero-Medina PJ, Navarrete-Sahagún V, Gómez-Olmos I, Velázquez-Suárez NY, De la CL, Gutiérrez-Lomelí M (2021) Biofilm formation by multidrug-resistant serotypes of salmonella isolated from fresh products: effects of nutritional and environmental conditions. Appl Sci 11:3581. https://doi.org/10.3390/app11083581
Baugh S, Ekanayaka AS, Piddock V, LJ, Webber MA, (2012) Loss of or inhibition of all multidrug resistance efflux pumps of Salmonella enterica serovar typhimurium results in impaired ability to form a biofilm. J Antimicrob Chemother 67(10):2409–2417. https://doi.org/10.1093/jac/dks228
Beuchat LR (2002) Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect 4(4):413–423. https://doi.org/10.1016/S1286-4579(02)01555-1
Boddicker JD, Ledeboer NA, Jagnow J, Jones BD, Clegg S (2002) Differential binding to and biofilm formation on HEp-2 cells by Salmonella enterica serovar typhimurium is dependent upon allelic variation in the fimH gene of the fim gene cluster. Mol Microbiol 45(5):1255–1265. https://doi.org/10.1046/j.1365-2958.2002.03121.x
Brockmann SO, Piechotowski I, Kimmig P (2004) Salmonella in sesame seed products. J Food Prot 67(1):178–180. https://doi.org/10.4315/0362-028X-67.1.178
Bruno VM, Hannemann S, Lara-Tejero M, Flavell RA, Kleinstein SH, Galan JE (2009) Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog 5(8):e1000538. https://doi.org/10.1371/journal.ppat.1000538
Caygill CP, Hill MJ, Braddick M, Sharp JC (1994) Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 343:83–84. https://doi.org/10.1016/S0140-6736(94)90816-8
Chaudhuri D, Chowdhury AR, Biswas B, Chakravortty D (2018) Salmonella typhimurium infection leads to colonization of the mouse brain and is not completely cured with antibiotics. Front Microbiol 9:1632. https://doi.org/10.3389/fmicb.2018.01632
Chen L, Wen Y (2011) The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci 3(2):73. https://doi.org/10.4248/IJOS11022
Chen L, Gu L, Geng X, Xu G, Huang X, Zhu X (2020) A novel cis antisense RNA AsfD promotes Salmonella enterica serovar Typhi motility and biofilm formation. Microb Pathog 142:104044. https://doi.org/10.1016/j.micpath.2020.104044
Chiu C, Su L, Chu C (2004) Salmonella enterica serotype choleraesuis: epidemiology, pathogenesis, clinical disease, and treatment. Clin Microbiol Rev 17(2):311–322. https://doi.org/10.1128/CMR.17.2.311-322.2004
Choi J, Shin D, Ryu S (2007) Implication of quorum sensing in Salmonella enterica serovar typhimurium virulence: the luxS gene is necessary for expression of genes in pathogenicity island. Infect Immun 75(10):4885–4890. https://doi.org/10.1128/IAI.01942-06
Choi J, Shin D, Kim M, Park J, Lim S, Ryu S (2012) LsrR-mediated quorum sensing controls invasiveness of Salmonella typhimurium by regulating SPI-1 and flagella genes. PLoS ONE 7(5):37059. https://doi.org/10.1371/journal.pone.0037059
Chorianopoulos NG, Giaouris ED, Kourkoutas Y, Nychas GJ (2010) Inhibition of the early stage of Salmonella enterica serovar Enteritidis biofilm development on stainless steel by cell-free supernatant of a Hafnia alvei culture. Appl Environ Microbiol 76(6):2018–2022. https://doi.org/10.1128/AEM.02093-09
Conceiçao RCS, Sturbelle RT, Timm CD, Leivas Leite FP (2015) Inducers and autoinducers on Salmonella enterica serovar Typhimurium motility, growth and gene expression. Cienc Rural 45:2201–2206. https://doi.org/10.1590/0103-8478cr20150295
Crawford RW, Gibson DL, Kay WW, Gunn JS (2008) Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect Immun 76(11):3541e9. https://doi.org/10.1128/IAI.00786-08
Crawford RW, Rosales-Reyes R, Ramirez-Aguilar Mde L, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS (2010) Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Nat Acad Sci USA 107:4353–4358. https://doi.org/10.1073/pnas.1000862107
Cui L, Wang X, Huang D, Zhao Y, Feng J, Lu Q, Pu Q, Wang Y, Cheng G, Wu M, Dai M (2020) CRISPR-cas3 of Salmonella upregulates bacterial biofilm formation and virulence to host cells by targeting quorum-sensing systems. Pathogens 9:53. https://doi.org/10.3390/pathogens9010053
Culler HF, Couto SCF, Higa JS, Ruiz RM, Yang MJ, Bueris V, Franzolin MR, Sircili MP (2018) Role of SdiA on biofilm formation by atypical enteropathogenic Escherichia coli. Genes 9(5):253. https://doi.org/10.3390/genes9050253
Cwiek K, Korzekwa K, Tabis A, Bania J, Bugla-Ploskonska G, Wieliczko A (2020) Antimicrobial resistance and biofilm formation capacity of Salmonella enterica serovar enteritidis strains isolated from poultry and humans in Poland. Pathogens 9(8):643. https://doi.org/10.3390/pathogens9080643
Da Re S, Ghigo J (2006) A CsgD-independent pathway for cellulose production and biofilm formation in Escherichia coli. J Bacteriol 188(8):3073–3087. https://doi.org/10.1128/JB.188.8.3073-3087.2006
Da Silva DP, Schofield MC, Parsek MR, Tseng B (2017) An update on the sociomicrobiology of quorum sensing in Gram-negative biofilm development. Pathogens 6(4):51. https://doi.org/10.3390/pathogens6040051
Davies D (2003) Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2(2):114–122. https://doi.org/10.1038/nrd1008
De Almeida FA, Pimentel-Filho NDJ, Pinto UM, Mantovani HC, De Oliveira LL, Dantas Vanetti MC (2017) Acyl homoserine lactone-based quorum sensing stimulates biofilm formation by Salmonella Enteritidis in anaerobic conditions. Arch Microbiol 199(3):475–486. https://doi.org/10.1007/s00203-016-1313-6
Desaia SK, Padmanabhana A, Harshea S, Zaidel-Barb R, Kenney LJ (2019) Salmonella biofilms program innate immunity for persistence in Caenorhabditis elegans. Proc Nat Acad Sci USA 116(25):12462–12467. https://doi.org/10.1073/pnas.1822018116
Di Domenico EG, Cavallo I, Pontone M, Toma M, Ensoli F (2017) Biofilm producing Salmonella Typhi: chronic colonization and development of gallbladder cancer. Int J Mol Sci 18(9):1887. https://doi.org/10.3390/ijms18091887
Dutta U, Garg PK, Kumar R, Tandon RK (2000) Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am J Gastroenterol 95(3):784–787. https://doi.org/10.1111/j.1572-0241.2000.01860.x
Dykes GA, Sampathkumar B, Korber DR (2003) Planktonic or biofilm growth affects survival, hydrophobicity and protein expression patterns of a pathogenic Campylobacter jejuni strain. Int J Food Microbiol 89(1):1–10. https://doi.org/10.1016/S0168-1605(03)00123-5
Escobedo GG, Gunn JS (2013) Identification of Salmonella enterica serovar typhimurium genes regulated during biofilm formation on cholesterol gallstone surfaces. Infect Immun 81(10):3770–3780. https://doi.org/10.1128/IAI.00647-13
Escobedo GG, Marshall JM, Gunn JS (2011) Chronic and acute infection of the gall bladder by Salmonella Typhi: Understanding the carrier state. Nat Rev Microbiol 9(1):9–14. https://doi.org/10.1038/nrmicro2490
Fàbrega A, Vila AJ (2013) Salmonella enterica Serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev 26(2):308–341. https://doi.org/10.1128/CMR.00066-12
Farahani RK, Ehsani P, Ebrahimi-Rad M, Khaledi A (2018) Molecular detection, virulence genes, biofilm formation, and antibiotic resistance of Salmonella enterica Serotype enteritidis isolated from poultry and clinical samples. Jundishapur J Microbiol 11(10):69504. https://doi.org/10.5812/jjm.69504
Flemming HC, Wingender J (2002) Extracellular polymeric substances: structure, ecological functions, technical relevance. In: Bitton G (ed) Encyclopedia of environmental microbiology. Wiley, NewYork, pp 1223–1231. https://doi.org/10.1002/0471263397.env292
Gong J, Xu M, Zhu C, Miao J, Liu X, Xu B, Zhang J, Yu Y, Jia X (2013) Antimicrobial resistance, presence of integrons and biofilm formation of Salmonella pullorum isolates from eastern China (1962_2010). Avian Pathol 42(3):290–294. https://doi.org/10.1080/03079457.2013.788129
González JF, Alberts H, Lee J, Doolittle L, Gunn JS (2018) Bioflm formation protects Salmonella from the antibiotic ciprofoxacin in vitro and in vivo in the mouse model of chronic carriage. Sci Rep 8:222. https://doi.org/10.1038/s41598-017-18516-2
Gunn JS, Marshall JM, Baker S, Dongol S, Charles RC, Ryan ET (2014) Salmonella chronic carriage: epidemiology, diagnosis and gallbladder persistence. Trends Microbiol 22(11):648–655. https://doi.org/10.1016/j.tim.2014.06.007
Hamilton S, Bongaerts JMR, Mulholland F, Cochrane B, Porter J, Lucchini S, Lappin-Scott HM, Hinton CDJ (2009) The transcriptional programme of Salmonella enterica serovar typhimurium reveals a key role for tryptophan metabolism in biofilms. BMC Genomics 10:599. https://doi.org/10.1186/1471-2164-10-599
Heydari S, Eftekhar F (2015) Biofilm formation and β-lactamase production in burn isolates of Pseudomonas aeruginosa. Jundishapur J Microbiol 8(3):e15514. https://doi.org/10.5812/jjm.15514
House D, Bishop A, Parry C, Dougan G, Wain J (2001) Typhoid fever: pathogenesis and disease. Curr Opin Infect Dis 14(5):573–578. https://doi.org/10.1097/00001432-200110000-00011
Jason Chin KC, Taylor TD, Hebrard M, Anbalagan K, Dashti MG, Phua KK (2017) Transcriptomic study of Salmonella enterica subspecies enterica serovar typhi biofilm. BMC Genomics 18(1):836. https://doi.org/10.1186/s12864-017-4212-6
Jennings LK, Storek KM, Ledvina HE, Coulon C, Marmont LS, Sadovskaya I, Filloux A (2015) Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc Nat Acad of Sci 112(36):11353–11358. https://doi.org/10.1073/pnas.1503058112
Jesudhasan PR, Cepeda ML, Widmer K, Dowd SE, Soni KA, Hume ME, Zhu J, Pillai SD (2010) Transcriptome analysis of genes controlled by luxS/autoinducer-2 in Salmonella enterica serovar typhimurium. Foodborne Pathog Dis 7(4):399–341. https://doi.org/10.1089/fpd.2009.0372
Ji Y, Li W, Zhang Y, Chen L, Zhang Y, Zheng X, Ni B (2017) QseB mediates biofilm formation and invasion in Salmonella enterica serovar Typhi. Microb Pathog 104:6–11. https://doi.org/10.1016/j.micpath.2017.01.010
Joseph B, Otta SK, Karunasagar I, Karunasagar I (2001) Biofilm formation by salmonella spp on food contact surfaces and their sensitivity to sanitizers. Int J Food Microbiol 64(3):367–372. https://doi.org/10.1016/S0168-1605(00)00466-9
Khambhati K, Patel J, Saxena V, Parvathy A, Jain N (2021) Gene regulation of biofilm-associated functional amyloids. Pathogens 10:490. https://doi.org/10.3390/pathogens10040490
Kim S-H, Wei C-I (2009) Molecular characterization of biofilm formation and attachment of Salmonella enterica serovar Typhimurium DT104 on food contact surfaces. J Food Prot 72(9):1841–1847. https://doi.org/10.4315/0362-028X-72.9.1841
Koshiol J, Wozniak A, Cook P, Adaniel C, Acevedo J, Azocar L, Hsing AW, Roa JC, Pasetti MF, Miquel JF, Levine MM, Ferreccio C (2016) Salmonella enterica serovar Typhi and gallbladder cancer: a case-control study and meta-analysis. Cancer Med 5(11):3310–3235. https://doi.org/10.1002/cam4.915
Krishna D, Dhanashree B (2020) Antibiogram, virulence genes, and biofilm-forming ability of clinical Salmonella enterica Serovars: an in vitro study. Microb Drug Resist. https://doi.org/10.1089/mdr.2020.0419
Latasa C, Roux A, Toledo-Arana A, Ghigo J-M, Gamazo C, Penades J-R, Lasa I (2005) BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar Enteritidis. Mol Microbiol 58(5):1322–1339. https://doi.org/10.1111/j.1365-2958.2005.04907.x
Lee K, Lee J, Roy PK, Mizan MFR, Hossain MI, Park SH, Ha S (2020) Viability of Salmonella Typhimurium biofilms on major food-contact surfaces and eggshell treated during 35 days with and without water storage at room temperature. Poult Sci 99(9):4558–4565. https://doi.org/10.1016/j.psj.2020.05.055
Lehmacher A, Bockemuhl J, Aleksic S (1995) Nationwide outbreak of human salmonellosis in Germany due to contaminated paprika and paprika-powdered potato chips. Epidemiol Infect 115(3):501–511. https://doi.org/10.1017/S0950268800058660
Machado I, Silva LR, Giaouris ED, Melo LF, Simoes M (2019) Quorum sensing in food spoilage and natural-based strategies for its inhibition. Food Res Int 127:108754. https://doi.org/10.1016/j.foodres.2019.108754
Majtan J, Majtanova L, Xu M, Majtan V (2008) In vitro effect of subinhibitory concentrations of antibiotics on biofilm formation by clinical strains of Salmonella enterica serovar typhimurium isolated in Slovakia. J Appl Microbiol 104(5):1294–1301. https://doi.org/10.1111/j.1365-2672.2007.03653.x
Marin C, Hernandiz A, Lainez M (2009) Biofilm development capacity of Salmonella strains isolated in poultry risk factors and their resistance against disinfectants. Poult Sci 88(2):424–431. https://doi.org/10.3382/ps.2008-00241
Mehmood A, Liu G, Wang X, Meng G, Wang C, Liu Y (2019) Fungal quorum-sensing molecules and inhibitors with potential antifungal activity: a review. Molecules 24(10):1950. https://doi.org/10.3390/molecules24101950
Mon KKZ, Saelao P, Halstead MM, Chanthavixay G, Chang H-C, Garas L, Maga EA, Zhou H (2015) Salmonella enterica serovars enteritidis infection alters the indigenous microbiota diversity in young layer chicks. Front Vet Sci 2:61. https://doi.org/10.3389/fvets.2015.00061
Moreira CG, Weinshenker D, Sperandio V (2010) QseC mediates Salmonella enterica serovar typhimurium virulence in vitro and in vivo. Infect Immun 78(3):914–926. https://doi.org/10.1128/IAI.01038-09
Moshiri J, Kaur D, Hambira CM, Sandala JL, Koopman JA, Fuchs JR, Gunn JS (2018) Identification of a small molecule anti-biofilm agent against Salmonella enterica. Front Microbio 9:2804. https://doi.org/10.3389/fmicb.2018.02804
Mukhopadhyay B, Sur D, Sen Gupta S, Ganguly NK (2019) Typhoid fever: control & challenges in India. Indian J Med Res 150(5):437–447. https://doi.org/10.4103/ijmr.IJMR_411_18
Nagaraja V, Eslick G (2014) Systematic review with meta-analysis: The relationship between chronic Salmonella typhi carrier status and gall-bladder cancer. Aliment Pharmacol Ther 39(8):745–750. https://doi.org/10.1111/apt.12655
Nakano M, Yamasaki E, Ichinose A, Shimohata T, Takahashi A, Akada JK, Nakamura K, Moss J, Hirayama T, Kurazono H (2012) Salmonella enterotoxin (Stn) regulates membrane composition and integrity. Dis Model Mech 5:515–521. https://doi.org/10.1242/dmm.009324
Nath G, Singh YK, Kumar K, Gulati AK, Shukla VK, Khanna AK, Tripathi SK, Jain AK, Kumar M, Singh TB (2008) Association of carcinoma of the gallbladder with typhoid carriage in a typhoid endemic area using nested PCR. J Infect Dev Ctries 2(4):302–307. https://doi.org/10.3855/jidc.226
Ogasawara H, Yamamoto K, Ishihama A (2011) Role of the biofilm master regulator CsgD in cross-regulation between biofilm formation and flagellar synthesis. J Bacteriol 193:2587–2597. https://doi.org/10.1128/JB.01468-10
Papavasileiou K, Papavasileiou E, Tseleni-Kotsovili A, Bersimis S, Nicolaou C, Ioannidis A, Chatzipanagiotou S (2010) Comparative antimicrobial susceptibility of biofilm versus planktonic forms of Salmonella enterica strains isolated from children with gastroenteritis. Eur J Clin Microbiol Infect Dis 29:1401–1405. https://doi.org/10.1007/s10096-010-1015-y
Parry CM, Threlfall EJ (2008) Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr Opin Infect Dis 21(5):531–538. https://doi.org/10.1097/QCO.0b013e32830f453a
Pellock BJ, Teplitski M, Boinay RP, Bauer WD, Walker GC (2002) A luxR homolog controls production of symbiotically active extracellular polysaccharide II by Sinorhizobium meliloti. J Bacteriol 184(18):5067–5076. https://doi.org/10.1128/JB.184.18.5067-5076.2002
Perrett CA, Karavolos MH, Humphrey S, Mastroeni P, Argudo IM, Spencer H, Bulmer D, Winzer K, McGhie E, Koronakis V, Williams P, Khan CM, Jepson MA (2009) LuxS-based quorum sensing Does not affect the ability of Salmonella enterica serovar typhimurium to express the SPI-1 Type 3 secretion system, induce membrane ruffles, or invade epithelial cells. J Bacterio 191(23):7253–7259. https://doi.org/10.1128/JB.00727-09
Prouty AM, Schwesinger WH, Gunn JS (2002) Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun 70(5):2640–2649. https://doi.org/10.1128/IAI.70.5.2640-2649.2002
Remy B, Mion S, Plener L, Elias M, Chabriere E, Daude D (2018) Interference in bacterial quorum sensing: a biopharmaceutical perspective. Front Pharmacol 9:203. https://doi.org/10.3389/fphar.2018.00203
Romeu M-J, Rodrigues D, Azeredo J (2020) Effect of sub-lethal chemical disinfection on the biofilm forming ability, resistance to antibiotics and expression of virulence genes of Salmonella Enteritidis biofilm-surviving cells. Biofouling 36(1):101–112. https://doi.org/10.1080/08927014.2020.1719077
Ruby T, McLaughlin L, Gopinath S, Monack D (2012) Salmonella’s long-term relationship with its host. FEMS Microbiol Rev 36(3):600–615. https://doi.org/10.1111/j.1574-6976.2012.00332.x
Scher K, Romling U, Yaron S (2005) Effect of heat, acidification, and chlorination on Salmonella enterica serovar typhimurium cells in a biofilm formed at the air-liquid interface. Appl Environ Microbiol 71(3):1163–1168. https://doi.org/10.1128/AEM.71.3.1163-1168.2005
Sheng X, Wang W, Chen L, Zhang H, Zhang Y, Xu S, Huang X (2019) Mig-14 may contribute to Salmonella enterica serovar Typhi resistance to polymyxin B by decreasing the permeability of the outer-membrane and promoting the formation of biofilm. Int J Med Microbiol 309(2):143–150. https://doi.org/10.1016/j.ijmm.2019.01.001
Shi L, Wu Y, Yang C, Ma Y, Zhang QZ, Huang W, Zhao K-Q (2019) Effect of nicotine on Staphylococcus aureus biofilm formation and virulence factors. Sci Rep 9(1):1–13. https://doi.org/10.1038/s41598-019-56627-0
Simm R, Morr M, Kader A, Nimtz M, Romling U (2004) GGDEF and EAL domains inversely regulate cyclic d-GMP levels and transition from sessility to motility. Mol Microbiol 53:1123–1134. https://doi.org/10.1111/j.1365-2958.2004.04206.x
Song J, Gao X, Gal´an JE (2013) Structure and function of the Salmonella typhi chimaeric A2B5 typhoid toxin. Nature 499(7458):350–354. https://doi.org/10.1038/nature12377
Surette MG, Bassler BL (1998) Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc Natl Acad Sci 95(12):7046–7050. https://doi.org/10.1073/pnas.95.12.7046
Swofford CA, Dessel NV, Forbes NS (2015) Quorum-sensing Salmonella selectively trigger protein expression within tumors. Proc Natl Acad Sci 112(11):3457–3462. https://doi.org/10.1073/pnas.1414558112
Tabak M, Scher K, Chikindas ML, Yaron S (2019) The synergistic activity of triclosan and ciprofloxacin on biofilms of Salmonella typhimurium. FEMS Microbiol Let 301(1):69–76. https://doi.org/10.1111/j.1574-6968.2009.01804.x
Tezela BU, Ak,celikb N, Y€ukselc FN, Karatugc NT, Ak,celikc M (2016) Effects of sub-MIC antibiotic concentrations on biofilm production of Salmonella infantis. Biotechnol Biotechnol Equip 30(6):1184–1191. https://doi.org/10.1080/13102818.2016.1224981
Trampari E, Holden ER, Wickham GJ, Ravi A, De Oliveira ML, Savva GM, Webber MA (2021) Exposure of Salmonella biofilms to antibiotic concentrations rapidly selects resistance with collateral tradeoffs. NPJ Biofilms Microbi 7(1):3. https://doi.org/10.1038/s41522-020-00178-0
Vestby LK, Moretro T, Langsrud S, Even H, Nesse L (2009) Biofilm forming abilities of Salmonella are correlated with persistence in fish meal- and feed factories. BMC Vet Res 5(20):1005–1012. https://doi.org/10.1186/1746-6148-5-20
Vuotto C, Longo F, Pascolini C, Donelli G, Balice M-P, Libori M-F, Varaldo P-E (2017) Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J Appl Microbiol 123(4):1003–1018. https://doi.org/10.1111/jam.13533
Walawalkar YD, Vaidya Y, Nayak V (2016) Response of Salmonella Typhi to bile-generated oxidative stress: implication of quorum sensing and persister cell populations. Pathog Dis 74(8):090. https://doi.org/10.1093/femspd/ftw090
Wang HH, Ye KP, Zhang QQ, Dong Y, Xu XL, Zhou GH (2013) Biofilm formation of meat-borne Salmonella enterica and inhibition by the cell-free supernatant from Pseudomonas aeruginosa. Food Control 32(2):650–658. https://doi.org/10.1007/s13197-018-3222-y
White AP, Gibson DL, Grassl GA, Kay WW, Finlay BB, Vallance BA, Surette MG (2008) Aggregation via the red, dry, and rough morphotype is not a virulence adaptation in Salmonella enterica serovar typhimurium. Infect Immun 76(3):1048–1058. https://doi.org/10.1128/IAI.01383-07
Widmer KW, Jesudhasan PR, Dowd SE, Pillai SD (2007) Differential expression of virulence-related genes in a Salmonella enterica serotype Typhimurium luxS mutant in response to autoinducer AI-2 and poultry meat-derived AI-2 inhibitor. Foodborne Pathog Dis 4(1):5–15. https://doi.org/10.1089/fpd.2006.40
Widmer KW, Jesudhasan P, Pillai SD (2012) Fatty acid modulation of autoinducer (AI-2) influenced growth and macrophage invasion by Salmonella Typhimurium. Foodborne Pathog Dis 9(3):211–217. https://doi.org/10.1089/fpd.2011.0949
Xu H, Lee HY, Ahn J (2010) Growth and virulence properties of biofilm-forming Salmonella enterica serovar typhimurium under different acidic conditions. Appl Environ Microbiol 76(24):7910–7917. https://doi.org/10.1128/AEM.01508-10
Young D, Hussell T, Dougan G (2002) Chronic bacterial infections: living with unwanted guests. Nat Immunol 3(11):1026–1032. https://doi.org/10.1038/ni1102-1026
Zhang X-S, García-Contreras R, Wood T-K (2007) YcfR (BhsA) influences Escherichia coli biofilm formation through stress response and surface hydrophobicity. J Bacteriol 189(8):3051–3062. https://doi.org/10.1128/JB.01832-06
Zhao X, Liu R, Tang H, Osei-Adjei G, Xu S, Zhang Y, Huang X (2018) A 3′ UTR-derived non-coding RNA RibS increases expression of cfa and promotes biofilm formation of Salmonella enterica serovar Typhi. Res Microbiol 169(6):279–288. https://doi.org/10.1016/j.resmic.2018.04.007
Zogaj X, Nimtz M, Rohde M, Bokranz W, Römling U (2001) The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol 39(6):1452–1463. https://doi.org/10.1046/j.1365-2958.2001.02337.x
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rana, K., Nayak, S.R., Bihary, A. et al. Association of quorum sensing and biofilm formation with Salmonella virulence: story beyond gathering and cross-talk. Arch Microbiol 203, 5887–5897 (2021). https://doi.org/10.1007/s00203-021-02594-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02594-y