Abstract
With an upsurge in multidrug resistant bacteria backed by biofilm defence armours, there is a desperate need of new antibiotics with a non-traditional mechanism of action. Targeting bacteria by misguiding them or halting their communication is a new approach that could offer a new way to combat the multidrug resistance problem. Quorum sensing is considered to be the achilles heel of bacteria that has a lot to offer. Since, both quorum sensing and biofilm formation have been related to drug resistance and pathogenicity, in this study we synthesised new derivatives of citral with antiquorum sensing and biofilm disrupting properties. We previously reported antimicrobial and antiquorum sensing activity of citral and herein we report the synthesis and evaluation of citral and its derivatives (CD1-CD3) for antibacterial, antibiofilm and antiquorum sensing potential against Chromobacterium violaceum using standard methods. Preliminary results revealed that CD1 is the most active of all the derivatives. Qualitative and quantitative evaluation of antiquorum sensing activity at sub-inhibitory concentrations of these compounds also revealed high activity for CD1 followed by CD2, CD3 and citral. These compounds also inhibit biofilm formation at subinhibitory concentrations without causing any bacterial growth inhibition. These results were replicated by RT-qPCR with down regulation of the quorum sensing genes when C. violaceum was treated with these test compounds. Overall, the results are quite encouraging, revealing that biofilm and quorum sensing are interrelated processes and also indicating the potential of these derivatives to impede bacterial communication and biofilm formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibiotics have been deemed as one of the greatest discoveries of last century that have saved more lives than anything else on the planet. Although, a plethora of mechanism of action of these drugs are known, fundamentally these drugs either kill or inhibit the growth of bacteria, thus imposing and inflicting evolutionary pressure on the bacteria and therefore promoting and driving their resistance to antibiotics. Among the various defence strategies of bacteria, the development of biofilms as bacterial defence armour is considered dangerously challenging (Koo et al. 2017). The multifactorial nature of biofilm development and drug tolerance imposes great challenges for the use of conventional antimicrobials and indicates the need for development of new antibiotics or treatment strategies (Bisht and Wakeman 2019). An effective strategy that could impose less evolutionary pressure on the microbes and disrupt the formation of biofilms is to target the bacterial cell-to-cell communication, also known as quorum sensing (Brackman and Coenye 2015; Roy et al. 2018; Lan et al. 2019). Quorum sensing controls a multitude of different physiological functions such as antibiotic production (Slater et al. 2003), biofilm differentiation (Coenye 2010), cell division (Chan and Chua 2010), sporulation (Jabbari et al. 2011), conjugation (White and Winans 2007), bioluminescence (Bassler and Losick 2006), secretion of virulence factors; including exoproteases, siderophores and exotoxins (Falcão et al. 2004; Rasmussen and Givskov 2006) as well as many other primary and secondary metabolites (Van Houdt et al. 2006; Rasmussen and Givskov 2006). Thus, quorum sensing allows a single prokaryote to behave like a multicellular organism (Zhu et al. 2011), and therefore targeting quorum sensing is now considered to be an effective and long-lasting strategy to tackle the multi-drug resistance and biofilm formation.
So far different natural and synthetic compounds have been successfully studied for the antiquorum sensing ability, however most of these quorum sensing inhibitory molecules have been identified as unsuitable for human use due to their high toxicity (Zhu et al. 2011). Therefore, developing semi-synthetic analogues or derivatives of natural products with desired properties is gaining much interest (Lan et al. 2019). In our previous study, we reported that citral possess good antimicrobial and antiquorum sensing activity that could be further ameliorated by synthesizing its derivatives with different functionalities (Ahmad et al. 2014). In continuation of that work, we herein report the synthesis and evaluation of three citral derivatives to develop new non-traditional antibacterial molecules that could target the bacterial quorum sensing and disrupt the bacterial biofilms.
Material and methods
Chemistry
Organic reagents and solvents were purchased from Sigma Aldrich and Merck (Germany) and used without further purification. Thin Layer Chromatography (TLC) was done using commercially available precoated aluminium sheets (silica gel 60 F254, Merck Germany) and visualization done under UV light. Heraeus Vario EL III analyser was used for elemental analysis. FTIR spectra were recorded on Bruker ALPHA FT-IR spectrometer (Eco-ATR). NMR spectra (1H and 13C) were recorded on Bruker AVANCE 400 spectrometer using DMSO-d6 or CDCl3 as solvent with TMS as internal standard. Splitting patterns are designated as follows; s, singlet; d, doublet; dd, doublet of doublets; t, triplet; m, multiplet. Chemical shift values are given in ppm. MICROMASS QUATTRO II triple quadrupole mass spectrometer was used to get the ESI–MS spectra.
Synthesis of citral derivatives (CD1-CD3)
The outline synthesis of citral derivatives (CD1-CD3) is given in Scheme 1.
3,7-dimethylocta-2,6-dienal oxime (CD1)
To a solution of citral (1 eq) and hydroxylamine hydrochloride (1.25 eq) in water (5 ml), a solution of sodium bicarbonate (1.25 eq) in water (10 ml) was added gradually with stirring, and the mixture was stirred for further 5 h. An organic layer was formed, which was separated and the aqueous layer was extracted with ether and added to the organic layer. The organic layer was dried with NaSO4 and evaporated.
Oil; Yield 80%; Anal. Calc. For C10H17NO: C 71.81, H 10.25, N 8.37%; found: C 71.79, H 10.26, N 8.39%; IR maxcm−1: 3250 (NO–H), 2864 (C–H), 1632 (C = N), 936 (N–O stretch); 1H NMR (DMSO-d6) (ppm): 8.95 (s, 1H, N–OH), 7.81 (s, 1H, CH = N–OH), 6.01 (d, 1H, = CH–CH=N, J = 11.7 Hz), 5.13–5.09 (t, 1H, = CH–CH2), 2.36–2.32 (t, 2H, CH2-CH2), 2.14–2.11 (t, 2H, CH2–CH2), 1.81 (s, 3H, CH3), 1.65 (s, 3H, CH3), 1.61 (s, 3H, CH3); 13CNMR (DMSO-d6) (ppm): 146.2 (C=N), 144.9, 132.2, 123.4, 121.7, 39.2, 26.2, 24.6, 21.2, 19.8; ESI–MS m/z: [M+ + 1] 168.15.
3,7-dimethylocta-2,6-dien-1-ylidene)hydrazine-1-carboxamide (CD2)
Citral (1 eq) was mixed with semicarbazide (1 eq) in ethanol with few drops of 10% NaOH under reflux for 5 h. Completion of the reaction was monitored by TLC. After completion of the reaction, the organic layer was extracted, washed, dried with NaSO4 and evaporated under vacuo.
Yield 75%; Anal. Calc. For C11H19N3O: C 63.13, H 9.15, N 20.08%; found: C 63.25, H 9.20, N 20.25%; IR νmaxcm−1: 3550, (NH), 3360, 3180 (NH2), 3010 (C–H) 1530 (C=N), 1650 (C=C), 1165 (C–N), 1085 (C=O); 1H NMR (DMSO-d6) d(ppm): 9.26 (1H, s, −NH), 6.95 (1H, d, –CH=N, 8.0 Hz), 6.90 (s, 2H, NH2), 5.97 (d, 1H, − CH, 4.0 Hz), 5.13–5.09 (t, 1H, = CH–CH2), 2.36–2.32 (t, 2H, CH2–CH2), 2.15–2.11 (t, 2H, CH2–CH2), 1.81 (s, 3H, CH3), 1.64 (s, 3H, CH3), 1.61 (s, 3H, CH3); 13CNMR (DMSO-d6) d(ppm): 156.9, 144.0, 141.8, 132.2, 123.4, 122.0, 39.4, 26.2, 24.9, 19.8, 17.9; ESI–MS m/z: [M+ + 1] 210.20.
3,7-dimethylocta-2,6-dien-1-ylidene)hydrazinecarbothioamide (CD3)
Citral (1 eq) was mixed with thiosemicarbazide (1 eq) in ethanol with few drops of 10% NaOH under reflux for 5 h. Completion of the reaction was monitored by TLC. After completion of the reaction, the organic layer was extracted, washed, dried with NaSO4 and evaporated under vacuo.
Yield 75%; Anal. Calc. For C11H19N3S: C 58.63, H 8.50, N 18.65%; found: C 58.85, H 8.20, N 18.85%; IR νmaxcm−1: 3545, (NH), 3386, 3180 (NH2), 3021 (C–H) 1534 (C=N), 1650 (C=C), 1168 (C–N), 1080 (C=S); 1H NMR (DMSO-d6) d(ppm): 9.32 (1H, s, − NH), 8.84 (s, 2H, NH2), 7.29 (1H, d, –CH=N, 8.0 Hz), 5.98 (d, 1H, − CH, 8.0 Hz), 5.13 (t, 1H, = CH–CH2), 2.36–2.33 (t, 2H, CH2–CH2), 2.15–2.11 (t, 2H, CH2–CH2), 1.82 (s, 3H, CH3), 1.64 (s, 3H, CH3), 1.61 (s, 3H, CH3);13CNMR (DMSO-d6) d(ppm): 179.9, 144.1, 141.6, 132.2, 123.4, 121.9, 39.4, 26.5, 24.9, 19.8, 17.9; ESI–MS m/z: [M+ + 1] 226.15.
Bacterial strain and growth conditions
An existing stock culture of Chromobacterium violaceum ATCC12472 stored at -80 °C supplemented with 20% glycerol in the Department of Clinical Microbiology and Infectious Diseases, University of the Witwatersrand, Johannesburg, was used in this study. For experimental purpose, the bacterial culture was revived by growing in Luria–Bertani (LB) agar plates at 30 °C for 24 h. All culture media and other chemicals used in this study are of high analytical grade.
Antimicrobial susceptibility testing
To determine the antibacterial activity of citral and its newly synthesised derivatives, minimum inhibitory concentration (MIC) for C. violaceum ATCC12472 was determined by broth microdilution susceptibility testing as per the recommended guidelines of Clinical and Laboratory Standards Institute (CLSI) reference document M07-A10 (Clinical and Laboratory Standards Institute 2015). The stock solutions of 250 µg/ml all the test compounds were prepared using 1% dimethyl sulfoxide (DMSO) and the range of concentrations tested was 62.5–0.031 µg/ml. In every set of experiment, cell free (sterility) and compound free (growth) controls were included. The MICs were defined as the lowest concentration of test compounds that resulted in the inhibition of visible growth. Results were calculated as a mean of experiments performed in triplicate.
Antiquorum sensing activity
Qualitative evaluation of antiquorum activity in C. violaceum
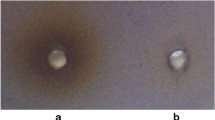
Citral and its three newly synthesised derivatives (CD1–CD3) were screened for their antiquorum activity by qualitatively measuring the violacein pigment production by biosensor strain C. violaceum ATCC12472, following the method described previously (Ahmad et al. 2015a). Briefly, 1.5 × 108 CFU ml−1 C. violaceum ATCC12472 cell suspension was mixed with molten LB agar at 45 ºC and gently poured into petri dishes and allowed to solidify. Sterile filter discs (Mast Diagnostics, Merseyside, UK) infused with 20 µl (MIC) of each test compound were placed on solid agar and incubated at 30 °C for 18 h. Sterile disc impregnated with 20 µl of 1% DMSO was used as a negative control. Following incubation, diameter of the clear zones representing growth inhibition and the opaque zones against a purple background representing violacein inhibition were measured in millimetres. For data analysis, the average diameters of the clear and turbid (i.e., opaque) zones were recorded. All the tests were repeated thrice and the results were reported as mean ± SD.
Quantitative evaluation of antiquorum activity in C. violaceum
Following the qualitative assay, quantification of violacein pigment inhibition was done in untreated and treated C. violaceum cells, using the microtiter plate method as described previously (Ahmad et al. 2015a). Briefly, C. violaceum ATCC12472 cells were sub-cultured on LB agar plates at 30 ºC for 24 h and a single colony from these plates was inoculated into 5 ml LB broth and incubated at 30 °C for 24 h with shaking. To confirm the capability of the test compounds to inhibit violacein production, 0.5 × MQSIC and MQSIC values of all the test compounds were added to 100 μl of LB broth in a microtiter plate. From the above prepared C. violaceum suspension, 100 µl were added to all the wells. In all the experiments, 1% DMSO was added as negative control. A culture control was included in each plate to confirm viability along with a media control to confirm sterility. Plates were incubated at 30 ºC for 24 h with shaking and the quantity of violacein was determined by measuring absorbance at OD585nm. The percentage of violacein suppression was determined using a formula:
The amount of violacein production in the control sample was employed to calculate the minimum quorum sensing inhibitory concentration (MQSIC) of test compounds and was determined as the minimum concentration at which violacein production was inhibited by ≥ 50%.
Biofilm inhibition assay
Biofilm inhibition assay was done using 96-well flat-bottom microtiter plate assay (Ravichandran et al. 2018). C. violaceum ATCC12472 cells were grown in LB broth for 24 h and 48 h at 30 ºC with 0.25 × MIC, 0.5 × MIC and 1 × MIC values of the test compounds. Following incubation, microtitre plates were washed with PBS (pH 7.4) to remove the free-floating planktonic cells and biofilms were stained using 200 μl of 0.1% crystal violet solution (Sigma—Aldrich St. Louis, MO, U.S.A.). After 15 min, crystal violet solution was removed and 200 μl of 95% ethanol was added. The biofilm was then quantified by measuring the absorbance at OD470 nm using iMark microplate reader (Bio-Rad laboratories, CA, USA).
Gene expression
Effect of the test compounds on the expression levels of the genes for quorum sensing (cviL, vioA, vioB, vioD and vioE gene) was evaluated by RT-qPCR. Briefly, C. violaceum cells at a concentration of 5 × 106 cells/ml were exposed to MIC of test compounds followed by incubation at 30 °C for 3 h. Untreated cells were used as negative control. Total RNA was extracted using High Pure RNA Isolation Kit (Roche, Basel, Switzerland), following manufacturer’s instructions. Concentration of total RNA was measured using Nanodrop 2000 spectrophotometer (Thermo Scientific, MA, USA). Thereafter, cDNA was synthesised using RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, MA, USA), according to manufacturer’s instructions. Following cDNA synthesis, RT-qPCR was performed using 2 × FastStart Essential DNA Green Master (Roche, Basel, Switzerland), 10 pmol of each primer (Anatech, Randburg, South Africa) in RocheLight® Cycler Nano instrument Real-time PCR system (Roche, Basel, Switzerland). The primer sequences used to amplify the above-mentioned genes and housekeeping genes are shown in Table 1. The following cycling conditions were employed for all amplifications: hot start at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 20 s, annealing at 52 °C for 20 s and extension at 72 °C for 20 s. Crossing-point analysis was employed to calculate the relative mRNA concentrations and specificity of amplicons was confirmed by melting curve analysis. Fold difference was calculated using formula 2−(ΔΔCt), where ΔCt was the mean Ct value of the target gene minus the mean of housekeeping genes, and ΔΔCt was the ΔCt of the tested cells minus ΔCt of the control cells.
Statistical analysis
GraphPad Prism software, version 8.1.0. was used for statistical analysis. Two-way ANOVA followed by Dunnett’s multiple comparisons test were applied to data. All the experiments were performed in triplicates (n = 3), and data were presented as mean ± standard deviation (SD). Value of P < 0.0001 was considered as statistically significant.
Results
Chemistry
The outline synthesis of citral derivatives (CD1–CD3) is given in Scheme 1. Characteristic FTIR bands provide significant indications for the formation of the oxime (CD1). The absence of a band at/or around 2665 cm−1 due to the proton of the aldehyde functional group in citral and the appearance of characteristic bands at 3260 cm−1 and 1620 cm−1 due to ν(NO–H) and ν(C=N), respectively, confirmed the formation of the oxime CD1. A singlet at 7.30 ppm due to imine (C–H=N) proton showed condensation between the carbonyl group of citral and amino group of hydroxylamine hydrochloride. The signal at 8.97 ppm assigned to (N–OH) proton further confirmed the formation of CD1. The structure of CD1 was further supported by 13C NMR spectra. The absence of any peak at 192 ppm which is characteristic of carbonyl carbon of the aldehyde and the presence of a signal at 149 ppm due to (C=N) confirmed the formation of CD1. Similarly the structure of semicarbazone and thiosemicarbazone derivatives (CD2 and CD3) was confirmed from their spectra. Semicarbazones and thiosemicarbazones may exhibit keto-enol or thione–thiol tautomerism, since they have a keto (C=O) and thione (C=S) group adjacent to a proton. It has been stated that the thione group (C=S) is relatively unstable in the monomeric form and tends to turn into a stable C–S single bond by tautomerism, if there is at least one hydrogen atom adjacent to the C=S bond. However, CD3 showed intense, strong bands in the region 1085 and 1088 cm–1 due to ν(C=S) stretch and no band near 2570 cm–1 due to ν(C–SH), suggesting that these compounds remain in the thione form. Semicarbazone CD2 showed similar spectrum which confirms the absence of any tautomerism. The spectra of these compounds also exhibit a strong band at 1530 and 1550 cm–1 region due to the ν(C=N) stretch of the azomethine linkage. The 1HNMR, 13CNMR and ESI MS spectra were also in good agreement with the structures of the compounds.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing of citral and its derivatives confirmed that these test compounds inhibit the growth of C. violaceum within the MIC range of 0.122–31.25 µg/ml (Table 2). All the test compounds showed high antimicrobial as well as antiquorum sensing activities with varying MIC values. Based on the MIC values, order of potency of the test compounds was CD1 > CD2 > CD3 > C.
Antiquorum sensing
Qualitative antiquorum sensing activity
Qualitative disc diffusion assay for antiquorum sensing activity of citral and its derivatives revealed their antiquorum properties at MIC (Table 2). The clear and turbid zones formed around sterile discs observed after performing the qualitative biosensor assay demonstrated inhibition of bacterial growth and antiquorum activity of test compounds, respectively. CD1 demonstrated the highest antiquorum sensing activity by effecting the formation of opaque zones with an average diameter of 22 mm, while parent compound C exerted the lowest antiquorum sensing efficacy resulting in the formation of turbid zones with an average diameter of about 13 mm (Table 2).
Quantitative antiquorum sensing activity
To further confirm and quantify the antiquorum activity of the test compounds, production of a quorum sensing related pigment, violacein, by C. violaceum was measured spectrophotometrically in treated and untreated cells. From the results, it is evident that all the test compounds significantly inhibited the production of violacein pigment at varying concentrations (Table 2). CD1 being the most active compound while inhibiting the growth and quorum sensing activity of C. violaceum effected the highest violacein suppression at MQSIC (94.17%) and 0.5 × MQSIC (78.1%) (Fig. 1). Other compounds also displayed high antiquorum sensing efficacy with violacein suppression ranging from 53 to 69% at 0.5 × MQSIC and 76% to 83% at MQSIC (Fig. 1). At 0.5 × MQSIC and MQSIC, where significant reduction in the production of violacein pigment was measured (P ≤ 0.0001), the numbers of cfu/ml present remained constant. For all experiments, the solvent controls showed similar cell growth and violacein production as observed in control cells, denoting that 1% DMSO neither inhibit cell growth nor inhibit violacein production.
Quantitative inhibition of violacein production by citral and its synthesised derivatives. C. violaceum cells were incubated with 0.5 × MQSIC and MQSIC values of test compounds for 24 h. Control bars (CN) indicate untreated cells, accepted as 0% inhibition. Data are presented from three independent experiments using means ± S.D. ****p ≤ 0.0001
Biofilm inhibition activity
A significant inhibition in biofilm formation was observed in C. violaceum cells after treated with different concentrations of test compounds for 24 or 48 h (Fig. 2). After 24 h of incubation, test entities showed inhibition in biofilm formation in the range of 28.84%–58.08%, 18.8%–35.38% and 9.7%–23.06% at concentrations of 1 × MIC, 0.5 × MIC and 0.25 × MIC, respectively. However, after 48 h of incubation, the higher rate of inhibition in biofilm formation was observed and the values were in the range of 38.68%–68.27%, 27.81%–49.41% and 20.64%–36.21% at 1 × MIC, 0.5 × MIC and 0.25 × MIC of test compounds, respectively. At sub-MIC values (0.5 × MIC and 0.25 × MIC) of test compounds, no reduction in bacterial growth was observed indicating that bacterial killing was not responsible for the lowered biofilm formation, whereas at MIC values significant reduction in bacterial growth was observed. The rate of inhibition in biofilm formation by these test compounds was depended on concentration and treatment time. Test compound CD1 showed the highest percentage of biofilm formation inhibition followed by CD2, CD3 and parent compound citrus, respectively.
Effect of citral and its synthesised derivatives on C. violaceum biofilm formation. C. violaceum cells were incubated with 0.25 × MIC, 0.5 × MIC and 1 × MIC values of test compounds for 24 and 48 h. Control bars (CN) indicate untreated cells, accepted as 0% inhibition. Data are presented from three independent experiments using means ± S.D. ****p ≤ 0.0001
Effect on quorum sensing associated genes
In a parallel investigation, an experiment was conducted to assess if administration of the test compounds would alter the expression levels of genes involved in quorum sensing. For this study, total RNA was extracted at 48 h post-administration and the relative expression of mRNAs corresponding to cviL, vioA, vioB, vioD and vioE genes was evaluated by RT-qPCR. Relative to untreated cells, compounds-treated bacterial cells significantly decreased expression of genes implicated in quorum sensing in C. violaceum, with CD1 being the most active compound followed by CD2, CD3 and citral, respectively (Fig. 3). All the test compounds at their MIC values downregulated the expression of all target genes, with a fold range of 3.0 to 4.3-fold, 2.3 to 2.8-fold, 1.8 to 2.2-fold and 1.5 to 1.7-folds by CD1, CD2, CD3 and citral, respectively, compared to control (untreated cells) that were set to 1.0 (Fig. 3). From these results, it can be concluded that citral and its derivatives downregulated the expression of above-mentioned genes which in turn inhibited N-acyl-L-homoserine lactone (AHL) synthase and thereby quorum sensing in C. violaceum.
Effect of citral and its synthesised derivatives at their respective MIC values on the expression of genes involved in quorum sensing. Cells without drug treatment (CN) were used as negative control. Data are presented from three independent experiments using means of fold changes ± S.D. ****p ≤ 0.0001
Discussion and conclusion
Quorum sensing, a communication system used by microbes based on the secretion and detection of external signal molecules, is highly regulated process and controls numerous functions in bacteria including pathogenicity and biofilm formation. Biofilm-associated bacteria are less likely to be cleared by our immune cells and they are more tolerant to antimicrobial therapies than non-biofilm-associated bacteria (Roy et al. 2018; Lebeaux et al. 2014). It has therefore become a challenge to eradicate biofilms using conventional antibiotics and to completely suppress the infection, despite high dosage and long-term treatment. Therefore, immediate measures are required for the development of antibiofilm drugs to fully cure biofilm-related infections. Since biofilm formation and quorum sensing are central and often interconnected features of bacterial lifestyle and it is now known that quorum sensing enables bacteria to turn on and off the secretion of EPS so as to increase their competitive ability against other strains and species within biofilms. Targeting quorum sensing is therefore an acknowledged strategy to stop bacteria from communicating and hence developing biofilms as their defence armour against any antibiotics (Rasko et al. 2008; Burt et al. 2014).
In our previous study, we reported the antimicrobial and antiquorum sensing properties of citral (Ahmad et al. 2015a). In continuation to that study, we further modified citral to change its functionality in the quest to get compounds with improved antimicrobial and antiquorum sensing properties. In this study, citral showed potent antimicrobial activity against the growth of C. violaceum and these results are in congruence with the previous findings where citral has been reported to possess high antibacterial activity (Shi et al. 2016). Modification of this compound further resulted in a significant increase of its antibiotic property by decreasing the MIC value up to 256-fold against C. violaceum when compared to the parent compound citral. These findings corroborate the work of other researchers where semi-synthetic modifications of natural compounds have been reported to increase their antimicrobial activity (Ahmad et al. 2015b; Shi et al, 2016).
Biofilm formation in bacteria is directly linked to the drug resistance and is currently a serious medical problem. Our results have shown that these derivatives significantly inhibit the biofilm formation at subinhibitory concentrations without killing the bacteria. This also signifies that the bacterial killing was not responsible for the reduction of biofilm formation. Our results are congruent with the previous findings, where natural compounds and synthetic derivatives are reported to inhibit biofilm formation at lower concentrations (Burt et al. 2014). Similarly these compounds also inhibit quorum sensing property of C. violaceum at sub-inhibitory concentrations where growth is not inhibited and thereby representing different mechanism of these compounds for antiquorum sensing other than growth inhibition or bacterial cell death. Inhibiting biofilm and quorum sensing at sub-inhibitory concentrations indicated that the mechanism of these compounds to inhibit biofilm formation can also be linked to quorum sensing inhibition. In addition, administration of these compounds to bacterial cells had a marked effect on the expression levels of genes implicated in quorum sensing activity in C. violaceum. Specifically, expression level of cviL, vioA, vioB, vioD and target genes vioE (Stauff and Bassler 2011; Liu et al. 2013; Devescovi et al. 2017) was significantly down regulated in treated cells as compared to untreated bacterial cells. This was in agreement with a previous study that reported a marked suppression in the expression of these target genes following administration of antiquorum sensing compounds in C. violaceum (Liu et al. 2013).
In contrast to conventional antibacterial regimens which generally aim to treat disease symptoms, these antiquorum sensing compounds are advantageous in that they abolish cell-to-cell communication, thus, preventing establishment of infection (Williams 2007; Sarkar and Chakraborty 2008; Swem et al. 2009; Dandekar et al. 2012; Rahman et al. 2017). Additionally, since these compounds are synthesised from natural plant material, they may potentially serve as a cheaper source of therapy, particularly for patients in underdeveloped and developing countries.
The major limitations of the current study include the lack of strain diversity, in which only a single C. violaceum bacterial pathogen was employed for all experiments as well as deficit of not performing cell culture and animal studies. Additionally, future studies will also evaluate the antiquorum activity of citral and its derivative compounds against different C. violaceum strains. This will include assessment of antibacterial activity in drug susceptible and drug resistant C. violaceum pathogens. The advantage of inhibiting cell-to-cell communication in microorganisms following administration of citral and its derivatives may also be interrogated in other bacterial pathogens of clinical significance such as Streptococcus pyogenes, Staphylococcus aureus and Vancomycin-Resistant Enterococci. Testing of antiquorum activity against diverse bacterial strains will generate reliable and reproducible data. Ultimately, this may have useful clinical applications with regard to suppressing the expression of virulence genes and associated proteins, inhibiting host infection and mitigating bacterial proliferation particularly in drug-resistant strains.
Experiments in various cell culture and animal models aimed at examining the impact of administering citral compounds are urgently required. In addition, the direct or indirect role of these synthetic molecules in regulating the host innate and adaptive immune responses as well as cytotoxicity profile is imperative for understanding drug pharmacokinetics. Importantly, intensive in vitro and in vivo studies may potentially unravel critical pathway activated after administration of antiquorum QS compounds, discovery of new molecular drug targets and generation of novel and efficient vaccines and antimicrobial agents. The relatively cheap synthetic method of natural compounds tested in the current study may potentially have enormous clinical impact in regions such as sub-Saharan Africa, where the public and private medical institutions are unable to provide effective healthcare services due to expensive drugs and an overwhelming number of infected patients with limited economic resources.
In conclusion, treatment of C. violaceum culture with citral and derivatives led to a marked reduction in growth at higher concentrations and biofilm and quorum sensing in lower concentrations. Furthermore, administration of these regimens also significantly changed the expression profile of target genes that play a crucial role in quorum sensing in C. violaceum reporter strain. Collectively, the promising preliminary data obtained from this study demonstrate that these compounds may be potentially employed to counter cell-to-cell communication between bacterial cells and their biofilm productions, thus, preventing bacterial replication and subsequent infection.
Data availability
Not applicable.
References
Ahmad A, van Vuuren S, Viljoen A (2014) Unravelling the complex antimicrobial interactions of essential oils–the case of Thymus vulgaris (thyme). Molecules 19(3):2896–2910
Ahmad A, Viljoen AM, Chenia HY (2015a) The impact of plant volatiles on bacterial quorum sensing. Lett Appl Microbiol 60(1):8–19
Ahmad A, Wani MY, Khan A, Manzoor N, Molepo J (2015b) Synergistic interactions of eugenol-tosylate and its congeners with fluconazole against Candida albicans. PLoS ONE 10(12):e0145053
Bassler BL, Losick R (2006) Bacterially speaking. Cell 125(2):237–246
Bisht K, Wakeman CA (2019) Discovery and therapeutic targeting of differentiated biofilm subpopulations. Front Microbiol 10:1908
Brackman G, Coenye T (2015) Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des 21(1):5–11
Burt SA, Ojo-Fakunle VTA, Woertman J, Veldhuizen EJA (2014) The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE 9(4):e93414
Chan YY, Chua KL (2010) Growth-related changes in intracellular spermidine and its effect on efflux pump expression and quorum sensing in Burkholderia pseudomallei. Microbiology 156(4):1144–1154
Clinical and Laboratory Standards Institute (2015) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 10th edn. Approved standard M07-A10. Fort Wayne, IN: National Committee for Clinical Laboratory Standards.
Coenye T (2010) Social interactions in the Burkholderia cepacia complex: biofilms and quorum sensing. Future Microbiol 5(7):1087–1099
Dandekar AA, Chugani S, Greenberg EP (2012) Bacterial quorum sensing and metabolic incentives to cooperate. Science 338(6104):264–266
Devescovi G, Kojic M, Covaceuszach S, Cámara M, Williams P, Bertani I, Subramoni S, Venturi V (2017) Negative regulation of violacein biosynthesis in Chromobacterium violaceum. Front Microbiol 8:349
Falcão JP, Sharp F, Sperandio V (2004) Cell-to-cell signaling in intestinal pathogens. Curr Issues Intest Microbiol 5(1):9–17
Jabbari S, Heap JT, King JR (2011) Mathematical modelling of the sporulation-initiation network in Bacillus subtilis revealing the dual role of the putative quorum-sensing signal molecule PhrA. Bull Math Biol 73(1):181–211
Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L (2017) Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 15(12):740–755
Lan Lu, Wei Hu, Tian Z, Yuan D, Yi G, Zhou Y, Cheng Q, Zhu J, Li M (2019) Developing natural products as potential anti-biofilm agents. Chin Med 14:11
Lebeaux D, Ghigo JM, Beloin C (2014) Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev 78(3):510–543
Liu Z, Wang W, Zhu Y, Gong Q, Yu W, Lu X (2013) Antibiotics at subinhibitory concentrations improve the quorum sensing behavior of Chromobacterium violaceum. FEMS Microbiol Lett 341(1):37–44
Rahman RT, Lou Z, Yu F, Wang P, Wang H (2017) Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko (Amommum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi J Biol Sci 24(2):324–330
Rasko DA, Moreira CG, Li DR, Reading NC, Ritchie JM, Waldor MK, Williams N, Taussig R, Wei S, Roth M, Hughes DT, Huntley JF, Fina MW, Falck JR, Sperandio V (2008) Targeting QseC signaling and virulence for antibiotic development. Science 321(5892):1078–1080
Rasmussen TB, Givskov M (2006) Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol 296(2–3):149–161
Ravichandran V, Zhong L, Wang H, Yu G, Zhang Y, Li A (2018) Virtual screening and biomolecular interactions of CviR-based quorum sensing inhibitors against Chromobacterium violaceum. Front Cell Infect Microbiol 8:292
Roy R, Tiwari M, Donelli G, Tiwari V (2018) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9(1):522–554
Sarkar S, Chakraborty R (2008) Quorum sensing in metal tolerance of Acinetobacter junii BB1A is associated with biofilm production. FEMS Microbiol Lett 282(2):160–165
Shi C, Song K, Zhang X, Sun Y, Sui Y, Chen Y, Jia Z, Sun H, Sun Z, Xia X (2016) Antimicrobial activity and possible mechanism of action of citral against Cronobacter sakazakii. PLoS ONE 11(7):e0159006
Slater H, Crow M, Everson L, Salmond GP (2003) Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Mol Microbiol 47(2):303–320
Stauff DL, Bassler BL (2011) Quorum sensing in Chromobacterium violaceum: DNA recognition and gene regulation by the CviR receptor. J Bacteriol 193(15):3871–3878
Swem LR, Swem DL, ƠLoughlin CT, Gatmaitan R, Zhao B, Ulrich SM, Bassler BL (2009) A quorum-sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Mol Cell 35(2):143-153
Van Houdt R, Moons P, Hueso Buj M, Michiels CW (2006) N-acyl-L-homoserine lactoe quorum sensing controls butanediol fermentation in Serratia plymuthica RVH1 and Serratia marcescens MG1. J Bacteriol 188(12):4570–4572
White CE, Winans SC (2007) Cell-cell communication in the plant pathogen Agrobacterium tumefaciens. Philos Trans R Soc Lond B Biol Sci 362(1483):1135–1148
Williams P (2007) Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 153(12):3923–3938
Zhu H, He CC, Chu QH (2011) Inhibition of quorum sensing in Chromobacterium violaceum by pigments extracted from Auricularia auricular. Lett Appl Microbiol 52(3):269–274
Funding
AA acknowledges grant support from the South African National Research Foundation (NRF) Research Development Grant for Y-Rated Researchers (RDYR180418322304; Grant No: 116339), and University Research Committee Grant for 2019—Friedel Sellschop Award (Grant No: AZMD019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
All authors declare no conflict of interest.
Consent for Publication
Not applicable.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Batohi, N., Lone, S.A., Marimani, M. et al. Citral and its derivatives inhibit quorum sensing and biofilm formation in Chromobacterium violaceum. Arch Microbiol 203, 1451–1459 (2021). https://doi.org/10.1007/s00203-020-02127-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02127-z