Abstract
Background
Microbial infections cause serious health problems especially with the rising antibiotic resistance which accounts for about 700,000 human deaths annually. Antibiotics which target bacterial death encounter microbial resistance with time, hence, there is an urgent need for the search of antimicrobial substances which target disruption of virulence factors such as biofilm and quorum sensing (QS) with selective pressure on the pathogens so as to avoid resistance.
Methods
Natural products are suitable leads for antimicrobial drugs that can inhibit bacterial biofilms and QS. Twenty compounds isolated from the medicinal plant Gambeya lacourtiana were evaluated for their antibiofilm and anti-quorum sensing effects against selected pathogenic bacteria.
Results
Most of the compounds inhibited violacein production in Chromobacterium violaceum CV12472 and the most active compound, Epicatechin had 100% inhibition at MIC (Minimal Inhibitory Concentration) and was the only compound to inhibit violacein production at MIC/8 with percentage inhibition of 17.2 ± 0.9%. Since the bacteria C. violaceum produces violacein while growing, the inhibition of the production of this pigment reflects the inhibition of signal production. Equally, some compounds inhibited violacein production by C. violaceum CV026 in the midst of an externally supplied acylhomoserine lactone, indicating that they disrupted signal molecule reception. Most of the compounds exhibited biofilm inhibition on Staphyloccocus aureus, Escherichia coli and Candida albicans and it was observed that the Gram-positive bacteria biofilm was most susceptible. The triterpenoids bearing carboxylic acid group, the ceramide and epicatechin were the most active compounds compared to others.
Conclusion
Since some of the compounds disrupted QS mediated processes in bacteria, it indicates that this plant is a source of antibiotics drugs that can reduce microbial resistance.
Similar content being viewed by others
Introduction
Bacterial infections lead to various diseases due to the development of pathogenic bacteria in humans or animals. They seriously threaten public health, with an estimated death of over 10 million people per year by 2050 [1]. With time, viruses, bacteria and fungi become resistant to the therapeutic effects of the drugs that they were previously susceptible to [2]. This effect is referred to as antimicrobial resistance and the statistics show that an estimated annual 700,000 human deaths occur as a result of antibiotic resistance [3]. Inappropriate and misuse of antimicrobials contribute to the emergence of resistance in bacteria and it is worse in developing countries since patients can access antibiotics without prescription [4]. Antibiotics which target the inhibition and death of bacterial and fungal cells are falling out of use since they are the faced with resistance. Targeting microbial cell-to-cell communication systems (quorum-sensing) provides a possible solution to overcome this phenomenon. QS mediates the generation, diffusion and reception of small signal molecules that trigger virulence, resistance genes, toxin production, non-tolerance to antibiotics, drug efflux pumps and extracellular polysaccharide synthesis which constitutes the biofilm barrier [5, 6]. Investigating the QS effects of phytochemicals during can shape the development of drugs that target QS-signal and receptors which contributes to antibiotic resistance, motility and biofilm formation [7, 8]. Biofilm must be considered synonymously with antibiotic resistance because of its proficiency in transferring resistance genes between bacterial species and colonies as well as its impermeability and insusceptibility to antibiotics as well as efflux pump systemic elimination of antibiotics [9]. Antibiofilm and quorum-sensing (QS) inhibition are new methods currently employed as suitable strategies to combat microbial resistance and reduce severity of infections [10,11,12]. For this reason, most researchers are currently engaged in investigating plant products in the search of new therapeutic antibiotic agents that are capable of inhibiting QS processes and control infections without promoting the development of resistant microbial strains [13, 14]. Various medicinal plants are used to treat infectious diseases especially in low-income countries with efficacy. Phytoconstituents such as saponins, alkaloids, flavonoids, terpenoids and tannins have various bioactive functions including antibacterial activity with different mechanisms of action and less chances for microbial resistance [15].
Gambeya genus (Sapotaceae) is a pantropical genus of about 80 species widely distributed in West and Central Africa. They are used in folk medicine to treat sterility and various diseases including wounds and vaginal infections [16,17,18]. Some species such as Gambeya africana and Gambeya boukokoensis are reported to possess antitumor, anti-inflammatory, antibacterial, antinociceptive, antioxidant, antiplasmodial, antiplatelet, hypoglycemic, hypolipidemic and hepatoprotective activities [16,17,18]. Phytochemical investigation of some Gambeya species from Africa have led to the isolation of diverse classes of compounds, including phytosteroids, saponins, pentacyclic triterpenoids, flavonoids, alkaloids and bi-flavonoids [16,17,18]. Gambeya lacourtiana (De Wild) Aubr. & Pellegr is mostly distributed from Cameroon to the Central African Republic, Gabon and Democratic Republic of Congo. Its vernacular names include ‘abam’, ‘longhi’, ‘longhi rouge’ (French) [17]. In Cameroon, the population in the upper Nyong Valley use the stem bark and leaves of Gambeya lacourtiana to treat male sexual impotence and wound infections [17]. It is also administered orally for the treatment of uterine heamorrhage, chlamydia and other vaginal infections. The phytochemical investigation of the fruits of G. lacourtiana led to the isolation and characterization of pentacyclic triterpenoids, phytosteroids, ceramide, cerebroside, glycolipid, chlorophyll and carbohydrate [16, 18]. The fact this plant is popularly used in treating wounds, chlamydia and vaginal infections suggests that it possesses antimicrobial activities.
In our continuous search of bioactive natural products, with new modes of action that can reduce the chances of antibiotic resistance, the chemical constituents of G. lacourtiana were evaluated for their anti-quorum sensing, antimicrobial and anti-biofilm activities. These assays involving the inhibition of virulence factors in pathogenic bacteria were conducted at low concentrations, usually below minimal inhibitions.
Materials and methods
Extraction and isolation of chemical compounds
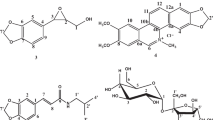
The fruits, leaves and stem bark of G. lacourtiana were collected in Mbalmayo (Latitude 3°23’ 0.01” North, Longitude 11°36’ 0.00” East) in the Centre Region of Cameroon, in June 2017 and identified by Mr. Victor Nana a botanist at the National Herbarium of Cameroon, where a voucher specimen is deposited under the reference YA0011679. The plant parts studied as well as the voucher specimen are provided on Fig. 1. The plant materials were extracted by maceration and purified using chromatographic methods to yield the compounds which were characterized using 1 H NMR and 13 C NMR and their structures are given in Fig. 2.
The fruits were chopped into pieces, dried and powdered to obtain 1428.6 g which was extracted by maceration with 5.0 L of methanol for 72 h at room temperature. The solvent was evaporated under vacuum to afford 109.4 g of methanol crude extract. Successive solvent-solvent extraction of the crude extract using n-hexane, methylene chloride and n-butanol afforded 63.7 g, 6.4 and 9.0 g respectively. A portion of (60.0 g) of the n-hexane extract was subjected to column chromatography (CC) over silica gel and eluted with a mixture of n-hexane-CH2Cl2 (0-100%) followed by CH2Cl2-MeOH (0-100%) to give 100 fractions of 125 mL each which were pooled on the basis of TLC into six sub-fractions (F1 to F6). Sub-fraction F1 (9.2 g), precipitated as a white powder which was further rechromatographed over silica gel with an isocratic system (n-hexane/CH2Cl2 9/1, v/v) and afforded Amyrincapraote (12.1 mg). Sub-fraction F2 (2.0 g) purified on silica gel column with isocratic system (n-hexane/CH2Cl2 1/4, v/v), yielded Acetylerythrodiol (11.3 mg) and Erythrodiol 3-O-palmitate (13.5 mg) while sub-fraction F3 (6.0 g) afforded 3-O-acetylbetulin (15.0 mg) and Acetylerythrodiol (20.0 mg) over silica gel using the same eluent phase. Purification of sub-fraction F6 (7.2 g), over silica gel column eluted with a mixture of CH2Cl2-MeOH (0–25%) afforded Lacourtianamide (9.5 mg) and Lacourtianoside II (25.2 mg).
The 1038.4 g of G. lacourtiana powdered leaves was extracted at room temperature in 10.0 L of a mixture of CH2Cl2:MeOH (1:1) to afford 112.9 g of crude extract. The crude extract was further extracted successively with n-hexane, ethyl acetate and n-butanol and 35.0 g, 5.8 and 8.6 g respectively of extracts were obtained. 30.0 g of the hexane extract was subjected to a column chromatography (CC) over silica gel and eluted with a mixture of n-hexane-CH2Cl2 and CH2Cl2-MeOH gradients to give 120 fractions of 125 mL each which were combined according to their TLC profiles into four sub-fractions (F1 to F4). Sub-fraction F1 (2.2 g), eluted with (n-hexane/CH2Cl2 90/10 to 80/20, v/v) precipitated few hours later at the ambient temperature, giving 3-O-acetyllupeol (100.0 mg). Sub-fraction 2 (3.3 g) was subjected to further CC using an isocratic system (n-hexane/CH2Cl2 4/1, v/v) to afford Lupeol (25.0 mg) and Stigmasterol (8.0 mg). Sub-fraction 3 (1.5 g) precipitated as a white powder which was filtered and washed with n-hexane/EtOAc 4/1 to yield Erythrodiol (19.8 mg). A portion of 5.0 g of ethyl acetate extract was subjected to column chromatography (CC) over silica gel and eluted with a mixture of n-hexane-ethyl acetate gradients polarity. A total of 90 fractions of 125 mL each were collected and combined according to TLC profile monitoring to two sub-fractions (F1 to F2). Sub-fraction F2 (0.9 g) precipitated as a bluish powder and was filtered and washed with n-hexane to afford Methyl pheophorbide-a (10.0 mg). A portion of n-butanol (8.0 g) was subjected to CC over silica gel and eluted with a mixture of EtOAc-MeOH (0-100%). 50 fractions of 125 mL each were collected and regrouped based on TLC into four sub-fractions (F1 to F3). Successive CC on F1 (3.2 g) with ethyl acetate-methanol (0–20%) led to the isolation of Epicatechin (23.0 mg) and 22,23-Dihydrospinasterol 3-O-β-D-glucopyranoside (18.8 mg).
2507.2 g of powdered stem barks were extracted thrice by maceration, using 8.0 L of methanol for 72 h at room temperature to give 235.8 g of MeOH extract. The crude extract was further extracted successively with n-hexane, ethyl acetate and n-butanol giving 41.0 g, 5.0 and 50.4 g of extracts respectively. A portion of the n-hexane extract (40.0 g) was subjected to flash chromatography (FC) over silica gel while eluting with a mixture of n-hexane-CH2Cl2 (0-100%) followed by CH2Cl2-MeOH (0–50%). A total 12 fractions of 500 mL each were collected and combined according to TLC profile into four sub-fractions (F1 to F4). Sub-fraction F1 (3.3 g) obtained by FC (n-hexane/CH2Cl2 25/75; v/v) was further subjected to silica gel column chromatography (CC) with isocratic system (n-hexane/CH2Cl2 17/3) to yield Taraxerone (9.2 mg). Sub-fraction F3 (0.9 g) obtained by FC (n-hexane/CH2Cl2 25/75; v/v) precipitated and was filtered to afford Spinasterol (12.9 mg). A portion of the ethyl acetate extract (4.8 g) was chromatographed over silica gel and eluted with a mixture n-hexane-ethyl acetate (0-100%) and ethyl acetate-MeOH (0–50%) gradient. A total of 60 fractions of 125 mL each were collected and combined according to TLC profile monitoring to two sub-fractions (F1 to F3). F3 fraction precipitated in the form of a white solid after purification on CC using n-hexane/EtOAc (1/9, v/v) to afford 6 (15.1 mg). Successive CC on F1 (0.6 g) using a mixture of n-hexane/EtOAc (0–20%) gradient yielded, Myrianthic acid (10.0 mg), Pomolic acid (9.1 mg) and Ursolic acid (12.7 mg). The sub-fractions F2 precipitated on standing and yielded Spinasterol 3-O-β-D-glucopyranoside (8.0 mg).
Microbial strains and test compounds solution preparations
The microorganisms used in this study were Staphylococcus aureus ATCC 25,923, Escherichia coli ATCC 25,922 and Candida albicans ATCC 10,239 for antimicrobial and antibiofilm assays. Chromobacterium violaceum CV12472 and Chromobacterium violaceum CV026 were used in the violacein and quorum sensing inhibitions respectively. Pseudomonas aeruginosa PA01 was used in the anti-swarming motility assay.
The stock solution of each test compound at a concentration of 10 mg/mL was prepared by dissolving the compounds in H2O:DMSO (95:5%, v:v) and serial dilutions were made from each stock solution using distilled H2O to further minimize the DMSO used in the stock solution.
Determination of minimum inhibitory concentrations
Minimal inhibitory concentration (MIC) of each compound was determined by the broth dilution method described previously [19, 20]. The MIC is the lowest extract concentration that yielded no visible microbial growth. The test medium was Mueller-Hinton broth and the density of bacteria was 5 × 105 colony-forming units (CFU)/mL. Cell suspensions (100 µL) were inoculated into the wells of 96-well microtitre plates in the presence of compounds with different final concentrations (1, 0.5, 0.25, 0.125, 0.0625, 0.03125 mg/mL). The inoculated microplates were incubated at 37ºC for 24 h before being read.
Inhibitory effect of compounds on microbial biofilm formation
The ability of the compounds at MIC and sub-MIC concentrations including 1, ½, ¼, and 1/8 MIC to inhibit biofilm by test microorganisms were evaluated with a microplate biofilm assay [21, 22]. Briefly, 1% of overnight cultures of isolates were added into 200 µL of fresh Tryptose-Soy Broth (TSB) supplemented with 0.25% glucose and cultivated in the presence and absence of compounds without agitation for 48 h at 37 ºC. The wells containing TSB + cells only served as control. After incubation, remove planktonic bacteria were removed by gently washing with distilled water. The biofilms were subsequently stained by filling wells with 200 µL of 0.1% crystal violet solution and then allowed for 10 min at room temperature. Wells were rinsed once more with distilled water using micro-pipette to remove the unabsorbed crystal violet. A volume of 200 µL of 33% glacial acetic acid (for Gram-positive bacteria) or ethanol 70% (for Gram-negative bacteria or fungi) were filled into the wells. After shaking 125 µL was pipetted from each of the wells into a sterile tube and volume was adjusted to 1 mL using distilled water. Finally, optical density (OD) of each well was measured at 550 nm (Thermo Scientific Multiskan FC, Vantaa, Finland). Percentage of inhibition of biofilm by the tested extracts was calculated using the formula:
Bioassay for quorum-sensing inhibition (QSI) activity using C. violaceum CV026
Inhibition of quorum sensing was determined as described elsewhere [23, 24] with little modifications. 5 mL of lukewarm molten Soft Top Agar (1.3 g agar, 2.0 g tryptone, 1.0 g sodium chloride, 200 mL deionized water) were seeded with 100 µL of an overnight culture of CV026, and 20 µL of 100 µg/mL acylhomoserine lactone (C6HSL) was added as exogenous hormone source. This was mixed gently and poured carefully over the surface of sterile solidified LBA plate as an overlay. 5 mm diameter wells were made on each plate after solidification of the overlay and each of the wells were filled with 50 µL of MIC and sub-MIC concentrations of filter sterilized compounds. Each experiment was done in triplicate and the plates were incubated in upright position at 30 °C for 3 days after which the diameters of the quorum sensing inhibition zones were measured. A white or cream-colored halo around this well against a purple lawn of activated CV026 bacteria was an indication of QSI and its diameter was measured in millimeters.
Violacein inhibition assay using C. violaceum CV12472
The compounds were subjected to qualitative analysis of QSI potentials for their ability to inhibit violacein production by C. violaceum ATCC 12,472 [13]. Overnight cultures (10 µL) of C. violaceum (adjusted to 0.4 OD at 600 nm) were added into sterile microtiter plates containing 200 µL of Luria-Bertani (LB) broth and incubated in the presence and absence of MIC and sub-MICs of extracts. LB broth containing C. violaceum ATCC 12,472 was used as a positive control. These plates were incubated at 30 °C for 24 h and observed for the reduction in violacein pigment production. The absorbance was read at 585 nm. The percentage of violacein inhibition was calculated by following the formula:
Swarming motility inhibition on Pseudomonas aeruginosa PA01
Swarming motility inhibition was evaluated according to a method previously described [25, 26]. Summarily, overnight cultures of P. aeruginosa PA01 strain were point inoculated at the center of swarming plates consisting of 1% peptone, 0.5% NaCl, 0.5% agar and 0.5% of filter-sterilized D-glucose with various concentrations of compounds (100, 75 and 50 µg/mL) and the plate without the compounds was maintained as control. Plates were incubated at an appropriate temperature in an upright position for 18 h. The swarming migration was recorded by following swarm fronts of the bacterial cells.
Statistical analyses
Descriptive statistics were applied on the data obtained. Each experiment was done in triplicate and the means of three parallel measurements were deduced. The values given are means ± SEM (Standard error of the mean) for three measurements. One-way ANOVA (analysis of variance) was used to compare differences amongst the means and were considered statistically significant p < 0.05.
Characteristics of isolated compounds
Acetylerythrodiol (1): White powder; m.p. 237–239 °C; 1 H NMR (CDCl3, 500 MHz): δH 0.82 (s, Me-24), 0.92 (s, Me-23), 0.95 (s, Me-25), 0.96 (s, Me-26), 0.97 (s, Me-29), 1.06 (s, Me-27), 1.12 (s, Me-30), 1.91 (1 H, dd, J = 13.5 and 4.3 Hz, H-18), 3.15 (1 H, d, J = 10.8 Hz, H-28a), 3.48 (1 H, d, J = 10.8 Hz, H-28b), 4.43 (1 H, m, H-3), 5.12 (1 H, t, J = 3.6 Hz, H-12), 1.98 (s, Me-2′); 13 C NMR (CDCl3, 125 MHz): δC 38.3 (C-1), 23.6 (C-2), 80.9 (C-3), 37.7 (C-4), 55.2 (C-5), 18.2 (C-6), 32.5 (C-7), 39.8 (C-8), 47.5 (C-9), 36.8 (C-10), 23.5 (C-11), 122.3 (C-12), 144.2 (C-13), 41.7 (C-14), 25.5 (C-15), 22.0 (C-16), 36.9 (C-17), 42.3 (C-18), 46.4 (C-19), 30.9 (C-20), 34.1 (C-21), 31.0 (C-22), 28.0 (C-23), 16.7 (C-24), 15.6 (C-25), 16.7 (C-26), 25.9 (C-27), 69.7 (C-28), 33.2 (C-29), 23.6 (C-30), 171.1 (C-1′), 21.3 (C-2′).
Amyrincapraote (2): White powder; 1 H NMR (CDCl3, 500 MHz): δH 0.76 (s, Me-24), 0.80 (s, Me-23), 0.83 (s, Me-30), 0.89 (s, Me-29), 0.90 (s, Me-26), 1.06 (s, Me-25), 1.25 (s, Me-27), 1.94 (1 H, dd, J = 13.5 and 4.3 Hz, H-18), 4.43 (1 H, m, H-3), 5.11 (1 H, t, J = 3.3 Hz, H-12), 0.82 (t, J = 3.5 Me-6′), 2.22 (2 H, t, J = 7.4 Hz, H-2′); 13 C NMR (CDCl3, 125 MHz): δC 38.3 (C-1), 23.6 (C-2), 80.6 (C-3), 37.8 (C-4), 55.3 (C-5), 18.3 (C-6), 32.6 (C-7), 39.8 (C-8), 47.6 (C-9), 36.8 (C-10), 23.5 (C-11), 121.7 (C-12), 145.2 (C-13), 41.7 (C-14), 26.1 (C-15), 26.9 (C-16), 32.5 (C-17), 47.2 (C-18), 46.8 (C-19), 31.1 (C-20), 34.7 (C-21), 37.2 (C-22), 28.1 (C-23), 16.8 (C-24), 15.6 (C-25), 16.8 (C-26), 25.9 (C-27), 28.4 (C-28), 33.4 (C-29), 23.7 (C-30), 173.7 (C-1′), 34.8 (C-2′), 24.9 (C-3′), 31.4 (C-4′), 22.3 (C-5′), 13.9 (C-6′).
Erythrodiol 3-O-palmitate (3): White powder; m.p. 121–123 °C; 1 H NMR (CDCl3, 500 MHz): δH 0.86 (s, Me-24), 0.87 (s, Me-23), 0.90 (s, Me-25), 0.94 (s, Me-26), 0.96 (s, Me-29), 1.17 (s, Me-27), 1.30 (s, Me-30), 1.91 (1 H, dd, J = 13.5 and 4.3 Hz, H-18), 3.15 (1 H, d, J = 10.9 Hz, H-28a), 3.48 (1 H, d, J = 10.9 Hz, H-28b), 4.44 (1 H, m, H-3), 5.12 (1 H, t, J = 3.6 Hz, H-12), 0.81 (t, J = 6.8 Hz, Me-16′), 1.54 (2 H, m, H-3′), 2.22 (2 H, t, J = 7.8 Hz, H-2′); 13 C NMR (CDCl3, 125 MHz): δC 38.3 (C-1), 23.6 (C-2), 80.5 (C-3), 37.8 (C-4), 55.2 (C-5), 18.2 (C-6), 32.5 (C-7), 39.8 (C-8), 47.6 (C-9), 36.8 (C-10), 23.5 (C-11), 122.3 (C-12), 144.2 (C-13), 41.7 (C-14), 25.5 (C-15), 22.0 (C-16), 36.9 (C-17), 42.3 (C-18), 46.4 (C-19), 30.9 (C-20), 34.1 (C-21), 31.0 (C-22), 28.0 (C-23), 16.7 (C-24), 15.6 (C-25), 16.8 (C-26), 25.9 (C-27), 69.7 (C-28), 33.2 (C-29), 23.6 (C-30), 173.7 (C-1′), 34.9 (C-2′), 25.2 (C-3′), 29.2–29.7 (C-4′-C-13′), 31.9 (C-14′), 22.7 (C-15′), 14.1 (C-16′).
Erythrodiol (4): White powder; m.p. 231 °C; 1 H NMR (CDCl3, 500 MHz): δH 0.78 (s, Me-24), 0.80 (s, Me-23), 0.86 (s, Me-25), 0.87 (s, Me-26), 0.94 (s, Me-29), 0.99 (s, Me-27), 1.21 (s, Me-30), 1.91 (1 H, dd, J = 13.5 and 4.3 Hz, H-18), 3.15 (1 H, d, J = 10.8 Hz, H-28a), 3.48 (1 H, d, J = 10.8 Hz, H-28b), 3.48 (1 H, 1 H, dd, J = 11.5 and 4.6 Hz, H-3), 5.22 (1 H, t, J = 3.6 Hz, H-12); 13 C NMR (CDCl3, 125 MHz): δC, 38.6 (C-1), 27.2 (C-2), 79.0 (C-3), 38.7 (C-4), 55.2 (C-5), 18.3 (C-6), 32.6 (C-7), 39.8 (C-8), 47.6 (C-9), 36.9 (C-10), 23.5 (C-11), 122.4 (C-12), 144.2 (C-13), 41.7 (C-14), 25.5 (C-15), 22.0 (C-16), 36.9 (C-17), 42.3 (C-18), 46.5 (C-19), 31.0 (C-20), 34.0 (C-21), 31.0 (C-22), 28.0 (C-23), 15.5 (C-24), 15.6 (C-25), 16.7 (C-26), 25.9 (C-27), 69.7 (C-28), 33.2 (C-29), 23.6 (C-30).
3-O-acetylbetulin (5): White powder; m.p. 260-260.5 °C; 1 H NMR (CDCl3, 500 MHz): δH 0.72 (1 H, d, J = 10.6 Hz, H-5), 0.77 (s, Me-25), 0.79 (s, Me-23), 0.79 (s, Me-24), 0.90 (s, Me-27), 0.95 (s, Me-26), 1.22 (1 H, d, J = 2.7 Hz, H-9), 1.62 (s, Me-30), 2.32 (1 H, td, J = 10.9 and 5.8 Hz, H-19), 3.26 (1 H, d, J = 10.7 Hz, H-28a), 3.73 (1 H, d, J = 10.7 Hz, H-28b), 4.40 (1 H, dd, J = 10.9 and 5.4 Hz, H-3), 4.51 (1 H, d, J = 1.9 Hz, H-29a), 4.61 (1 H, d, J = 1.9 Hz, H-29b), 1.97 (s, Me-2′); 13 C NMR (CDCl3, 125 MHz): δC 38.4 (C-1), 23.7 (C-2), 80.9 (C-3), 37.8 (C-4), 55.4 (C-5), 18.2 (C-6), 34.2 (C-7), 40.9 (C-8), 50.3 (C-9), 37.0 (C-10), 20.8 (C-11), 25.2 (C-12), 37.3 (C-13), 42.7 (C-14), 27.0 (C-15), 29.2 (C-16), 47.8 (C-17), 48.7 (C-18), 47.8 (C-19), 150.5 (C-20), 29.7 (C-21), 33.9 (C-22), 27.9 (C-23), 15.9 (C-24), 16.5 (C-25), 16.2 (C-26), 14.7 (C-27), 60.6 (C-28), 109.7 (C-29), 19.1 (C-30), 171.0 (C-1′), 21.3 (C-2′).
Betulinic acid (6): White powder; m.p. 277–279 °C; 1 H NMR (CDCl3, 500 MHz): δH 0.71 (1 H, d, J = 9.5 Hz, H-5), 0.78 (s, Me-24), 0.96 (s, Me-25), 0.99 (s, Me-23), 0.99 (s, Me-26), 1.00 (s, Me-27), 1.71 (s, Me-30), 2.34 (1 H, dd, J = 11.6 and 2.6 Hz, H-13), 3.02 (1 H, td, J = 10.8 and 4.9 Hz, H-18), 3.21 (1 H, dd, J = 11.4 and 4.8 Hz, H-3), 3.04 (1 H, dd, J = 11.1 and 4.8 Hz, H-19), 4.63 (s, H-29a), 4.76 (s, H-29b); 13 C NMR (CDCl3, 125 MHz): δC 38.7 (C-1), 27.4 (C-2), 79.0 (C-3), 38.9 (C-4), 55.3 (C-5), 18.3 (C-6), 34.3 (C-7), 40.7 (C-8), 50.5 (C-9), 37.2 (C-10), 20.9 (C-11), 25.5 (C-12), 38.4 (C-13), 42.4 (C-14), 30.6 (C-15), 32.2 (C-16), 56.3 (C-17), 46.9 (C-18), 49.3 (C-19), 150.4 (C-20), 29.7 (C-21), 37.0 (C-22), 28.0 (C-23), 15.3 (C-24), 16.0 (C-25), 16.1 (C-26), 14.7 (C-27), 180.4 (C-28), 109.7 (C-29), 19.4 (C-30).
3-O-acetyllupeol (7): White powder; m.p. 216–218 °C; 1 H NMR (CDCl3, 500 MHz): δH 0.69 (1 H, d, J = 9.5 Hz, H-5), 0.77 (s, Me-28), 0.81 (s, Me-24), 0.82 (s, Me-23), 0.83 (s, Me-25), 0.92 (s, Me-27), 1.16 (s, Me-26), 1.66 (s, Me-30), 1.67 (dd, J = 11.6 and 2.6 Hz, H-13), 1.35 (1 H, td, J = 10.8 and 4.9 Hz, H-18), 2.35 (dd, J = 11.1 and 4.8 Hz, H-19), 4.40 (1 H, dd, J = 11.4 and 4.8 Hz, H-3), 4.55 (s, H-29a), 4.86 (s, H-29b), 2.03 (s, Me-2′); 13 C NMR (CDCl3, 125 MHz): δC 38.4 (C-1), 23.7 (C-2), 81.0 (C-3), 37.8 (C-4), 55.4 (C-5), 18.2 (C-6), 34.3 (C-7), 40.9 (C-8), 50.4 (C-9), 37.1 (C-10), 21.0 (C-11), 25.1 (C-12), 38.1 (C-13), 42.9 (C-14), 27.5 (C-15), 35.6 (C-16), 43.0 (C-17), 48.3 (C-18), 48.0 (C-19), 150.9 (C-20), 29.9 (C-21), 40.0 (C-22), 28.0 (C-23), 16.5 (C-24), 16.2 (C-25), 16.0 (C-26), 14.5 (C-27), 18.0 (C-28), 109.3 (C-29), 19.3 (C-30), 170.8 (C-1′), 21.3 (C-2′).
Lupeol (8): White powder; m.p. 212–214 °C; 1 H NMR (CDCl3, 500 MHz): δH 0.78 (s, Me-25), 0.79 (s, Me-24), 0.86 (s, Me-26), 0.95 (s, Me-27), 0.98 (s, Me-23), 1.71 (s, Me-30), 2.40 (1 H, td, J = 11.1 and 5.8 Hz, H-19), 3.21 (1 H, dd, J = 11.4 and 4.9 Hz, H-3), 4.59 (1 H, d, J = 2.3 Hz, H-29a), 4.71 (1 H, d, J = 2.3 Hz, H-29b); 13 C NMR (CDCl3, 125 MHz): δC 38.6 (C-1), 27.6 (C-2), 79.0 (C-3), 38.6 (C-4), 55.3 (C-5), 18.2 (C-6), 33.2 (C-7), 41.9 (C-8), 50.2 (C-9), 37.4 (C-10), 20.6 (C-11), 23.7 (C-12), 32.5 (C-13), 42.5 (C-14), 27.5 (C-15), 40.4 (C-16), 48.6 (C-17), 53.8 (C-18), 48.0 (C-19), 151.0 (C-20), 27.6 (C-21), 44.5 C-22), 28.2 (C-23), 15.7 (C-24), 16.8 (C-25), 16.1 (C-26), 15.3 (C-27), 16.8 (C-28), 109.3 (C-29), 19.4 (C-30).
Myrianthic acid (9): White powder, m.p. > 300 °C; 1 H NMR (acetone-d6, 500 MHz): δH 0.75 (s, Me-26), 0.78 (s, Me-24), 0.91 (d, J = 6.7 Hz, Me-30), 0.99 (s, Me-25), 1.18 (s, Me-29), 1.34 (s, Me-27), 1.49 (1 H, m, H-16a), 2.50 (1 H, s, H-18), 2.60 (1 H, td, J = 13.3 and 4.6 Hz, H-16b), 3.37 (1 H, d, J = 11.0 Hz, 23a), 3.50 (1 H, d, J = 11.0 Hz, 23b), 3.60 (1 H, d, J = 2.5 Hz, H-3), 3.89 (1 H, m, H-2), 5.27 (1 H, t, J = 3.5 Hz, H-12); 13 C NMR (acetone-d6, 125 MHz): δC 41.0 (C-1), 65.6 (C-2), 77.7 (C-3), 41.4 (C-4), 42.6 (C-5), 17.7 (C-6), 32.4 (C-7), 41.1 (C-8), 47.0 (C-9), 37.7 (C-10), 23.4 (C-11), 127.7 (C-12), 138.8 (C-13), 39.8 (C-14), 28.3 (C-15), 25.3 (C-16), 47.3 (C-17), 53.5 (C-18), 72.1 (C-19), 41.8 (C20), 26.0 (C-21), 37.6 (C-22), 70.0 (C-23), 16.3 (C-24), 16.1 (C-25), 16.5 (C-26), 23.7 (C-27), 179.5 (C-28), 26.0 (C-29), 15.5 (C-30).
Pomolic acid (10): White powder; m.p. 272–274 °C; 1 H NMR (CD3OD, 500 MHz): δH 0.92 (s, Me-23), 1.03 (s, Me-24), 1.07 (s, Me-25), 1.08 (s, Me-26), 1.14 (d, J = 6.9 Hz, Me-30), 1.28 (s, Me-27), 1.90 (s, Me-29), 2.62 (1 H, s, H-18), 3.47 (1 H, dd, J = 11.2 and 4.1 Hz, H-3), 5.75 (1 H, t, J = 3.6 Hz, H-12); 13 C NMR (CD3OD, 125 MHz): δC 38.7 (C-1), 27.5 (C-2), 79.1 (C-3), 38.5 (C-4), 55.3 (C-5), 18.4 (C-6), 32.8 (C-7), 40.0 (C-8), 47.3 (C-9), 36.9 (C-10), 23.7 (C-11), 129.1 (C-12), 138.0 (C-13), 41.1 (C-14), 28.2 (C-15), 25.5 (C-16), 47.9 (C-17), 53.2 (C-18), 73.1 (C-19), 41.1 (C-20), 26.0 (C-21), 37.5 (C-22), 28.2 (C-23), 15.2 (C-24), 15.5 (C-25), 16.6 (C-26), 24.6 (C-27), 180.6 (C-28), 27.3 (C-29), 16.2 (C-30).
Ursolic acid (11): White powder; m.p. 284 °C; 1 H NMR (C5D5N, 500 MHz): δH 0.86 (1 H, d, J = 11.9 Hz, H-5), 0.95 (d, J = 6.2 Hz, Me-30), 1.00 (d, J = 6.4 Hz, Me-29), 1.22 (s, Me-27), 1.24 (s, Me-23), 1.55 (2 H, dd, J = 14.0 and 6.5 Hz, H-1), 1.63 (1 H, dd, J = 10.1 and 7.6 Hz, H-9), 2.12 (2 H, td, J = 13.3 and 4.2 Hz, H-16), 2.33 (2 H, td, J = 13.4 and 4.6 Hz, H-15), 2.63 (1 H, d, J = 11.3 Hz, H-18), 3.45 (1 H, dd, J = 10.1 and 5.8 Hz, H-3), 5.49 (1 H, t, J = 3.3 Hz, H-12); 13 C NMR (C5D5N, 125 MHz): δC 38.9 (C-1), 27.9 (C-2), 77.9 (C-3), 39.2 (C-4), 55.6 (C-5), 18.6 (C-6), 33.4 (C-7), 39.7 (C-8), 47.8 (C-9), 37.1 (C-10), 23.4 (C-11), 125.4 (C-12), 139.1 (C-13), 42.3 (C-14), 28.5 (C-15), 24.7 (C-16), 47.8 (C-17), 53.3 (C-18), 39.3 (C-19), 39.2 (C-20), 30.9 (C-21), 37.2 (C-22), 28.6 (C-23), 17.3 (C-24), 15.5 (C-25), 17.2 (C-26), 23.7 (C-27), 179.7 (C-28), 16.4 (C-29), 21.2 (C-30).
Taraxerone (12): White powder; m.p. 238–240 °C; 1 H NMR (CDCl3, 500 MHz): δH 0.76 (s, Me-29), 0.84 (s, Me-30), 0.85 (s, Me-23), 0.89 (s, Me-24), 1.00 (s, Me-25), 1.01 (s, Me-26), 1.02 (s, Me-28), and 1.07 (s, Me-27), 1.85 (1 H, dd, J = 14.8 and 3.1 Hz, H-16a), 2.01 (1 H, dt, J = 12.9 and 3.3 Hz, H-16b), 2.26 (1 H, ddd; J = 15.8; 6.4 and 3.3 Hz, H-2a), 2.51 (1 H, ddd; J = 15.8; 11.8 and 7.1 Hz, H-2b), 5.49 (1 H, dd, J = 8.2 and 3.2 Hz, H-15); 13 C NMR (CDCl3, 125 MHz): δC 38.3 (C-1), 34.1 (C-2), 217.5 (C-3), 47.6 (C-4), 55.8 (C-5), 19.9 (C-6), 35.1 (C-7), 38.9 (C-8), 48.7 (C-9), 35.8 (C-10), 17.4 (C-11), 37.7 (C-12), 37.7 (C-13), 157.6 (C-14), 117.2 (C-15), 36.6 (C-16), 37.5 (C-17), 48.8 (C-18), 40.6 (C-19), 28.8 (C-20), 33.6 (C-21), 33.1 (C-22), 26.1 (C-23), 21.5 (C-24), 14.8 (C-25), 29.9 (C-26), 25.6 (C-27), 29.8 (C-28), 33.3 (C-29), 21.3 (C-30).
22,23-Dihydrospinasterol 3-O-β-D-glucopyranoside (13): White powder; m.p. 284 °C; 1 H NMR (DMSO-d6, 500 MHz): δH 0.48 (s, Me-18), 0.72 (s, Me-19), 0.89 (d, J = 5.9 Hz, Me-21), 0.90 (1 H, m, H-24), 3.53 (1 H br s, H-3), 5.10 (1 H br s, H-7), 3.00 (1 H br s, H-4′), 3.09 (1 H br s, H-3′), 3.09 (1 H br s, H-5′), 3.40 (1 H, m, H-6′a), 3.62 (1 H, br d, J = 5.9 Hz, H-6′b), 3.87 (1 H br s, H-2′), 4.20 (1 H, d, J = 7.1 Hz, H-1′); 13 C NMR (DMSO-d6, 500 MHz): δC 37.0 (C-1), 31.8 (C-2), 76.7 (C-3), 43.4 (C-4), 40.5 (C-5), 29.6 (C-6), 117.6 (C-7), 139.5 (C-8), 49.1 (C-9), 34.4 (C-10), 21.4 (C-11), 39.3 (C-12), 43.3 (C-13), 54.8 (C-14), 23.0 (C-15), 28.3 (C-16), 55.9 (C-17), 12.1 (C-18), 13.2 (C-19), 36.4 (C-20), 19.3 (C-21), 33.8 (C-22), 26.0 (C-23), 45.6 (C-24), 29.2 (C-25), 19.4 (C-26), 19.2 (C-27), 23.1 (C-28), 12.3 (C-29). 101.1 (C-1′), 73.9 (C-2′), 77.1 (C-3′), 70.6 (C-4′), 77.2 (C-5′), 61.6 (C-6′).
Spinasterol (14): White powder; m.p. 171–173 °C; 1 H NMR (CDCl3, 500 MHz): δH 0.48 (s, Me-18), 0.73 (s, Me-19), 0.96 (d, J = 6.6 Hz, Me-21), 1.32 (1 H, dd, J = 11.3 and 4.0 Hz, H-5), 1.46 (1 H, m, H-24), 1.90 (1 H, d, J = 2.9 Hz, H-12a), 1.94 (1 H, d, J = 4.3 Hz, H-12b), 1.95 (1 H, m, H-20), 3.53 (1 H t, J = 4.5 Hz, H-3), 4.96 (1 H dd, J = 15.1 and 8.6 Hz, H-23), 5.09 (1 H, m, H-7), 5.09 (1 H, m, H-22); 13 C NMR (CDCl3, 500 MHz): δC 36.8 (C-1), 31.2 (C-2), 71.0 (C-3), 37.9 (C-4), 40.1 (C-5), 29.6 (C-6), 117.4 (C-7), 138.2 (C-8), 49.5 (C-9), 34.0 (C-10), 21.2 (C-11), 39.4 (C-12), 43.2 (C-13), 55.4 (C-14), 22.9 (C-15), 28.5 (C-16), 55.7 (C-17), 12.0 (C-18), 13.0 (C-19), 40.7 (C-20), 21.2 (C-21), 138.1 (C-22), 129.4 (C-23), 51.2 (C-24), 31.8 (C-25), 19.0 (C-26), 21.2 (C-27), 25.3 (C-28), 12.0 (C-29).
Spinasterol 3-O-β-D-glucopyranoside (15): White powder; m.p. 279–281 °C; 1 H NMR (C5D5N, 500 MHz): δH 0.51 (s, Me-18), 0.73 (s, Me-19), 0.77 (m, Me-26), 0.77 (m, Me-29), 0.82 (d, J = 6:5 Hz; Me-27), 0.99 (d, J = 6:5 Hz; Me-21), 3.54 (1 H, m, H-3), 5.02 (1 H, dd, J = 9:0; 15.0 Hz, H-23), 5.11 (1 H, m, H-7), 5.16 (1 H, dd, J = 9:0; 15.0 Hz, H-22), 2.88 (1 H, t, J = 9:0 Hz, H-5′), 3.05 (1 H, overlapped, H-2′), 3.05 (1 H, overlapped, H-3′), 3.05 (1 H, overlapped, H-4′), 3.40 (1 H, dd, J = 5:0; 11.0 Hz, H-6′a), 3.63 (1 H, d, J = 11:0; H-6′b), 4.21 (1 H, d, J = 8:0 Hz; H-1′); 13 C NMR (C5D5N, 500 MHz): δC 37.3 (C-1), 30.0 (C-2), 77.1 (C-3), 34.6 (C-4), 40.2 (C-5), 30.0 (C-6), 117.9 (C-7), 139.6 (C-8), 49.6 (C-9), 34.8 (C-10), 21.8 (C-11), 39.6 (C-12), 43.5 (C-13), 55.3 (C-14), 23.4 (C-15), 29.0 (C-16), 56.1 (C-17), 12.3 (C-18), 13.1 (C-19), 41.2 (C-20), 21.7 (C-21), 138.7 (C-22), 129.7 (C-23), 51.5 (C-24), 32.2 (C-25), 19.2 (C-26), 21.3 (C-27), 25.7 (C-28), 12.6 (C-29), 102.3 (C-1′), 75.4 (C-2′), 78.7 (C-3′), 71.8 (C-4′), 78.6 (C-5′), 62.9 (C-6′).
Stigmasterol (16): White powder; m.p. 175–177 °C; 1 H NMR (CDCl3, 500 MHz): δH 0.74 (s, Me-19), 0.81 (d, J = 6.7 Hz, Me-26), 0.83 (d, J = 6.7 Hz, Me-27), 0.86 (t, J = 7.2 Hz, Me-28), 0.92 (d, J = 6.5 Hz, Me-21), 1.05 (s, Me-18); 3.53 (1 H, tdd, J = 4.6, 4.5 and 3.7 Hz, H-3), 4.98 (1 H, m, H-22), 5.14 (1 H, m, H-23), 5.34 (1 H, t, J = 6.5 Hz, H-5); 13 C NMR (CDCl3, 125 MHz): δC 37.2 (C-1), 31.9 (C-2), 71.8 (C-3), 42.3 (C-4), 140.8 (C-5), 121.7 (C-6), 31.7 (C-7), 31.9 (C-8), 50.2 (C-9), 36.5 (C-10), 21.1 (C-11), 39.7 (C-12), 42.2 (C-13), 56.9 (C-14), 24.4 (C-15), 28.9 (C-16), 56.0 (C-17), 19.4 (C-18), 12.1 (C-19), 40.5 (C-20), 21.1 (C-21), 138.3 (C-22), 129.3 (C-23), 51.3 (C-24), 31.9 (C-25), 21.2 (C-26), 19.0 (C-27), 25.4 (C-28), 12.3 (C-29).
Lacourtianamide (17): White powder; 1 H NMR (C5D5N, 500 MHz): δH 0.85 (t, J = 5.6 Hz, Me-25), 1.13–1.48 (br s, H-11-H-22), 1.26 (2 H, m, H-24), 1.27 (2 H, m, H-23), 1.27 (2 H, m, H-6), 1.92 (1 H, m, H-5a), 1.99 (2 H, m, H-10), 2.17 (2 H, m, H-7), 2.24 (1 H, m, H-5b), 4.29 (1 H, m, H-4), 4.36 (1 H, m, H-3), 4.43 1 H, dd, J = 9.8 and 5.0 Hz, H-1a), 4.52 (1 H, m, H-1b), 5.12 (1 H, m, H-2), 5.49 (1 H, dt, J = 15.4 and 6.4 Hz, H-9), 5.54 (1 H, dt, J = 15.4 and 6.4 Hz, H-8), 8.58 (1 H, d, J = 9.0 Hz, N-H), 0.85 (t, J = 5.6 Hz, Me-19′), 1.13–1.48 (br s, H-5′-H-16′), 1.26 (2 H, m, H-18′), 1.27 (2 H, m, H-17′), 1.75 (2 H, m, H-4′), 2.02 (1 H, m, H-3′a), 2.25 (1 H, m, H-3′b), 4.62 (1 H, br s, H-2′); 13 C NMR (C5D5N, 125 MHz): δC 61.8 (C-1), 52.7 (C-2), 76.6 (C-3), 72.8 (C-4), 33.9 (C-5), 26.4 (C-6), 33.1 (C-7), 130.7 (C-8), 130.6 (C-9), 32.8 (C-10), 29.3–30.1 (C11-C-22), 31.9 (C-23), 22.7 (C-24), 14.1 (C-25), 175.0 (C-1′), 72.2 (C-2′), 35.5 (C-3′), 25.6 (C-4′), 29.3–30.1 (C-5′-C-16′), 31.9 (C-17′), 22.7 (C-18′), 14.1 (C-19′).
Lacourtianoside II (18) Yellow powder; 1 H NMR (CDCl3/CD3OD 1:1, 500 MHz): δH 0.87 (3 H, t, J = 6.9 Hz, H-37), 4.06 (1 H, m, H-1a), 3.21 (1 H, m, H-3′ ), 3.30 (1 H, br d, J = 6.9 Hz, H-4′) 3.39 (1 H, m, H-5′ ), 3.70 (1 H, dd, J = 11.9 and 4.8 Hz, H-6′b), 3.82 (1 H, m, H-1b), 3.87 (1 H, dd, J = 11.9 and 1.9 Hz, H-6′a), 4.03 (1 H, dd, J = 7.8 and 3.6 Hz, H-2′ ), 4.27 (1 H, d, J = 7.8 Hz, H-1′); 13 C NMR (CDCl3/CD3OD 1:1, 125 MHz): δC 68.5 (C-1), 34.3 (C-2), 29.5–29.2 (C-3-C-35), 31.8 (C-35), 22.5 (C-36), 37 (C-37), 103.1 (C-1′), 71.8 (C-2′), 73.4 (C-3′), 70.0 (C-4′), 76.4 (C-5′), 61.4 (C-6′).
Epicatechin (19): White amorphous powder; m.p. 235–237 °C; 1 H NMR (CD3OD, 500 MHz): δH 2.77 (1 H, dd, J = 16.7 and 2.5 Hz, H-4b), 2.86 (1 H, dd, J = 16.7 and 4.6 Hz, H-4a), 4.17 (1 H, br s, H-3), 4.81 (1 H, br s, H-2), 5.91 (1 H, d, J = 2.1 Hz, H-8), 5.94 (1 H, d, J = 2.1 Hz, H-6), 6.75 (1 H, d, J = 8.1 Hz, H-5′), 6.79 (1 H, dd, J = 8.1 and 2.0 Hz, H-6′), 6.97 (1 H, d, J = 2.0 Hz, H-2′); 13 C NMR (CD3OD, 125 MHz): δC 78.5 (C-2), 66.1 (C-3), 27.8 (C-4), 98.7 (C-4a), 156.3 (C-5), 95.0 (C-6), 156.6 (C-7), 94.5 (C-8), 155.9 (C-8a), 130.9 (C-1′), 113.9 (C-2′), 144.5 (C-3′), 144.4 (C-4′), 114.5 (C-5′), 118.0 (C-6′).
Methyl pheophorbide-a (20): Blue amorphous powder; m.p. 226–228 °C; 1 H NMR (CDCl3, 500 MHz): δH 1.70 (t; J = 7.6 Hz, Me-82), 3.20 (s, Me-71), 3.41 (s, Me-21), 3.61 (s, MeO-175), 3.65 (2 H, q, J = 7.6, H-81), 3.70 (s, Me-121), 3.92 (s, MeO-135), 4.24 (1 H, dt, J = 9.0 and 2.6 Hz, H-17), 6.18 (1 H, dd, J = 11.5 and 1.5 Hz, H-32), 6.27 (1 H, dd, J = 17.9 and 1.5 Hz, H-32′), 7.97 (1 H, dd, J = 17.9 and 11.5 Hz, H-31), 8.58 (1 H, s, H-20), 9.34 (1 H, s, H-5), 9.49 (1 H, s, H-10); 13 C NMR (CDCl3, 125 MHz): δC 142.1 (C-1), 131.9 (C-2), 12.1 (C-21), 136.2 (C-3), 128.9 (C-31), 122.8 (C-32), 136.3 (C-4), 97.6 (C-5), 155.7 (C-6), 136.5 (C-7), 11.2 (C-71), 145.2 (C-8), 19.5 (C-81), 17.4 (C-82), 151.0 (C-9), 104.5 (C-10), 137.9 (C-11), 129.1 (C-12), 12.1 (C-121), 128.9 (C-13), 189.6 (C-131), 64.7 (C-132), 169.6 (C-133), 52.9 (C-135), 149.7 (C-14), 105.2 (C-15), 161.2 (C-16), 51.1 (C-17), 29.4 (C-171), 23.1 (C-172), 173.3 (C-173), 51.7 (C-175), 50.1 (C-18), 22.7 (C-181),172.2 (C-19), 93.1 (C-20).
Results
Violacein production, swarming motility and biofilm formation are amongst the important quorum-sensing mediated processes in bacteria. The disruption of quorum-sensing communication networks in bacterial colonies is an effective strategy to eliminate or reduce resistance to antibiotics and it is not intended to kill bacteria but to prevent the expression of their virulence factors and pathogenicity [27]. For this reason, violacein inhibition, anti-QS, swarming inhibition and antibiofilm assays were performed at concentrations below the minimal inhibitory concentration (sub-MIC).
Inhibition of violacein production in C. violaceum CV12472
When Chromobacterium violaceum grows, it produces a violet pigment called violacein which plays the role of a signal molecule and indicates proper and normal functioning of this bacterium. Violacein production is mediated by QS process in C. violaceum CV12472. The inhibition of violacein at sub-MIC concentrations is significant as it indicates the potential of the compounds to prevent signal molecule production in bacteria. Prior to evaluation of violacein inhibition, MIC values were determined and almost all compounds inhibited C. violaceum CV12472 within test concentrations except Lacourtianoside II as shown on Table 1. For the active compounds, MIC values varied from 0.25 mg/mL for the most active compound Epicatechin to 1.00 mg/mL. Epicatechin had the highest violacein inhibition of 100% at MIC and was the only compound to inhibit violacein production at MIC/8 with percentage inhibition of 17.2 ± 0.9%. Some other compounds were also active at MIC/4 concentration including Acetylerythrodiol (14.2 ± 0.28%), Pomolic acid (9.7 ± 0.2%), Ursolic acid (7.5 ± 0.4%), Lacourtianamide (5.5 ± 0.1%), Epicatechin (39.8 ± 0.6%) and Methyl pheophorbide-a (8.4 ± 0.5%). All compounds inhibited violacein at MIC except compound 18 and at MIC/2 except compounds 22,23-Dihydrospinasterol 3-O-β-D-glucopyranoside, Spinasterol 3-O-β-D-glucopyranoside and Lacourtianoside II.
Quorum sensing inhibition in mutant strain C. violaceum CV026
The mutant strain C. violaceum 026 does not produce violacein when growing unless if an acylhomoserine lactone (AHL) is supplied to it from an external source. However, in this assay the hormone is supplied in the presence of the test compounds so as to evaluate the ability of the compounds to prevent the response of the bacteria cells to the AHL. This assay was done at sub-MIC concentrations and the zones of inhibition in millimetres are reported on Table 2. Erythrodiol, Lupeol, Taraxerone, 22,23-Dihydrospinasterol 3-O-β-D-glucopyranoside, Spinasterol, Spinasterol 3-O-β-D-glucopyranoside, Stigmasterol and Lacourtianoside II could not inhibit C. violaceum CV026 within the tested concentrations while the other compounds inhibited this bacterium with MIC values varying from 0.50 mg/mL to 1.00 mg/mL. No compound showed anti-QS activity at MIC/8 and only the most active compound, Epicatechin showed inhibition of QS with inhibition zones of 9.5 ± 0.1 mm at MIC/4. At MIC/2, Acetylerythrodiol (9.1 ± 0.4 mm), Betulinic acid (8.5 ± 0.2 mm), Myrianthic acid (9.8 ± 0.4 mm), Pomolic acid (10.5 ± 0.7 mm), Ursolic acid (11.3 ± 0.5 mm), Lacourtianamide (9.1 ± 0.5 mm), Epicatechin (11.5 ± 0.5 mm) and Methyl pheophorbide-a (10.3 ± 0.1 mm) were able to inhibit QS as per the inhibition zones shown on Fig. 3. However, at MIC concentration, most of the compounds inhibited QS.
Inhibition of swarming motility on P. aeruginosa PA01
P. aeruginosa PA01 is an opportunistic bacterium that inhabits many environments capable of utilizing several motility strategies to move and colonize surfaces and establishing biofilms. Swarming motility constitutes an important process in this bacterium and for this reason, inhibiting swarming is a good strategy to eliminate its pathogenicity. The inhibition of swarming movement in P. aeruginosa PA01 was carried out at 100, 75 and 50 µg/mL and reported on Table 3. All the compounds showed swarming inhibitions at 100 µg/mL concentration and this activity further varied in a concentration dependent manner at 75 and 50 µg/mL. The most active compound was Epicatechin whose anti-swarming activity varied from 58.84 ± 1.28% at 100 µg/mL to 20.52 ± 0.14% at 50 µg/mL. Pomolic acid also exhibited good activity at 100 µg/mL (51.61 ± 1.24%) and at 50 µg/mL (22.64 ± 0.44%) as well as Ursolic acid at 100 µg/mL (54.74 ± 1.08%) and at 50 µg/mL (15.92 ± 0.46%) and Lacourtianamide at 100 µg/mL (50.94 ± 1.05%) and at 50 µg/mL (17.52 ± 0.34%). Acetylerythrodiol, Amyrincapraote, Erythrodiol 3-O-palmitate, 3-O-acetylbetulin, 3-O-acetyllupeol, 22,23-Dihydrospinasterol 3-O-β-D-glucopyranoside, Spinasterol 3-O-β-D-glucopyranoside, Lacourtianoside II had weak activities as they only inhibited swarming at MIC.
Antimicrobial activity
The antimicrobial effects of the compounds were evaluated on three strains: one Gram-positive (S. aureus), one Gram-negative (E. coli) and one yeast (C. albicans) and the minimal inhibitory concentration of each compound on each of the microorganisms are given on Table 4. The Gram-positive bacteria S. aureus was susceptible to all the compounds except to 22,23-Dihydrospinasterol 3-O-β-D-glucopyranoside, Spinasterol 3-O-β-D-glucopyranoside, Stigmasterol and Lacourtianoside II. The other compounds had MIC values on S. aureus varying from 0.25 mg/mL for the most active compound, Lacourtianamide to 1.00 mg/mL for Acetylerythrodiol, 3-O-acetyllupeol, Pomolic acid, Ursolic acid, Spinasterol and Methyl pheophorbide-a. All other compounds had MIC values on S. aureus to be 0.50 mg/mL. The Gram-negative bacteria E. coli was the least susceptible to the compounds and only Epicatechin had a MIC value of 0.50 mg/mL against E. coli, while the other active compounds Acetylerythrodiol, Amyrincapraote, Erythrodiol 3-O-palmitate, 3-O-acetylbetulin, Betulinic acid, 3-O-acetyllupeol, Myrianthic acid, Ursolic acid, Lacourtianamide, Methyl pheophorbide-a, all had MIC values of 1.00 mg/mL. The other compounds could not inhibit E. coli within the tested concentrations. Against the yeast cells C. albicans, Erythrodiol 3-O-palmitate, Erythrodiol and 3-O-acetylbetulin were not active within tested concentrations. The most active compounds against C. albicans were Acetylerythrodiol, 3-O-acetyllupeol, Pomolic acid, Ursolic acid, Lacourtianamide, Epicatechin and Methyl pheophorbide-a which had MIC values of 0.50 mg/mL. The other compounds inhibited C. albicans with MIC values of 1.00 mg/mL.
Inhibition of biofilms by test compounds
The capacity of the compounds to inhibit biofilms in S. aureus, E. coli and C. albicans were evaluated at sub-MIC concentrations and percentage inhibitions reported on Table 5. It is always necessary to evaluate the effect of samples on planktonic bacterial and also in their biofilm forms at sub-MIC concentrations so as to have a proper reflection of their potential to eliminate the bacteria. All the compounds were able to inhibit bacterial biofilms in S. aureus at MIC and MIC/2 concentrations except 22,23-Dihydrospinasterol 3-O-β-D-glucopyranoside, Spinasterol, Spinasterol 3-O-β-D-glucopyranoside, Stigmasterol and Lacourtianoside II. At MIC/4 concentration, S. aureus biofilm formation was inhibited by Acetylerythrodiol (11.3 ± 0.4%), Betulinic acid (09.4 ± 0.7%), Myrianthic acid (22.8 ± 0.4%), Lacourtianamide (19.8 ± 0.9%), Epicatechin (28.4 ± 0.9%) and Methyl pheophorbide-a (14.6 ± 0.8%). At MIC/8, only Myrianthic acid (08.3 ± 0.2%), Lacourtianamide (07.0 ± 0.2%) and Epicatechin (11.7 ± 0.3%) were able to inhibit biofilm formation and these compounds exhibited the highest antibiofilm activity on S. aureus. The Gram-positive bacteria E. coli was the most difficult biofilm to inhibit as the percentage inhibitions were low and no inhibition was observed at MIC/4 and MIC/8. At MIC/2, only Erythrodiol (11.6 ± 0.8%), Betulinic acid (17.4 ± 0.5%), Lupeol (06.2 ± 0.1%), Ursolic acid (16.3 ± 0.2%), Lacourtianamide (05.7 ± 0.3%) and Epicatechin (25.5 ± 0.8%) were able to inhibit E. coli biofilm formation. Ursolic acid and Epicatechin had the highest biofilm inhibitions on E. coli. Against C. albicans, Erythrodiol, 22,23-Dihydrospinasterol 3-O-β-D-glucopyranoside, Spinasterol 3-O-β-D-glucopyranoside and Lacourtianoside II could not inhibit biofilm formation while at MIC/8 no biofilm inhibition was observed for the active compounds. At MIC/4, only Ursolic acid (08.8 ± 0.5%), Lacourtianamide (09.8 ± 0.4%) and Methyl pheophorbide-a (06.3 ± 0.1%) could inhibit biofilm formation in C. albicans. Ursolic acid, Lacourtianamide and Methyl pheophorbide-a had the highest biofilm inhibition percentages on C. albicans compared to the other compounds.
Discussion
Plant-derived compounds have different structural diversities, with different modes of action and are safer and cheaper than synthetic compounds, and this can be seen with the large number of discovered drugs from natural origin [28]. Various secondary metabolites from plants including phenolic compounds, alkaloids, β-lactam, macrolides, lectins, terpenoids, peptides and lipoglycopeptides, have been shown to possess antimicrobial activities with different modes of action such as inhibition of quorum sensing, efflux pump effects, biofilm inhibition and anti-motilities [29,30,31,32]. Violacein inhibition is easily measurable and reflects an anti-QS process in bacteria [33]. Violacein production contributes to the virulence factors in the C. violaceum CV12472 Gram-negative bacterium and its inhibition reflects the blocking of signal molecules that promotes communication within the bacterial colony. It can be seen from the results obtained that pentacyclic triterpenoids, the ceramide and epicatechin were able to inhibit violacein production in C. violaceum CV12472. Pentacyclic triterpenoids of the class of lupane and oleanane have demonstrated violacein inhibition and those containing the acid function were more potent, indicating that pentacyclic triterpenoids could be suitable scaffolds for the development of quorum quenching antimicrobials on various pathogens [34]. The violacein pigment production is mediated by QS system of genes dependent on CviR and it is helps microorganisms to coordinate processes such as population density and involves production and response to acylhomoserine lactones [35]. Since the bacteria C. violaceum produces violacein while growing, the inhibition of the production of this pigment reflects the inhibition of signal production. The mutant strain C. violaceum CV026 is unable to produce AHL and therefore can only produce violacein when an external AHL is supplied. The results in this study indicates that some compounds at certain concentrations were able to prevent C. violaceum CV026 from producing violaceum even when an AHL was supplied, and this reflects inhibition of signal reception by such compounds. QS inhibition can occur in multiple ways either by prevention of AHL signal production, disruption of AHL signal dissemination or through the interruption of AHL signal reception [36]. The reduction in the production of violacein pigment by C. violaceum CV12472 and the anti-QS zones against C. violaceum CV026 present as halos are visible on Fig. 3.
This is beneficial and can help to reduce bacterial virulence. QS communication systems help the bacteria to protect themselves from pressure exerted by antibiotic drugs and also to form self-protecting biofilm matrices on surfaces making treatments difficult [37,38,39,40]. Triterpenoids are able to impede various virulence factors including biofilm formation which increases microbial resistance to antibiotics and biofilms are regulated by QS through the inter-bacterial communication networks mediated by small signal molecules production and diffusion [41]. In one of such assays, triterpenoids of lupane and oleanane classes with carboxylic acid functional groups are very active and this can be justified as Betulinic acid, Myrianthic acid, Pomolic acid and Ursolic acid were amongst the active terpenoid compounds in all the assays. Equally, the most active compound could be considered to be epicatechin which was able to effectively inhibit QS and virulence factors with good inhibition of violacein pigment production, swarming motility and biofilm formation. Some plant extracts rich in epicatechin and other catechin derivatives have been shown to inhibit violacein, biofilms and swarming in bacteria [14].
Some pathogenic bacteria use coordinated flagella-driven movements called swarming on solid and semisolid surfaces to colonize surfaces, increase virulence and resistance antibiotics and this is a suitable way of bacteria to adapt to environmental challenges using signalling networks [42]. Most of the compounds were able to inhibit swarming motility against the model bacteria P. aeruginosa PA01. This ability to prevent swarming movement can be of interest for the development of new class of antimicrobials that can block coordinated behaviour in bacterial colonies including the movement towards nutrients, attachment and colonisation of surfaces and subsequent biofilm formation [43, 44]. It is beneficial to find new antimicrobial substances that can inhibit these QS regulated swarming motility which is a virulence factor exhibited by P. aeruginosa for adhesion unto surfaces and nutrient rich areas and to form biofilm [45]. Epicatechin showed the highest motility inhibition on P. aeruginosa PA01, and this confirms its ability to inhibit biofilms since swarming movement is involved in the early stages of biofilm formation. In one study, pure isolated catechin and an epicatechin-rich extract were able to inhibit pyocyanin pigment production as well as elastase synthesis and biofilm formation in P. aeruginosa PA01 thereby quenching QS-dependent virulence factors in this bacterium [46].
Swarming motility is also necessary for the dispersion of biofilms. Biofilms consist of extracellular matrix made up of self-produced substances, such as lipids, proteins and polysaccharides that protects the bacteria from disinfectants, antibiotics and host defence systems and contribute to increase resistance, and different antimicrobials that are capable of inhibiting biofilms and reducing virulence without killing the bacteria are important antimicrobial therapeutics [47, 48]. The ability of the test compounds to inhibit biofilm formation at low concentrations (sub-MIC), is a very good indication of their possible potential application to overcome microbial resistance developed by biofilms embedded pathogen cells. The results shown in this study therefore corroborates with previous studies that report inhibition of QS systems and biofilms by various triterpenoid scaffolds against many pathogens with promising potential [49, 50]. Various triterpenoids constitute bioactive phytochemicals that affects microbial biofilms and some structurally similar lupane and oleanane type triterpenoids with carboxylic acid groups substantially depleted biofilms to various extents, reducing surface and exo-polysaccharides of biofilm matrices [51]. It should be noted that bacteria within biofilms can’t be eliminated by ordinary antibiotics and these biofilm communities will continue to exercise pathogenicity and virulence even when planktonic communities are inhibited or killed. Biofilm-associated infections are chronic and are very difficult to treat with conventional antibiotics since most antibiotics must enter the cells meanwhile biofilms prevent antibiotics from entering bacterial cells. The development of non-biocidal strategies such as QS and biofilm inhibitions to combat bacterial infections is very crucial since it avoids antibiotic resistance contrary to the use of conventional antibiotics that can possibly lead to antimicrobial drug resistance [52].
Conclusion
Conventional antibiotics which aim at killing bacteria or inhibiting their growth are usually challenged with antibiotic resistance and the fall out of use within short times of use. It is necessary to search for new antimicrobial substances which can overcome resistance and eliminate virulence factors and pathogenicity of bacteria. One important method to achieve this is to inhibit QS bacterial-communication systems. For this reason, twenty compounds isolated from the medicinal plant G. lacourtiana were evaluated for their inhibitory effects on QS and biofilm and the results indicated good potential especially for triterpenoids with carboxylic acid groups, the ceramide and epicatechin. Compounds from G. lacourtiana could serve as cheap starting materials for the development of antimicrobial drug which can possibly overcome microbial resistance since some of the compounds are capable of disrupting QS mediated processes in bacteria and biofilms.
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhang L, Wang Y, Wang J, Wang Y, Chen A, Wang C, Mo W, Li Y, Yuan Q, Zhang Y. A photon-responsive antibacterial nanoplatform for synergistic photothermal-pharmaco-therapy of skin infection. ACS Appl Mater Interfaces. 2018;1–37. https://doi.org/10.1021/acsami.8b18146.
Walusansa A, Asiimwe S, Nakavuma JL, Ssenku EJ, Katuura E, Kafeero HM, Aruhomukama D, Nabatanzi A, Anywar G, Tugume AK, Kakudidi EK. Antibiotic-resistance in medically important bacteria isolated from commercial herbal medicines in Africa from 2000 to 2021: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2022;11:11. https://doi.org/10.1186/s13756-022-01054-6.
Famuyide IM, Aro AO, Fasina FO, Eloff JN, McGaw LJ. Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south african Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement Altern Med. 2019;19:141. https://doi.org/10.1186/s12906-019-2547-z.
Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017;6:47. https://doi.org/10.1186/s13756-017-0208-x.
Bauerle T, Fischer A, Speck T, Bechinger C. Self-organization of active particles by quorum sensing rules. Nat Commun. 2018;9:8. https://doi.org/10.1038/s41467-018-05675-7.
Zhao X, Yu Z, Ding T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms. 2020;8(3):425. https://doi.org/10.3390/microorganisms8030425.
Cabuhat KSP, Moron-Espiritu LS. Quorum sensing orchestrates Antibiotic Drug Resistance, Biofilm formation, and motility in Escherichia coli and Quorum quenching Activities of Plant-derived Natural Products. Rev J Pure Appl Microbiol. 2022;16(3):1538–49. https://doi.org/10.22207/JPAM.16.3.52.
Ikome HN, Tamfu AN, Abdou JP, Fouotsa H, Nangmo PK, Lah FCW, Tchinda AT, Ceylan O, Frederich M, Nkengfack AE. Disruption of Biofilm formation and Quorum sensing in pathogenic Bacteria by Compounds from Zanthoxylum Gilletti (De Wild) P.G. Waterman. Appl Biochem Biotechnol. 2023. https://doi.org/10.1007/s12010-023-04380-6.
Bowler P, Murphy C, Wolcott R. Biofilm exacerbates antibiotic resistance: is this a current oversight in antimicrobial stewardship? Antimicrob Resist Infect Control. 2020;9:162. https://doi.org/10.1186/s13756-020-00830-6.
Ngenge A, Ceylan O, Fru G, Arab Y, Emin D, Ozturk M. Antimicrobial, antibiofilm, anti-quorum sensing and motility inhibition activities of essential oil from seeds of food spice Xylopia aethiopica (Dunal) A. Rich. On some pathogenic bacteria. Res J Biotechnol. 2021;16:68–76.
Beddiar H, Boudiba S, Benahmed M, Tamfu AN, Ceylan Ö, Hanini K, Kucukaydin S, Elomri A, Bensouici C, Laouer H, et al. Chemical composition, anti-quorum sensing, enzyme inhibitory, and antioxidant properties of phenolic extracts of Clinopodium nepeta L. Kuntze. Plants. 2021;10:1955. https://doi.org/10.3390/plants10091955.
Ngenge TA, Kucukaydin S, Ceylan O, Duru ME. Evaluation of enzyme inhibition and anti-quorum sensing potentials of Melaleuca alternifolia and Citrus sinensis essential oils. Nat Prod Com. 2021;16(9):1–8. https://doi.org/10.1177/1934578X211044565.
Tamfu AN, Ceylan O, Fru GC, Ozturk M, Duru ME, Shaheen F. Antibiofilm, antiquorum sensing and antioxidant activity of secondary metabolites from seeds of Annona senegalensis, Persoon. Mic Pathogen. 2020;144:104191. https://doi.org/10.1016/j.micpath.2020.104191.
Tamfu AN, Ceylan O, Kucukaydin S, Duru ME. HPLC-DAD phenolic profiles, antibiofilm, anti-quorum sensing and enzyme inhibitory potentials of Camellia sinensis (L.) O. Kuntze and Curcuma longa L. LWT-Food Sci Technol. 2020;133:110150. https://doi.org/10.1016/j.lwt.2020.110150.
Kebede T, Gadisa E, Tufa A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: a possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE. 2021;16(3):e0249253. https://doi.org/10.1371/journal.pone.0249253.
Talla MR, Jouda JB, Mawabo KI, Tegasne C, Happi MG, Lenta NB, Kapche FWDG, Frese M, Wandji J, Sewald N. Chemical constituents of the fruits of Gambeya lacourtiana (Sapotaceae). Phytochem Lett. 2020;38:84–9. https://doi.org/10.1016/j.phytol.2020.05.009.
Talla MR, Jouda JB, Mbazoa DC, Kapche FWDG, Lenta NB, Sewald N, Wandji J. Pentacyclic triterpenoids and other constituents isolated from the leaves of Gambeya lacourtiana and their antibacterial activity. Biochem Syst Ecol. 2021;98:104322. https://doi.org/10.1016/j.bse.2021.104322.
Talla MR, Jouda JB, Mawabo KI, Tegasne C, Happi MG, Lenta NB, Kapche FWDG, Sewald N, Wandji J. One new constituent from the stem bark of Chrysophyllum lacourtianum De Wild. (Sapotaceae). Nat Prod Res. 2021;1–8. https://doi.org/10.1080/14786419.2021.1986493.
Ceylan O, Tamfu AN, Doğaç Y, Teke M. Antibiofilm and anti-quorum sensing activities of polyethylene imine coated magnetite and nickel ferrite nanoparticles. 3 Biotech. 2020;10:1–12. https://doi.org/10.1007/s13205-020-02509-6.
Arab Y, Sahin B, Ceylan O, Zellagui A, Olmez OT, Kucukaydin S, Tamfu AN, Ozturk M, Gherraf N. Assessment of in vitro activities and chemical profiling of Senecio hoggariensis growing in Algerian Sahara. Biodiversitas. 2022;23:3498–506. https://doi.org/10.13057/biodiv/d230724.
Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms, Cur. Protoc. Microbiol. John Wiley & Sons, Inc.; 2005. https://doi.org/10.1002/9780471729259.mc01b01s00.
Tamfu AN, Munvera AM, Botezatu AVD, Talla E, Ceylan O, Fotsing MT, Mbafor JT, Shaheen F, Dinica RM. Synthesis of benzoyl esters of β-amyrin and lupeol and evaluation of their antibiofilm and antidiabetic activities. Results Chem. 2022;4:100322. https://doi.org/10.1016/j.rechem.2022.100322.
Koh KM, Tham FY. Screening of traditional chinese medicinal plants for quorum-sensing inhibitors activity. J Microbiol Immunol Infect. 2011;44:144–8. https://doi.org/10.1016/j.jmii.2009.10.001.
Kocak G, Tamfu AN, Bütün V, Ceylan O. Synthesis of quaternary piperazine methacrylate homopolymers and their antibiofilm and anti-quorum sensing effects on pathogenic bacteria. J Appl Polym Sci. 2021;138:1–14. https://doi.org/10.1002/app.50466.
Packiavathy IASV, Agilandeswari P, Musthafa KS, Pandian SK, Ravi AV. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res Int. 2012;45:85–92. https://doi.org/10.1016/j.foodres.2011.10.022.
Tamfu AN, Kucukaydin S, Ceylan O, Sarac N, Duru ME. Phenolic composition, enzyme inhibitory and anti-quorum sensing activities of cinnamon (Cinnamomum zeylanicum Blume) and Basil (Ocimum basilicum Linn). Chem Afric. 2021;4(4):759–67. https://doi.org/10.1007/s42250-021-00265-5.
Kapadia C, Kachhdia R, Singh S, Gandhi K, Poczai P, Alfarraj S, Ansari MJ, Gafur A, Sayyed RZ. Pseudomonas aeruginosa inhibits quorum-sensing mechanisms of soft rot pathogen Lelliottia amnigena RCE to regulate its virulence factors and biofilm formation. Front Microbiol. 2022;13:977669. https://doi.org/10.3389/fmicb.2022.977669.
Ginovyan M, Petrosyan M, Trchounian A. Antimicrobial activity of some plant materials used in armenian traditional medicine. BMC Complement Altern Med. 2017;17(1):50. https://doi.org/10.1186/s12906-017-1573-y.
Upadhyay A, Upadhyaya I, Kollanoor-Johny A, Venkitanarayanan K. Combating pathogenic microorganisms using plant-derived antimicrobials: a minireview of the mechanistic basis. Biomed Res Int. 2014;761741. https://doi.org/10.1155/2014/761741.
Boudiba S, Tamfu AN, Berka B, Hanini K, Hioun S, Allaf K, Boudiba L, Ceylan O. Anti-quorum sensing and antioxidant activity of essential oils extracted from Juniperus species, growing spontaneously in Tebessa Region (East of Algeria). Nat Prod Com. 2021;16(6):1–11. https://doi.org/10.1177/1934578X211024039.
Ye L, Zhang J, Xiao W, Liu S. Efficacy and mechanism of actions of natural antimicrobial drugs. Pharmacol Ther. 2020;216:107671. https://doi.org/10.1016/j.pharmthera.2020.107671.
Tamfu AN, Ceylan O, Cârâc G, Talla E, Dinica RM. Antibiofilm and anti-quorum sensing potential of cycloartane-type triterpene acids from cameroonian grassland propolis: phenolic profile and antioxidant activity of crude extract. Molecules. 2022;7(15):4872. https://doi.org/10.3390/molecules27154872.
Tamfu AN, Kucukaydin S, Quradha MM, Ceylan O, Ugur A, Duru ME. Ultrasound assisted extraction of Syringa vulgaris Mill., Citrus sinensis L. and Hypericum perforatum L.: phenolic composition, enzyme inhibition and anti-quorum sensing activities. Chem Afric. 2022;5:237–49. https://doi.org/10.1007/s42250-022-00315-6.
Bhattacharya SP, Mitra A, Bhattacharya A, Sen A. Quorum quenching activity of pentacyclic triterpenoids leads to inhibition of biofilm formation by Acinetobacter baumannii. Biofouling. 2020;36:922–37. https://doi.org/10.1080/08927014.2020.1831480.
McLean RJC, Pierson LS, Fuqua C. A simple screening protocol for the identification of quorum signal antagonists. J Microbiol Methods. 2004;351–60. https://doi.org/10.1016/j.mimet.2004.04.016.
Hentzer M, Givskov M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest. 2003;112:1300–7. https://doi.org/10.1172/JCI20074.
Tamfu AN, Ceylan O, Kucukaydin S, Ozturk M, Duru ME, Dinica RM. Antibiofilm and enzyme inhibitory potentials of two annonaceous food spices, african pepper (Xylopia aethiopica) and african nutmeg (Monodora myristica), Foods. 2020; 9(12):1768, https://doi.org/10.3390/foods9121768.
Venkatramanan M, Ganesh PS, Senthil R, Akshay J, Ravi AV, Langeswaran K, Vadivelu J, Nagarajan S, Rajendran K, Shankar EM. Inhibition of quorum sensing and biofilm formation in Chromobacterium violaceum by fruit extracts of Passiflora edulis. ACS Omega. 2020;5(40):25605–16. https://doi.org/10.1021/acsomega.0c02483.
Alfred TN, Ceylan O, Kucukaydin S, Olmez OT, Godloves CF, Sylvain SK, Yeskaliyeva B, Duru ME, Ozturk M. HPLC-DAD and GC-MS characterization of cameroonian honey samples and evaluation of their antibiofilm, anti-quorum sensing and antioxidant activities. Bull Env Pharmacol Life Sci. 2020;9(10):132–42.
Popova M, Gerginova D, Trusheva B, Simova S, Tamfu AN, Ceylan O, Clark K, Bankova V. A preliminary study of chemical profiles of honey, cerumen, and propolis of the african stingless bee Meliponula ferruginea. Foods. 2021;10:997. https://doi.org/10.3390/foods10050997.
Gilabert M, Marcinkevicius K, Andujar S, Schiavone M, Arena ME, Bardón A. Sesqui- and triterpenoids from the liverwort Lepidozia chordulifera inhibitors of bacterial biofilm and elastase activity of human pathogenic bacteria. Phytomedicine. 2015;22(1):77–85. https://doi.org/10.1016/j.phymed.2014.10.006.
Rütschlin S, Böttcher T. Inhibitors of bacterial swarming behavior. Chemistry. 2020;26(5):964–79. https://doi.org/10.1002/chem.201901961.
Alain KY, Tamfu AN, Kucukaydin S, Ceylan O, Pascal AD, Félicien A, Dominique SC, Duru ME, Dinica RM. Phenolic profiles, antioxidant, antiquorum sensing, antibiofilm and enzyme inhibitory activities of selected Acacia species collected from Benin. LWT. 2022;12:114162. https://doi.org/10.1016/j.lwt.2022.114162.
Tamfu AN, Boukhedena W, Boudiba S, Deghboudj S, Ceylan O. Synthesis and evaluation of inhibitory potentials of microbial biofilms and quorum-sensing by 3-(1, 3-dithian-2-ylidene) pentane-2, 4-dione and ethyl-2-cyano-2-(1, 3-dithian-2-ylidene) acetate. Pharmacia. 2022;69:973–80. https://doi.org/10.3897/pharmacia.69.e87834.
Lakshmanan D, Nanda J, Jeevaratnam K. Inhibition of swarming motility of Pseudomonas aeruginosa by methanol extracts of Alpinia officinarum Hance. And Cinnamomum tamala T. Nees and Eberm. Nat Prod Res. 2018;32:1307–11. https://doi.org/10.1080/14786419.2017.1340289.
Vandeputte OM, Kiendrebeogo M, Rajaonson S, Diallo B, Mol A, El Jaziri M, Baucher M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl Environ Microbiol. 2010;76(1):243–53. https://doi.org/10.1128/AEM.01059-09.
Chang AW, Dowd SE, Brackee G, Fralick JA, Vediyappan G. Inhibition of Staphylococcus aureus biofilm formation by gurmarin, a plant-derived cyclic peptide. Front Cell Infect Microbiol. 2022;12:1017545. https://doi.org/10.3389/fcimb.2022.1017545.
Wei G, He Y. Antibacterial and antibiofilm activities of novel cyclic peptides against methicillin-resistant Staphylococcus aureus. Int J Mol Sci. 2022;23:8029. https://doi.org/10.3390/ijms23148029.
Rajkumari J, Borkotoky S, Murali A, Suchiang K, Mohanty SK, Busi S. Attenuation of quorum sensing controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by pentacyclic triterpenes, betulin and betulinic acid. Microb Pathog. 2018;118:48–60. https://doi.org/10.1016/j.micpath.2018.03.012.
Silva G, Primon-Barros M, Macedo AJ, Gnoatto SCB. Triterpene derivatives as relevant scaffold for new antibiofilm drugs. Biomolecules. 2019;9:58. https://doi.org/10.3390/biom9020058.
Bhattacharya SP, Bhattacharya A, Sen A. A comprehensive and comparative study on the action of pentacyclic triterpenoids on Vibrio cholerae biofilms. Microb Pathog. 2020;149:104493. https://doi.org/10.1016/j.micpath.2020.104493.
Adeyemo RO, Famuyide IM, Dzoyem JP, Joy ML. Anti-biofilm, antibacterial, and anti-quorum sensing activities of selected south african plants traditionally used to treat diarrhoea. Evidence-Based Com Alt Med. 2022;1307801. https://doi.org/10.1155/2022/1307801.
Acknowledgements
The authors are grateful to the Higher Teacher Training College of the University of Yaoundé 1, Ula Ali Kocman Vocational School, Mugla Sitki Kocman University, Bielefeld University and the School of Chemical Engineering and Mineral Industries, University of Ngaoundere.
Funding
This work was supported by Yaoundé-Bielefeld Bilateral Graduate School Natural Products with Anti-parasite and Anti-bacterial Activity (YaBiNaPA) project, financially supported by Deutscher Akademischer Austauschdienst (DAAD) [grant number 57316173].
Author information
Authors and Affiliations
Contributions
R.M.T particpated in Methodology, Data curation; Experimentation, Writing original draft; Writing review & editing. A.N.T. paricipated in Conceptualization, Data curation, Methodology, Experimentation, Writing original draft, Writing review & editing, Validation and Formal analysis. B.N.K.W. participated in Methodology, Data curation, experimentation and Writing original draft. O.C., C.D.M., G.D.W.F.K. participated in Conceptualization, Data curation, Formal analysis, Visualization, Review & editing, Supervision and Resources. B.N.L., N.S. and J.W. participated in Conceptualization, Review & editing, Resources, Supervision and Funding acquisition. B.N.L. participated in Project administration. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study on Gambeya lacourtiana is part of a PhD work and was performed in accordance with the relevant regulations of the University of Yaounde I in compliance with the IUCN Policy Statement on Research Involving Species at Risk of Extinction. The University of Yaounde I issued the permission to collect the plant with the aid of the National Herbarium of Cameroon.
Consent for publication
N/A.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Talla, R.M., Tamfu, A.N., Wakeu, B.N.K. et al. Evaluation of anti-quorum sensing and antibiofilm effects of secondary metabolites from Gambeya lacourtiana (De Wild) Aubr. & Pellegr against selected pathogens. BMC Complement Med Ther 23, 300 (2023). https://doi.org/10.1186/s12906-023-04115-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-04115-4