Abstract
In this study, the antimicrobial mechanism of cinnamaldehyde (CIN) against Gram-negative Escherichia coli ATCC 25922 (E. coli) based on membrane and gene regulation was investigated. Treatment with low concentration (0, 1/8, 1/4, 3/8 MIC) of CIN can effectively suppress the growth of E. coli by prolonging its lag phase and Raman spectroscopy showed obvious distinction of the E. coli after being treated with these concentration of CIN. The determination of relative conductivity indicated that CIN at relatively high concentration (0, 1, 2, 4 MIC) can increase the cell membrane permeability, causing the leakage of cellular content. Besides, the content of malondialdehyde (MDA) and the activity of total superoxide dismutase (SOD) of E. coli increased with increasing treatment concentration of CIN, implying that CIN can cause oxidative damage on E. coli cell membrane and induce the increase of total SOD activity to resist this oxidative harm. Moreover, quantitative real-time RT-PCR (qRT-PCR) analysis revealed the relationship between expression of antioxidant genes (SODa, SODb, SODc) and treatment CIN concentration, suggesting that SOD, especially SODc, played a significant role in resistance of E. coli to CIN. The underlying inactivation processing of CIN on E. coli was explored to support CIN as a potential and natural antimicrobial agent in food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Escherichia coli is deemed as a kind of significant food-borne pathogens, which has caused serious disease outbreaks in recent years. Food may be contaminated with this microorganism by being tainted with animal waste in water or soil (Baskaran et al. 2010; Hong et al. 2016), so people try to find suitable disposing methods to solve this problem. However, traditional strategies such as sterilization of ultraviolet, thermal processing, and chemical technique have marked disadvantages, which may induce negative impacts on nutrition and even health problems (Hong et al. 2016; Tian et al. 2012). Thus, natural and effective sterilization methods have received great attention with the aim to keep nutrition and control food-related undesirable microorganisms.
Essential oils were traditionally used to enhance flavor and preserve human foods as a group of natural food additives, which catered to people’s pursuit of green and natural food (Boubaker et al. 2016; Raut and Karuppayil 2014). Importantly, essential oils are considered safe and approved to be used in foods, so they have gradually become a hot area of food preservation in recent years (Pérez-Alfonso et al. 2012; Zhou et al. 2014). Cinnamaldehyde (CIN), presenting as a major component of extraction of cinnamon bark (Cinnamomum verum), was reported that it was one of the most effective essential oils against undesirable food microorganisms (Wang et al. 2016a; Yin et al. 2015b) and can be a kind of feed additives to control microbial contamination (Yin et al. 2015a). Membrane fluidity is a basic condition to maintain normal cell activity (Tourdot-Maréchal et al. 2000; Wei et al. 2018). Shen et al. revealed that CIN can impact the morphology of cell, causing membrane disruption and leakage of cell content (Shen et al. 2015). Besides, our previous research has verified that Escherichia coli changed its fluidity to resist the harmful effect of CIN by decreasing the proportion of unsaturated fatty acids (He et al. 2017). Enzymes are essential components to maintain vital activities of microorganisms, and it was reported that CIN can drastically decrease the enzyme activities (Lee et al. 2002). Although the strong antimicrobial activity of CIN has been confirmed, its specific behavior of sterilization and the response of microbes to it are still lacking. Therefore, additional researches should be carried out for its wider and better application in future.
In this work, the disruption and oxidation of CIN on cytoplasmic membrane of Escherichia coli (E. coli) were explored. Moreover, to detect the resistance of E. coli to the oxidative damage, the expressions of antioxidant genes (SODa, SODb, SODc) were determined to identify the roles they played. In short, the antimicrobial mechanism of CIN on E. coli was detected more deeply to benefit that CIN can be commonly and safely used in food industry and agriculture.
Materials and methods
Materials
CIN (purity, 99.0%) was supplied by Aladdin Chemistry Co, Ltd. (Shanghai, China). It was dissolved in ethanol (purity, 99.7%) to achieve its stock solution (5.0 g/L) and then stored at 4 °C. The microbial strains, Gram-negative E. coli ATCC 25922 was obtained from Microbial Culture Collection Center of Guangdong Institute of Microbiology (Guangzhou, China).
Culture conditions
A plate containing tryptic soy broth with 0.6% (w/v) of yeast extract (TSA-YE) was inoculated a loopful of strains onto and then incubated on an orbital shaker (120 rpm, HY-5, JinBo Equipment Industry Co., Jiangsu, China) at 37 °C for 18–24 h. A loopful of colony from the precultured plate was transferred to 200 mL fresh sterile TSA-YE liquid medium and cultured at 37 °C for 12 h with shaking at 120 rpm. 3 mL of this broth was added into sterile test tubes added with 1 mL, 25% glycerol, and stored at -80 °C. The suspension in test tubes was then transferred to 100 mL fresh sterile TSA-YE liquid mediums (OD600 ≈ 0.09), and cultured with CIN at different concentrations [0, 0.29 mM (1/8 MIC), 0.59 mM(1/4 MIC) and 0.88 mM (3/8 MIC)] for the following antimicrobial tests.
Growth curves of E. coli with CIN treatment
The minimum inhibitory concentration (MIC) of CIN toward E. coli was indicated to be 0.31 g/L (2.35 mM) (Shen et al. 2015). Our research about the effect of CIN on growth curves of E. coli was consistent with a previous method (Zeng et al. 2012). The cultures in test tubes were transferred into 100 mL fresh sterile TSA-YE liquid mediums with equal volumes of ethanol (control) and CIN at different concentrations (1/8 MIC, 1/4 MIC, 3/8 MIC). These mediums were incubated on a rotary shaker at 37 °C, and measured every half hour for 4.5 h.
Raman spectroscopy analysis
The E. coli cell suspension was obtained according to the above conditions and treated with a series of concentrations of CIN (0, 1/8, 1/4, 3/8 MIC) as samples to prepare for the Raman measurement. In this work, a LabRAM HR Evolution spectrometer system (Horiba, Japan) using a 633-nm near-infrared diode laser source and equipped with a Leica DMLB microscope (Wetzlar, Germany) was used to detect the Raman scattering signals from these samples with range from 600 to 1800 cm−1 in extended mode. All samples were exposed 10 s and c. 10 mW Laser power according to a previous research (Liu et al. 2009). Then, the Raman data were obtained using the Delight version 3.2.1 (D-Squared Development Inc., LaGrande, OR, USA) software. Instrumental noises and polynomial subtract removes baseline offsets were eliminated smoothly in accordance with a previous study (He et al. 2008).
Principal component analysis (PCA) is a mathematical algorithm to analyze the connection among different data, indicating their similarities or differences. This technique focuses on the more significant factors, removing influence of random varieties (Goodacre et al. 1998). In this work, PCA was employed to suggest changes of cell compositions after treatment with different concentrations of CIN to exhibit their potential connection.
Relative conductivity determination
This experiment was based on a previous method using a conductivity meter (DDS-11A, Shanghai Leici Instrument Inc., Shanghai, China) to measure the electrolyte leakage (Wang et al. 2017). Briefly, the cells were first cultivated at the stationary phase. After these cell suspensions were centrifuged at 4000×g for 5 min, and washed twice with 5.0% glucose, the suspension was diluted and separated equally into four flasks. Then, CIN was added to the bacteria suspension to achieve different treatment concentrations (0, 1/8, 1/4, 3/8, 1/2, 1, 2 and 4 MIC). A 5 mL sample from each flask was used for the measurement at 0, 0.5, 1, 1.5, 2, 2.5 and 6.5 h. The change of permeability of E.coli cell membrane influenced by different concentration of CIN was according to the measurement of relative conductivity.
where E1 and E2 represent the conductivity of the initial suspension and the suspension treated with different concentration of CIN for different treatment time, respectively. Additionally, E0 is the conductivity of the sample in 5.0% glucose finally treated in boiling water for 10 min.
Determination of oxidation indexes
The content of malondialdehyde (MDA) and the activity of superoxide dismutase (SOD) are significant indexes to suggest the harm caused by the oxidative damage and the resistance of microorganisms to the oxidant harm from external environment (Yun et al. 2016a). The stationary-phase E. coli bacterial suspension was cultured with different concentrations of CIN (0, 1/2, 3/4, 1 MIC) and detected according to the instruction of Malondialdehyde (MDA) Assay Kit and Superoxide Dismutase (SOD) Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Relevant RNA extraction and quantitative real-time RT-PCR (qRT-PCR) analysis
Following the instruction of FastQuantity RT Kit (KR106) (Tiangen biochemical technology Co., Ltd., Beijing, China), total RNA was extracted from the E. coli cells at stationary phase with different treated concentration of CIN (0, 1/8, 1/4, 3/8 MIC). Then the purity, concentration and integrity of RNA extraction were determined by Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., USA), Nanodrop 2000 (Thermo Scientific Inc., Wilmington, USA) and agarose gel electrophoresis, respectively.
Total RNA extraction (200 ng) was used to carry out the first-strand cDNA synthesis reverse transcription according to the FastQuant RT Kit (with gDNase) (Tiangen biochemical technology Co., Ltd., Beijing, China). Then, ABI step one plus (ABI, USA) was used to detect the transcription levels of relevant genes (SODa, SODb, SODc) to process qRT-PCR analysis. Our complete sequences of genes were provided by NCBI database and all primers listed in Table 1 were obtained from Jidiao Biotech Co. Ltd. (Guangzhou, China). The relative expression was checked by 20 µL qPCR reaction mix, which consisted of 10 µL qPCR Master Mix, 0.6 µL of 10 µM forward primer, 0.6 µL of 10 µM reverse primer, 4 µL of template cDNA, and ddH2O was used to maintain the total volume to 20 µL. The conditions of qRT-PCR were as follows: 1 cycle at 95 °C for 90 s; 40 cycles at 95 °C for 5 s, 60 °C for 15 s and 72 °C for 20 s. The method of 2−∆∆Ct 162 was used to determine the relative change of gene expression (Livak and Schmittgen 2001).
Statistical analysis
All tests were measured in triplicates and all data were performed as mean ± standard deviation, using statistical program SPSS (Statistical Package for the Social Sciences, version 22.0, IBM, NY, USA). One-way analysis of variance (ANOVA) was applied to analyze the comparison of difference between data groups. Significant differences were set if P < 0.05.
Results and discussion
The effect of CIN on the growth of E. coli
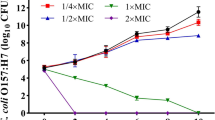
The negative influence of CIN on E.coli, performed by corresponding growth curves at different concentrations of CIN (0, 1/8, 1/4, 3/8 MIC), was investigated. As shown in Fig. 1, CIN has obviously inhibitory effect on the growth of E. coli, which can be inferred from these growth curves. Compared with the optical density at 600 nm of the control group at 2 h (OD600 = 1.460 ± 0.035), the value of OD600 at 2 h decreased from 1.250 ± 0.001 to 0.529 ± 0.003 with the concentration of CIN increasing from 1/8 MIC to 3/8 MIC. Besides, the lag phase was gradually prolonged when E. coli was exposed to increasing concentration of CIN. The lag phase of E. coli with absence of CIN was nearly 1.5 h, while it was extended to 2 h with 1/8 MIC concentration of CIN, further longer lag phase when the concentration of CIN was 1/4 and 3/8 MIC. A previous research has found that the growth of microorganism can be significantly inhibited by thymol (Wang et al. 2016b), and our result obviously verified the similar depression effect of CIN on E. coli growth.
Raman spectroscopy analysis
This spectra showed different absorption bands from 600 to 1800 cm−1, suggesting the change of cell compositions. Previous studies have indicated these bands represented different cell compositions, including lipids, proteins, carbohydrates and nucleic acids (Liu et al. 2009). For instance, absorption bands at 977 and 1453 cm−1 represent lipids. Bands around 853, 1005, 1252 and 1665 cm−1 are consistent with proteins. Those bands around 1035 cm−1 and Raman swifts (726, 783, 936, 1101, 1340 and 1577 cm−1) are carbohydrates and nucleic acids, respectively. As shown in Fig. 2a, the shape of Raman spectroscopy of the cells with absence of CIN was in good agreement with others in different concentrations (1/8, 1/4, 3/8 MIC). However, the messages in Fig. 2b illustrated that with increasing concentration of CIN, the Raman spectral intensity of nearly all kinds of cell substances increased, suggesting that CIN had negative impact on E. coli membrane, casing the slight leakage of cellular content and the effect was more obvious as concentration of CIN increasing. PCA analysis was performed to process the data of Raman spectroscopy and the score plot was generated to show their potential connection. As shown in Fig. 3c, the first two principal components accounted for 90% and 6% of total variance in E. coli cell, respectively. Clear segregation was observed among the samples treated with different concentration of CIN, which suggested that the exposure of E. coli to CIN has caused systematic changes in cell compositions.
Raman spectra of E. coli cells treated with different concentrations of CIN (0, 1/8, 1/4, 3/8 MIC). The Raman shift of measurement was in range from 600 to 1800 cm−1. a Separated curves; b aggregated curves; c principal component analysis (PCA) based on the data of Raman spectra of E. coli cells treated with different conditions
Cell membrane permeability
Microorganism cell membrane plays an important role in maintaining cell morphology, information exchange and material movement in and out of cell, so it is an essential part for the maintenance of life activities. The damage of cell membrane will cause leakage of cellular content, leading to the value of electric conductivity increasing (Wang et al. 2016b). Therefore, the measurement of relative electrical conductivity is a reliable method to explore the permeability of cell membrane. As shown in Fig. 3, relative electrical conductivity of bacterial suspension with 1, 2 and 4 MIC of CIN at 2.5 h were 35.537 ± 0.242, 58.093 ± 0.477 and 59.904 ± 0.256%, respectively, which were obviously higher than that of the control group with 0 MIC CIN (4.672 ± 0.674%) and gradually increased with the extension of processing time, implying the increase of cell membrane permeability at higher treated concentration of CIN. Shen et al. used SEM and TEM techniques to exhibit the morphology of E. coli after CIN treatment, showing that CIN can cause distortion and rupture of E. coli cell membrane (Shen et al. 2015). Xu et al. verified that the integrity of membrane of A. alternata was destroyed after being treated with CIN (Xu et al. 2018). However, CIN at relatively low concentration (1/8, 1/4, 3/8, 1/2 MIC) induced slight increase of relative electrical conductivity, suggesting that these concentrations of CIN cannot lead to serious membrane disruption. In short, it can be inferred that high concentration of CIN had significant influence on cell membrane, leading to increase of membrane permeability and rupture of cell membrane.
Oxidation damage of CIN in E. coli
Malondialdehyde (MDA), is normally generated as a by-product during the oxidation of cell membrane, and its content reflects the peroxidation degree of cell membrane lipid (Yun et al. 2016b). As shown in Fig. 4a, the content of MDA exhibited an increasing trend with the increase of CIN concentration and the treatment time. For example, the content of MDA was 1.848 ± 0.210 nmol/mgprot after the cells were treated with 1/2 MIC CIN for 2 h, which was obviously higher than the content of MDA from samples with the absence of CIN (0.435 ± 0.051 nmol/mgprot), and it generally increased to 2.322 ± 0.154 and 2.580 ± 0.256 nmol/mgprot when E. coli being treated with 3/4 and 1 MIC CIN, respectively. Besides, the MDA content exhibited a marked increase with increasing treatment time. Our result illustrated that CIN can cause oxidative injury in E. coli membrane, resulting in the increase of MDA content with the concentration of CIN increasing. Moreover, a previous study has revealed that MDA was a significant product of the oxidation of unsaturated fatty acids (Lefèvre et al. 1998) and our previous study (He et al. 2017) has verified the decrease of the ratio of unsaturated fatty acids in E. coli cell membrane when it was exposed to CIN, so it can be inferred that the unsaturated fatty acids of membrane was one of important targets when CIN impacted on E. coli. Besides, it was reported that microorganism resisted the harm from external environment by adjusting the ratio of unsaturated fatty acids in cell membrane, which changed the fluidity of cell membrane to protect itself (Burns et al. 1979). The fluidity of cell membrane decreased when the ratio of unsaturated fatty acid decreased, which enhanced its resistance (Denich et al. 2003). Thus, except the stress response of microorganism itself (He et al. 2017), the oxidative damage of CIN may also be one of the important reasons for the change of the fluidity of E. coli cell membrane when it was exposed to CIN.
Superoxide dismutase (SOD) can effectively remove the excessive active oxygen to keep the balance of active oxygen metabolism, and its activity is commonly used to detect the resistance of microorganisms to the external oxidative harm (Knight and Knight 2001; Rohrdanz et al. 2001). As shown in Fig. 4b, the activity of total SOD without treatment of CIN for 1 h was 95.718 ± 0.090 U/mgprot, and increased to 102.983 ± 0.354, 108.460 ± 0.298, 113.192 ± 1.469 U/mgprot with 1/2, 3/4, 1 MIC CIN, respectively. Besides, it had a rapid increase during the first hour and then increased smoothly, exhibiting a total increase trend with the extension of treatment time. However, there was a slight decrease after the samples were treated in 1 MIC CIN for 3 h, which may due to the production of free radicals exceeding the scavenging ability of total SOD, leading to negative impacts on total SOD and decrease of its activity. It was reported that active oxygen radicals can effectively induce the activity of total SOD increasing (Greenberg et al. 1990), and it will be produced when the unsaturated fatty acids of the cell membrane are oxidized (Lefèvre et al. 1998). Therefore, it can be inferred that the mechanism of CIN oxidizing E. coli cell membrane is that CIN oxidizes the unsaturated fatty acids of the cell membrane, causing generation of large number of active oxygen radicals, which induces increasing SOD activity to resist this harm.
The relationship between regulation of SOD genes and the resistance of E .coli to CIN
To detect the relationship between the expression of antioxidant genes and the resistance of E .coli to CIN, SOD relevant genes (SODa, SODb, SODc) in E. coli were selected and their transcription levels were investigated after being treated with different concentration of CIN (0, 1/8, 1/4, 3/8 MIC). Previous studies have indicated that SOD was divided into Mn-SOD (SODa), Fe-SOD (SODb) and CuZn-SOD (SODc) according to its combination with metal ions and they played significant roles in different locations to maintain normal life activities of microbes (Battistoni 2003).
As shown in Fig. 5, the relative expression of SODc gradually increased with the concentration of CIN increasing (from 1 ± 0.05- to 3.78 ± 0.05-fold), showing up-regulated relationship between them. Meanwhile, for SODa and SODb, there was no clear relationship between their relative expression and CIN concentration. It was reported that microorganism can generate superoxide dismutase (SOD), catalasein (CAT), peroxidase (PO) and other protein to eliminate the oxidative damage of external environment (Knight and Knight 2001; Rohrdanz et al. 2001). Moreover, previous researches illustrated that SODc was revolved in the resistance of E. coli to H2O2, aiming to protect cell against the oxidative damage (Scotti et al. 2015), and SODc mutant strains suffered more serious damage compared to the normal strains (Pacello et al. 2008). Therefore, it can be inferred that the activity of SODc of E.coli was promoted and had a gradually increased trend with the increase of CIN concentration. Besides, SODc played a significant role in resistance of E. coli to the oxidative damage of CIN when E. coli was against to the harm of CIN according to our present work.
What’s more, SODc may also have a great significance to protect E.coli cell membrane from CIN. It was reported that SOD was divided into three kinds of enzymes (SODa, SODb, SODc). SODa and SODb mainly played important roles in protecting inside cells while SODc cleared free radicals on or outside membrane (Battistoni 2003). Previous study suggested that SODc gene was revolved in the synthesis of cell membrane (Kim et al. 2006), and according to the SEM images showing the difference between SODc mutants and normal strains when they suffered from the harm of H2O2, the result indicated that SODc probably played a significant role on the surface of cell membrane (Scotti et al. 2015). In our present work, it was found that one of the impacting locations of CIN on E. coli was cell membrane, which led to cell membrane distortion and oxidation. Then, the activity of total SOD, especially SODc, which mainly played an important role on the cell membrane or outside the cell, will increase to protect cell membrane from the oxidative damage caused by CIN.
Conclusions
This work has shown two key mechanisms of CIN impacting on E. coli membrane to exert its antibacterial behaviors: increasing the permeability of cell membrane and oxidizing the cell membrane. CIN at lower levels can effectively suppress the growth of E. coli cell while CIN at relative higher concentration can increase the permeability of cell membrane, leading to the leakage of cellular content. Analysis of the content of MDA and SOD indicated that cell membrane of E. coli would be oxidized when the cells were exposed to CIN and SOD would be produced increasingly to resist its oxidative harm. Besides, according to qRT-PCR analysis of SOD genes, it was explored that total SOD, especially SODc, played a significant role in resistance to the oxidative harm of CIN. In conclusion, CIN had excellent antibacterial effect against E. coli, especially its cell membrane and the corresponding genes were regulated to resist this damage. Moreover, this result implied that CIN may be a potential natural preservative and may get commonly used to enhance the safety in food industry and agriculture.
References
Baskaran SA, Amalaradjou MAR, Hoagland T, Venkitanarayanan K (2010) Inactivation of Escherichia coli O157:H7 in apple juice and apple cider by trans-cinnamaldehyde. Int J Food Microbiol 141:126–129
Battistoni A (2003) Role of prokaryotic Cu,Zn superoxide dismutase in pathogenesis. Biochem Soc Trans 31:1326–1329
Boubaker H et al (2016) Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Industrial Crops Products 86:95–101
Burns CP, Luttenegger DG, Dudley DT, Buettner GR, Spector AA (1979) Effect of modification of plasma membrane fatty acid composition on fluidity and methotrexate transport in L1210 Murine leukemia cells 1. Can Res 39:1726–1732
Denich TJ, Beaudette LA, Lee H, Trevors JT (2003) Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J Microbiol Methods 52:149–182
Goodacre R, Timmins EM, Burton R, Kaderbhai N, Woodward AM, Kell DB, Rooney PJ (1998) Rapid identification of urinary tract infection bacteria using hyperspectral whole-organism fingerprinting and artificial neural networks. Microbiology 144(Pt 5):1157
Greenberg JT, Monach P, Chou JH, Josephy PD, Demple B (1990) Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci USA 87:6181
He L, Kim NJ, Li H, Hu Z, Lin M (2008) Use of a fractal-like gold nanostructure in surface-enhanced raman spectroscopy for detection of selected food contaminants. J Agric Food Chem 56:9843–9847
He TF, Zhang ZH, Zeng XA, Wang LH, Brennan CS (2017) Determination of membrane disruption and genomic DNA binding of cinnamaldehyde to Escherichia coli by use of microbiological and spectroscopic techniques. J Photochem Photobiol B Biol 178:623–630
Hong J, Chen R, Zeng XA, Han Z (2016) Effect of pulsed electric fields assisted acetylation on morphological structural functional characteristics of potato starch. Food Chem 192:15–24
Kim YH et al (2006) The role of periplasmic antioxidant enzymes (superoxide dismutase and thiol peroxidase) of the Shiga toxin-producing Escherichia coli O157:H7 in the formation of biofilms. Proteomics 6:6181–6193
Knight H, Knight MR (2001) Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci 6:262
Lee HS, Kim BS, Kim MK (2002) Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. J Agric Food Chem 50:7700–7703
Lefèvre G, Beljeanleymarie M, Beyerle F, Bonnefontrousselot D, Cristol JP, Thérond P, Torreilles J (1998) Evaluation of lipid peroxidation by measuring thiobarbituric acid reactive substances. Annales De Biologie Clinique 56:305
Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M (2009) Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J Appl Microbiol 107:1193–1201
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T) Method. Methods 25:402–408
Pacello F, Ceci P, Ammendola S, Pasquali P, Chiancone E, Battistoni A (2008) Periplasmic Cu, Zn superoxide dismutase and cytoplasmic Dps concur in protecting Salmonella enterica serovar Typhimurium from extracellular reactive oxygen species. Biochim Biophys Acta 1780:226
Pérez-Alfonso CO, Martínez-Romero D, Zapata PJ, Serrano M, Valero D, Castillo S (2012) The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int J Food Microbiol 158:101–106
Raut JS, Karuppayil SM (2014) A status review on the medicinal properties of essential oils. Ind Crops Prod 62:250–264
Rohrdanz E, Schmuck G, Ohler S, Kahl R (2001) The influence of oxidative stress on catalase and MnSOD gene transcription in astrocytes. Brain Res 900:128–136
Scotti R, Nicolini L, Stringaro A, Gabbianelli R (2015) A study on prophagic and chromosomal sodC genes involvement in Escherichia coli O157:H7 biofilm formation and biofilm resistance to H2O2. Annali Dellistituto Superiore Di Sanità 51:62–66
Shen S, Zhang T, Yuan Y, Lin S, Xu J, Ye H (2015) Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 47:196–202
Tian J, Huang B, Luo X, Zeng H, Ban X, He J, Wang Y (2012) The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem 130:520–527
Tourdot-Maréchal R, Gaboriau D, Beney L, Diviès C (2000) Membrane fluidity of stressed cells of Oenococcus oeni. Int J Food Microbiol 55:269–273
Wang LH, Wang MS, Zeng XA, Gong DM, Huang YB (2016a) An in vitro investigation of the inhibitory mechanism of β-galactosidase by cinnamaldehyde alone and in combination with carvacrol and thymol. Biochim Biophys Acta 1861
Wang LH, Zhang ZH, Zeng XA, Gong DM, Wang MS (2016b) Erratum to: Combination of microbiological, spectroscopic and molecular docking techniques to study the antibacterial mechanism of thymol against Staphylococcus aureus: membrane damage and genomic. DNA Bind Anal Bioanal Chem 409:3055–3055
Wang LH, Wang MS, Zeng XA, Xu XM, Brennan CS (2017) Membrane and genomic DNA dual-targeting of citrus flavonoid naringenin against Staphylococcus aureus. Integr Biol 9
Wei JN, Zeng XA, Tang T, Jiang Z, Liu YY (2018) Unfolding and nanotube formation of ovalbumin induced by pulsed electric field. Innovative Food Sci Emerg Technol 45:249–254
Xu L, Tao N, Yang W, Jing G (2018) Cinnamaldehyde damaged the cell membrane of Alternaria alternata and induced the degradation of mycotoxins in vivo Industrial. Crops Products 112:427–433
Yin HB, Chen CH, Kollanoor-Johny A, Darre MJ, Venkitanarayanan K (2015a) Controlling Aspergillus flavus and Aspergillus parasiticus growth and aflatoxin production in poultry feed using carvacrol and trans-cinnamaldehyde. Poult Sci 94:2183
Yin HB, Chen CH, Kollanoorjohny A, Darre MJ, Venkitanarayanan K (2015b) Controlling Aspergillus flavus and Aspergillus parasiticus growth and aflatoxin production in poultry feed using carvacrol and trans-cinnamaldehyde. Poult Sci 94:2183–2190
Yun O, Liu ZW, Zeng XA, Han Z (2016a) Salmonella typhimurium resistance on pulsed electric fields associated with membrane fluidity and gene regulation. Innov Food Sci Emerg Technol 36:252–259
Yun O, Zeng XA, Brennan CS, Han Z (2016b) Effect of pulsed electric field on membrane lipids and oxidative injury of Salmonella typhimurium. Int J Mol Sci 17
Zeng WC, He Q, Sun Q, Zhong K, Gao H (2012) Antibacterial activity of water-soluble extract from pine needles of Cedrus deodara. Int J Food Microbiol 153:78–84
Zhou H, Tao N, Jia L (2014) Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 37:277–283
Acknowledgements
This research was supported by the National “Thirteenth Five” Key Research Project (2017YFD0400502), the 111 Project (B17018), the National Natural Science Foundation of China (21576099) and the S&T projects of Guangdong Province (2017B020207001 and 2015A030312001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jorge Membrillo-Hernández.
Rights and permissions
About this article
Cite this article
He, TF., Wang, LH., Niu, Db. et al. Cinnamaldehyde inhibit Escherichia coli associated with membrane disruption and oxidative damage. Arch Microbiol 201, 451–458 (2019). https://doi.org/10.1007/s00203-018-1572-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-018-1572-5