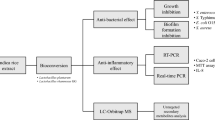
Abstract
Many studies have demonstrated that natural plant extracts have inhibitory effects on microorganisms. The purpose of this study was to investigate the inhibitory effect of phenolic acids from rice straw (PAs) on Escherichia coli and their synergistic effect in combination with antibiotics. PAs can inhibit the growth of E. coli effectively by inducing the formation of H2O2; PA-treated cells had a tenfold greater intracellular H2O2 concentration than the control group. The synergistic effect caused by the interaction of PAs and antibiotics on inhibiting the growth of E. coli was significant. This effect may be caused by a PA-induced change in the permeability of E. coli cell membrane. The treatment with PAs made the extracellular K+ concentration reached 15 mg/L within 30 min, while the K+ concentration in the control group was very low and did not change significantly over time. Similarly to the extracellular K+, the extracellular protein concentration exceeded 150 mg/L in the PA treatment group, while it remained very low in the control group. Due to the increased cell permeability, more antibiotics can enter the cell. Hence, this study may provide a novel method of improving the safe use of antibiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial infections are a worldwide problem endangering human health. Many formerly sensitive pathogens have developed resistance to antibiotics due to their misuse and overuse [1]. According to the US Centers for Disease Control and Prevention, approximately two million people worldwide suffer from microbial infections each year and 23,000 die from them [2, 3].
Phenolic compounds are considered to be effective antimicrobial agents. Pomegranate polyphenol extracts can inhibit the growth of pathogens, such as Staphylococcus aureus and Escherichia coli [4]. Catechin extracted from kombucha had inhibitory effects on enterotoxigenic E. coli, Vibrio cholerae, Shigella flexneri, and Salmonella typhimurium [5]. Lignin-derived phenolic acids (PAs) had inhibitory effects on Clostridium acetobutylicum [6]. Extracting phenolic compounds from low-cost raw materials and discussing their inhibitory mechanism would be beneficial to the application of bioantimicrobial agents.
Lignocellulosic biomass is the most abundant biomass resource, which consists primarily of cellulose, hemicellulose, and lignin [7, 8]. Rice straw is one of the most abundant sources of lignocellulosic biomass in the world. According to the Food and Agriculture Organization, approximately 600–900 million tons of rice straw is produced worldwide each year [9]. Rice straw is usually burned in the open air, which not only wastes this resource but also causes environmental pollution and seriously endangers human health. The bioconversion of rice straw into bioethanol could effectively mitigate these problems [10]. In addition to the main components cellulose, hemicellulose, and lignin, rice straw contains polyphenolic compounds, such as PAs of 13.6 mg/g, tannins of 1.9 mg/g, and flavonoids of 4.46 mg/g [11, 12]. Other lignocellulosic biomass also contain different types of phenolic compounds. For example, the phenolic compounds in wheat straw were coumaric acid and ferulic acid [13]; soybean contains caffeic acid, naringenin, and daidzein[14]; corn cob mainly contains coumaric acid [15]. The phenolic compounds could be considered high value-added products in bio-refinery process to reduce the production cost of bioethanol [16].
Antibiotics exhibit a strong inhibitory effect against pathogens. However, in recent years, the abuse and overuse of antibiotics made pathogens become drug- or even multi-drug-resistant, seriously threatening human health and lives [17]. Recent studies have also shown that phenolic compounds and antibiotics had a strong synergistic effect on pathogens. Combining phenolic compounds, such as gallic acid and hamamelitannin, with antibiotics more effectively inhibited the growth and biofilm viability of E. coli than individual antibiotics [18]. Equol has a synergistic effect with meropenem on carbapenem-resistant E. coli [19]. PAs derived from rice straw exhibited strong antimicrobial activity by destroying the permeability of cell membranes of S. aureus [20, 21]. Therefore, we assume that the destruction of cell membrane permeability by PAs may enable antibiotics to enter pathogenic cells more easily, thus improving the effect of antibiotics and reducing the amount used.
In this study, E. coli was used as a test species, because it is one of the most common foodborne and intestinal pathogens [22, 23]. The objectives of this paper were to (1) determine the inhibitory effect of PAs from rice straw on E. coli and (2) explore the synergistic effects of PAs acting with antibiotics. Fully understanding the synergistic effect of PAs and antibiotics could improve the utilization value of straw-based PAs, and expand new directions and possibilities for straw resource utilization.
Materials and Methods
Preparation of Phenolic Acids from Rice Straw
Rice straw was harvested from Chongming Island, Shanghai, China. After drying, rice straw was ground in a grinder, and then, a standard 1-mm diameter sieve was used to produce coarsely ground rice straw. Twenty gram of coarsely ground rice straw was treated with 200 mL of 2% NaOH at 121 °C for 20 min. The pH of the resulting solution was adjusted to 4, followed by centrifugation at 8000 rpm for 5 min. Then, the supernatant was treated with equal volumes of ethyl acetate for 30 min on a shaker at 100 rpm for three times. After the extraction, ethyl acetate was removed by rotary evaporation at 60 °C and 60 rpm to obtain PA fraction, which was dissolved in methanol. The composition of PAs was qualified and quantified using high-performance liquid chromatography (HPLC) as described in previous studies [24]. The free PAs in the samples were analyzed using a HPLC system (EX1600, Exformma, USA) equipped with a UV detector and an Eclipse SDB C18 column (250 mm × 4.6 mm, Agilent, USA). Elution was performed using mobile phase A (0.5% acetic acid in ultrapure water) and mobile phrase B (5% ultrapure water and 0.5% acetic acid in methanol). The following linear gradient was used: 5% B at 0–5 min, 5–25% B at 5–10 min, 25–40% B at 10–30 min, 40–50% B at 30–45 min, 50–100% B at 45–55 min, 100% B at 55–60 min, 100–5% B at 60–65 min. The column oven temperature was set at 35 °C, and the samples (diluted tenfold) were eluted with water at a flow rate of 1 mL min−1. The injection volume was 20 μL with the UV detector set to an absorbance wavelength of 280 nm.
Strain and Culture Conditions
The strain used in this study was E. coli ATCC 43,894. Before the experiment, the strain was pre-cultured in Luria–Bertani (LB) medium at 37 °C and 200 rpm to achieve logarithmic growth. The bacteria were harvested by centrifugation (5000 rpm, 5 min), and then washed twice with sterile water and resuspended in 25 g/L LB broth mixed with 2.5 g/L glucose. The initial OD600 was adjusted to 0.01 to complete the inhibition curves, synergy analysis, resazurin assay, colony-forming unit (CFU) assay, and cell metabolism assay. For the intracellular protein leak and intracellular K+ leak assays, the strain was suspended in sterile water and the initial OD600 was adjusted to 0.3.
Colony-Forming Unit (CFU) Assay
The CFU assay was used to determine the number of living cells in the culture. A 5 mL bacterial suspension with an initial OD600 value of 0.01 was placed in a large test tube and 50 µL of PAs was added to give a final concentration of 0.4 g/L. The culture was placed on a shaking table at 37 °C and 200 rpm for 12 h. Then, 100 μL of the culture with appropriate dilutions was spread on a solid LB plate and placed in an incubator at 37 °C for 18–24 h. Then, the number of individual colonies that formed on the plate was counted.
Analysis of Antibacterial Activity and Determination of the Minimum Inhibitory Concentration (MIC)
The antibacterial activity of PAs against E. coli was investigated according to the method described by Tao, with some modifications [25]. Briefly, 50 μL of PAs was added at different concentrations to 5 mL of cell suspension (25 g/L LB broth mixed with 2.5 g/L glucose) at an initial OD600 of 0.01 to obtain final concentrations ranging from 0.1 to 0.5 g/L. The concentration gradient was 0.1 g/L. These cultures were placed in an incubator at 37 °C and shaken at 200 rpm for 24 h. Samples were taken every 3 h to measure the OD600 value with a microplate reader. A total of 50 µL of PA-free methanol was used as a blank control. The MIC was determined according to the method described by Barman [26].
Synergistic Effect of PAs and Antibiotics
The synergistic effects of PAs with ampicillin and kanamycin were assessed according to previous studies with some modifications [27]. A total of 50 µL of PAs and 50 µL ampicillin or kanamycin were added to 5 mL of bacterial suspension with an initial OD600 value of 0.01 to obtain the desired concentration. The suspension was then placed on a shaker at 37 °C and 200 rpm for 12 h. Samples were taken to measure the OD600 value with a microplate reader. The actual and theoretical growth inhibition rates were then calculated according to the method described by Gu et al. [27], and the synergistic effect of PAs and antibiotics to the bacterial solution was determined. PAs (50 µL) in 50 µL of sterile water, 50 µL of ampicillin in 50 µL of sterile water, and 50 µL of kanamycin in 50 µL of sterile water were used as single drug controls, respectively. A culture containing 100 µL of sterile water was used as a blank control.
Cell Viability and Metabolic Assays
Metabolic Assays
The concentrations of glucose and acetic acid were determined using HPLC as described previously [28]. The experimental method was the same as described in the “Analysis of antibacterial activity and determination of the minimum inhibitory concentration (MIC)” section. The supernatant was obtained by passing 1 mL of culture through a 0.22-µm filter, after which the glucose and acetic acid concentrations were measured. Intracellular NAD+/NADH and NADP+/NADPH levels were measured with an NAD+/NADH quantification kit and NADP+/NADPH quantification kit (Sigma-Aldrich), respectively.
Viability Assays
ATP assay kit (Promega Corporation, Shanghai, China) was used according to the manufacturer’s instructions, to determine the concentrations of ATP. The ratio of ADP to ATP was determined with an ADP/ATP ratio assay kit (Sigma-Aldrich). All experiments were performed in triplicate.
The oxidative stress in cells was evaluated by detecting the level of intracellular H2O2 with a hydrogen peroxide assay kit (Beyotime, China). Briefly, 50 µL of PAs was added to 5 mL of bacterial suspension with an initial OD600 value of 0.3 to obtain a final concentration of 0.4 g/L. The solution was then placed on a shaker at 37 °C and 200 rpm for 12 h. A 1 mL aliquot of the culture was washed three times with sterile PBS (0.01 M, pH 7.4) and then resuspended in PBS, following which the H2O2 was detected.
Resazurin Assay
The resazurin assay was performed to indicate cell viability according to the method described by Skogman with slight modifications [29]. Briefly, the bacteria were collected by centrifugation from 1 mL of culture and the remaining medium was thoroughly washed with sterile PBS (0.01 M, pH 7.4) three times. The bacteria were then resuspended with sterile water of the same volume. An aliquot (100 μL) of the washed cells was added to 100 μL of 0.0125 g/L resazurin and placed in the dark at 37 °C for 30 min. Then, 100 μL of the reaction solution was transferred to a new white plate and the fluorescence value (λex570/λem590) was measured immediately. A higher fluorescence value represented greater cell viability.
Cytomembrane Permeability Analysis
The effect of PAs on the membrane permeability of E. coli cells was evaluated by detecting extracellular K+ and protein [24]. Briefly, 15 mL of bacterial suspension with an initial OD600 value of 0.3 was placed in a 50-mL centrifuge tube and 150 µL of PAs was added to produce a final concentration of 0.4 g/L. The culture was placed on a shaking table at 37 °C and 150 rpm. Samples were removed at 10, 30, 60, and 90 min and passed through 0.22-µm filters to obtain a supernatant. To detect K+, the supernatant was diluted with autoclaved ultrapure water (fivefold) and measured at 766.5 nm using flame photometry. The ZEEnit 700P (Analytic Jena, Germany) atomic absorption spectroscopy system was used for K+ quantification. The Bradford protein assay kit (Beyotime, China) was used to detect proteins.
Intracellular Antibiotic Content Assay
PAs (50 μL) and 50 μL ampicillin or kanamycin were added to 100 mL of bacterial suspension with an initial OD600 value of 0.01 and diluted to produce final concentrations of 0, 0.1, 0.2, and 0.3 mg/L (ampicillin); 0.2 g/L PAs + 0.15 mg/L ampicillin; 0, 5, 10, and 15 mg/L kanamycin; and 0.15 g/L PAs + 5 mg/L kanamycin. These solutions were placed on a shaker at 37 °C and 200 rpm for 12 h. Samples were taken to measure their OD600 values with a microplate reader. Afterward, the bacterial suspensions were centrifuged before being washed with ultrapure water three times. After that, 1 mL of ultrapure water was added to the samples, and the cell walls were broken by placing the samples at − 80 °C for 10 min and 100 °C for 10 min. The above process was repeated three times. The samples were then centrifuged.
An HPLC system equipped with a UV detector and an Eclipse XDB C18 column (250 mm × 4.6 mm, Agilent, Palo Alto, CA, USA) was employed to analyze the ampicillin content. The separation conditions were as described by Abdel et al. [30]. The mobile phase was composed of the following: mobile phase A: 50 mM KH2PO4 (pH 3.5), and mobile phase B: acetonitrile. Mobile phase B changes from 20 to 80% linearly within 10 min. The kanamycin contents were analyzed according to the method described by Zhang et al. [31].
Results and Discussion
Chemical Composition of PAs
The chemical composition of PAs was determined by HPLC and the results are shown in Table 1. The composition of PAs was relatively complex, mainly containing vanillic acid, vanillin, p-coumaric acid (CA), and ferulic acid (FA). These components accounted for 0.36, 0.18, 3.96, and 1.50 mg/g rice straw, respectively. These results are consistent with those described in previous studies [11, 21, 32], in which CA and FA were considered to be the main antibacterial constituents [21].
Inhibitory Effect of PAs on E. coli
Previous research has shown that rice straw polyphenols exhibit significant inhibitory effects on cellulase production by Trichoderma reesei, ethanol fermentation by Pichia stipitis, and lactic acid fermentation by Rhizopus oryzae [11, 20, 33]. Moreover, PAs from rice straw were shown to have strong inhibitory effects on S. aureus, a gram-positive bacteria, and could be used as a plant-based antimicrobial agent [20, 21]. To prove the broad-spectrum antibacterial activity of PAs, the inhibitory effect of PAs on E. coli, a gram-negative bacterium, and the synergistic effect of PAs with antibiotics were investigated in this study.
The time-inhibition curves of PAs, CA, and FA for E. coli ATCC 43,894 are shown in Fig. 1. The antibacterial effects of PAs, CA, and FA on E. coli were concentration-dependent (Fig. 1A–C). The MICs of the PAs and CA were 0.4 g/L and 0.6 g/L, respectively, while that of FA exceeded 1.0 g/L (Table 2). Compared with the MIC of FA on E. coli, the MIC of CA was closer to that of the PAs. Glucose consumption, fluorescence intensity (λex570/λem590), and CFU values showed similar trends (Fig. 1D–F); i.e., the degree of influence of PAs on cell viability was in the order PAs > CA > FA. The results indicated that PAs could effectively inhibit the growth of gram-negative bacteria, and CA was the main bioactive component, playing the most important role in suppressing E. coli.
Influence of PAs on H2O2 Formation by E. coli
H2O2 is an important reactive oxygen species, which reflects the redox state of cells. Studies have shown that bactericidal antibiotics can induce the formation of large amounts of H2O2 in cells after treatment, destroying various cell tissues and leading to cell lysis and death [34]. Therefore, the intracellular H2O2 levels were measured in this study (Fig. 2). As expected, cells treated with PAs, CA, or FA had higher H2O2 levels than that in the control group. In particular, PA-treated cells had a tenfold greater intracellular H2O2 concentration than the control group, which was higher than CA and FA treatment alone, demonstrating that PAs can significantly change the redox state in cells.
Many studies have shown that there is a common mechanism activated by antibiotics to induce cell death; i.e., antibiotics induce the formation of hydroxyl radicals as an end product of oxidative damage in cells that causes cell death [32]. Therefore, PAs were proposed to act synergistically with antibiotics due to the mechanism of causing cellular death.
Synergistic Effect of Interaction Between PAs and Antibiotics on E. coli
To evaluate the synergistic effect of antibiotics and PAs, ampicillin and kanamycin were used. Ampicillin (0.15 mg/L) and PAs (0.2 g/L) inhibited the growth of E. coli by 59% and 57%, respectively (Fig. 3A). The theoretical growth inhibition rate was calculated using the following formula: theoretical growth inhibition rate = 1 − growth rate of PAs × growth rate of antibiotic[27]. For example, the theoretical growth inhibition rate caused by the mixture of ampicillin and PAs should have been (1 − 41% × 43%) = 82%, where 41% and 43% were the growth rates of E. coli under the ampicillin and PA treatments, respectively. However, the actual growth inhibition rate caused by the mixture of ampicillin and PAs against E. coli was 96%, which was higher than the theoretical growth inhibition rate (82%). Similarly, the growth inhibition rates caused by kanamycin and PAs at 5 mg/L and 0.15 g/L were 55% and 48%, respectively (Fig. 3B). However, the actual growth inhibition rate caused by the combination of kanamycin and PAs was 97%, which was higher than the theoretical growth inhibition rate (76%). These results indicated a synergistic effect between PAs, ampicillin, and kanamycin on E. coli.
Synergistic growth inhibition effect between PAs and antibiotics on E. coli. A Synergistic effect between PAs and ampicillin on E. coli growth. The dashed line represents the theoretical growth inhibition rate caused by the mixture of ampicillin and PAs. B Synergistic effect between PAs and kanamycin on E. coli growth. C Synergistic effect between PAs and ampicillin on ATP in E. coli. D Synergistic effect between PAs and kanamycin on ATP in E. coli. E Synergistic effect between PAs and ampicillin on CFU of E. coli. F Synergistic effect between PAs and kanamycin on CFU of E. coli
Figure 3 C and D indicate that under ampicillin (0.15 mg/L), kanamycin (5 mg/L), and PA (0.2 g/L and 0.15 g/L) stress, there was an insignificant decrease in ATP relative to the control group. However, the combinations of PAs and antibiotics (0.15 mg/L ampicillin + 0.2 g/L PAs and 5 mg/L kanamycin + 0.15 g/L PAs) had stronger inhibitory effects than the PAs, ampicillin, or kanamycin alone, and there was a more obvious decreasing trend in ATP production
The viable bacteria assay showed a similar result. Figure 3E shows that after culturing, the Lg CFU value of the control group increased to 8.96, while the Lg CFU values were 7.81 and 8.34 for those samples containing PAs and ampicillin alone, indicating that PAs (0.2 g/L) and ampicillin (0.15 mg/L) alone were unable to inhibit the growth of E. coli completely. However, the combination of PAs and ampicillin led to a reduction of the Lg CFU value to 5.81, which was lower than the control value. The combination of PAs and kanamycin led to a similar result (Fig. 3F). However, the combination of PAs and kanamycin led to a decrease in the Lg CFU value (5.34) than the combination of PAs and ampicillin, indicating a different synergistic effect between the PAs and each antibiotic.
Influence of the Combination on H2O2 Formation by E. coli
Previous studies have shown that PAs can inhibit the growth of bacteria mainly by increasing the accumulation of intracellular H2O2, which leads to a change in cell membrane permeability. Figure 4 A and B show that under the stresses of ampicillin (0.15 mg/L), PAs (0.2 and 0.15 g/L), and kanamycin (5 mg/L), the contents of H2O2 were 7.86, 5.63 and 3.45, and 3.62 times higher than that of the control group, respectively. However, when PAs were combined with ampicillin and kanamycin, the intracellular H2O2 contents were 10.1 and 14.7 times higher than that of the control group, respectively. These findings indicated that the combination of PAs and antibiotics could promote the accumulation of intracellular H2O2 more than PAs or antibiotics alone.
The formation of more H2O2 could be attributed to the change of the intracellular redox status of cells induced by the antibiotics and PAs [21]. The NAD+/NADH ratio increased and the NADP+/NADPH ratio decreased under PA and antibiotic stress (Fig. 5A–D). These results demonstrated that more NADH than NADPH was involved in the intracellular redox reactions under such conditions. The combination of PAs and antibiotics led to a greater increase in the NAD+/NADH ratio and decrease in the NADP+/NADPH ratio than the PAs or antibiotics alone, indicating a synergistic effect of combining the PAs and antibiotics.
A marked depletion of NADH can generate ferrous iron, and this reaction consumes ATP, which is mainly produced by the tricarboxylic acid cycle [35]. The increasing trend of ADP/ATP ratio was most evident in the combination of PAs and the antibiotics (Fig. 5E, F). A common mechanism of cell death is stimulation of the Fenton reaction, which consumes ATP and NADH and increases the hydroxyl radical concentration, resulting in oxidative damage to cells [34]. The formation of peroxides is a mechanism of cell death commonly induced by bactericidal antibiotics [36]. Therefore, it may be proposed that the synergistic effect of PAs and antibiotics could be due to the increased formation of H2O2 resulting from their combined effects.
Influence of PAs on Membrane Permeability of E. coli
The increased formation of H2O2 may be attributed to a change in the permeability of the cell membrane caused by PAs [21]. Many studies have indicated that some natural plant antimicrobials can increase the membrane permeability of bacteria to release intracellular K+ and protein [37].
To explore whether PAs disrupt the membrane permeability of cells, the extracellular K+ and protein concentrations were measured (Fig. 6A). The cells treated with PAs, CA, and FA suffered from a rapid release of K+ within 30 min, and the extracellular K+ concentration reached 15 mg/L. However, the K+ concentration in the control group was very low and did not change significantly over time.
Similarly to the extracellular K+ detected above, the cells treated with PAs, CA, and FA also suffered from protein leakage (Fig. 6B). In the control group, extracellular protein concentrations remained low and changed little over time, while the extracellular protein concentrations detected in the PA treatment groups increased rapidly within 60 min and then remained unchanged. The highest extracellular protein concentration exceeded 150 mg/L in the PA treatment group, followed by 150 mg/L in the CA treatment group and 100 mg/L in the FA treatment group. These results indicated that PAs seriously damaged the membrane permeability of E. coli. After treatment with the combination of PAs and antibiotics (0.2 g/L PAs + 0.15 mg/L ampicillin, 0.15 g/L PAs + 5 mg/L kanamycin), the content of antibiotics in the E. coli cells significantly increased relative to the control group and the ampicillin or kanamycin treatment groups (Fig. 6C, D). As the concentration of antibiotics increased, the OD600 value of E. coli decreased over 12 h of cultivation, and the antibiotic content per unit OD600 of E. coli cells increased. These results indicated that more antibiotics permeated into cells even though fewer antibiotics were added because of the increased membrane permeability caused by the PAs.
Conclusions
In this study, a change in cell membrane permeability caused by PAs was found to improve the uptake of antibiotics into E. coli cells. Because PAs and antibiotics exhibited similar effects on the formation of H2O2, their combination resulted in a good synergistic effect on inhibiting the growth of E. coli. For that reason, the antibiotic could be reduced significantly. These results may provide a novel way of safely using antibiotics in the future.
References
Si, H., Hu, J., Liu, Z., & Zeng, Z.-L. (2008). Antibacterial effect of oregano essential oil alone and in combination with antibiotics against extended-spectrum beta-lactamase-producing Escherichia coli. FEMS Immunology and Medical Microbiology, 53(2), 190–194.
Servick, K. (2015). THE DRUG PUSH As fears of drug-resistant bacteria loom, governments try to coax companies back to the field. Science, 348(6237), 850–853.
Solomon, S. L., & Oliver, K. B. (2014). Antibiotic resistance threats in the United States: Stepping back from the brink. American Family Physician, 89, 938–941.
Anahi Portillo-Torres, L., Bernardino-Nicanor, A., Alberto Gomez-Aldapa, C., Gonzalez-Montiel, S., Rangel-Vargas, E., Roberto Villagomez-Ibarra, J., Gonzalez-Cruz, L., Cortes-Lopez, H., & Castro-Rosas, J. (2019). Hibiscus acid and chromatographic fractions from Hibiscus sabdariffa calyces: Antimicrobial activity against multidrug-resistant pathogenic bacteria. Antibiotics-Basel, 8(4), 218.
Bhattacharya, D., Bhattacharya, S., Patra, M. M., Chakravorty, S., Sarkar, S., Chakraborty, W., Koley, H., & Gachhui, R. (2016). Antibacterial activity of polyphenolic fraction of kombucha against enteric bacterial pathogens. Current Microbiology, 73(6), 885–896.
Luo, H., Liu, Z., Xie, F., Bilal, M., & Peng, F. (2021). Lignocellulosic biomass to biobutanol: Toxic effects and response mechanism of the combined stress of lignin-derived phenolic acids and phenolic aldehydes to Clostridium acetobutylicum. Industrial Crops and Products, 170, 113722.
Garrote, G., Dominguez, H., & Parajo, J. C. (2002). Autohydrolysis of corncob: Study of non-isothermal operation for xylooligosaccharide production. Journal of Food Engineering, 52(3), 211–218.
Saha, B. C. (2003). Hemicellulose bioconversion. Journal of Industrial Microbiology and Biotechnology, 30(5), 279–291.
Devendra, L. P., & Pandey, A. (2016). Hydrotropic pretreatment on rice straw for bioethanol production. Renewable Energy, 98, 2–8.
Hu, J., Xue, Y., Guo, H., Gao, M.-T., Li, J., Zhang, S., & Tsang, Y. F. (2017). Design and composition of synthetic fungal-bacterial microbial consortia that improve lignocellulolytic enzyme activity. Bioresource Technology, 227, 247–255.
Zheng, W., Zheng, Q., Xue, Y., Hu, J., & Gao, M.-T. (2017). Influence of rice straw polyphenols on cellulase production by Trichoderma reesei. Journal of Bioscience and Bioengineering, 123(6), 731–738.
Ma, X., Chen, X., Wang, X., Choi, S., Zhang, T.-A., Hu, J., Tsang, Y. F., & Gao, M.-T. (2019). Extraction of flavonoids from the saccharification of rice straw is an integrated process for straw utilization. Applied Biochemistry and Biotechnology, 189(1), 249–261.
Holopainen-Mantila, U., Marjamaa, K., Merali, Z., KaSper, A., de Bot, P., Jaaskelainen, A. S., Waldron, K., Kruus, K., & Tamminen, T. (2013). Impact of hydrothermal pre-treatment to chemical composition, enzymatic digestibility and spatial distribution of cell wall polymers. Bioresource Technology, 138, 156–162.
Oskoueian, E., Abdullah, N., Hendra, R., & Karimi, E. (2011). Bioactive compounds, antioxidant, xanthine oxidase inhibitory, tyrosinase inhibitory and anti-inflammatory activities of selected agro-industrial by-products. International Journal of Molecular Sciences, 12(12), 8610–8625.
Hernandez, M., Ventura, J., Castro, C., Boone, V., Rojas, R., Ascacio-Valdes, J., & Martinez-Avila, G. (2018). UPLC-ESI-QTOF-MS2-based identification and antioxidant activity assessment of phenolic compounds from red corn cob (Zea mays L.). Molecules, 23(6), 1425.
Xue, Y., Wang, X., Chen, X., Hu, J., Gao, M.-T., & Li, J. (2017). Effects of different cellulases on the release of phenolic acids from rice straw during saccharification. Bioresource Technology, 234, 208–216.
Coates, A. R. M., Hu, Y., Holt, J., & Yeh, P. (2020). Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Anti-Infective Therapy, 18(1), 5–15.
Hossain, M. A., Park, H. C., Park, S. W., Park, S. C., & Kang, J. J. P. (2020). Synergism of the combination of traditional antibiotics and novel phenolic compounds against Escherichia coli. Pathogens, 9(10), 811.
Kim, H. R., & Eom, Y. B. (2021). Synergistic activity of equol and meropenem against carbapenem-resistant Escherichia coli. Antibiotics-Basel, 10(2), 13.
Wang, X., Tsang, Y. F., Li, Y., Ma, X., Cui, S., Zhang, T.-A., Hu, J., & Gao, M.-T. (2017). Inhibitory effects of phenolic compounds of rice straw formed by saccharification during ethanol fermentation by Pichia stipitis. Bioresource Technology, 244, 1059–1067.
Cui, S., Ma, X., Wang, X., Zhang, T.-A., Hu, J., Tsang, Y. F., & Gao, M.-T. (2019). Phenolic acids derived from rice straw generate peroxides which reduce the viability of Staphylococcus aureus cells in biofilm. Industrial Crops and Products, 140, 111561.
Gutierrez-Larrainzar, M., Rua, J., Caro, I., de Castro, C., de Arriaga, D., Rosario Garcia-Armesto, M., & del Valle, P. (2012). Evaluation of antimicrobial and antioxidant activities of natural phenolic compounds against foodborne pathogens and spoilage bacteria. Food Control, 26(2), 555–563.
Ma, Y., Ding, S., Fei, Y., Liu, G., Jang, H., & Fang, J. (2019). Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control, 106, 106712.
Wei, H., Wang, Y., Jin, Z., Yang, F., Hu, J., & Gao, M.-T. (2020). Utilization of straw-based phenolic acids as a biofugicide for a green agricultural production. Journal of Bioscience and Bioengineering, 131(1), 53–60.
Tao, R., Wang, C., Ye, J., Zhou, H., & Chen, H. (2016). Polyprenols of Ginkgo biloba enhance antibacterial activity of five classes of antibiotics. Biomed Res. Int, 4191938.
Barman, R., Mondal, T., Sarkar, J., Sikder, A., & Ghosh, S. (2020). Self-assembled polyurethane capsules with selective antimicrobial activity against gram-negative E. coli. ACS Biomaterials Science & Engineering, 6(1), 654–663.
Gu, H., Zhu, Y., Peng, Y., Liang, X., Liu, X., Shao, L., Xu, Y., Xu, Z., Liu, R., & Li, J. (2019). Physiological mechanism of improved tolerance of Saccharomyces cerevisiae to lignin-derived phenolic acids in lignocellulosic ethanol fermentation by short-term adaptation. Biotechnology for Biofuels, 12(1), 268–268.
Shi, J., Wang, Y., Wei, H., Hu, J., & Gao, M.-T. (2020). Structure analysis of condensed tannin from rice straw and its inhibitory effect on Staphylococcus aureus. Industrial Crops and Products, 145, 112130.
Skogman, M. E., Vuorela, P. M., & Fallarero, A. (2012). Combining biofilm matrix measurements with biomass and viability assays in susceptibility assessments of antimicrobials against Staphylococcus aureus biofilms. Journal of Antibiotics, 65(9), 453–459.
Abdel Hady, K. K., Abdel Salam, R. A., Hadad, G. M., & Abdel Hameed, E. A. (2020). Simultaneous HPLC determination of vildagliptin, ampicillin, sulbactam and metronidazole in pharmaceutical dosage forms and human urine. J. Iran Chem, 1–10.
Zhang, X., Liu, C., Li, Y., Song, J., & Zhao, X. (2016). Determination of kanamycin with the fading method of Congo red. Applied Chemical Industry, 45(7), 1376–1378. 1381.
Chen, X., Wang, X., Xue, Y., Zhang, T.-A., Hu, J., Tsang, Y. F., & Gao, M.-T. (2018). Tapping the bioactivity potential of residual stream from its pretreatments may be a green strategy for low-cost bioconversion of rice straw. Applied Biochemistry and Biotechnology, 186(3), 507–524.
Chen, X., Wang, X., Xue, Y., Zhang, T.-A., Li, Y., Hu, J., Tsang, Y. F., Zhang, H., & Gao, M.-T. (2018). Influence of rice straw-derived dissolved organic matter on lactic acid fermentation by Rhizopusoryzae. Journal of Bioscience and Bioengineering, 125(6), 703–709.
Belenky, P., Ye, J. D., Porter, C. B. M., Cohen, N. R., Lobritz, M. A., Ferrante, T., Jain, S., Korry, B. J., Schwarz, E. G., Walker, G. C., & Collins, J. J. (2015). Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Reports, 13(5), 968–980.
Kohanski, M., Dwyer, D., Hayete, B., Lawrence, C. A., & Collins, J. J. (2007). A common mechanism of cellular death induced by bactericidal antibiotics. Cell, 130(5), 797–810.
Wright, G. D. (2007). On the road to bacterial cell death. Cell, 130(5), 781–783.
Xiang, F., Bai, J., Tan, X., Chen, T., Yang, W., & He, F. (2018). Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var. argyi cv. Qiai. Industrial Crops and Products, 125, 582–587.
Funding
This work was supported by the National Key R&D Program of China (2017YFE0116300), the National Natural Science Foundation of China (32071640), and the Special Fund for Agroscientific Research in the Public Interest (No. 201503135–14).
Author information
Authors and Affiliations
Contributions
Huanran Wei, Zheng Jin: conceptualization, methodology, writing original draft, writing—review and editing, visualization. Min-Tian Gao: conceptualization, methodology, supervision, funding acquisition, writing—review and editing. Jiajun Hu: methodology. Yazhu Wang, Fan Yang, Ying Xiao, Yipeng Jiang: methodology, writing—review and editing.
Corresponding author
Ethics declarations
Ethics Approval
No related ethical issues.
Consent to Participate
The authors promise that the work described has not been published previously, that it is not under consideration for publication elsewhere, and that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out.
Consent for Publication
The authors promise that if the manuscript is accepted, it will not be published elsewhere in the same form, in English or in any other language, without the written consent of the publisher.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huanran Wei and Zheng Jin have made equal contributions to this article and they are co-first authors
Rights and permissions
About this article
Cite this article
Wei, H., Jin, Z., Wang, Y. et al. Antibacterial Effect of Phenolic Acids Derived from Rice Straw and in Combination with Antibiotics Against Escherichia coli. Appl Biochem Biotechnol 194, 2931–2945 (2022). https://doi.org/10.1007/s12010-021-03650-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03650-5