Abstract
This review presents biocontrol agents employed to alleviate the deleterious effect of the pathogen Fusarium graminearum on maize. The control of this mycotoxigenic phytopathogen remains elusive despite the elaborate research conducted on its detection, identification, and molecular fingerprinting. This could be attributed to the fact that in vitro and greenhouse biocontrol studies on F. graminearum have exceeded the number of field studies done. Furthermore, along with the variances seen among these F. graminearum suppressing biocontrol strains, it is also clear that the majority of research done to tackle F. graminearum outbreaks was on wheat and barley cultivars. Most fusariosis management related to maize targeted other members of Fusarium such as Fusarium verticillioides, with biocontrol strains from the genera Bacillus and Pseudomonas being used frequently in the experiments. We highlight relevant current techniques needed to identify an effective biofungicide for maize fusariosis and recommend alternative approaches to reduce the scarcity of data for indigenous maize field trials.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Fungal pathogens pose a great challenge to grain production in several regions of the world. The threat is reported in many continents with the members of the Fusarium spp. still frequently encountered as causative agents of fusariosis. The dominant species of Fusarium that cause maize rots worldwide are F. verticillioides, F. graminearum, F. culmorum, and F. proliferatum; the more recent but less significant species include F. subglutinans, F. sporotrichioides, and F. temperatum (Czembor et al. 2015; Summerell and Leslie 2011). Significant genetic and morphological diversity was observed within species associated with F. graminearum across geographic regions (Przemieniecki et al. 2014; van der Lee et al. 2015) and this prompted researchers to establish the F. graminearum species complex (FGSC). Species within the FGSC cause head blight diseases and serious rots of several cereal crops, such as maize, barley, and wheat worldwide (Sampietro et al. 2012; Yang et al. 2013). They are still responsible for the periodic epidemics of fusariosis that result in significant economic losses due to reduction in grain yield and quality.
Production of maize in developing countries occupies nearly 100 million hectares and 70% of the total maize produced in the developing world, where demand is expected to double by 2050, comes from low and lower middle income countries (Cairns et al. 2012). Members of the FGSC such as F. graminearum sensu stricto belonging to lineage seven secrete toxins that include nivalenol (NIV), deoxynivalenol (DON), and zearalenone (ZEA), and the presence of these phytopathogens or their toxins in cereal grains pose a threat to public health. The toxic effect on animals and humans of these mycotoxins in several geographic regions globally is a cause for concern (McMullen et al. 2012; Varga et al. 2015).
Fusarium graminearum clade, comprising at least 16 phylogenetically distinct species, was divided into various species using nucleic acid based techniques (Aoki et al. 2012; O’Donnell et al. 2004; Wang et al. 2011). FGSC were identified based on evolutionary mechanisms and a simultaneous analysis of multiple sequences (loci) using diagnostic methods involving genealogical concordance phylogenetic species recognition (GCPSR) loci and multilocus genotyping assay (MLGT) loci (O’Donnell et al. 2004, 2008). The GCPSR approach supports the determination of similarities and boundaries between fungal species while the MLGT method relies on an analysis of single nucleotide polymorphism (SNPs). Both methods generate a marker database used to monitor taxon migration, variances within a population, and the mycotoxin dispersal within species (Zhang et al. 2012).
Controlling the emergence of fusariosis or rots on maize caused by F. graminearum with chemicals has been difficult, largely due to the nature of the pathogen and the prevailing climatic conditions (Bacon and Hinton 2007). For example, F. graminearum enters the maize through the silk-channel for ear rot infections and also enters maize ears through injuries inflicted on kernels by insects or birds (Sutton 1982; Zhang et al. 2012). Earlier studies showed that the acuteness of ear rot symptoms increases during cool temperatures (below 23 °C) accompanied by rainfall, and that only F. graminearum produces DON under wet conditions (Doohan et al. 2003) (Table 1).
The application of chemical fungicides on maize seedlings prior to planting has not been effective in fusariosis management, rather it leads to significant increases in mycotoxin concentrations in plants (Pereira et al. 2009; Small et al. 2012a, b). The ascomycota F. graminearum causes fusariosis with different symptoms (ear rot, root rot, and leaf rot) in maize, resulting in poor grain yield and accumulation of fungal mycotoxins (DON, NIV, and ZEA) in the grain (Wang et al. 2011).
A thorough study of the problem and effective control strategies of this disease in relation to maize production is still necessary. Most research has leaned toward using biological control as an alternative for alleviating plant diseases rather than chemical control (Babalola and Glick 2012; Heydari and Pessarakli 2010), and large numbers of bacterial species, predominantly Pseudomonas and Bacillus strains, have been frequently identified to be highly antagonistic against agents of fusariosis (Pérez-Montaño et al. 2014).
The most common approach utilized for biocontroller innovation chain was proposed by (Bailey et al. 2010), and involves (a) screening and early discovery of strains, (b) proof of field applicability, (c) fermentation development procedures, (d) formulation and application into technological platforms, and lastly (e) implementation into farming systems. To date, many of the studies do not pass the screening stages; few studies have identified or reported commercialized biocontrollers for FGSC. Often, laboratory assessment data that are temporary screening methods are the only reports which are readily available, while field experimental studies are not readily available. Even when available, most reports show no relationship between the reactions in vitro and in planta.
Several factors affect the efficacy of potential biocontrol agents in field experiments ranging from culture formulations, dosage of microbial inoculants, crop cultivars, experimental site, and changing weather conditions. The compatibility of a PGPR strain with commonly used fungicides, spermosphere, and rhizosphere competence are pre-requisites for reproducible biological control activity during field studies. Reports involving field studies showing the successful use of an antagonist during plant disease management are not readily available (Xu et al. 2009). In planta studies often give a realistic indication of the biocontrol measure achievable in real-time environmental situations. This article primarily discusses the strategies used in finding biocontrol agents that are able to suppress maize fusariosis caused by F. graminearum. It further highlights the efforts made at providing biocontrollers for the management of F. graminearum maize fusariosis.
Screening approaches used for selecting Fusarium graminearum biocontrollers
When selecting for a biocontrol agent (BCA), it is paramount to have a clear understanding of the expected result. Will the BCA only disrupt infection stages or will it eliminate toxin production and reduce disease severity? Will the BCA stop the onset of disease? Previous reports demonstrate that there is a positive linear relationship between the occurrence of fusariosis and toxin contamination (Wegulo 2012; Wegulo et al. 2015). Köhl et al. (2011), concluded that most screening approaches that have been employed have focused on antagonistic efficacy shown by a potential biological control strain during in vitro or greenhouse tests as the criteria for their selection. Many did not highlight other characteristics of the potential biocontrol strain that would be relevant for commercial exploitation during their screening approaches (Köhl et al. 2011; Walsh et al. 2001), in their reviews proposed screening approaches and commercialization strategies that could be adopted for selecting BCAs. Figure 1 describes the sequence of events that take place from the isolation stage of a potential biocontrol strain to its commercialisation. This sequence that could lead to successful commercialization specifically targets biocontrollers for the management of maize fusariosis and is the discourse of this review.
Since most potential antagonists are selected following the screening steps described in Fig. 1, it is likely that a large number of microbial isolates can be found showing antagonism in experimental studies yet they are not suitable for commercial use. A potential biocontrol strain may be too expensive for mass fermentation production; its inoculum during product formulation may not have a long shelf life, and its target end users may be too few for its large-scale production. A good screening approach would include commercial aspects in the early stage of selecting a potential biocontrol strain, ensuring the strain meets the specifications needed for commercial application (Kamilova and de Bruyne 2013).
Potential biocontrol strains evaluated in vitro
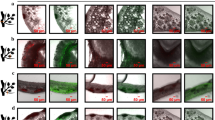
The progression in determining the biocontrol potential of a rhizospheric isolate for the inhibition of fungal phytopathogens includes in vitro tests, such as the dual culture agar plate test. In this test, the inhibitory or cidal capability of the antagonists or their metabolites is investigated. The in vitro tests are mostly used to select for the most effective isolates, which are then utilized in further plant bioassay conducted with crop seedlings. This initial step narrows down the total number of microbial isolates that show presumptive antagonistic potential. The inhibition is identified by zones of clearing seen around the pathogen. For example, Fig. 2a shows the inhibition of the mycelia growth of F. graminearum by some Bacillus spp,. and Fig. 2b shows the inhibitory effect of the secondary metabolite of Bacillus strain BS10.5 against same pathogens. These zones of clearing on the agar plates signify the rate of susceptibility of the pathogen.
The in vitro culture selection process is a necessary systematic, comprehensive method for high throughput screening of microbes for antifungal activity (Shehata et al. 2016b). Although laborious, the method captures the majority of the microbes, both weak and strong, that can suppress pathogen proliferation in planta. This provides strong evidence that a BCA performing very well in planta, was first identified or chosen from a numerous pool of potential strains. However, the possibility of overlooking strains that could have strong field viability and efficacy, but exhibiting weak in vitro antagonistic potential is not always considered (Schöneberg et al. 2015). In vitro tests could also include monitoring seed assays in petri dishes or conical flasks for a specific length of time for disease manifestation or improved plant growth (Abd El Daim et al. 2015).
In most in vitro tests, the rhizobacteria strains utilized include Azotobacter, Bacillus, Azospirillum, Pseudomonas, and Spirulina species (Wang et al. 2007). The study by Abdulkareem et al. (2014) showed that Azotobacter chroococcum, Bacillus pumilus, Azospirillum sp., and B. subtilis isolated from the cucumber rhizosphere reduced the mycelia growth of F. graminearum in a dual culture test. The Azospirillum sp. produced volatile inhibitory metabolites, but the secretion of the metabolites was dependent on the growth phases. The antagonistic effect of the Azospirillum sp. disappeared at day 7, however, that of the B. subtilis continued after day 7. Similarly, Bacillus strains (As.43.3, As.43.4, and IB) inhibiting F. graminearum and producing lipopeptides, iturin, fengycin, surfactin, were employed by Wang et al. (2007) and Dunlap et al. (2011) in their in vitro assay. (Cordero et al. 2012), reported that Pseudomonas MGR39 inhibited F. graminearum by producing hydrogen cyanide (HCN) and pyrrolnitrin, which were detected biochemically and by polymerase chain reaction (PCR) amplification. Active phenolic acids and siderophores were also identified to be responsible for the antifungal activity against some FGSC during the in vitro study by Laslo et al. (2012) and Pagnussatt et al. (2014). From the studies reviewed, the selection processes could have been less laborious if differential and selective chromogenic detection methods that target specific genera were included in the isolation stages. It was also evident that the growth requirements for the candidate biocontrol strains varied and played a significant role in the production of the active metabolites. The growth phases and conditions must be carefully considered during the in vitro selection process.
In vitro molecular approaches for detecting biocontrol strains, mechanisms of action, and application of plant–microbe interaction
Following the culture selection stage described above, detection of novel candidates harboring novel or known antimicrobial compounds and detection of novel antimicrobial compounds in known biocontrol candidates is another important in vitro selection process. Understanding the mode of action of the antimicrobials secreted by biocontrol candidates cannot be understated. A combination of action mechanisms, which are not limited to antibiotic secretion, ethylene production regulation, iron sequestration and nutrient competition, hydrogen cyanide production, solubilisation of phosphate, release of lytic enzymes, hormone phytostimulation, acetoin, and butanediol production, are employed by biocontrol organisms to suppress plant pathogens. These mechanisms of action have been adequately reviewed (Ali et al. 2015; Babalola 2010; Lugtenberg and Kamilova 2009; Raaijmakers and Mazzola 2012).
The mechanism of action employed by beneficial organisms is largely dependent on the plant host responses. Crop plants and their secretions play an important role in microbial community structure. Hence, there is a need to develop techniques that assist in understanding the interactions that exist between the plant hosts and their resident microbe (Huang et al. 2014). To better understand plant–microbe interactions, novel and current techniques such as microarray systems, quantitative real-time PCR (qRT-Pcr), subtractive hybridization, serial analysis of gene expression (SAGE), northern blotting, and next-generation sequencing (NGS) have been employed. These technologies have given better insights into gene expression, have helped select robust biocontrol candidates, and also increased the knowledge of the capabilities possessed by well-known biocontrol candidates (Knief 2014).
For example, using molecular genetics (mutant selection, transposon mutagenesis), or genomics (PCR techniques), one can deliberately target candidate strains harboring two or more biosynthetic genes while discarding strains lacking the presence of known biosynthetic gene clusters (Mousa and Raizada 2015; Mousa et al. 2015; Shehata et al. 2016a). Metagenomics studies evaluating microbial diversity of resident flora of symptomatic/diseased crop/plant parts (stalks, stems, and roots), rhizosphere, endosphere, and phyllosphere for suppressiveness have also been employed to run a comparison between low fusarium-colonized samples and highly colonized ones (Köhl et al. 2015). Presently, genomic and structural analyses have also become valuable for unraveling the functional assets of beneficial organisms. For example, transposon mutagenesis and whole genome mining of Paenibacillus polymyxa strain A26 revealed that the mode of action of P. polymyxa against F. graminearum and F. culmorum was not limited to production of fusaricidin but also involved polymyxins and other novel non-ribosomal lipopeptides (Abd El Daim et al. 2015).
In recent years, efficiently designed RNA interference (RNAi) plant protection approaches involving the manipulation of major biosynthetic pathways in invading pathogens are being employed. The RNAi technique is a gene attenuation method used to develop transgenic pathogen resistant crops and identify gene function in microbes. These plants possess the RNAi machinery which they use as a defense mechanism (RNA silencing or co-suppression mechanism) against invading pathogens (Cairns et al. 2016; Schumann et al. 2013; Wang and Jin 2016). To silence an endogenous gene in a plant, an effective method would be to transform the plant with a gene construct that encodes a hairpin RNA (hpRNA) (Helliwell and Waterhouse 2003). The constructs are expressed in plants through plasmid delivery, or viral or bacterial vectors, and specifically target the invading microbe they are supposed to silence (Wani et al. 2010). Currently, improved RNAi methods are being exploited in plant management strategies, some of which target the Fusarium spp. (Chen et al. 2015; Koch et al. 2016; Schumann et al. 2013).
The phenome-based functional analysis approach has also been recently employed for understanding the functionality of candidate BCA being selected for the management of maize fusariosis. It has also been used in the identification of putative transcription factors (TFs), characterization of functional genes, and identification of unique traits in phytopathogens such as F. graminearum (Son et al. 2011). Another breakthrough that revolutionized the application of NGS in plant–microbe interaction is the use of RNA sequencing (RNA-seq) in transcriptome research. During RNA-seq, a transcriptome, being a collection of all the transcripts (RNAs) present in a given cell, is evaluated qualitatively and quantitatively at a particular moment of cell development or during a specific physiological condition (Wang et al. 2009), yielding unbiased transcripts with dynamic detection range (Marguerat and Bahler 2010; Pinto et al. 2011). RNA-seq analysis can be used for both exploratory and quantitative assessment. Furthermore, RNA-seq is useful in understanding the transcriptional profile of BCAs (identification of genes involved in production of secondary metabolites) and understanding the resistance elicited by host crops against invading phytopathogens. With the use of RNA-seq, activation of antibiotic genes in BCAs and defense-related genes in maize artificially infected with F. graminearum were shown to be time dependent (Kröber et al. 2016; Zhang et al. 2015). Furthermore, RNA-seq has been used to determine the mechanisms that regulate microbial metabolite synthesis (either in pathogen or BCA) and metabolite function in the microbial cell, which has become a major priority for plant pathologists (Mwita et al. 2016; Sieber et al. 2014).
Information on how a specific genera of bacteria attaches to, colonies and proliferates on plant, or plant parts will assist in determining if a strain from such genera would be an effective BCA. Unraveling the plant microbiome is crucial to identifying microbes that can be exploited for plant growth promotion and bioprotection (Huang et al. 2014). For example, F. graminearum persists in soil and infects both systemically and superficially (Martinez-Alvarez et al. 2016). Therefore, an effective BCA for its control must exhibit both endophytic and exophytic action mechanisms. Babalola (2010) affirmed that effective reduction of disease symptoms by a BCA is dependent on (a) how aggressively such BCA colonizes plant parts and (b) how it dominates the surrounding ecological niche. Also, plant–microbe interactions are dependent on several abiotic factors. Having knowledge of when environmental factors such as pH, temperature, or moisture favors the growth of the pathogen or BCA on the host plant is crucial to disease management. It is evident that when structural and functional assets of host plants, candidate BCAs, and invading phytopathogens are known or better understood, effective disease management strategies can be proffered. The in vitro techniques and approaches mentioned above are valuable in detecting biocontrol strains, identifying the mechanisms they employ against phytopathogens, and understanding plant–microbe interactions.
Greenhouse evaluation of promising biocontrol strains
Following in vitro assessments, most research proceeds to conduct greenhouse experiments to ascertain the appropriateness of a selected BCA for crop protection. The greenhouse-controlled assay provides a promising strain of BCA with a stable environmental condition for survival and proliferation. Here, commercial potting soil or synthetic mixes of loamy sand, sandy clay loam, or sandy loam soil may be used as the soil medium. The soil could be pasteurized or heat-treated to create an axenic or gnotobiotic condition. Light intensity, water availability, soil amount, seed type, and pot size are also controlled. Greenhouse experiments are employed to evaluate any potential BCAs that have passed the in vitro test. Results obtained from the greenhouse show more reliability and are most suitable to produce a BCA that can pass on to commercialisation. Some potential isolates from the in vitro studies become less effective, or very ineffective, during the greenhouse experiments. Bacillus mojavensis strain RRC 101, a patented endophyte, was antagonistic in vitro against F. graminearum and seven other related species, but became ineffective against F. pseudograminearum and F. verticillioides during growth room assay (Bacon and Hinton 2007).
Trichoderma spp., Stenotrophomonas maltophilia, Pseudomonas corrugata, P. polymyxa strain (C1-8-b, W1-14-3, 1D6, 4G12, and 4G4), B. subtilis strains (MAA03, MR-11 and MRF), and P. fluorescens strain MAA10 were utilized in different growth chamber and greenhouse experiments (Dal Bello et al. 2002; He et al. 2009; Moussa et al. 2013; Pandey et al. 2001). The ability of the isolates to control maize phytopathogens (Pythium ultimum, P. arrhenomanes, and F. graminearum, F. moniliforme, Macrophomina phaseolina) varied extensively. The experiments conducted in vitro and in the greenhouse showed that the isolates varied in their ability to inhibit Fusarium mycelial growth and reduce toxin production respectively. During the greenhouse assays, all the isolates evaluated for disease reduction exhibited suppressive effect, but their level of aggression during proliferation and colonization of the root zones differed. Furthermore, some of the candidate strains were also found to exhibit high resistance to antibiotics, a potential that could enhance their environmental survival rate. The use of the strains either in combination or separately also significantly affected the plant growth parameters evaluated.
Most of the reports from the greenhouse experiments gave adequate information on the effectiveness and the mechanism of action of the BCA applied. For example, two greenhouse trials conducted by Mousa et al. (2015), during summers of 2012 and 2013 showed that endophytic Citrobacter sp. and P. polymyxa spp. suppressed the F. graminearum and symptoms in maize. Only 4 highly potent isolates out of 215 isolated from three varieties of maize (wild, traditional, and modern maize) exhibited broad antifungal spectrum during in vitro analysis. Three P. polymyxa strains (1D6, 4G12, and 4G4) from the four isolated from the wild maize cultivar, harbored the biosynthetic gene responsible for the synthesis of fusaricidin, an anti-fusarium compound. The Citrobacter strain (3H9) from modern maize was the least potent of the four. However, it caused a higher DON reduction during storage. Without the data to prove the efficacy of a potential BCA for crop protection or disease suppression under controlled environment, the claims for the suitability of such BCA might be labeled speculative. Greenhouse experiments provide data showing strong evidence for such claims.
Field trials conducted with potential biocontrol strains
Field trials under natural conditions help in selecting BCAs that are more suitable for further biotechnological studies. Under field trials, fluctuations in climatic conditions are naturally expected and it is significant to analyze how environmental factors will affect the effectiveness and survival of BCAs. The viability and biocontrol capacity of several antagonists are reduced when applied in the field compared to the in vitro and greenhouse assays. During the field trials, the effectiveness of a BCA in suppressing disease severity and toxin production, its ability to tolerate environmental conditions and remain viable both during field application and in storage is crucial. Few articles have reported the progression from in vitro tests directly to the field experiments. By bypassing the greenhouse, is it possible to determine the degree of suppression likely to be exhibited by a potential biocontrol strain in the real-time environmental situation?
Lysobacter enzymogenes strain C3, an antagonist of F. graminearum was also reported by Jochum et al. (2006), to improve the growth of several wheat cultivars through induced resistance in the greenhouse. After establishing the effectiveness of the bacterium in suppressing the pathogenic effects of F. graminearum, the authors further carried out four field experiments to ascertain the suitability of the strains as a biocontrol agent for FHB. The data revealed that when L. enzymogenes C3 was used singly as a crop treatment, it showed inconsistencies in the field. However, its potency was restored when combined with the fungicide tebuconazole. A similar study by Luongo et al. (2005) determined the efficacy of several saprophytic fungi isolated from necrotic plant tissues, stubbles, straw, seed surfaces, phyllosphere, or roots of cereal crops to suppress the sporulation and saprophytic colonization of several pathogenic Fusarium spp. on wheat and maize crop residues. F. graminearum was part of the toxigenic fusarium tested against. The bioassay (in vitro petri dish test and preliminary field test) involved applying several of the antagonists to pieces of maize stalks and flowering maize ears. From the saprophytic fungi, the most effective antagonist against F. graminearum was T. harzianum. Clonostachys rosea exhibited the strongest inhibition against all the six Fusarium spp. by reducing their sporulation. The antagonists C. cladosporioide and F. equiseti showed appreciable inhibition range against F. proliferatum and F. verticillioides but not on F. culmorum nor F. graminearum. The mode of action proposed for C. rosea was competitive colonization.
Another field experiment with adequate data was carried out by Zhao et al. (2014). In the study, application of the strain Bacillus SG6 caused significant changes in the parameters considered (such as crop yield, FHB index, and DON production). Bacillus SG6 also effectively inhibited the growth and sporulation of F. graminearum in vitro. When compared during the field trial with carbendazim (the chemical fungicide widely used in China for the control of FHB), its effectiveness was more pronounced. The strain SG6 was reported to harbor five genes (bmyB, fenD, ituC, srfAA, and bacA) known for the secretion of antimicrobial peptides. Through ultrastructural microscopy, the authors further showed that the SG6 inhibited F. graminearum by either disrupting its hyphae or lysing its cell wall. They observed that although the majority of these field studies were targeted against F. graminearum, many of the studies involved protection of wheat and barley. The reason for these biases was, however, not stated.
Efficacy and stability of biocontrol strains
Regarding the survivability and effectiveness of a BCA in the field, producers and end users of the BCAs give different reports. End users complain that BCAs are inconsistent under natural conditions compared to the expected information given by the scientist introducing the BCA. Because the scientist proved the effectiveness of the BCA in the experimental tests, he assumes high reliability of the product. However, most BCAs that have been proven stable and effective had to pass a series of evaluative tests by the end users even after the field trials carried out by the scientists. The scientists must, therefore, prepare to carry out experimental trials to suit the needs of expected end users. During these trials, scientists should take into consideration critical factors that could influence the effectiveness and stability of the BCA. Such factors include the soil environment in which the BCA will be introduced, genotype of plant for which the BCA is being manufactured, delivery mode by which the BCA would be applied, the beneficial, neutral, or detrimental effects the BCA would have on its host environment (Kamilova and de Bruyne 2013). The durability of a BCA is measured by its degradation or loss of effectiveness during field or on the farm applications. Geographical instability has been recognized to be responsible for the ineffectiveness and inconsistences of several commercial biocontrol strains outside their indigenous environment (Abiala et al. 2015; Ahmad et al. 2008; Bardin et al. 2015). For example, three B. subtilis strains and four yeast strains were earlier found to antagonize F. graminearum during greenhouse-controlled experiment conducted by Khan et al. (2004). However, only the yeasts Cryptococcus sp. OH 181.1, Cryptococcus sp. OH 71.4, and C. nodaensis OH182.9 exhibited geographical stability during the field trials that were later conducted.
Semi-controlled experimental conditions
Most of the studies described in the greenhouse assays above utilized sterilized soils. This approach is deemed necessary and suitable because it helps detect and compare the effectiveness of the BCA in the treated plants and untreated plants under the identifiable external factors introduced. Greenhouse-controlled experiments require a shorter time to determine the results of the experiment. The experimental location is also easier to manage. In contrast, field trials which involve the use of natural unsterilized soils during experimental periods to assess the relationship between the plant, the phytopathogen, environmental factors (biotic and abiotic), and the biocontrol strain being introduced are quite expensive and require a minimum of 2 years and two experimental sites (Vacheron et al. 2016a). In as much as these field trials are necessary for predicting the real-time efficiency of the potential biocontrollers, another approach to circumvent the need for the expensive field trials might be the use of unsterilized soils in semi-controlled trials. The unique factor that might make semi-controlled trials widely accepted in the near future will be because they combine components of both greenhouse experiments and field trials.
In semi-controlled experiments, soils are taken from the locality from which field trials should be carried out and used also in greenhouse pot experiments under natural atmospheric conditions such as open rainfed conditions and exposure to direct sunlight (Mao et al. 1998; Mehnaz et al. 2010). Since the semi-controlled trial will be a combination of several aspects of the two in planta experiments (greenhouse tests and field trials), it should provide a picture closer to what happens on the farm. Though the quantity of soil is controlled, the quality is not controlled. In addition, the quantity and concentration of BCA and phytopathogen inoculum being introduced during the experiment still remains controlled (Abiala et al. 2015). The background is that even though the close monitoring of growth conditions is partially eliminated during semi-controlled experiments, the potential biocontrollers will be proliferating along with both the indigenous microbes present in the soil and the introduced phytopathogens.
Soil biophysiological parameters and management practices have been shown to have diverse effects on the soil microbial community (Akhtar and Malik 2000; Babalola 2010), and soil type remains a major determinant of community structure for microbial communities. Furthermore, bacterial inoculants in unsterilised soil stimulated better growth effects in maize compared to sterilized soil (Johnston-Monje et al. 2014; Singh et al. 2007). Despite the role soil amendment plays in the efficacy and survival of BCAs during greenhouse experiments, the effect of its inclusion in the design of semi-controlled biocontrol trials is unknown. Should soil amendment be included in the design of semi-controlled biocontrol trials?
Another key requirement of a biocontrol strain targeted for use against soil-borne phytopathogens is its competence in the rhizosphere. It must compete adequately with indigenous microbial populations within the environment of the rhizosphere and colonize the root surface (Ambrosini et al. 2015; Khabbaz et al. 2015). The use of untreated soil could also help ascertain the survivability of the potential strain and its effect on non-target indigenous microbial populations present in such soil.
Once the competence of biocontrol strain is proven in the semi-controlled trial, thus bypassing the several expensive preliminary field trials, the biocontrol strain can then be introduced for further trials. Most traditional farmers and large-scale maize producers are reluctant to engage biocontrol trials in their field, perhaps due to the fear of a disease outbreak. Employing semi-controlled trials should be more suitable for researchers to prove that their novel biocontrol product will eradicate the outbreak or emergence of the said plant disease.
Another aspect of the semi-controlled experimental conditions, which should reduce cost and time of selecting a candidate biocontrol strain for further biotechnological application, is the use of established plant growth-promoting strains. In recent times, more authors reported conducting their in vitro analysis, greenhouse experiments, and field trials with microbial strains previously isolated by other researchers. This enables them to bypass the laborious stage of sampling, isolation, and identification of potential isolates and determination of the mechanism of action of such isolate (Dunlap et al. 2011; Grosu et al. 2015).
Effect of the mode and condition of application of candidate biocontrol agent during bioprotection experiments
The decision on the mode of application of a BCA during in planta studies and subsequent industrial production remains a challenge encountered when in search of a suitable BCA. Treatment types, inoculum dosage, formulation types, vehicle of delivery, storage of the microbial formulations, and application times have a significant effect on the effectiveness of a candidate BCA designed for crop protection. A major limitation identified for a BCA is the continuing environmental fluctuations it encounters in planta and the challenges encountered in developing a stable product formulation. Some of the utilized vehicles of delivery and storage in recent times include liquid and mineral carriers, protectants, organic carriers, desiccants, stabilizers, UV protectants, binders, and stabilizers (Babalola 2010; Schisler and Slininger 1997). But the copyright issues protecting and guiding production industries have made it difficult to get information regarding the recipe for commercialized microbial formulations. All this must considered when designing in planta biocontrol experiments for crops.
From the in planta reports reviewed, it is seen that the microbial formulations types and the mode of application of bioinoculants affected the growth and development of the host crop. However, these results are not always comprehensive. What is experienced when seeds are coated or soaked prior to application? Do the plants or their roots show significant increases in leaf numbers, weight and length, when seeds are soaked compared to when seeds are coated? Soil drenched treatments have been reported to cause drastic reduction in the shoot length. In contrast, spray-dryers delivering dry powder or freeze-dried microbial formulations have been reported to reduce production costs and increase processing rates (Palazzini et al. 2016). Palazzini et al. (2016) utilized physiologically improved cells (vegetative cells) and also a spore treatment for the field trial they conducted with B. subtilis RC 218 which led to improved processing rates. How can the mode of applying bioinoculants be improved upon?
Perspectives to ensuring the effectiveness of biocontrol agents
Table 2 describes a list of candidate biocontrollers from different microbial phyla and genera used in various experiments that involved bioprotecting maize against F. graminearum. Few of these BCAs have been adopted for commercial use. Lack of effectiveness or reduced effectiveness of BCA formulations during field trials and commercialisation have been reported and these inconsistencies have often been attributed to several factors such as variations in climatic conditions, innate potentials of the pathogen, instability of BCA during storage, and application (Bardin et al. 2015; Ruocco et al. 2011). Others factors include geographical diversity found in the F. graminearum species and the geographical instability of its potential biocontrollers (Zhang et al. 2012).
Velivelli et al. (2014) and Varga et al. (2015) suggested that to ensure that the performance of a potential biocontroller that has undergone in vitro and in planta experiments is consistent, such a biocontroller should be tested in multi-geographical sites, under different climates and against a diverse range of pathogens and crops. The potential BCA should also be isolated from the soil environment in which it will be used (Howell 2003; Small et al. 2012a). The economic and financial implications of such a project will, however, be enormous. Another challenge to monitoring effectiveness would be what to monitor and when should monitoring take place. Should control strategies targeting disease severity, reduction in pathogen population and mycotoxin production be implemented before cropping, during growth stages or postharvest? Few field reports on the control of F. graminearum mycotoxin contamination by BCAs are available probably because research focusing on such areas are still in the developmental stages (Wegulo et al. 2015).
The high cost of carrying out in planta studies is a major reason why most of the potential strains identified in various geographical zones during in vitro and greenhouse studies are yet to be used for in planta studies or formulated for commercialisation (Ash 2010; Bailey et al. 2010; Köhl et al. 2011; Ruocco et al. 2011). But how will the farmer/end-user know the effectiveness of a BCA, if the scientist is not able to carry out thorough field trials? How will the industry be convinced to invest a large amount to support commercialisation if the data from field trials are not comprehensive? The unwillingness of the end users or farmers to try out something new, and the difficulty in getting funds or industrial partners are major challenges encountered prior to the formulation of a potential microbial strain to a commercialized state. To ensure the efficiency of a BCA, Schisler and Slininger (1997) also proposed that researchers should focus on bioefficacy and growth kinetics of the candidate BCA during their feasibility studies. They also divided their proposed screening process into three categories: (1) the necessity of choosing an appropriate pathosystem; (2) the importance of having an appropriate method for microbe isolation, and (3) the necessity of determining the appropriate isolate characterization and performance evaluation procedures.
Another way of ensuring effectiveness of a BCA is to identify the spectrum of microbial pathogens such BCA can suppress. Most biocontrollers that show broad spectrum bioactivity in vitro against phytopathogens have not been reported to be effectively broad spectrum in planta. A broad spectrum antagonist does not only target its main pathogen, but the antagonist is expected to suppress other closely related species or genera. How will this alter the microbial community structure and the environment? Several questions will need to be answered in the coming years. Researchers will need to further re-examine whether a narrow spectrum approach to biocontrol provides a better option for alleviating the challenges faced in cereal disease management. Is it possible that the effectiveness of a BCAs is host plant dependent? The recent debate on the effectiveness of BCAs or plant extracts having a single mechanism of action in contrast to those having multiple mechanisms must also be further investigated (Bardin et al. 2015; Vacheron et al. 2016b).
Although, most of the earliest effective field trials on cereal grains were solely performed to identify management strategies for single mycotoxigenic fungus (Chandra Nayaka et al. 2009), our review shows that the majority of studies affecting major cereal grains in the past decade not only focused on alleviating fusariosis through a broad spectrum antagonism approach (Table 3), but they also paid more attention to wheat bioprotection. Could this bias toward broad spectrum, cereal bioprotection investigations reported in Table 3 be attributed to the higher production and consumption rate of wheat in contrast to other cereals? Formenti et al. (2012) confirmed the differences that exist in efficacy of broad spectrum fungicides against different fungal pathogens. The authors showed that the variability seen in the suppression of F. verticillioides and A. flavus using the same fungicide was significantly different. The diverse strain found in the FGSC complex could make the broad application of an effective biocontrol strain in multiple geographical zones almost impossible.
The approaches and technologies mentioned above obviously come with their merits and demerits. However, a combination of these approaches should ensure rapid selection of biocontrol candidates and further enhance current plant disease management strategies that will help reduce fusariosis and increase maize yield. Most of the novel approaches to understanding host–pathogen relationships in plants are now geared towards identifying plant-mediated responses to pathogen or BCAs such as induced systemic resistance (ISR). However, few reports are available on transcriptomics studies involving cereals, fusarium, and rhizobacteria. The combination of the technologies will allow the identification of the functional genes in both the host plant and the colonizing microbe (pathogen or BCA). Where the attenuation or amplification of the identified functional gene is required (either in host or colonizer), such can be readily implemented.
Because of the growing need for a competent biocontrol strain in field trials and the continued reservations for genetically modified BCAs in some parts of the world, culture-dependent selection methods for screening candidate strains will still be widely employed. But rather than every disease management project embarking on a search for its own highly effective biocontroller, efforts should be directed at applying already identified indigenous candidates. Academically promising strains that have passed multiple pilot tests should be developed, commercialized, and adopted in the market. An alternative, less laborious approach for providing a commercialized F. graminearum biosuppressor could bypass several of the laborious stages in identifying and selecting a potential strain (Fig. 3). It has been employed in some semi-controlled experiments. This approach, however, obviously omits the major initial laborious steps. In addition, it will only be effective if the goal of the research is to utilize a native BCA strain in its region of isolation.
Another area that must be given attention during field trials is the role of plant microbiome in the effectiveness of bioinoculants. Since field experiments are done under a natural uncontrolled environment, the effect of the soil microbiome on bioinoculants might be responsible for the geographic instability experienced among BCAs during field experiments. Reports of the complex synergistic coexistence, mutualistic, and saprophytic association found within the plant microbiome are available (Berendsen et al. 2012; Lundberg et al. 2012; Mendes et al. 2013). Furthermore, the plant microbiome has actually been implicated in the suppressive attribute found in some soils. In disease-suppressive ecosystems, plants are able to resist phytopathogenic attack without any direct human input. Consequently, disease-suppressive soils allow proper growth and development of plants even with the existence of phytopathogens and without chemical pesticides (Berendsen et al. 2012; Kyselková and Moënne-Loccoz 2012; Mendes et al. 2013; Michelsen et al. 2015). Reports on the role of suppressive soils and entire plant microbiomes in the effectiveness of introduced BCAs are, however, not readily available. The microbiome found in disease-suppressive soils are diverse, with complex interactive pathogen control mechanisms (Chaparro et al. 2012; Mendes et al. 2013). However, do these beneficial microbes conferring disease-suppressive ability on soil truly enhance the activity of bioinoculants, or reduce their effectiveness? How is the introduced BCA able to tolerate or out-compete the resident beneficial microbes responsible for the disease suppressiveness for it to be effective? In geographical zones with crop disease outbreaks, is it possible to measure soil productivity in correlation with the productivity of introduced BCAs? These are some of the questions that must be answered when a search for an applicable BCA for crop disease management is underway.
A collaborative effort of diverse scientific disciplines appears to be the future for the management of plant diseases and the biofungicide industry. Right from the start, the manufacturing companies and industry should become involved in the process of selecting the correct biological control agent, all the way through to the selection of a commercial brand. The collaboration should not be lacking in funds during the pilot studies and validation processes. The funds and infrastructures should be sourced or provided by major stakeholders involved in plant disease management. In addition, better results will be recorded in the field of crop bioprotection if intercontinental collaborations between crop scientist and stakeholders involved in policy implementation are strengthened. Such bilateral collaborations involving plant bioprotection projects and data exchange might provide better background for tackling the issue of geographical instabilities experienced with non-indigenous BCAs. This sort of exchange or collaboration only becomes the stakeholder’s priority when there are disease outbreaks.
Concluding remarks
In this review, we have investigated several means of screening for potential biological agents that could be used to control F. graminearum fusariosis in maize. We are of the opinion that a narrow spectrum biofungicide might be the most effective in controlling the continued emergent, rapid spread of fusariosis in maize. Therefore, there is a need for further studies that will concentrate solely on pathogen specificity of a BCA. There are still drawbacks in the field application, commercialisation, and end-user adoption of currently available potential BCAs; therefore, this area should be zealously pursued.
References
Abd El Daim IA, Häggblom P, Karlsson M, Stenström E, Timmusk S (2015) Paenibacillus polymyxa A26 Sfp-type PPTase inactivation limits bacterial antagonism against Fusarium graminearum but not of F. culmorum in kernel assay. Front Plant Sci 6:368. https://doi.org/10.3389/fpls.2015.00368
Abdulkareem M, Aboud HM, Saood HM, Shibly MK (2014) Antagonistic activity of some plant growth rhizobacteria to Fusarium graminearum. Int J Phytopathol 3:49–54
Abiala M, Odebode A, Hsu S, Blackwood C (2015) Phytobeneficial properties of bacteria isolated from the rhizosphere of maize in southwestern Nigerian soils. Appl Environ Microbiol 81:4736–4743
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181. https://doi.org/10.1016/j.micres.2006.04.001
Akhtar M, Malik A (2000) Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: a review. Bioresour Technol 74:35–47. https://doi.org/10.1016/S0960-8524(99)00154-6
Ali GS, Norman D, El-Sayed AS (2015) Chapter ten—soluble and volatile metabolites of plant growth-promoting rhizobacteria (pgprs): role and practical applications in inhibiting pathogens and activating induced systemic resistance (ISR). In: Harsh B, Janine S (eds) Advances in botanical research, vol 75. Academic Press, Cambridge, pp 241–284. https://doi.org/10.1016/bs.abr.2015.07.004
Ambrosini A, Souza R, Passaglia LMP (2015) Ecological role of bacterial inoculants and their potential impact on soil microbial diversity. Plant Soil 400:193–207. https://doi.org/10.1007/s11104-015-2727-7
Aoki T, Ward TJ, Kistler HC, O’Donnell K (2012) Systematics, phylogeny and trichothecene mycotoxin potential of Fusarium head blight cereal pathogens. JSM Mycotoxins 62:91–102. https://doi.org/10.2520/myco.62.91
Ash G (2010) The science, art and business of successful bioherbicides. Biol Control 52:230–240
Babalola OO (2010) Beneficial bacteria of agricultural importance. Biotechnol Lett. https://doi.org/10.1007/s10529-010-0347-0
Babalola OO, Glick BR (2012) Indigenous African agriculture and plant associated microbes: current practice and future transgenic prospects. Sci Res Essays 7:2431–2439
Bacon CW, Hinton DM (2007) Potential for control of seedling blight of wheat caused by Fusarium graminearum and related species using the bacterial endophyte Bacillus mojavensis. Biocontrol Sci Technol 17:81–94. https://doi.org/10.1080/09583150600937006
Bailey K, Boyetchko S, Längle T (2010) Social and economic drivers shaping the future of biological control: a Canadian perspective on the factors affecting the development and use of microbial biopesticides. Biol Control 52:221–229
Bardin M, Ajouz S, Comby M, Lopez-Ferber M, Graillot B, Siegwart M, Nicot PC (2015) Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front Plant Sci 6:566
Beattie S, Schwarz PB, Horsley R, Barr J, Casper HH (1998) The effect of grain storage conditions on the viability of Fusarium and deoxynivalenol production in infested malting barley. J Food Prot 61:103–106
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Cairns JE et al (2012) Chapter one—maize production in a changing climate: impacts, adaptation, and mitigation strategies. In: Donald LS (ed) Advances in agronomy, vol 114. Academic Press, Cambridge, pp 1–58. https://doi.org/10.1016/B978-0-12-394275-3.00006-7
Cairns TC, Studholme DJ, Talbot NJ, Haynes K (2016) New and improved techniques for the study of pathogenic fungi. Trends Microbiol 24:35–50
Chan Y-K, Savard ME, Reid LM, Cyr T, McCormick WA, Seguin C (2009) Identification of lipopeptide antibiotics of a Bacillus subtilis isolate and their control of Fusarium graminearum diseases in maize and wheat. Biocontrol 54:567–574. https://doi.org/10.1007/s10526-008-9201-x
Chandra Nayaka S, Udaya Shankar AC, Reddy MS, Niranjana SR, Prakash HS, Shetty HS, Mortensen CN (2009) Control of Fusarium verticillioides cause of ear rot of maize by Pseudomonas fluorescens. Pest Manag Sci 65:769–775
Chaparro JM, Sheflin AM, Manter DK, Vivanco JM (2012) Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils 48:489–499
Chen L, Wang N, Wang X, Hu J, Wang S (2010) Characterization of two anti-fungal lipopeptides produced by Bacillus amyloliquefaciens SH-B10. Bioresour Technol 101:8822–8827. https://doi.org/10.1016/j.biortech.2010.06.054
Chen H, Chen Z, Zhang Y, Zhu W (2014) Identification of antifungal peptides from Bacillus subtilis BS-918. Anal Lett 47:2891–2899. https://doi.org/10.1080/00032719.2014.928882
Chen Y, Gao Q, Huang M, Liu Y, Liu Z, Liu X, Ma Z (2015) Characterization of RNA silencing components in the plant pathogenic fungus Fusarium graminearum. Sci Rep 5:12500
Cordero P, Cavigliasso A, Príncipe A, Godino A, Jofré E, Mori G, Fischer S (2012) Genetic diversity and antifungal activity of native Pseudomonas isolated from maize plants grown in a central region of Argentina. Syst Appl Microbiol 35:342–351. https://doi.org/10.1016/j.syapm.2012.04.005
Czembor E, Stępień Ł, Waśkiewicz A (2015) Effect of environmental factors on Fusarium species and associated mycotoxins in maize grain grown in poland. PLoS One. https://doi.org/10.1371/journal.pone.0133644
Dal Bello G, Monaco C, Simon M (2002) Biological control of seedling blight of wheat caused by Fusarium graminearum with beneficial rhizosphere microorganisms. World J Microbiol Biotechnol 18:627–636
Díaz Herrera S, Grossi C, Zawoznik M, Groppa MD (2016) Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol Res 186–187:37–43. https://doi.org/10.1016/j.micres.2016.03.002
Doohan FM, Brennan J, Cooke BM (2003) Influence of climatic factors on Fusarium species pathogenic to cereals. Eur J Plant Pathol 109:755–768. https://doi.org/10.1023/a:1026090626994
Dunlap CA, Schisler DA, Price NP, Vaughn SF (2011) Cyclic lipopeptide profile of three Bacillus subtilis strains; antagonists of Fusarium head blight. J Microbiol 49:603–609
Formenti S, Naresh M, Pietri A, Battilani P (2012) In vitro impact on growth, fumonisins and aflatoxins production by Fusarium verticillioides and Aspergillus flavus using anti-fungal compounds and a biological control agent. Phytopathologia Mediterranea 51:247–256
Gong AD et al (2015) Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS One 10:e0116871. https://doi.org/10.1371/journal.pone.0116871
Greenhalgh R, Neish G, Miller J (1983) Deoxynivalenol, acetyl deoxynivalenol, and zearalenone formation by Canadian isolates of Fusarium graminearum on solid substrates. Appl Environ Microbiol 46:625–629
Grosu AI, Sicuia O-A, Dobre A, Voaideş C, Cornea CP (2015) Evaluation of some Bacillus spp. strains for the biocontrol of Fusarium graminearum and F. culmorum in Wheat. Agric Agric Sci Proc 6:559–566. https://doi.org/10.1016/j.aaspro.2015.08.085
He J, Boland GJ, Zhou T (2009) Concurrent selection for microbial suppression of Fusarium graminearum, Fusarium head blight and deoxynivalenol in wheat. J Appl Microbiol 106:1805–1817. https://doi.org/10.1111/j.1365-2672.2009.04147.x
Helliwell C, Waterhouse P (2003) Constructs and methods for high-throughput gene silencing in plants. Methods 30:289–295
Heydari A, Pessarakli M (2010) A review on biological control of fungal plant pathogens using microbial antagonists. J Biol Sci 10:273–290
Homdork S, Fehrmann H, Beck R (2000) Influence of different storage conditions on the mycotoxin production and quality of fusarium-infected wheat grain. J Phytopathol 148:7–15
Howell C (2003) Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis 87:4–10
Hu W, Gao Q, Hamada MS, Dawood DH, Zheng J, Chen Y, Ma Z (2014) Potential of Pseudomonas chlororaphis subsp. aurantiaca strain Pcho10 as a biocontrol agent against Fusarium graminearum. Phytopathology 104:1289–1297
Huang X-F, Chaparro JM, Reardon KF, Zhang R, Shen Q, Vivanco JM (2014) Rhizosphere interactions: root exudates, microbes, and microbial communities. Botany 92:267–275
Jimenez M, Manez M, Hernandez E (1996) Influence of water activity and temperature on the production of zearalenone in corn by three Fusarium species. Int J Food Microbiol 29:417–421
Jochum C, Osborne L, Yuen G (2006) Fusarium head blight biological control with Lysobacter enzymogenes strain C3. Biol Control 39:336–344
Johnston-Monje D, Mousa WK, Lazarovits G, Raizada MN (2014) Impact of swapping soils on the endophytic bacterial communities of pre-domesticated, ancient and modern maize. BMC Plant Biol 14:233. https://doi.org/10.1186/s12870-014-0233-3
Jung B, Park S-Y, Lee Y-W, Lee J (2013) Biological efficacy of Streptomyces sp. strain BN1 against the cereal head blight pathogen Fusarium graminearum. Plant Pathol J 29:52
Kamilova F, de Bruyne R (2013) Plant growth promoting microorganisms: the road from an academically promising result to a commercial product. In: de Bruijn FJ (ed) Molecular microbial ecology of the rhizosphere, vol 1 & 2. Wiley, Hoboken, pp 677–686
Khabbaz SE, Zhang L, Cáceres LA, Sumarah M, Wang A, Abbasi PA (2015) Characterisation of antagonistic Bacillus and Pseudomonas strains for biocontrol potential and suppression of damping-off and root rot diseases. Ann Appl Biol. https://doi.org/10.1111/aab.12196
Khan NI, Schisler DA, Boehm MJ, Lipps PE, Slininger PJ (2004) Field testing of antagonists of Fusarium head blight incited by Gibberella zeae. Biol Control 29:245–255. https://doi.org/10.1016/s1049-9644(03)00157-9
Knief C (2014) Analysis of plant microbe interactions in the era of next generation sequencing technologies. Front Plant Sci 5:216
Koch A et al (2016) An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog 12:e1005901
Köhl J, Postma J, Nicot P, Ruocco M, Blum B (2011) Stepwise screening of microorganisms for commercial use in biological control of plant-pathogenic fungi and bacteria. Biol Control 57:1–12
Köhl J, Lombaers C, Moretti A, Bandyopadhyay R, Somma S, Kastelein P (2015) Analysis of microbial taxonomical groups present in maize stalks suppressive to colonization by toxigenic Fusarium spp.: a strategy for the identification of potential antagonists. Biol Control 83:20–28. https://doi.org/10.1016/j.biocontrol.2014.12.007
Kröber M, Verwaaijen B, Wibberg D, Winkler A, Pühler A, Schlüter A (2016) Comparative transcriptome analysis of the biocontrol strain Bacillus amyloliquefaciens FZB42 as response to biofilm formation analyzed by RNA sequencing. J Biotechnol 231:212–223. https://doi.org/10.1016/j.jbiotec.2016.06.013
Kyselková M, Moënne-Loccoz Y (2012) Pseudomonas and other microbes in disease-suppressive soils. In: Lichtfouse E (ed) Organic fertilisation, soil quality and human health. Springer, Dordrecht, pp 93–140. https://doi.org/10.1007/978-94-007-4113-3_5
Laslo É, György É, Mara G, Tamás É, Ábrahám B, Lányi S (2012) Screening of plant growth promoting rhizobacteria as potential microbial inoculants. Crop Prot 40:43–48
Lawongsa P, Boonkerd N, Wongkaew S, O’Gara F, Teaumroong N (2008) Molecular and phenotypic characterization of potential plant growth-promoting Pseudomonas from rice and maize rhizospheres. World J Microbiol Biotechnol 24:1877–1884
Li Y, Geng X, Ji P, Pan C, Wei S (2016) Isolation and evaluation of a Bacillus methylotrophicus strain for control of corn stalk rot. Biocontrol Sci Technol 26:727–731. https://doi.org/10.1080/09583157.2016.1144047
Lisboa BB, Bayer C, Passaglia LMP, Camargo FAdO, Beneduzi A, Ambrosini A, Vargas LK (2015) Soil fungistasis against Fusarium graminearum under different crop management systems. Revista Brasileira de Ciência do Solo 39:69–77
Liu Y et al (2015) Identification and antagonistic activity of endophytic bacterial strain Paenibacillus sp. 5 L8 isolated from the seeds of maize (Zea mays L., Jingke 968). Ann Microbiol 66:653–660
Lori G, Henning C, Violante A, Alippi H, Varsavsky E (1990) Relation between the production of deoxynivalenol and zearalenone and the mycelial growth of Fusarium graminearum on solid natural substrates. Microbiologia (Madrid Spain) 6:76–82
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. https://doi.org/10.1146/annurev.micro.62.081307.162918
Lundberg DS et al (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488:86
Luongo L, Galli M, Corazza L, Meekes E, Haas LD, Van Der Plas CL, Köhl J (2005) Potential of fungal antagonists for biocontrol of Fusarium spp. in wheat and maize through competition in crop debris. Biocontrol Sci Technol 15:229–242. https://doi.org/10.1080/09583150400016852
Mao W, Lumsden RD, Lewis JA, Hebbar PK (1998) Seed treatment using pre-infiltration and biocontrol agents to reduce damping-off of corn caused by species of Pythium and Fusarium. Plant Dis 82:294–299. https://doi.org/10.1094/PDIS.1998.82.3.294
Marguerat S, Bahler J (2010) RNA-seq: from technology to biology. Cell Mol Life Sci. https://doi.org/10.1007/s00018-009-0180-6
Martinez-Alvarez JC, Castro-Martinez C, Sanchez-Pena P, Gutierrez-Dorado R, Maldonado-Mendoza IE (2016) Development of a powder formulation based on Bacillus cereus sensu lato strain B25 spores for biological control of Fusarium verticillioides in maize plants. World J Microbiol Biotechnol 32:75. https://doi.org/10.1007/s11274-015-2000-5
Martins MLG, Martins HM (2002) Influence of water activity, temperature and incubation time on the simultaneous production of deoxynivalenol and zearalenone in corn (Zea mays) by Fusarium graminearum. Food Chem 79:315–318
McMullen M, Bergstrom G, De Wolf E, Dill-Macky R, Hershman D, Shaner G, Van Sanford D (2012) A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis 96:1712–1728. https://doi.org/10.1094/PDIS-03-12-0291-FE
Mehnaz S, Kowalik T, Reynolds B, Lazarovits G (2010) Growth promoting effects of corn (Zea mays) bacterial isolates under greenhouse and field conditions. Soil Biol Biochem 42:1848–1856
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37:634–663. https://doi.org/10.1111/1574-6976.12028
Michelsen CF, Watrous J, Glaring MA, Kersten R, Koyama N, Dorrestein PC, Stougaard P (2015) Nonribosomal peptides, key biocontrol components for Pseudomonas fluorescens In5, isolated from a Greenlandic suppressive soil. mBio 6:e00079–e00015. https://doi.org/10.1128/mBio.00079-15
Mousa WK, Raizada MN (2015) Biodiversity of genes encoding anti-microbial traits within plant associated microbes. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00231
Mousa WK, Shearer CR, Limay-Rios V, Zhou T, Raizada MN (2015) Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation. Front Plant Sci 6:805. https://doi.org/10.3389/fpls.2015.00805
Moussa TAA, Almaghrabi OA, Abdel-Moneim TS (2013) Biological control of the wheat root rot caused by Fusarium graminearum using some PGPR strains in Saudi Arabia. Ann Appl Biol 163:72–81. https://doi.org/10.1111/aab.12034
Mwita L, Chan WY, Pretorius T, Lyantagaye SL, Lapa SV, Avdeeva LV, Reva ON (2016) Gene expression regulation in the plant growth promoting Bacillus atrophaeus UCMB-5137 stimulated by maize root exudates. Gene 590:18–28
O’Donnell K, Ward TJ, Geiser DM, Kistler HC, Aoki T (2004) Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet Biol 41:600–623
O’Donnell K et al (2008) Multilocus genotyping and molecular phylogenetics resolve a novel head blight pathogen within the Fusarium graminearum species complex from Ethiopia. Fungal Genet Biol 45:1514–1522
Pagnussatt FA, Del Ponte EM, Garda-Buffon J, Badiale-Furlong E (2014) Inhibition of Fusarium graminearum growth and mycotoxin production by phenolic extract from Spirulina sp. Pestic Biochem Physiol 108:21–26
Palazzini JM, Groenenboom-de Haas BH, Torres AM, Köhl J, Chulze SN (2013) Biocontrol and population dynamics of Fusarium spp. on wheat stubble in Argentina. Plant Pathol 62:859–866. https://doi.org/10.1111/j.1365-3059.2012.02686.x
Palazzini JM, Alberione E, Torres A, Donat C, Köhl J, Chulze S (2016) Biological control of Fusarium graminearum sensu stricto, causal agent of Fusarium head blight of wheat, using formulated antagonists under field conditions in Argentina. Biol Control 94:56–61. https://doi.org/10.1016/j.biocontrol.2015.12.009
Pan D, Mionetto A, Tiscornia S, Bettucci L (2015) Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotoxin Res 31:137–143
Pandey A, Palni LMS, Hebbar KP (2001) Suppression of damping-off in maize seedlings by Pseudomonas corrugata. Microbiol Res 156:191–194. https://doi.org/10.1078/0944-5013-00102
Pereira P, Nesci A, Etcheverry M (2009) Efficacy of bacterial seed treatments for the control of Fusarium verticillioides in maize. Biocontrol 54:103–111. https://doi.org/10.1007/s10526-007-9148-3
Pérez-Montaño F et al (2014) Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 169:325–336. https://doi.org/10.1016/j.micres.2013.09.011
Pinto A, Melo-Barbosa H, Miyoshi A, Silva A, Azevedo V (2011) Application of RNA-seq to reveal the transcript profile in bacteria. Genet Mol Res 10:1707–1718
Przemieniecki SW, Kurowski TP, Korzekwa K (2014) Chemotypes and geographic distribution of the Fusarium graminearum species complex. Environ Biotechnol 10:45–59
Raaijmakers JM, Mazzola M (2012).Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu Rev Phytopathol. https://doi.org/10.1146/annurev-phyto-081211-172908
Ruocco M, Woo S, Vinale F, Lanzuise S, Lorito M (2011) Identified difficulties and conditions for field success of biocontrol. 2. Technical aspects: factors of efficacy. In: Nicot PC (ed) Classical and augmentative biological control against diseases and pests: critical status analysis and review of factors influencing their success. IOBC/WPRS, Veldhoven, pp 45–57
Ryu D, Bullerman LB (1999) Effect of cycling temperatures on the production of deoxynivalenol and zearalenone by Fusarium graminearum NRRL 5883. J Food Prot 62:1451–1455
Sahab AF, Aly S, Hathout AS, Ziedan E-SH, Sabry BA (2014) Application of some plant essential oils to control Fusarium isolates associated with freshly harvested maize in Egypt. J Essent Oil Res 17:1146–1155. https://doi.org/10.1080/0972060X.2014.891447
Sampietro DA, Ficoseco MEA, Jimenez CM, Vattuone MA, Catalán CA (2012) Trichothecene genotypes and chemotypes in Fusarium graminearum complex strains isolated from maize fields of northwest Argentina. Int J Food Microbiol 153:229–233. https://doi.org/10.1016/j.ijfoodmicro.2011.10.029
Sarrocco S, Moncini L, Pachetti G, Moretti A, Ritieni A, Vannacci G (2013) Biological control of Fusarium head blight under field conditions. IOCB WPRS Bull 86:95–100
Schisler D, Slininger P (1997) Microbial selection strategies that enhance the likelihood of developing commercial biological control products. J Ind Microbiol Biotechnol 19:172–179
Schöneberg A, Musa T, Voegele R, Vogelgsang S (2015) The potential of antagonistic fungi for control of Fusarium graminearum and Fusarium crookwellense varies depending on the experimental approach. J Appl Microbiol 118:1165–1179
Schumann U, Smith NA, Kazan K, Ayliffe M, Wang M-B (2013) Analysis of hairpin RNA transgene-induced gene silencing in Fusarium oxysporum. Silence 4:3. https://doi.org/10.1186/1758-907x-4-3
Shali A et al (2010) Bacillus pumilus SG2 chitinases induced and regulated by chitin, show inhibitory activity against Fusarium graminearum and Bipolaris sorokiniana. Phytoparasitica. https://doi.org/10.1007/s12600-009-0078-8
Shan H et al (2013) Biocontrol of rice blast by the phenaminomethylacetic acid producer of Bacillus methylotrophicus strain BC79. Crop Prot 44:29–37. https://doi.org/10.1016/j.cropro.2012.10.012
Shehata HR, Ettinger CL, Eisen JA, Raizada MN (2016a) Genes required for the anti-fungal activity of a bacterial endophyte isolated from a corn landrace grown continuously by subsistence farmers since 1000 BC. Front Microbiol 7:1548. https://doi.org/10.3389/fmicb.2016.01548
Shehata HR, Lyons EM, Jordan KS, Raizada MN (2016b) Relevance of in vitro agar based screens to characterize the anti-fungal activities of bacterial endophyte communities. BMC Microbiol 16:1–7. https://doi.org/10.1186/s12866-016-0623-9
Shi C, Yan P, Li J, Wu H, Li Q, Guan S (2014) Biocontrol of Fusarium graminearum growth and deoxynivalenol production in wheat kernels with bacterial antagonists. Int J Environ Res Public Health 11:1094–1105
Sieber CMK et al (2014) The Fusarium graminearum genome reveals more secondary metabolite gene clusters and hints of horizontal gene transfer. PLoS One 9:e110311. https://doi.org/10.1371/journal.pone.0110311
Singh BK, Munro S, Potts JM, Millard P (2007) Influence of grass species and soil type on rhizosphere microbial community structure in grassland soils. Appl Soil Ecol 36:147–155. https://doi.org/10.1016/j.apsoil.2007.01.004
Small I, Flett B, Marasas W, McLeod A, Stander M, Viljoen A (2012a) Resistance in maize inbred lines to Fusarium verticillioides and fumonisin accumulation in South Africa. Plant Dis 96:881–888
Small I, Flett B, Marasas W, McLeod A, Viljoen A (2012b) Use of resistance elicitors to reduce Fusarium ear rot and fumonisin accumulation in maize. Crop Prot 41:10–16
Son H et al (2011) A phenome-based functional analysis of transcription factors in the cereal head blight fungus, Fusarium graminearum. PLoS Pathog 7:e1002310. https://doi.org/10.1371/journal.ppat.1002310
Summerell BA, Leslie JF (2011) Fifty years of Fusarium: how could nine species have ever been enough? Fungal Divers 50:135–144. https://doi.org/10.1007/s13225-011-0132-y
Sutton JC (1982) Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can J Plant Pathol 4:195–209. https://doi.org/10.1080/07060668209501326
Vacheron J, Combes-Meynet E, Walker V, Gouesnard B, Muller D, Moënne-Loccoz Y, Prigent-Combaret C (2016a) Expression on roots and contribution to maize phytostimulation of 1-aminocyclopropane-1-decarboxylate deaminase gene acdS in Pseudomonas fluorescens F113. Plant Soil 407:187–202. https://doi.org/10.1007/s11104-016-2907-0
Vacheron J, Moënne-Loccoz Y, Dubost A, Gonçalves-Martins M, Muller D, Prigent-Combaret C (2016b) Fluorescent Pseudomonas strains with only few plant-beneficial properties are favored in the maize rhizosphere. Front Plant Sci 7:1212. https://doi.org/10.3389/fpls.2016.01212
van der Lee T, Zhang H, van Diepeningen A, Waalwijk C (2015) Biogeography of Fusarium graminearum species complex and chemotypes: a review. Food Addit Contam Part A 32:453–460. https://doi.org/10.1080/19440049.2014.984244
Varga E et al (2015) New tricks of an old enemy: isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. Environ Microbiol 17:2588–2600
Velivelli SL, De Vos P, Kromann P, Declerck S, Prestwich BD (2014) Biological control agents: from field to market, problems, and challenges. Trends Biotechnol 32:493–496
Walsh UF, Morrissey JP, O’Gara F (2001) Pseudomonas for biocontrol of phytopathogens: from functional genomics to commercial exploitation. Curr Opin Biotechnol 12:289–295
Wang M, Jin H (2016) Spray-induced gene silencing: a powerful innovative strategy for crop protection. Trends Microbiol 25:4–6
Wang J, Liu J, Chen H, Yao J (2007) Characterization of Fusarium graminearum inhibitory lipopeptide from Bacillus subtilis IB. Appl Microbiol Biotechnol 76:889–894
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63
Wang J-H, Ndoye M, Zhang J-B, Li H-P, Liao Y-C (2011) Population structure and genetic diversity of the Fusarium graminearum species complex. Toxins (Basel) 3:1020–1037. https://doi.org/10.3390/toxins3081020
Wani SH, Sanghera GS, Singh NB (2010) Biotechnology and plant disease control-role of RNA interference. Am J Plant Sci 1:55
Wegulo SN (2012) Factors influencing deoxynivalenol accumulation in small grain cereals. Toxins (Basel) 4:1157–1180
Wegulo SN, Baenziger PS, Hernandez Nopsa J, Bockus WW, Hallen-Adams H (2015) Management of Fusarium head blight of wheat and barley. Crop Prot 73:100–107. https://doi.org/10.1016/j.cropro.2015.02.025
Wicklow DT, Poling SM (2009) Antimicrobial activity of pyrrocidines from Acremonium zeae against endophytes and pathogens of maize. Phytopathology 99:109–115
Xu Y, Wang G, Jin J, Liu J, Zhang Q, Liu X (2009) Bacterial communities in soybean rhizosphere in response to soil type, soybean genotype, and their growth stage. Soil Biol Biochem 41:919–925. https://doi.org/10.1016/j.soilbio.2008.10.027
Yang F, Jacobsen S, Jørgensen HJL, Collinge DB, Svensson B, Finnie C (2013) Fusarium graminearum and its interactions with cereal heads: studies in the proteomics era. Front Plant Sci. https://doi.org/10.3389/fpls.2013.00037
Zhang H et al (2012) Population analysis of the Fusarium graminearum species complex from wheat in china show a shift to more aggressive isolates. PLoS One 7:e31722. https://doi.org/10.1371/journal.pone.0031722
Zhang N et al (2015) Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genom 16:685. https://doi.org/10.1186/s12864-015-1825-5
Zhao Y et al (2014) Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS One 9:e92486. https://doi.org/10.1371/journal.pone.0092486
Acknowledgements
This work is based on the research supported by the National Research Foundation, South Africa (Grant UID: 81192).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict.
Additional information
Communicated by Yusuf Akhter.
Rights and permissions
About this article
Cite this article
Adeniji, A.A., Babalola, O.O. Tackling maize fusariosis: in search of Fusarium graminearum biosuppressors. Arch Microbiol 200, 1239–1255 (2018). https://doi.org/10.1007/s00203-018-1542-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-018-1542-y