Abstract
The present investigation aimed to understand the influence of two plant growth promoting cyanobacterial formulations (Anabaena-Mesorhizobium ciceri biofilm and Anabaena laxa), along with Mesorhizobium ciceri, on the symbiotic performance of five each of desi- and kabuli-chickpea cultivars. Inoculation with cyanobacterial formulations led to significant interactions with different cultivars, in terms of fresh weight and number of nodules, the concentration of nodular leghemoglobin, and the number of pods. The inoculant A. laxa alone was superior in its performance, recording 30–50% higher values than uninoculated control, and led to significantly higher nodule number per plant and fresh root weight, relative to the M. ciceri alone. Highest nodule numbers were recorded in the kabuli cultivars BG256 and BG1003. The kabuli cultivar BG1108 treated with the biofilmed Anabaena-M. ciceri inoculant recorded the highest concentration of leghemoglobin in nodules. These inoculants also stimulated the elicitation of defense- and pathogenesis-related enzymes in both the desi and kabuli cultivars, by two to threefolds. The analyses of Denaturing Gradient Gel Electrophoresis (DGGE) profiles revealed that microbial communities in nodules were highly diverse, with about 23 archaeal, 9 bacterial, and 13 cyanobacterial predominant phylotypes observed in both desi and kabuli cultivars, and influenced by the inoculants. Our findings illustrate that the performance of the chickpea plants may be significantly modulated by the microbial communities in the nodule, which may contribute towards improved plant growth and metabolic activity of nodules. This emphasizes the promise of cyanobacterial inoculants in improving the symbiotic performance of chickpea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil–plant–microbe interactions are critical to improving soil fertility and plant nutrient uptake, particularly significant in the current context of increasing costs and negative environmental consequences of fertilizer application in various crops (Bakker et al. 2012; Pii et al. 2016; Sarr et al. 2008). Cyanobacteria are an exceptional group of prokaryotic organisms, with several genera possessing the unique ability to perform carbon fixation (photosynthesis) and nitrogen fixation. These ecologically important photosynthetic organisms exhibit diverse modes of nutrition, ranging from obligate phototrophy to heterotrophy (Prasanna et al. 2009a, b). These organisms also display a remarkable capability to form symbiotic and facultative associations with diverse members of Gymnosperms, Pteridophytes, and Bryophytes. Cyanobacteria are promising as biofertilizing options, which can improve soil nutrient status, besides crop growth and yields (Nayak et al. 2004). Their associations with crop plants other than rice and wheat are less explored for deriving benefits for plant growth and development (Bidyarani et al. 2015; Karthikeyan et al. 2007; Rai et al. 2002). Cyanobacteria are also known to produce hydrolytic enzymes, whose activity correlates with fungicidal attributes and the ability to elicit defense enzymes in plants, therefore, represents promising biocontrol and fungal-disease suppressive options in agriculture (Gupta et al. 2013; Prasanna et al. 2009b, 2013b).
The combined inoculation of Anabaena oscilliarioides, Brevundimonas diminuta, and Ochrobactrum spp. improved rice yield by 21.2%, in comparison with the application of recommended dose of chemical fertilizers. A significant enhancement in the nitrogen, phosphorus, and potassium (NPK) contents in rice–wheat cropping system was also recorded (Rana et al. 2015). Synergism among the proteobacterial and cyanobacterial strains was also observed in terms of improvements in plant growth and soil microbiological parameters in wheat crop (Manjunath et al. 2011). Earlier, Prasanna et al. (2013a) reported a 20–45% enhancement in plant biomass weight by inoculation of Trichoderma viride–Bradyrhizobium biofilm over other microbial treatments. Anabaena laxa as an inoculant recorded statistically at par yield with that of T. viride-Bradyrhizobium biofilm in mungbean, while, in soybean, the Anabaena–T. viride biofilmed formulations proved superior, recording 12–25% enhancement in yield and microbial activities.
Chickpea (Cicer arietinum L.) is a significant leguminous crop which is a chief source of protein in the diets of the poor in several Asian and African countries (Gaur et al. 2010). Chickpea is important for sustaining cropping system productivity, and its diazotrophic nature contributes to nitrogen, facilitated through the formation of nodules as a result of a highly specific symbiotic association. Some of the hurdles faced mainly deal with the absence of suitable strains, poor numbers, and colonization in the rhizosphere, leading to ineffective nodule formation in several cropping systems (Kantar et al. 2007; Sheoran et al. 1997). Several legumes are a source of non-rhizobial and rhizobial endophytic bacteria which influence plant health and yield, particularly those from the root and nodule tissues, which also include symbiovars of rhizobia (Laranjo et al. 2012). Nodulation, due to co-inoculation with these nodule endophytes, was better than that of inoculation with rhizobia alone (Sturz et al. 2000). Bacterial endophytes have the potential to accelerate seedling germination and enhance plant growth by helping in nutrient acquisition by mobilization of phosphorus and fixing of atmospheric nitrogen, besides their antagonistic activity against root pathogens (Saini et al. 2015). A bacterial strain IC-76 that was isolated from chickpea nodules and characterized showed higher potential to produce plant growth promoting traits such as the production of indole acetic acid (IAA), siderophore, HCN, and β-1,3-glucanase (Gopalakrishnan et al. 2015). Chickpea nodulation has been a subject of focused research globally (Brígido et al. 2009; Israr et al. 2016; Singh et al. 2011; Zhang et al. 2014); however, reports are scanty on the interactions of chickpea crop with cyanobacterial inoculants (Bidyarani et al. 2016). Therefore, to gain a better understanding of cyanobacteria–chickpea interactions, our research study focused on the following questions: (1) Does cyanobacterial inoculation have any effect on the diversity of microbiota and metabolic activities of the root nodules? and (2) Are these effects correlated with improved growth of chickpea? The present investigation included five each of desi- and kabuli-chickpea cultivars and three microbial inoculants including M. ciceri.
Materials and methods
Organisms used in this study and their maintenance
The cyanobacterial strain Anabaena laxa (RPAN28) and bacterial strain Mesorhizobium ciceri belong to the germplasm of the Division of Microbiology, ICAR-Indian Agricultural Research Institute (IARI), New Delhi. Mesorhizobium ciceri (M.ciceri) was chosen as it is the routinely used inoculant at ICAR-IARI for chickpea. It was maintained in Yeast Mannitol Agar and incubated for 48 h at 30 ± 2 °C. Anabaena laxa (RPAN28) is a well-characterized potent biocontrol agent and plant growth promoting inoculant, which has been used in several crops, besides chickpea (Bidyarani et al. 2016; Prasanna et al. 2013a, b, 2015). This phototrophic strain was grown and maintained in Haffkine flasks, with BG-11 medium, under the optimized conditions of light and temperature (27 ± 2 °C; light:dark cycles 16 h:8 h; with intensity of white light, 50–55 μmol photons m−2 s−1). The development of cyanobacterial biofilm—Anabaena-Mesorhizobium ciceri (An-M.ciceri)—and its characterization has been given earlier (Bidyarani et al. 2016).
Preparation of formulations
Paddy straw compost after amendment with vermiculite (1:1) was used as carrier, as described earlier (Prasanna et al. 2013a, b). The chlorophyll content of cyanobacterial cultures and their biofilms was measured following the method of MacKinney (1941) and maintained as 100 μg g−1 carrier. A CFU of 108 to 109 was maintained per gram of the carrier for Mesorhizobium ciceri in the formulation and water-holding capacity was maintained at 60%, after amendment with cultures and requisite amount of water.
Experimental setup with chickpea crop
The field experiment was taken up in the fields of ICAR-Indian Agricultural Research Institute, New Delhi (latitude 28°40′ N and longitude 77°12′ E, altitude 228.6 m above the mean sea level), during Rabi season of 2014. The soil type of IARI is classified as well-drained old alluvial soil, belonging to the coarse sandy loam, non-acidic, mixed hypothermic family of the Typic Haplustepts. Analyses of soil before sowing revealed that the experimental field had pH of 7.5 (1:2.5 soil-and-water ratio), 225 kg ha−1 alkaline permanganate oxidizable N, 16.0 kg ha−1 available P, and 275 kg−1 N ammonium acetate exchangeable K, with 0.53% organic carbon as estimated by standard protocols (Prasad et al. 2006). Application of 100 kg diammonium phosphate per hectare (18% N and 46% P2O5) to the soil was done before sowing. The sowing was done on 1st December 2014 and sampling was done 60 DAS (days after sowing). Harvesting was undertaken on 27th April 2015. The mean annual rainfall of Delhi is 650 mm, with more than 80% generally during the southwest monsoon season (July–September), and the mean annual evaporation is 850 mm. The details of inoculants used are—Anabaena sp.—Rhizobium biofilm (An-M.ciceri biofilm), Anabaena laxa, Mesorhizobium ciceri (M. ciceri) IARI inoculant, and Control (no inoculation, only carrier).
A total of ten cultivars of chickpea were tested among which BG372, PUSA372, BGM547, BG362, and BG256 were desi varieties, and BG1003, BG1053, BGD128, BG1105, and BG1108 were kabuli varieties. Split plot design with three replications was used, in which a light irrigation was given at pre-sowing stage, followed by two pre flowering irrigations and one at pod development stage. The seeds to be used for each plot were coated with 5 g formulation using 1% carboxymethyl cellulose (CMC), as the sticking agent.
Parameters estimated in root nodules
Gas chromatography of ethylene formed was used as an index of nitrogenase activity or nitrogen-fixing potential and expressed as acetylene reducing activity (Nayak et al. 2004) of the fresh nodules. The values were compared with those of commercially available standard ethylene, and vials containing an equivalent volume of water served as controls. All the values presented represent the means of triplicate measurements.
Nodule parameters such as total nodule number/plant and fresh weight root/plant were evaluated. Leghemoglobin content in the nodules was estimated by the standardized method of Appleby and Bergersen (1980). The leghemoglobin content was given as µmol g−1 fresh weight of nodules, which was calculated using the following formula:
where D is the initial dilution.
Diversity of archaea, bacteria, and cyanobacteria in the nodules
The nodules were washed under running water and then surface sterilized with 70% ethanol, to remove adhering cells or soil. These nodules were treated with 0.1% HgCl2 for 2 min, followed by repeated washing with sterile distilled water for 1 min, under aseptic conditions. Such nodules were crushed after placing in sterile eppendorf tubes using sterile plastic pestles under aseptic conditions, and the extracted DNA was kept at −20 °C until further analysis. The total DNA was extracted from the nodules using Power Plant® DNA Isolation Kit (MoBio, Carlsbad, CA), following the manufacturer’s instructions from the selected chickpea cultivars treated with the microbial inoculants and control. The total DNA extracted from nodules was then amplified using QB-96 Gradient Thermal Cycler from Biotron Healthcare (India) Pvt. Ltd., Mumbai. The specific primers for the archaea (GC-Arc 344 F: 5′-CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCC-ACGGGGCGCAGCAGGCGCGA -3′ and 517 R: 5′-ATTACCGCGGCTGCTGG-3′), bacteria (F984GC: 5′-CGCCCGGGGCGCGCCCCGGGCGGGGCGGGGGCACGGGGGGAACGCGAAGAACCTTAC-3′ and R1378: 5′-CGGTGTGTACAAGGCCCGGGAACG-3′), and cyanobacteria ((GC-)CSIF: 5′-CGCCCGCCGCGCCCCGCGCCCGGCCCGCCGCCCCCGCCCC-G(T/C)CACGCCCGAAGTC(G/A)TTAC-3′ and 373R: 5′-CTAACCACCTGAGCTAAT-3′) were used for the amplification (Heuer et al. 1997; Bano et al. 2004; Janse et al. 2003). The total reaction volume of (25 µL) contained 12.5 µL RedTaq®ReadyMix (Sigma-Aldrich), 1 µL Bovine serum albumin (10 mg/L), 0.5 µL of each forward and reverse primer from the stock of 10 µM, and 10–15 ng DNA template. With the use of Invitrogen Quant-iT dsDNA assay kit (Eugene, Oregon, USA), the concentrations of PCR products were quantified in a Nanodrop 3300 fluorospectrometer (Thermo Scientific, Waltham, Massachusetts, USA).
The PCR product (250 ng) was loaded into each lane of Denaturing Gradient Gel Electrophoresis system using a DCode System (BioRad). The denaturing gradient of 30–60, 30–70, and 30–60% was used for cyanobacterial, bacterial, and archaeal community analyses, respectively. Gradients were formed with 8% (wt/vol) acrylamide stock (40% Acrylamide/Bis-acrylamide in 37.5:1 ratio) solutions contained denaturing solution 0 and 100% (7 M urea and 40% vol/vol formamide (Sigma-Aldrich). Electrophoresis was performed at a constant voltage of 38 V and temperature 60 °C. After 15 h of electrophoresis, gels were stained with ethidium bromide (0.5 µg/L) for 15 min and rinsed for 15 min with Milli-Q water. The gel was photographed with the Alpha Innotech AlphaImager Gel Imaging System. The software GeneTools version 1.4.1.0 (Syngene, Cambridge, United Kingdom) was used for band visualization of the digital gel images. Cluster analysis was done based on the Jaccard correlation coefficients, which was then used to construct the dendrogram using the method of unweighted pair-group agglomeration method with arithmetic average (UPGMA).
Activity of defense and antioxidant enzymes in shoot and root
The samples of fresh shoot and root after washing in running tap water were homogenized in 50 mM Tris HCl buffer, centrifuged and the supernatant enzyme extracts stored at −20 °C. Peroxidase (PO) activity was measured by the changes in absorbance at 470 nm, and one unit of enzyme was defined as the change in absorbance of 0.01 unit min−1. Polyphenol oxidase (PPO) activity was measured with catechol (2 mg ml−1), serving as the substrate, and recording the changes in absorbance at 546 nm. One unit of enzyme is defined as the change in absorbance of 0.01 IU min−1, recorded at 30 s intervals for 3 min. Both these procedures were modifications of the methods given by Jennings et al. (1969). Phenylalanine ammonia-lyase (PAL) activity was assayed in leaf and root extracts (100 μl) by the method given by Beaudoin-Eagan and Thorpe (1985). One unit of enzyme (IU) was defined as the change in absorbance min−1 g−1 fresh tissue weight. Laminarin was used as the substrate for measuring the activity of β-1, 3-glucanase (EGases, EC 3.2.1.39), following the modified protocol, as optimized by Prasanna et al. (2013a, b).
The fresh shoot/root tissues were homogenized in liquid nitrogen with phosphate buffer pH 7.0 for assay of antioxidant enzyme activity. The ability to inhibit photochemical reduction of NBT (nitrotetrazolium blue chloride) at 560 nm is taken as an index of superoxide dismutase activity (Beauchamp and Fridovich 1971). Catalase activity was measured based on the initial rate of disappearance of H2O2 at 240 nm (Bergmeyer 1970), using a reaction mixture containing 0.05 M Na–phosphate buffer (pH 7.0), mixed with 0.1 mM EDTA and 3% H2O2. The decrease in absorption was based on the rate of 1 mol H2O2 being destroyed per min, which represents one unit of catalase activity. The decrease in absorbance at 290 nm based on the ascorbate being oxidized is an index of Ascorbate peroxidase activity (Nakano and Asada 1981), which is expressed as IU/mg protein.
Determination of yield
The total number of pods per plant at mid-crop stage was taken as an index of yield.
Statistical analysis
The field experiment was used a split plot design with four treatments including control. The data were recorded in triplicate on all the parameters and analyzed using Analysis of Variance (ANOVA) and WINDOSTAT 8.0 statistical package. Pearson’s correlation analyses were done using Microsoft, Excel package.
Results
ARA and leghemoglobin content
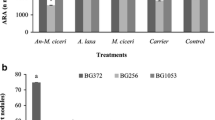
Analysis of variance (ANOVA) showed significant variations in acetylene reduction activity (ARA) and leghemoglobin content, total number of nodules and fresh root weight per plant among the inoculants, and the chickpea cultivars tested (Table 1). ARA was the highest due to the inoculation with M. ciceri alone (689.3 nmol ethylene g−1 nodules h−1), which was at par with that of A. laxa (683.2 nmol ethylene g−1 nodules h−1). Thus, the ARA in the microbially inoculated chickpea nodules was higher than that of uninoculated control, (494.9 nmol ethylene g−1 nodules h−1) by 38–39%. ARA showed a positive correlation with fresh root weight of the plant (0.258), polyphenol oxidase (PPO) activity of (root 0.324, shoot 0.239), and β, 1-3 endoglucanase activity (0.279).
Leghemoglobin content and a total number of nodules and fresh root weight per plant showed a significant variation among microbial inoculants as well as different chickpea cultivars tested (Table 1). Mean performance in terms of leghemoglobin content in the microbially inoculated- and control plant nodules ranged from 74.79 to 117.79 (µmol g−1 nodules) (Table 1). The highest value was recorded in the Anabaena-M. ciceri biofilm inoculated plants, which was at par with those of M. ciceri and A. laxa inoculated plants (112.59 and 108.99 µmol g−1 nodules, respectively). The leghemoglobin content of the inoculated treatments were significantly higher, as compared to control. A positive correlation was observed between the leghemoglobin contents and the activities of peroxidase (0.312), phenylalanine ammonia-lyase (0.28), and β-1,3 endoglucanase (0.24) in shoots and the contents of chlorophyll (Chl a 0.42) and carotenoids (0.42) in leaves. The total number of nodules per plant was higher in the A. laxa- followed by the M. ciceri-inoculated plants (Table 1). Similarly, higher fresh root weight was by the Anabaena-M. ciceri biofilm (5.08 g) inoculation, followed by A. laxa (5.06 g), which were 9.04 and 8.71% over control (4.66 g), respectively. The root biomass was positively correlated with pod yield (0.363) and plant defense enzyme activity. Among the inoculants tested, A. laxa exhibited a significant enhancement across the cultivars.
Diversity of nodule-associated microorganisms
The archaeal, bacterial, and cyanobacterial communities analyzed by the PCR-DGGE method showed the differential effect of the microbial inoculants tested on four selected cultivars—desi BG372 and BG256 (desi), and BG1003 and BG1053 (kabuli) (Figs. 1, 2, 3). Not only the presence but also intensities of each band, representing the abundance of individual phylotype, varied among the treatments. The ‘phylotypes’ deduced from the number of prominent bands were about 23, 9, and 13 in the archaeal, bacterial, and cyanobacterial communities, respectively.
Differences in the DGGE profiles of archaeal communities analyzed using specific primers in the nodules of selected chickpea cultivars, as influenced by the microbial formulations. Lanes represent: 1—BG372 (desi variety) Control; 2—BG372 (desi variety)-An-M.ciceri biofilm; 3—BG372 (desi variety)-Anabaena laxa; 4—BG372 (desi variety)-Mesorhizobium ciceri; 5—BG256 (desi variety) Control; 6—BG256 (desi variety)-An-M.ciceri biofilm; 7—BG256 (desi variety)-Anabaena laxa; 8—BG256 (desi variety)-Mesorhizobium ciceri; 9—BG1003 (kabuli) Control; 10—BG1003 (kabuli)-An-M.ciceri biofilm; 11—BG1003 (kabuli)-Anabaena laxa; 12—BG1003 (kabuli)-Mesorhizobium ciceri; 13—BG1053 (kabuli) Control; 14—BG1053 (kabuli)-An-M.ciceri biofilm; 15—BG1053 (kabuli)-Anabaena laxa and 16—BG1053 (kabuli)-Mesorhizobium ciceri
Differences in the DGGE profiles of bacterial communities analyzed using specific primers in the nodules of selected chickpea cultivars, as influenced by the microbial formulations. The treatment designations are provided in the legend of Fig. 1
Differences in the DGGE profiles of cyanobacterial communities analyzed using specific primers in the nodules of selected chickpea cultivars, as influenced by the microbial formulations. The treatment designations are provided in the legend of Fig. 1
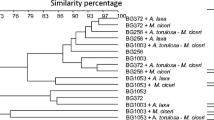
The multivariate cluster analysis of the DGGE profiles suggested about seven clusters within archaeal communities, five in bacterial communities, and six in cyanobacterial communities (Figs. 4, 5, 6). The microbial inoculants altered these communities, evidently from the changes in the presence and abundance (intensities) of phylotypes; each of the desi and kabuli cultivars tested had distinct communities. The desi cultivar BG256 under the control treatment had a distinctly different archaeal community (Fig. 4). The archaeal community of another desi cultivar BG372 treated with A. laxa was different from all other communities. Interestingly, the application of microbial inoculants was found to influence the archaeal community considerably from these clusters. The bacterial community of desi BG256 cultivar without any treatment (control) was distinctly different (Fig. 5). Similarly, the bacterial community profile of BG372 under control treatment was different from others; all other cultivars showed variations due to the microbial inoculation treatments. The inoculation with M. ciceri led to the distinction of kabuli from desi cultivars to some extent, evidently from their bacterial community profiles.
UPGMA dendrograms constructed using Jaccard similarity index generated from the PCR-DGGE of archaeal communities analyzed using specific primers in the nodules of the selected chickpea cultivars, as influenced by the microbial formulations. The treatment designations are provided in the legend of Fig. 1
UPGMA dendrograms constructed using Jaccard similarity index generated from the PCR-DGGE of bacterial communities analyzed using specific primers in the nodules of the selected chickpea cultivars, as influenced by the microbial formulations. The treatment designations are provided in the legend of Fig. 1
UPGMA dendrograms constructed using Jaccard similarity index generated from the PCR-DGGE of cyanobacterial communities analyzed using specific primers in the nodules of the selected chickpea cultivars, as influenced by the microbial formulations. The treatment designations are provided in the legend of Fig. 1
The cyanobacterial community profiles in these chickpea nodules also showed variations due to the application of microbial inoculants (Fig. 6). In contrast to those of archaeal and bacterial communities, the kabuli cultivar BG1003 without any microbial inoculation showed a distinctly different cyanobacterial community profile. The differential effects of cyanobacteria-based formulations on the kabuli and desi cultivars were evident to some extent. The effect of M. ciceri was distinctly different between the cyanobacterial communities of the desi and kabuli cultivars. The stronger effect of cyanobacteria-based formulations on the cyanobacterial communities of nodules was clearly observed in these DGGE profiles.
Plant physiological parameters
In the root tissues, PAL activity remarkably increased almost sevenfold due to the inoculation of M. ciceri and A. laxa relative to the uninoculated control (Table 2). Inoculation with A. laxa showed the highest increase in PAL activity in shoot samples, up to threefold over control. Peroxidase activity was between 0.56 and 0.69 IU g−1 root tissue. Highest peroxidase activity in root tissues was observed due to the inoculation with A. laxa. However, the highest peroxidase activity in the shoot tissues was observed due to the inoculation with the Anabaena-M. ciceri biofilm (0.62 IU g−1 tissue), followed by A. laxa which was at par with that of M. ciceri (0.61 IU g−1 tissue). All three inoculants performed significantly better than control (0.36 IU g−1). Polyphenol oxidase activity in the shoot tissues was higher than that of root tissues, and ranged from 0.39 to 0.73 IU g−1. The microbial inoculation led to significantly higher activities than control. The biofilm Anabaena-M. ciceri inoculant led to highest polyphenol oxidase activity (0.73 IU g−1) in the shoot tissues. All three microbial inoculants tested showed significantly higher polyphenol oxidase activity over control.
The activity of hydrolytic enzyme β 1,3 endoglucanase in root and shoot tissues revealed significant variations (Table 3). Highest β 1,3 endoglucanase enzyme activity in root tissues was due to the M. ciceri inoculant (0.86 IU g−1) followed by A. laxa (0.83 IU g−1) and the Anabaena-M.ciceri biofilm (0.66 IU g−1). All microbial inoculant treatments were superior to the control treatment. In shoot samples, the M. ciceri inoculant led to the highest activity (1.18 IU g−1), followed by the Anabaena-M.ciceri biofilm and A. laxa (1.13 and 1.10 IU g−1, respectively). These activities were significantly higher than that of control.
Photosynthetic pigment contents varied due to the microbial inoculant treatments as well as chickpea cultivars. The chlorophyll a content was higher due to the A. laxa inoculant (0.45 mg g−1), which was on par with the M. ciceri inoculant (0.44 mg g−1), followed by the Anabaena-M.ciceri biofilm (0.38 mg g−1) (Table 3). Chlorophyll b was the highest due to the Anabaena-M.ciceri biofilm (0.25 mg g−1), which was superior to A. laxa (0.23 mg g−1). Carotenoid content was highest due to the A. laxa inoculant (0.26 mg g−1) Chlorophyll b accumulation in leaves was higher in the kabuli cultivar—BG1053, followed by BG3372. The inoculation with Anabaena-M.ciceri biofilm influenced its concentration, followed by that of A. laxa.
Analyses of β,1-3 endoglucanase activity in shoot tissues revealed the significant interaction between the microbial inoculants and the cultivars tested. The activity of β,1-3 endoglucanase in root tissues was 50–70% more in the inoculated treatments than in control, with highest being observed with the M. ciceri treatment. In the shoot tissues, endoglucanase activity enhanced by more than threefold due to the microbial inoculation; M. ciceri alone led to the highest activity. Inoculation significantly enhanced the activity by two to threefolds, in both roots and shoots, respectively, across all the cultivars tested.
Antioxidant enzyme activity
Superoxide dismutase activity in the shoot tissues was the highest due to the M. ciceri inoculation (12.97 IU mg−1 protein), followed by the Anabaena-M.ciceri biofilm (12.10 IU mg−1 protein) and A. laxa (11.02 IU mg−1 protein) (Table 4). Catalase activity in the root tissues was higher in the A. laxa treatment (0.47 IU mg−1 protein) than in other treatments. Inoculation with A. laxa ranked highest for the catalase activity in the root tissues. The Anabaena-M. ciceri biofilm was superior to others in the catalase activity of shoots. Similarly, the ascorbate peroxidase activity due to the Anabaena-M. ciceri biofilm inoculation was significantly higher than that of control. However, the ascorbate peroxidase activity in the shoot tissues was highest due to the inoculation with M. ciceri (0.199 IU mg−1 protein). The catalase activity in the root tissues was positively correlated with ARA, while the ascorbate peroxidase activity was positively correlated with leghemoglobin. The SOD activity in shoot tissues of the inoculated treatments was 40–50% higher than control treatment.
Yield index
The number of pods per plant at mid-crop stage was taken as a representative of yield index. Analysis of variance (ANOVA) revealed a significant variation in terms of the number of pods at mid crop stage and crude protein (%), due to the different chickpea cultivars and microbial inoculants (Table 5); the interaction was also significant. The treatments M. ciceri and A. laxa performed significantly better than the control, and the total number of pods ranged from 38 to 58 per plant (Table 5). A positive correlation was recorded between the number of nodules per plant and yield of a total number of pods per plant (0.36). M. ciceri proved to be the most promising inoculant and showed more than 40% higher values than the uninoculated control.
Discussion
Cyanobacteria stimulate the growth of plants directly by providing nutrients, and indirectly by inhibiting the growth of phytopathogenic fungi, thereby influencing plant growth and yields positively (Prasanna et al. 2015). They represent valuable bio resources as they produce a number of bioactive compounds, enzymes, and pigments (Gupta et al. 2013), and are extensively utilized biofertilizers in agriculture (Prasanna et al. 2015; Venkataraman 1981). Cyanobacterial exudates comprise mainly of nitrogenous compounds, irrespective of their growth on elemental nitrogen or combined N sources (Nayak et al. 2004, Mandal et al. 1999). In the chickpea–Rhizobium symbiosis (Smith 1992), the process of nitrogen fixation involves nutrient exchange and mobilization, exudation of enzymes and stimulatory compounds which modify the rhizosphere and its microbiome, facilitating the development of specialized structures called nodules (Vessey 2003). Brígido et al. (2017) collected several native Portuguese chickpea Mesorhizobium isolates which showed not only the plant growth-promoting traits but also tolerance to metals such as Zn and Pb. Cyanobacteria are also known to secrete indole acetic acid, gibberellins-like compounds, sugars, and bioactive compounds with fungicidal activity including pathogenesis-related proteins which play both direct and indirect roles in plant growth promotion (Gupta et al. 2013; Prasanna et al. 2009b, 2012, 2013b; Younis et al. 2009).
Cyanobacteria form associations with vascular/non-vascular plants, which may be symbiotic or mutualistic or even associative (Prasanna et al. 2009a, b). In the present study, focus was towards evaluating the potential of three different inoculants which included two cyanobacterial inoculants—the biofilm of Anabaena-M. ciceri and A. laxa singly, and the inoculant of M. ciceri, a standard inoculant for chickpea. Significantly higher values of ARA (an index of nitrogen fixation) in the roots (with nodules) as compared to the uninoculated control were recorded that illustrated the persistence or influence on the metabolic activity by the inoculant or biofilms used in the present study. Similar findings exist on the potential of cyanobacterial biofilms as biofertilizers and biocontrol agents in various crops including rice, wheat, cotton, vegetables, and legumes (Prasanna et al. 2013a, b, 2015). Saini et al. (2004) observed an enhancement in nodule dry weight and acetylene reduction activity (ARA) in Rhizobium and vesicular arbuscular mycorrhizal (VAM) inoculation treatments in chickpea. Co-inoculation of Rhizobium and PSB (phosphate solubilizing bacteria) performed better than Rhizobium and PSB alone in terms of leghemoglobin content in the nodular tissues of chickpea crop. Figueredo et al. (2014) observed that Bacillus sp. enhanced the colonization of Bradyrhizobium spp. and indirectly played an important role in induced systemic resistance against Sclerotium rolfsii leading to increased peanut yields.
Leghemoglobin is a major factor which influences nitrogen fixation efficiency of legume crops (Tagore et al. 2013). Higher leghemoglobin content in nodules is often reflective of efficient symbiosis. The highest leghemoglobin values were recorded due to the inoculation with the Anabaena-M. ciceri biofilm. Leghemoglobin content positively correlated with ARA observations in both parameters, M. ciceri and Anabaena-M. ciceri biofilm which showed higher leghemoglobin and acetylene reduction activity. Deka and Azad (2006) reported that the leghemoglobin content has a positive correlation with N2 fixation and nitrogenase activity in nodules.
Cyanobacterial inoculation showed a positive impact on the increment of chlorophyll a content. Chlorophyll content positively correlated with defense- and antioxidant enzyme activities and the interaction of the varieties with inoculation was significant. The enhancement of chlorophyll a, b, and carotenoid content by the A. laxa inoculation, showed a positive association with nitrogen fixation, illustrated the source–site relationship in plant metabolism. Burjus et al. (2014) documented the positive response of total chlorophyll of plant to cyanobacterial inoculation. Cyanobacterial suspensions comprise biologically active compounds, such as plant growth regulators, which increase the content of leaf chlorophyll in response to the different fertilization treatments, eliciting a decrease in the rate of senescence and transpiration (Younis et al. 2009).
Microorganisms are the logical choice to boost enzyme activity of plant and soil due to their abundant biomass, higher metabolic activity, and extra-cellular enzyme production. Microorganisms elicit multiple antioxidant enzymes; such enzymes counteract the oxidative stress caused by ROS during plant defense. Enhanced activity of defense enzymes by co-inoculation with cyanobacteria has been earlier documented in tomato, leading to suppression of Fusarium wilt and representing the underlying bioprotection mechanism (Prasanna et al. 2013a). Inoculation of Anabaena–Azotobacter biofilmed formulation also showed significantly higher levels of peroxidase activity in maize (Prasanna et al. 2015). However, in chickpea, this is the first report. The positive interaction of cyanobacteria with crop plants in terms of enhanced photosynthetic pigment content and biomass reflects a direct effect. Defense enzymes are a part of the signaling cascade in plant–microbe interactions; an enhanced activity illustrates that these inoculants exert indirect effects also (Ibiang et al. 2017). Essa et al. (2015) documented the effect of cyanobacterial filtrates of N. ellipsosporum, A.oryzae, and Synechococcus spp. by enhancing the peroxidase activity, relative to the control, in the seedlings of Sorghum durra and Helianthus annus. These augmentations ranged from 54 to 106% compared to control. They also reported increased polyphenol oxidase activity of seedlings with the maximal increase in enzyme activity recorded with Synechococcus spp. Racchi (2013) showed a significant variation among the treatments, illustrating positive interactions, particularly illustrated by the activities of the main antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) in the potato leaves. A similar trend in defense enzyme activity was documented by Kumar et al. (2007) who observed that Pseudomonas inoculant Pf4-99 treated chickpea plants recorded increased phenolic content and PAL activity on challenge inoculation with M. phaseolina.. Hemissi et al. (2013) reported strong antagonism by Rhizobium strains PchAzm and Pch S.Nsir2 in response to Rhizoctonia solani, leading to a reduction in infection, and enhanced level of defense enzymes such as phenylalanine ammonia-lyase, peroxidase, and phenolic content of the plants.
The major endosymbionts of chickpea include Mesorhizobium ciceri and M. mediterraneum, while other species such as M. amorphae, M. tianshanense, M. huakuii, M. opportunistum, M. muleiense, M. loti, Sinorhizobium medicae, and Ensifer meliloti can also nodulate (Rouhrazi and Khodakaramian 2015). Though it was considered earlier that the chickpea-nodulating rhizobia are host specific, recent reports suggest the presence of several other endophytes. The nodules of chickpea are of an indeterminate type, which offers a competent biological niche for microbial colonization. Several bacteria possess the ability to colonize the intercellular spaces which is also facilitated by the Mesorhizobium cells. Zgadzaj et al. (2015) showed that the legume nodule represents a unique environmental niche, which is well adapted to accommodate host-selected soil microbes which lead to effective nitrogen fixation. The bacterial community profiles obtained in the present study also indicate the diversity of eubacteria within the nodules, with about nine predominant phylotypes. Sequence analysis of endophytic root nodule bacterial isolates from chickpea (Cicer arietinum L.) and mothbean (Vigna aconitifolia L.), illustrated a predominance of the genus Pseudomonas sp., while others genera included Rhizobium, Agrobacterium and Erwinia spp. (Sharma et al. 2012). There is a need to understand the phenotypic, functional diversity, and molecular diversity of mesorhizobia (Dudeja and Chaudhary 2008), along with other microbes that interact with plants to fully achieve the potential of efficient plant–microbe partnerships. Rajendran et al. (2012) observed that non-rhizobial nodule-associated bacteria (NAB) survive well and cause better nodulation and plant growth when co-inoculated with rhizobia. Co-inoculation of these isolates with rhizobial strain S. meliloti led to increase in the length of roots and shoots, chlorophyll content, nodulation efficiency, and nodule dry weight.
What was more interesting in the present study was the presence of about 23 archaeal phylotypes in the nodule microbiome of both desi and kabuli cultivars. Since the archaeal members were not inoculated, their presence in the nodule was intriguing. The diversity of archaeal members was extensive, evidently from the predominant phylotypes of about 23 in the nodules of the cultivars tested. Although the presence of bacteria other than rhizobia like Rhizobium radiobacter symbiovar trifolii was reported earlier (Rouhrazi and Khodakaramian 2015; Martinez-Hidalgo et al. 2015), this is the first report on the presence and diversity of archaea in the nodules. The microbiome of both the desi and kabuli cultivars had archaeal members, and their communities were considerably influenced by the microbial formulations used for inoculation. Likewise, the cyanobacterial communities were distinctly influenced by the application of microbial formulations. The nodules from the control and the M. ciceri inoculant treatments showed the presence of cyanobacterial members, suggesting their active role in the nodules. Future investigations are warranted on the roles of archaea, bacteria other than rhizobia, and cyanobacteria in the formation, as well as the metabolism of nodules. Based on the published literature, it can be considered as a first report on the diversity of archaea, bacteria, and cyanobacteria in the nodules of chickpea plants. The inoculation with different microbial formulations led to the distinct changes in these communities in the nodules of both desi and kabuli cultivars, illustrating that the performance of the chickpea plants may be significantly influenced by the microbiome.
In the present investigation, the cyanobacterial formulations such as the Anabaena-M.ciceri biofilm, and A. laxa as well as M. ciceri recorded plant growth promoting effect, along with induction of defense and oxidative stress enzyme activity, which led to a high quantity of biological-nitrogen fixation measured as ARA, enrichment of leghemoglobin, and high pod yield. These effects could be positively correlated with changes in the nodule microbiome. The present investigation highlights the potential of integrating cyanobacterial inoculants and biofilmed biofertilizers in the nutrient management strategies for legume crops.
References
Appleby CA, Bergersen FJ (1980) Preparation and experimental use of leghaemoglobin. In: Bergersen FJ (ed) Methods for evaluating biological nitrogen. Wiley, Chichester, pp 315–335
Bakker MG, Manter DK, Sheflin AM, Weir TL, Vivanco JM (2012) Harnessing the rhizosphere microbiome through plant breeding and agricultural management. Plant Soil 360:1–13
Bano N, Ruffin S, Ransom B, Hollibaugh JT (2004) Phylogenetic composition of Arctic Ocean archaeal assemblages and comparison with Antarctic assemblages. Appl Environ Microbiol 70:781–789
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beaudoin-Eagan LD, Thorpe TA (1985) Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol 78:438–441
Bergmeyer, N (1970) Methoden der enzymatischen analyse, vol 1. Akademie Verlag, Berlin, pp 636–647
Bidyarani G, Prasanna R, Chawla G, Babu S, Singh R (2015) Deciphering the factors associated with the colonization of rice plants by cyanobacteria. J Basic Microbiol 55:407–419
Bidyarani N, Prasanna R, Babu S, Hossain F, Saxena AK (2016) Enhancement of plant growth and yields in Chickpea (Cicer arietinum L.) through novel cyanobacterial and biofilmed inoculants. Microbiol Res 188:97–105
Brígido AAC, Laranjo M, Rodrigues S, Oliveira S (2009) Survey of chickpea Rhizobia diversity in Portugal reveals the predominance of species distinct from Mesorhizobium ciceri and Mesorhizobium mediterraneum. Microb Ecol 58:930–941
Brígido C, Glick BR, Oliveira S (2017) Survey of plant growth-promoting mechanisms in native Portuguese chickpea Mesorhizobium Isolates. Microb Ecol 73:900–915
Burjus SJ, Jawad ALM, Al-Ani NK (2014) Effect of two species of cyanobacteria as biofertilizer on characteristics and yield of chickpea plant. Iraqi J Sci 55:685–696
Deka AK, Azad P (2006) Screening for efficient strains of Bradyrhizobium. Indian J Pulses Res 19:79–82
Dudeja SS, Chaudhary P (2008) High and low nodulation in relation to molecular diversity of chickpea mesorhizobia in Indian soils. Arch Agron Soil Sci 54:109–120
Essa AMM, Ibrahim WM, Mahmud RM, Kassim NAE (2015) Potential impact of cyanobacterial exudates on seed germination and antioxidant enzymes of crop plan seedlings. Int J Curr Microbiol Appl Sci 4:1010–1024
Figueredo MS, Tonelli ML, Taurian T, Angelini J, Ibañez F, Valetti L, Muñoz V, Anzuay MS, Ludueña L, Fabra A (2014) Interrelationships between Bacillus sp. CHEP5 and Bradyrhizobium sp. SEMIA6144 in the induced systemic resistance against Sclerotium rolfsii and symbiosis on peanut plants. J Biosci 39:877–885
Gaur PM, Tripathi S, Gowda CLL, Ranga GV, Sharma HC, Pande S, Sharma M (2010) Chickpea seed production manual. International Crops Research Institute for the Semi-Arid Tropics, Patancheru
Gopalakrishnan S, Srinivas V, Alekhya G, Prakash B, Kudapa H, Rathore A, Varshney RK (2015) The extent of grain yield and plant growth enhancement by plant growth-promoting broad-spectrum Streptomyces sp. in chickpea. Springer Plus. doi:10.1186/s40064-015-0811-3
Gupta V, Ratha SK, Sood A, Chaudhary V, Prasanna R (2013) New insights into the biodiversity and applications of cyanobacteria (blue-green algae)-prospects and challenges. Algal Res 2:69–97
Hemissi I, Mabrouk Y, Mijri S, Saidi M, Bouaziz S (2013) Enhanced defence responses of chickpea plants against Rhizoctonia solani by pre-inoculation with Rhizobia. J Phytopathol 161:412–418
Heuer H, Krsek M, Baker P, Smalla K, Wellington EM (1997) Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 63:3233–3241
Ibiang YB, Mitsumoto H, Sakamoto K (2017) Bradyrhizobia and arbuscular mycorrhizal fungi modulate manganese iron, phosphorus and polyphenols in soyabean [Glycine max (L.) Merr.] under excess zinc. Environ Exp Bot. doi:10.1016/j.envexpbot.2017.01.011
Israr D, Mustafa G, Khan KS, Shahzad M, Ahmad N, Masood S (2016) Interactive effects of phosphorus and Pseudomonas putida on chickpea (Cicer arietinum L.) growth, nutrient uptake, antioxidant enzymes and organic acids exudation. Plant Physiol Biochem 108:304–312
Janse I, Meima M, Kardinaal WEA, Zwart G (2003) High-resolution differentiation of cyanobacteria by using rRNA-internal transcribed spacer denaturing gradient gel electrophoresis. Appl Environ Microbiol 69:6634–6643
Jennings PH, Brannaman BL, Zoheille FP (1969) Peroxidase and polyphenoloxidase activity and associated with Helminthosporium leaf spot of maize. Phytopathology 5:963–967
Kantar F, Hafeez FY, Shivakumar BG, Sundaram SPN, Tejera A, Aslam A, Bano A, Raja P (2007) Chickpea: Rhizobium management and nitrogen fixation. In: Yadav SS, Redden RJ, Chen W, Sharma B (eds) Chickpea Breeding and Management. CAB International, Wallingford, pp 179–192
Karthikeyan N, Prasanna RL, Nain L, Kaushik BD (2007) Evaluating the potential of plant growth promoting cyanobacteria as inoculants for wheat. Eur J Soil Biol 43:23–30
Kaushik BD (2004) Use of blue-green algae and Azolla biofertilizers in rice cultivation and their influence on soil properties. In: Jain PC (ed) Microbiology and biotechnology for sustainable development. CBS Publishers and Distributors, New Delhi, pp 166–184
Kumar V, Kumar A, Verma VC, Gond SK, Kharwar RN (2007) Induction of defense enzymes in Pseudomonas fluorescens treated chickpea roots against Macrophomina phaseolina. Indian Phytopathol 60:289–295
Laranjo M, Young JPW, Oliveira S (2012) Multilocus sequence analysis reveals multiple symbiovars within Mesorhizobium species. Syst Appl Microbiol 35:359–367
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:329–342
Mandal BPL, Vlek PLG, Mandal LN (1999) Beneficial effects of blue-green algae and Azolla, excluding supplying nitrogen, on wetland rice fields: a review. Biol Fertil Soils 28:329–342
Manjunath M, Prasanna R, Sharma P, Nain L, Singh R (2011) Developing PGPR consortia using novel genera-Providencia and Alcaligenes along with cyanobacteria for wheat. Arch Agron Soil Sci 57:873–887
Martinez-Hidalgo P, Flores-Feliz JD, Menendez E, Rivas R, Carro L, Mateos PF, Martinez-Molina E, Leon-Barrios M, Velazquez E (2015) Cicer canariense, an endemic legume to the Canary Islands, is nodulated in mainland Spain by fast-growing strains from Symbiovar trifolii phylogenetically related to Rhizobium leguminosarum. Syst Appl Microbiol 38:346–350
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in Spinach chloroplasts. Plant Cell Physiol 22:867–880
Nayak S, Prasanna R, Pabby A, Dominic TK, Singh PK (2004) Effect of BGA-Azolla biofertilizers on nitrogen fixation and chlorophyll accumulation at different depths in soil cores. Biol Fertil Soils 40:7–72
Pii Y, Marastoni L, Springeth C, Fontanella MC, Beone GM, Cesco S, Mimmo T (2016) Modulation of Fe acquisition process by Azospirillum brasilense in cucumber plants. Environ Exp Bot 130:216–225
Prasad R, Shivay YS, Kumar D, Sharma SN (2006) Learning by doing exercises in soil fertility. Division of Agronomy, Indian Agricultural Research Institute, New Delhi
Prasanna R, Jaiswal P, Nayak S, Sood A, Kaushik BD (2009a) Cyanobacterial diversity in the rhizosphere of rice and its ecological significance. Indian J Microbiol 49:89–97
Prasanna R, Nain L, Ancha R, Jadhav S, Joshi M, Kaushik BD (2009b) Rhizosphere dynamics of inoculated cyanobacteria and their growth-promoting role in rice crop. Egypt J Biol 11:26–36
Prasanna R, Joshi M, Rana A, Shivay YS, Nain L (2012) Influence of co-inoculation of bacteria cyanobacteria on crop yield and C–N sequestration in soil under rice crop. World J Microbiol Biotechnol 28:1223–1235
Prasanna R, Chaudhary V, Gupta V, Babu S, Kumar A, Singh R, Shivay YS (2013a) Cyanobacteria mediated plant growth promotion and bioprotection against Fusarium wilt in tomato. Eur J Plant Pathol 136:337–353
Prasanna R, Kumar A, Babu S, Chawla G, Choudhary V, Singh S, Gupta V, Nain L, Sexana AK (2013b) Deciphering the biochemical spectrum of novel cyanobacterium-based biofilms for use as inoculants. Biol Agric Hortic 29:145–158
Prasanna R, Hossain F, Babu S, Bidyarani N, Adak A, Verma S, Shivay SY, Nain L (2015) Prospecting cyanobacterial formulations as growth-promoting agents for maize hybrids. S Afr J Plant Soil 32:199–207
Racchi ML (2013) Antioxidant defenses in plants with attention to Prunus and Citrus spp. Antioxidants 2:340–369
Rai AN, Bergman B, Rasmussen U (2002) Cyanobacteria in symbiosis. Kluwer Academic Publishers, Dordrecht
Rajendran G, Patel MH, Joshi SJ (2012) Isolation and characterization of nodule-associated Exiguobacterium sp. from the root nodules of Fenugreek (Trigonella foenum-graecum) and their possible role in plant growth promotion. Int J Microbiol. doi:10.1155/2012/693982
Rana A, Kabi SR, Verma S, Adak A, Pal M, Shivay YS, Prasanna R, Nain L (2015) Prospecting plant growth promoting bacteria and cyanobacteria as options for enrichment of macro and micronutrients in grains in rice-wheat cropping sequence. Cogent Food Agric. doi:10.1080/23311932.2015.1037379
Rouhrazi K, Khodakaramian G (2015) Phenotypic and genotypic diversity of root-nodulating bacteria isolated from chickpea (Cicer arietinum L.) in Iran. Ann Microbiol 65:2219–2227
Saini VK, Bhandarib SC, Tarafdar JC (2004) Comparison of crop yield, soil microbial C. N and P, N-fixation, nodulation and mycorrhizal infection in inoculated and non-inoculated sorghum and chickpea crops Field Crops Res 89:39–47
Saini R, Dudeja SS, Giri R, Kumar V (2015) Isolation, characterization, and evaluation of bacterial root and nodule endophytes from chickpea cultivated in Northern India. J Basic Microbiol 55:74–81
Sarr PS, Khouma M, Sene M, Guisse A, Badiane AN, Yamakawa T (2008) Effect of Pearl millet-cowpea cropping systems on nitrogen recovery, nitrogen use efficiency and biological fixation using 15N tracer technique. Soil Sci Plant Nutr 54:142–147
Sharma S, Gaur RK, Choudhary DK (2012) Phenetic and Functional Characterization of Endophytic Root-nodule Bacteria Isolated from Chickpea (Cicer arietinum L.) and Mothbean (Vigna aconitifolia L.) of Arid-and Semi-arid Regions of Rajasthan, India. Pak J Biol Sci 15:889–894
Sheoran A, Khurana AL, Dudeja SS (1997) Nodulation competitiveness in the Rhizobium-chickpea nodulation variants symbiosis. Microbiol Res 152:407–412
Singh G, Sekhon HS, Sharma P (2011) Effect of irrigation and biofertilizer on water use, nodulation, growth and yield of chickpea (Cicer arietinum L.). Arch Agron Soil Sci 57:715–726
Smith R (1992) Legume inoculant formulation and application. Can J Microbiol 38:485–492
Sturz AV, Christie BR, Nowak J (2000) Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci 19:1–30
Tagore GS, Namdeo SL, Sharma SK, Kumar N (2013) Effect of Rhizobium and phosphate solubilizing bacterial inoculants on symbiotic traits, nodule leghemoglobin, and yield of chickpea genotypes. Int J Agron. doi:10.1155/2013/581627
Venkataraman GS (1981) Cyanobacteria for rice production. FAO Soils Bulletin No. 46, Rome
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Younis ME, Hasaneen MNA, Tourky SMN (2009) Plant growth, metabolism and adaptation in relation to stress conditions. XXIV. Salinity biofertility interactive effects on proline, glycine and various antioxidants in Lactuca sativa. Plant Omics 2:197–205
Zgadzaj R, James EK, Kelly S, Kawaharada Y, de Jonge N, Jensen DB, Madsen LH, Radutoiu SA (2015) Legume genetic framework controls infection of nodules by symbiotic and endophytic bacteria. PLoS Genet 11:e1005280
Zhang JJ, Yua T, Loub K, Maoc PH, Wanga ET, Chena WF, Chen WX (2014) Genotypic alteration and competitive nodulation of Mesorhizobium muleiense against exotic chickpea rhizobia in alkaline soils. Syst Appl Microbiol 37:520–524
Acknowledgements
This investigation received support partially from the Indian Council of Agricultural Research (ICAR) funded Network Project on Microorganisms “Application of Microorganisms in Agricultural and Allied Sectors” (AMAAS), New Delhi to RP and SERB Project, funded by DST, Government of India to BR. The authors are also thankful to the Division of Microbiology, ICAR-IARI, New Delhi for access to the facilities to undertake this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state no conflicts of interest.
Additional information
Communicated by Djamel DRIDER.
Rights and permissions
About this article
Cite this article
Prasanna, R., Ramakrishnan, B., Simranjit, K. et al. Cyanobacterial and rhizobial inoculation modulates the plant physiological attributes and nodule microbial communities of chickpea. Arch Microbiol 199, 1311–1323 (2017). https://doi.org/10.1007/s00203-017-1405-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-017-1405-y