Abstract
Purpose
Chickpea is generally cultivated after seed treatment with host-specific Mesorhizobium ciceri, the nitrogen-fixing bacterium forming root nodules. Some species of free-living cyanobacteria are capable of nitrogen fixation. We examined the rhizosphere microbiota changes and the potential for plant growth promotion by applying a free-living, nitrogen-fixing cyanobacterium and the biofilm formulation of cyanobacterium with M. ciceri, relative to M. ciceri applied singly, to two each of desi and kabuli varieties of chickpea.
Materials and methods
Denaturing gradient gel electrophoresis (DGGE) profiles of archaeal, bacterial and cyanobacterial communities and those of phospholipid fatty acids (PLFAs) were obtained to evaluate the changes of the microbial communities in the chickpea rhizosphere. Plant growth attributes, including the pod yields and the availabilities of soil macronutrients and micronutrients, were monitored.
Results and discussion
The DGGE profiles showed distinct and characteristic changes due to the microbial inoculation; varietal differences exerted a marked influence on the archaeal and cyanobacterial communities. However, bacterial communities were modulated more by the type of microbial inoculants. Abundance of Gram-negative bacteria (in terms of notional PLFAs) differed between the desi and the kabuli varieties inoculated with M. ciceri alone, and the principal component analysis of PLFA profiles confirmed the characteristic effect of microbial inoculants tested. Microbial inoculation led to increases in the 100-seed weight and differential effects on the concentrations of available nitrogen and phosphorus, and those of iron, zinc and copper, suggesting their increased cycling in the rhizosphere.
Conclusions
Microbial inoculation of chickpea brought out the characteristic changes in rhizosphere microbiota. Consequently, the growth promotion of chickpea and nutrient cycling in its rhizosphere distinctively differed. Further studies are needed to analyse the association and dynamic changes in the microbial communities to define the subset of microorganisms selected by chickpea in its rhizosphere and the influence of microbial inoculation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Legumes are the second most agronomically important crop, next only to cereals in the developing countries. Annually, India, China and Myanmar contribute about 25, 10 and 4%, respectively, to the global legume pulses production (Tagore et al. 2013). Among the legume crops, chickpea, a poor man’s legume crop, is cultivated in 12 million ha, with an average productivity of 911 kg ha−1 in about 50 countries (FAOSTAT 2010). Often, it is cultivated on marginal lands without any chemical fertilizer application. Nevertheless, its high protein concentration of 19 to 25% makes this legume crop an important diet source for humans and animals (FAOSTAT 2010; Gaur et al. 2015). The ability to fix atmospheric nitrogen is an important factor for its high protein concentration and also its ecological role in sustaining the cropping system productivity. Chickpea gets a substantial portion (4–85%) of its N requirement through symbiotic nitrogen fixation. The supply of nitrogen from their nodules influences the concentration of chlorophyll pigments, the amino acids and the amount of protein. The ineffective nodules and consequently the poor N supply can result in the growth rate reduction, chlorosis, an early onset of senescence in leaves and even low yield.
Chickpea is considered to have a highly specific association with a group of rhizobia that forms nodules and fixes the atmospheric nitrogen. Problems in nodule formation are due to the absence of suitable rhizobial strains or their small population size or their poor survival (Kantar et al. 2007). Most rhizobial species that have the ability to form a symbiotic association with legumes are traditionally considered belonging to one of the six genera (Mesorhizobium ciceri, Allorhizobium ciceri, Ensifer (Sinorhizobium ciceri), Mesorhizobium ciceri, Bradyrhizobium ciceri and Azorhizobium ciceri) of the subclass α-proteobacteria. Among these rhizobia, Mesorhizobium ciceri and Mesorhizobium mediterraneum are often considered to be very specific to chickpea. But, recent studies suggest the presence of several other rhizobia such as Mesorhizobium amorphae, Mesorhizobium tianshanense, Mesorhizobium loti, Sinorhizobium ciceri medicae, Sinorhizobium meliloti and other bacteria such as Agrobacterium tumefaciens in the nodules of chickpea (Romdhane et al. 2007; Rouhrazi and Khodakaramian 2015). Moreover, the rhizobial symbiosis due to the members of other subclass β-proteobacteria, especially by the strains of Burkholderia caribensis and Ralstonia taiwanensis, is found to be widespread in legumes (Moulin et al. 2001; Chen et al. 2003). The rhizosphere as the dynamic ecological niche for various types of soil flora and fauna is intimately related to both the soil fertility and the efficient production of crops (Vessey 2003; Zhou et al. 2015). Elkoca et al. (2007) reported that the chickpea seed inoculation with Mesorhizobium ciceri, Bacillus subtilis (OSU-142) and Bacillus megaterium (M-3) in dual and triple combinations could be an alternative to the nitrogen and phosphorus fertilizers. For the successful colonization of roots by microorganisms with beneficial traits, competition exists between the plants and the other native microorganisms and between the beneficial microorganisms and their competitors.
Cyanobacteria are a group of unique prokaryotic organisms with the ability to perform mutually compatible functions like nitrogen fixation and photosynthesis. They are ecologically important photosynthetic organisms with a wide range of nutritional capabilities ranging from obligate phototrophy to heterotrophy (Prasanna et al. 2009). Cyanobacteria as bioinoculants help to increase the availability of phosphates accumulated in the soil, besides enhancing plant growth by increasing the efficiency of biological nitrogen fixation and the availability of Fe and zinc (Zn) through the production of plant growth-promoting substances (Elkoca et al. 2007). Biofilm formation is known to be a dynamic and fine-tuned orchestrated process, involving quorum sensing and signalling that lead to successful attachment and colonization, as observed in several cyanobacteria. In maize, rice, wheat and cotton fields, beneficial biofilmed bioinoculants, comprising fungi, cyanobacteria and/or bacteria were found to grow attached to the plant roots of crops, facilitate the cycling of nutrients and also aid in the biocontrol of pests and disease, thereby improving productivity (Babu et al. 2015; Prasanna et al. 2012, 2015a, b, 2016). Microbial inoculation contributes to rapid colonization of the host rhizosphere through the changes in the microbial community diversity (Kennedy and Tchan 1992). Here, we tested this hypothesis by examining the changes in the microbial community structure in the chickpea rhizospheres, due to the application of a single free-living, nitrogen-fixing and plant growth-promoting cyanobacterium, a biofilmed combination of cyanobacterium-Mesorhizobium ciceri and efficient Mesorhizobium ciceri to two each of desi and kabuli varieties. Further, we ascertained the relationship between plant growth and soil functioning due to the application of these inoculants.
2 Experimental procedures
2.1 Organisms used in this study and their maintenance
The cyanobacterial strains of Anabaena laxa (RPAN8) and A. torulosa and the bacterial strain Mesorhizobium ciceri (routinely used inoculant for chickpea) were collected from the germplasm of the Division of Microbiology, Indian Council of Agricultural Research (ICAR)-Indian Agricultural Research Institute (IARI), New Delhi. The cyanobacterial cultures were grown using the BG-11 medium, while the Mesorhizobium ciceri culture was maintained using the yeast mannitol agar and incubated for 48 h at 30 ± 2 °C. The flasks containing cyanobacterial cultures were grown at 27 ± 2 °C, with white light (50–55 μmol photons m−2 s−1) in Haffkine flasks.
2.2 Preparation of formulations
The biofilmed bioinoculant using A. torulosa and M. ciceri was developed and characterized as reported earlier (Prasanna et al. 2014). Paddy straw compost was used after amendment with vermiculite (1:1) as the carrier. For the cyanobacterial cultures and its biofilm, the chlorophyll content was measured according to the method of MacKinney (1941) and maintained as 100 μg g−1 carrier; the bacterial partners were about 107 to 108 cfu in the biofilm. The water holding capacity of the carrier was maintained at 60% after amendment with cultures and a suitable amount of water.
2.3 Chickpea field experiment
The field experiment was conducted at the research farm of ICAR-IARI, New Delhi, during the rabi season of 2014. The geographical details are latitude 28° 37′–28° 39′ N and longitude 77° 9′–77° 11′ E, altitude 228.6 m above the mean sea level (Arabian Sea). The mean annual rainfall is 650 mm, out of which, more than 80% generally occurs during the southwest monsoon season (July–September), with the mean annual evaporation of 850 mm. The sandy clay loam soil of alluvial origin (Typic Haplustepts) with pH of 7.5 (1:2.5 soil/water ratio) had 225 kg ha−1 alkaline permanganate oxidizable nitrogen, 16.0 kg ha−1 available phosphorus, 275 kg ha−1 ammonium acetate exchangeable potassium and 0.53% organic carbon as estimated by standard protocols using the soil samples collected at 0–30 cm depth (Prasad et al. 2006). The desi (BG372 and BG 256) and kabuli (BG1003 and BG1053) varieties of chickpea were used. The microbial inoculants such as Anabaena torulosa-M. ciceri (biofilmed biofertilizer), A. laxa (cyanobacterial inoculant) and M. ciceri (IARI Mesorhizobium ciceri inoculant) were used to treat the seeds along with sticker (1% carboxymethyl cellulose); the inoculated seeds were air dried for an hour before sowing. The seeds for the control treatment were treated only with carrier and sticker, air dried and sown. The seeds were planted at a row spacing of 45 cm with 70 kg seed rate ha−1. The experiment was studied in split-plot design with three replications.
2.3.1 Rhizosphere soil sampling
The rhizosphere soil samples (0–30 cm depth) were collected after 90 days of plant growth from roots of five randomly selected plants. The plants were removed from the plots by carefully slicing the soil layers using a chrome-plated shovel. The roots were shaken vigorously, and firmly adhering soils were collected for each composite rhizosphere soil sample. The fresh rhizosphere soil samples were used the extraction of the lipid fractions. Another set was stored at −80 °C for soil DNA extraction.
2.3.2 PCR-DGGE profiling of archaeal, bacterial and cyanobacterial communities
Total soil DNA of the composite rhizosphere soil samples was extracted using PowerSoil DNA Isolation Kit (MoBio Inc., USA) as per the instructions of the manufacturer. The PCR amplification using specific primers for archaea (ARC344F attached with a 40-bp GC clamp and 517; Bano et al. 2004), bacteria (F984GC and R1378; Heuer et al. 1997) and cyanobacteria [(GC-)CSIF and 373R; Janse et al. 2003)] was done according to the method of Muyzer et al. (1993). The reaction mixture of 25 μL contained 1× Taq buffer, 2.5 mM MgCl2, 0.3 mM, each of the deoxynucleotide triphosphate, 10 pmol of each primer, 1 U Taq DNA polymerase, 50 ng of template DNA and MilliQ to give final volume. The amplification was carried out in a Peqlab Primus 96 Thermal cycler, following the protocols described in Table 1. The PCR-amplified products were examined in a 1.2% (w/v) agarose gel using the mass ladders (Fermentas) and then quantified using Invitrogen Quant-iT dsDNA Assay Kit (Eugene, Oregon, USA) in a NanoDrop 3300 fluorospectrometer (Thermo Scientific, Waltham, Massachusetts, USA).
The PCR-denaturing gradient gel electrophoresis (DGGE) profiling was done using 6% polyacrylamide gel in a Bio-Rad DCode™ Universal Mutation Detection System (Bio-Rad, Hercules, CA). The gel of 1 mm thick was cast using acrylamide/N,N-methylene bisacrylamide (40%) and formamide (40% v/v), 7 M urea and 1× TAE. The denaturing gradient gel was run for 16 h at 60 °C and 38 V. The gels that were stained with an ethidium bromide solution (0.5 mg mL−1) were viewed in AlphaImager 1220 (Alpha Innotech Corporation, California). The gel images were analysed using GeneTools version 1.4.1.0 (Syngene, Cambridge, United Kingdom); the dendrograms after the cluster analysis were constructed using the unweighted pair-group agglomeration methods with arithmetic average (UPGMA) with Dice coefficient.
2.3.3 Analysis of PLFAs for characterizing microbial communities
The fresh soil samples (5 g) were used to extract the lipid fractions according to Buyer et al. (2010). The modified Bligh-Dyer extraction procedure was followed with a nitrogen stream for evaporation. The lipid fractions were separated on a solid-phase extraction column, eluted with methanol and evaporated under nitrogen. Trans-esterified phospholipids as fatty acid methyl esters were extracted with hexane, evaporated and analysed by gas chromatography with the MIS Sherlock (MIDI, Inc., Newark, DE, USA). The separation of fatty acid methyl esters (FAMEs) was done using the column of a 25-m long × 0.2 mm internal diameter × 0.33 μm film thickness; the oven temperature was 190 °C initially, with ramping to 285 °C at the rate of 10 °C min−1 and then to 310 °C at 60 °C min−1, followed by a hold at 310 °C for 2 min. The injector temperature was 250 °C while the FID was at 300 °C. Methyl nonadecanoate was used as an internal standard for the calculation of FAME concentrations, expressed as nanomole per gram of soil. The notional ‘microbial groups’ as suggested by Frostegård and Bååth (1996), Zelles (1999) and Ringelberg et al. (1997) were characterized in these soils. The concentrations and types of phospholipid fatty acids (PLFAs) in these soils were ordinated using principal component analysis, based on correlation to explore the variability among the treatments.
2.4 Plant growth and yield attributes
Plant growth and yield parameters such as the plant height, the number of primary and secondary branches per plant, the number of pods per plant, the number of seeds per plant (seed yield per plant) and plot (seed yield per plot) and the 100-grain weight were recorded at the stage of harvest. The total nitrogen content (%) was determined, and the contents of crude protein in seeds were estimated by the Snell and Snell method (1949), using the Nessler’s reagent. The contents of crude protein were calculated using a factor of 6.25 with the concentrations of nitrogen.
2.5 Soil nutrient concentrations
The concentrations of available nitrogen were estimated by the Subbiah and Asija method (1956). The soil samples were distilled with alkaline potassium permanganate solution, and the concentrations of liberated ammonium served as an index of the available nitrogen status. The method of Olsen et al. (1954) was employed to determine the concentrations of available phosphorus (P) by recording the intensity of blue colour at 600 nm. The concentrations of soluble iron (Fe), Copper (Cu) and zinc (Zn) were measured after extraction of soil with 0.005 M diethyl triamine penta-acetic acid (DTPA) and 0.1 M triethanol amine (TEA) and 0.01 M CaCl2 buffered to pH 7.3 with dilute hydrochloric acid (Lindsay and Norvell 1978). Data were recorded using an atomic absorption spectrophotometer (Optima 3000 Perkin-Elmer).
2.6 Statistical analysis
Data for the various parameters were analysed using WINDOW STAT (version 8.0) and XL STAT (version 2014.5.3) statistical packages. The community pattern analyses of archaea, bacteria and cyanobacteria were done using the representative gels of each microbial group, which had consistent profile patterns. Presence or absence of distinct and reproducible bands in each of the individual profiles was converted into binary data using the GeneTools software. The pooled binary data were used to construct a composites dendrogram using the UPGMA trees using Dice coefficient. The principal component analysis (PCA) of the Pearson (n) type for all the PLFAs quantified above the detection level in triplicate samples was performed using XL STAT.
3 Results
3.1 DGGE profiles of chickpea rhizosphere due to microbial inoculation
The DGGE profiles of archaeal 16S ribosomal RNA (rRNA) gene fragments in the rhizospheres of chickpea formed five distinct clusters (Fig. 1 and Fig. S1, Electronic Supplementary Material). The influences of inoculation with A. laxa alone, M. ciceri alone and A. torulosa + M. ciceri were more on desi varieties (cv. BG256 and cv. BG372), albeit their effects on cv. BG1003. Along with the uninoculated controls of cv. BG256 and cv. BG1003, these two varieties had similar DGGE archaeal profiles due to microbial inoculation. The kabuli variety BG1053 had distinctly different profiles due to inoculation with A. laxa alone and with M. ciceri alone. The profiles of cv. BG372 and BG1053 inoculated with M. ciceri and A. laxa clustered together. In general, the desi and kabuli varieties had distinct influences on the archaeal communities, evident from the DGGE profiles.
There were four separate clusters among the DGGE bacterial 16S rRNA profiles of chickpea rhizospheres, with or without inoculation (Fig. 2 and Fig. S2, Electronic Supplementary Material). The clusters did not delineate the effect of different microbial inoculants or the influences of desi or kabuli varieties. The DGGE cyanobacterial profiles of chickpea rhizospheres showed six different clusters (Fig. 3 and Fig. S3, Electronic Supplementary Material) due to biofilmed A. torulosa-M. ciceri and A. laxa inoculants and desi and kabuli varieties; the effect was more due to the latter than the former. The DGGE cyanobacterial profiles of cv. BG372 without inoculation and with the biofilmed A. torulosa-M. ciceri formed a distinct cluster. Cyanobacterial inoculants, either alone or biofilmed with M. ciceri, showed characteristic influences on certain varieties such as cv. BG1053 and cv. BG256 and BG372.
3.2 PLFA profiling of rhizosphere soils of chickpea without or with inoculation
The peaks of fatty acids having area/height ratios of greater than 0.017 and less than 0.070 were identified and were used to discriminate the microbial communities in the chickpea rhizosphere. The concentrations of total PLFAs ranged from 154 to 436 nmol g−1, and the application of rhizobial inoculant alone led to higher concentrations (396–436 nmol g−1) in both the desi and the kabuli varieties of chickpea (Table 2). The seed inoculation with A. laxa alone and biofilmed A. torulosa-M. ciceri led to increased concentrations of total PLFA, compared to the uninoculated control. The absolute concentrations of straight fatty acids were more due to the inoculation with M. ciceri alone and biofilmed A. torulosa-M. ciceri. Nevertheless, the percentage of straight fatty acids to total PLFAs declined to the levels of 13–18% in the rhizosphere of chickpea inoculated with M. ciceri, while it was between 16 and 26% in other chickpea rhizospheres. The branched fatty acids constituted about 8.6 to 10.7%, while the monounsaturated fatty acids (MUFAs) were about 3 to 5.3% in the chickpea rhizospheres. The inoculation with M. ciceri alone distinctly influenced both the branched and the monounsaturated fatty acids. The proportional representation of polyunsaturated fatty acids (PUFAs) was more than that of all other fatty acids. The effect of desi and kabuli varieties was distinct within the respective treatments, except the uninoculated control. The concentrations of dimethyl acetal (DMA) were relatively lower compared to the straight, branched, monounsaturated and polyunsaturated fatty acids. The concentrations of both 18:1 ω9c and 18:2 ω6,9c increased several folds (more than 10) due to the inoculation with M. ciceri alone.
The abundance of the notional microbial groups was estimated from the concentrations of biomarker fatty acids (Table 3). Gram-positive bacteria constituted about 11–17% of the total microbial groups. The absolute values of fatty acids representing Gram-positive bacteria were more in the rhizospheres of chickpea inoculated with M. ciceri. But its proportional representation declined relative bacteria to that of control plants. The abundance of Gram-negative increased and was distinctly different between the desi (31.5–32.4%) and kabuli (33.5–35.2% of the total PLFAs) varieties inoculated with M. ciceri alone. Increases in the Gram-negative bacterial composition were about fourfolds compared to that of control. The biofilmed A. torulosa-M. ciceri also increased the abundance of Gram-negative bacteria more than the application of A. laxa, except in the rhizosphere of cv. BG1053. Though the proportional representation by anaerobes did not vary among the treatments, the absolute concentrations of fatty acids signifying anaerobes clearly increased due to the inoculation with M. ciceri alone. Similarly, the abundances of fungi and eukaryotes in terms of absolute concentrations of PLFAs were more due to the inoculation with M. ciceri alone as well as the biofilmed A. torulosa-M. ciceri. Interestingly, there were marginal increases in the proportional representations of fungi but decreases in eukaryotes due to the inoculation of M. ciceri.
The data of PLFAs extracted from the rhizospheres of chickpea with or without microbial inoculation were used for the principal component analysis to discriminate the effects of different inoculants. The first two principal components accounted for 50.24% of the total variances (Fig. 4). Interestingly, there was a distinct effect of M. ciceri inoculant alone on both the desi and the kabuli varieties of chickpea. The biofilmed inoculant had a lesser effect on cv. BG1053 since both the PLFA patterns of control and biofilmed A. torulosa-M. ciceri inoculant treatments clustered together. The PLFA patterns of desi varieties (cv. BG256 and cv. BG372) were highly similar. The biofilmed A. torulosa-M. ciceri had relatively comparable influences on these two desi varieties. Inoculation with A. laxa had a distinctly different effect on desi varieties, while the influences were comparable on the kabuli varieties.
3.3 Growth parameters and yield of chickpea
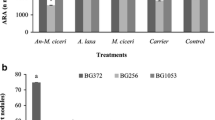
Plant height of both desi and kabuli varieties was influenced by the inoculants such as A. laxa and M. ciceri, albeit with some differences in the tested varieties (Table 4). In general, the kabuli varieties cv. BG1003 and cv. BG 1053 responded better to microbial inoculation. The number of primary, secondary and tertiary branches was more due to the biofilmed A. torulosa-M. ciceri except that of cv. BG1053 control (without inoculation) which had about 11 secondary branches. The number of pods in cv. BG372 without inoculation (20 per plant) was the least, while it was highest in cv. BG256 inoculated with M. ciceri (70 per plant). The seed yield per plant ranged from 1.5 to 4.5 g, and the seed yield per plot was highest in cv. BG1053 (1203 g), followed by cv. BG372 (1026 g) when both were inoculated with M. ciceri. There were no appreciable increases in seed yield of desi varieties due to inoculation with A. torulosa + M. ciceri, compared to the respective uninoculated controls. The 100-seed weight of chickpea varieties tested ranged from 22.9 to 46.3 g (Fig. 5). While the highest was by cv. BG1053 inoculated with A. laxa alone, the least was by cv. BG256 inoculated with biofilmed A. torulosa-M. ciceri. The contents of crude proteins were between 13.6 and 29.17%. Inoculation with M. ciceri invariably led to increased contents of crude proteins in these varieties. Only two cultivars, i.e., cv. BG256 and cv. BG1003 responded to the inoculation with the biofilmed A. torulosa-M. ciceri or A. laxa alone with increased contents of crude proteins. Other two varieties showed decreased contents of crude proteins due to these inoculants. Though there were increases in the 100-seed weight of cv. BG372 inoculated with biofilmed A. torulosa-M. ciceri, and the A. laxa inoculated cv. BG1053 and BG1003, the contents of crude proteins were relatively lower (Fig. 5).
3.4 Soil nutrient availability in chickpea rhizosphere due to microbial inoculation
The biofilmed A. torulosa + M. ciceri improved the concentrations of available nitrogen in both desi and kabuli varieties tested (144–163 kg N ha−1 soil) (Table 5). Inoculation with A. laxa alone increased it significantly in the rhizospheres of cv. BG372 and BG1003 (194 kg N ha−1). The concentrations of available nitrogen increased due to inoculation with M. ciceri alone, albeit marginally in cv. BG256 and cv. BG1003, compared to the respective uninoculated controls. The differential effects of these inoculants were observed with the concentrations of available phosphorus, which ranged from 14.9 to 32.9 kg ha−1 soil. Inoculation with M. ciceri increased the available phosphorus concentrations in desi cv. BG372 and kabuli cv. BG1003 and BG1053. In contrast, the concentrations of iron which ranged from 0.94 to 2.51 mg kg−1 decreased due to inoculation with M. ciceri. Similar declines in the concentrations of zinc were observed due to microbial inoculation, more so with M. ciceri alone. The desi variety cv. BG372 was found to be most responsive to microbial inoculation, in terms of increased zinc and copper availability in the rhizosphere soil (Table 5). The A. laxa inoculant brought about a distinct enhancement in the availabilities of Zn and Cu in the rhizosphere soil, as compared to other inoculants. The concentrations of copper which ranged from 0.67 to 3.41 mg kg−1 marginally increased due to inoculants, as evidenced in cv. BG1053 (kabuli)
4 Discussion
4.1 Microbial communities in the chickpea rhizosphere
Typical association of chickpea with different species of Mesorhizobium ciceri has been well reported using the culture media-based methods (Nour et al. 1994; Kim et al. 2014; Esfahani et al. 2016). In the present study, we used the PCR-DGGE profiles to characterize the changes of the archaeal, bacterial and cyanobacterial communities in the chickpea rhizosphere. The changes in the archaeal communities due to microbial inoculants were evident from the DGGE archaeal profiles of both desi and kabuli types. The kabuli variety BG1053 had distinctly different archaeal communities due to inoculation with A. laxa alone and with M. ciceri alone. Though archaeal members are commonly found in soils, the first report on the presence of nonthermophilic members of Crenarchaeota, by their cultivation in enrichment cultures, in the roots of tomatoes was illustrated by Simon et al. (2005). What is interesting in the results of PCR-DGGE profiling in the present study is that microbial inoculants of chickpea altered the archaeal community structure in its rhizosphere, suggesting their probable associations with the growth promotion of chickpea plants. Both desi and kabuli varieties showed distinct influences on archaeal communities, and hence, the observed modulation of soil archaeal communities as a result of inoculation with different microbial inoculants was inclusive of varietal effects.
The bacterial DGGE profile of cv. BG1053 with M. ciceri was found to be distinctly different from other profiles. Though there are suggestions about species-specific development of bacterial communities (Marschner et al. 2004; Trabelsi et al. 2011), alterations due to different microbial inoculants were clearly evident in the present study. The varietal specific effect of desi and kabuli types could not be delineated, partly because of the limited number of varieties tested, but the influences of microbial inoculants tested were strongly evident from their DGGE bacterial profiles. However, the genotype effect of chickpea on the microbiota in its rhizosphere may require expensive sequencing-based methods to discern the differences in their influences on, not only predominant but also other members of importance.
Cyanobacterium-based microbial inoculants have been examined for their plant growth promotion effect in chickpea earlier (Svitlana 2013; Burjus et al. 2014). In the present study, we tested the cyanobacterium-based inoculants to discern the effect of these inoculants on the native microbial community structures. The cyanobacterial inoculants, either alone or biofilmed with M. ciceri, characteristically influenced the profiles of cv. BG1053 and desi cv. BG256 and BG372. The differences in the cyanobacterial communities were not significantly different between the desi and the kabuli varieties. This could be probably because of the inherent difficulties in the PCR-DGGE method wherein the number and intensities of phylotypes as bands are only considered for comparison. Cyanobacteria are an important member of the core microbiome of Arabidopsis thaliana (Lundberg et al. 2012). Chaparro et al. (2014) reported that cyanobacteria along with other phyla such as Acidobacteria, Actinobacteria and Bacteroidetes formed distinct patterns at four different development stages in Arabidopsis. The results of the present study provide evidence regarding the distinct influences of desi and kabuli varieties on the archaeal and cyanobacterial communities and that of microbial inoculants in the bacterial DGGE communities in the chickpea rhizospheres. We analysed the PLFA profiles of the chickpea rhizosphere soils, and the increases in the total concentrations of PLFA suggested the positive, significant effect of microbial inoculation on the active microbial biomass contents (Table 1). The notional microbial groups identified from the biomarker PLFAs (Frostegard and Baath 1996; Ringelberg et al. 1997; Zelles 1999) showed the higher abundance of Gram-negative bacteria due to the inoculation with M. ciceri alone. It is likely that the early colonization of Gram-negative M. ciceri in the roots had led to either its own enrichment or enrichment of other Gram-negative bacterial members. It can also be surmised that the shift from a Gram-positive-dominated community to Gram-negative populations is indicative of a progressive change from oligotrophic to more copiotrophic conditions, as a result of inoculation with aggressive inoculants, which compete with the native flora for the nutrients released from dead cells (Yao et al. 2000). The PLFAs of eukaryotes, fungi and anaerobes were also abundant in the rhizospheres of both desi and kabuli chickpea types due to the inoculation with M. ciceri alone.
In the present study, the biplot of PLFA profiles showed the distinctive effect of M. ciceri inoculant on the chickpea rhizospheres (Fig. 4). This provides a strong evidence of M. ciceri altering the rhizosphere microbial communities, besides its capability to form the nodules in chickpea. Earlier studies by Schweiger and Tebbe (2000) and Roesti et al. (2006) in alfalfa and Phaseolus vulgaris, respectively, showed distinct shifts in the α- and γ-proteobacterial communities as a result of rhizobial inoculation. The variations among the desi and kabuli types on the PLFA profiles were noted, albeit with few exceptions, due to the microbial inoculants tested. Probably, the abilities of M. ciceri to influence the rhizosphere microbial communities and to form nodules determine the successful symbiotic relationship with a particular genotype of chickpea. Since the PLFA-based techniques provide quantitative information on the composition of certain notional, microbial groups, compared to the DNA-based fingerprinting methods such as DGGE method (Ramsey et al. 2006), the specific influences of microbial groups such as Gram-negative bacteria were clearly evident in the present study.
Plants and microorganisms are known to have significant influences on the rhizosphere microbial communities. Earlier reports suggest the development of specific rhizosphere microbial communities in relation to soil type, nutrition and plant species (Marschner et al. 2004; Berg and Smalla 2009). Ellouze et al. (2013) demonstrated that chickpea genotypes not only determined the soil microbiome but affected the subsequent durum wheat crop. What is interesting in the present study is that the rhizosphere microbial communities are greatly influenced by the microbial inoculants that were used to treat the seeds before sowing. Their effects were persistent late in the developmental stages of chickpea. This also provides ample evidence to the rhizosphere microbiome engineering mediated by the seed treatment with microbial inoculants.
4.2 Influences on growth and yield of chickpea due to microbial inoculants
The plant rhizosphere is a dynamic ecological niche for various types of soil microflora/macroflora and fauna due to the availability of nutrients, which in turn is intimately related to soil fertility and successful production of crops (Vessey 2003). In the present study, the influence of microbial inoculants on plant height was variety specific and impacted the number of primary branches and the secondary branches to a minimal extent. But, the microbial inoculants characteristically influenced the number of pods, the seed yield per plant and the seed yield per plot. The response of cv. BG372 and cv. BG1053 to the microbial inoculant M. ciceri in terms of seed yield per plot was better than that of cv. BG256 and cv. BG1003. The number of pods in cv. BG372 enhanced by twofolds through microbial inoculation. Inoculation with Mesorhizobium ciceri is often found to increase the number of nodules and total N concentration (Elkoca et al. 2015). Namwar et al. (2011) documented that the highest plant height, number of primary and secondary branches, number of pods per plant and number of grains per plant were obtained from the highest level of nitrogen fertilizer (100 kg urea ha−1) and Mesorhizobium ciceri inoculation. The application of 75 and 100 kg urea ha−1 showed no significant difference in these traits. Often, yield increases obtained in the treated plants were attributed to the production of plant growth promoting substances by rhizobacteria (Kennedy and Tchan 1992). In our investigation, also, the inoculation with M. ciceri recorded higher pod yield per plant than that of other inoculants and brought about twofold–sixfold enhancement in seed yield/plot in cv. BG372 and BG1053. Earlier, Rokhadi et al. (2011) showed that the combined inoculation with Azospirillum spp. + Azotobacter chroococcum 5 + Mesorhizobium ciceri SWR17 + Pseudomonas fluorescens P21 led to the maximum dry weight of root nodules. Recently, Imran et al. (2015) showed that the inoculation with the indole acetic acid producing Ochrobactrum ciceri alone or co-inoculated with M. ciceri led to increases in nodules, biomass, grain yield and harvest index in both the desi and the kabuli genotypes in two different soil types. Singh et al. (2015) observed that plant growth exhibited a linear relationship with inoculation of rhizospheric bacterial communities possessing increasing levels of species or plant growth promoting trait diversity. However, in the present study, single inoculants were as effective as the biofilmed combination.
Both the cyanobacterial inoculations such as A. laxa and the biofilmed A. torulosa-M.ciceri increased the 100-seed weight, which was higher than that of M. ciceri or uninoculated control. Our results suggest that the choice of microbial inoculants can determine the quality of seeds produced in chickpea. Despite the increases in the 100-seed weight of cv. BG372 inoculated with biofilmed A. torulosa-M. ciceri, and the A. laxa inoculated cv. BG1053 and BG1003, the contents of crude proteins declined relatively (Fig. 5). The differential effect of inoculants on chickpea growth and yield was reported earlier by Mirza et al. (2007). The combination of the Mesorhizobium ciceri strain Rn1 with Enterobacter strains A and B was effective for cv. Parbat and NIFA88 while that of Mesorhizobium ciceri strain Rr2 and Enterobacter strain B was ineffective for cv. Parbat.
Available soil nitrogen and soil pH are important determinants of the distribution of different rhizobial species in soils (Van Cauwenberghe et al. 2015). The external application of chemical N influences the soil microbial community diversity due to nitrogen being the major nutrient to microorganisms and also indirectly altered plant and soil properties (Zhang et al. 2015). In the present study, the application of M. ciceri, A. laxa and the biofilmed A. torulosa + M. ciceri increased the available nitrogen in the rhizospheres. But, M. ciceri alone lowered the concentrations of iron. The leghaemoglobin, a haemoprotein (iron containing), is critical to the nitrogenase function in nodules (Virtanen et al. 1947). Lower levels of iron in the rhizosphere indicated their efficient uptake by chickpea plants inoculated with M. ciceri. In an earlier report, Rokhzadi and Toashih (2011) observed that the combination of Azospirillum + Azotobacter significantly improved the concentration of phosphorus in shoots. The potential of microorganisms in improving soil fertility or crop productivity has not been successful to the desired extent because of the oligotrophic nature of soils. Plants are the natural hot spots of microbial activities. Though a number of formulations are available in the market, their spectrum of activity with respect to time and crops is limited (Adesemoye and Kloepper 2009). Cyanobacteria are known to possess the ability to form associations with vascular/nonvascular plants and produce growth-promoting substances (Prasanna et al. 2009). Our earlier studies illustrated the potential of biofilmed cyanobacterial inoculants for plant growth promotion in rice, wheat, maize, tomato, cotton and several other crops (Prasanna et al. 2012, 2014, 2015b). The sequestering of iron and zinc by cyanobacteria using metallothioneins is well investigated (Barnett et al. 2012), and cyanobacterial biofilmed inoculants were found to enhance the mobilization of micronutrients, such as zinc to grains in rice (Adak et al. 2016; Prasanna et al. 2015a). The earlier report on the biofilms of Bradyrhizobium ciceri-Penicillium suggested their stimulatory effect on nodulation in soybean (Jayasinghearchchi and Seneviratne 2004). Thus, the selection of microbial inoculants, individually or combinations as biofilms, has to be based on the response of a chickpea genotype in a particular soil.
5 Conclusions
Although a significant contribution to the plant growth promotion in general and nitrogen nutrition in particular is made by the rhizobia associated with their nodules and the rhizospheric microorganisms, this may also be related to the ability of chickpea-specific nodulating rhizobia such as M. ciceri that alter the rhizosphere microbial community structure. In the present investigation, similar influences of cyanobacterial inoculants such as the A. laxa and the biofilmed A. torulosa-M. ciceri which resulted in a significant increment in plant growth and soil nutrient availability suggest that these inoculants interact not only with the host plants but also with other microorganisms in the rhizosphere. Besides, the genotype of chickpea selected for the cultivation, either desi or kabuli varieties, was found to be an important determinant for the choice of microbial inoculants. Future research should focus on influences of microbial inoculation, especially along with Mesorhizobium ciceri recommended inoculant, on the soil and plant microbiomes of chickpea in many growing areas.
References
Adak A, Prasanna R, Babu S, Bidyarani N, Verma S, Pal M, Shivay YS, Nain L (2016) Micronutrient enrichment mediated by plant-microbe interactions and rice cultivation practices. J Plant Nutr 39:1216–1232
Adesemoye AO, Kloepper JW (2009) Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol 85:1–12
Babu S, Bidyarani N, Chopra P, Monga D, Kumar R, Prasanna R, Kranthi S, Adak A, Saxena AK (2015) Evaluating microbe-plant interactions and varietal differences for enhancing biocontrol efficacy in root rot challenged cotton crop. Eur J Plant Pathol 142: 345-362
Bano N, Ruffin S, Ransom B, Hollibaugh JT (2004) Phylogenetic composition of arctic ocean archaeal assemblages and comparison with antarctic assemblages. Appl Environ Microbiol 70:781–789
Barea J-M, Pozo MJ, Azcon R, Concepcion A-A (2005) Microbial co-operation in the rhizosphere. J Expl Bot 56:1761–1778
Barnett JP, Millard A, Ksibe AZ, Scanlan DJ, Schmid R, Blindaue CA (2012) Mining genomes of marine cyanobacteria for elements of zinc homeostasis. Front Microbiol 3:142–147
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13
Burjus SJ, Jawad ALM, Al-Ani NK (2014) Effect of two species of cyanobacteria as biofertilizers on characteristics and yield of chickpea plant. Iraqi J Sci 55:685–696
Buyer JS, Teasdale JR, Roberts DP, Zasada IA, Maul JE (2010) Factors affecting soil microbial community structure in tomato cropping systems. Soil Biol Biochem 42:831–841
Chaparro JM, Badri DV, Vivanco JM (2014) Rhizosphere microbiome assemblage is affected by plant development. The ISME J 8:790–803
Chen W-M, Moulin L, Bontemps C, Vandamme P, Béna G, Boivin-Masson C (2003) Legume symbiotic nitrogen fixation by beta-proteobacteria is widespread in nature. J Bacteriol 185:7266–7272
Elkoca E, Kantar F, Sahin F (2007) Influence of nitrogen fixing and phosphorus solubilizing bacteria on the nodulation, plant growth, and yield of chickpea. J Plant Nutr 31:157–171
Elkoca E, Kocli T, Gunes A, Turan M (2015) The symbiotic performance and plant nutrient uptake of certain nationally registered chickpea ( Cicer arietinum L.) cultivars of Turkey. J Plant Nutr 38:1427–1443
Ellouze W, Hamel C, Vujanovic V, Gan Y, Bouzid S, St-Arnaud M (2013) Chickpea genotypes shape the soil microbiome and affect the establishment of the subsequent durum wheat crop in the semiarid North American Great Plains. Soil Biol Biochem 63:129–141
Esfahani MN, Kusano M, Nguyen KH, Watanabe Y, Ha CV, Saito K, Sulieman S, Herrera-Estrella L, Phan Tran L-S (2016) Adaptation of the symbiotic Mesorhizobium-chickpea relationship to phosphate deficiency relies on reprogramming of whole-plant metabolism. Proc Natl Acad Sci U S A 113:E4610–E4619
FAOSTAT (2010) FAO statistical database. From Food and Agricultural Organization, Rome, Italy. www.faostat.fao.org/site/339/default.aspx#ancor
Frostegård Å, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Gaur R, Jeena G, Shah N, Gupta S, Pradhan S, Tyagi AK, Jain M, Chattopadhyay D, Bhatia S (2015) High density linkage mapping of genomic and transcriptomic SNPs for synteny analysis and anchoring the genome sequence of chickpea. Sci Rep. doi:10.1038/srep13387
Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH (1997) Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 63:3233–3241
Imran A, Mirza MS, Shah TM, Malik KA, Hafeez FY (2015) Differential response of kabuli and desi chickpea genotypes toward inoculation with PGPR in different soils. Front Microbiol 6:1–14
Janse I, Meima M, Kardinaal WEA, Zwart G (2003) High-resolution differentiation of cyanobacteria by using rRNA-internal transcribed spacer denaturing gradient gel electrophoresis. Appl Environ Microbiol 69:6634–6643
Jayasinghearachchi HS, Seneviratne G (2004) A bradyrhizobial-Penicillium spp. biofilm with nitrogenase activity improves N2 fixing symbiosis of soybean. Biol Fertil Soils 40:432–434
Kantar F, Elkoca E,Ogutcu H, Algur OF (2003) Chickpea yields in relation to Rhizobium inoculation from wild chickpea at high altitudes. J Agron Crop Sci 189:1-7
Kennedy IR, Tchan Y-T (1992) Biological nitrogen fixation in non-leguminous field crops: recent advances. Plant Soil 141:93–118
Kim DH, Kaashyap M, Rathore A, Das RR, Parupalli S, Upadhyaya HD, Gopalakrishnan S, Gaur PM, Singh S, Kaur J, Yasin M, Varshney RK (2014) Phylogenetic diversity of Mesorhizobium in chickpea. J Biosci 39:513–517
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci Soc America 42:421–428
Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, del Rio TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488:86–90
Mackinney G (1941) Absorption of light by chlorophyll. J Biol Chem 140:315–323
Marschner P, Marschner P, Crowley D, Crowley D, Yang CH, Yang CH (2004) Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 261:199–208
Mirza BS, Mirza MS, Bano A, Malik KA (2007) Coinoculation of chickpea with Mesorhizobium ciceri isolates from roots and nodules and phytohormone-producing Enterobacter strains. Aust J Expl Agric 47:1008–1015
Moulin L, Munive A, Dreyfus B, Boivin-Masson C (2001) Nodulation of legumes by members of the beta-subclass of proteobacteria. Nature 411:948–950
Muyzer G, Waal ED, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Namvar A, Sharifi RS, Khandan T, Moghadam MJ (2013) Seed inoculation and inorganic nitrogen fertilization effects on some physiological and agronomical traits of chickpea (Cicer arietinum L.) in irrigated condition. J Central Eur Agric 14:28–40
Nour SM, Fernandez MP, Normand P, Cleyet-Marel J-C (1994) Rhizobium ciceri sp. nov., consisting of strains that nodulate chickpeas (Cicer arietinum L.) Int J Syst Bacteriol 44:511–522
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Dept Agric Circ 339, USDA, Washington
Prasanna R, Jaiswal P, Nayak S, Sood A, Kaushik BD (2009) Cyanobacterial diversity in the rhizosphere of rice and its ecological significance. Indian J Microbiol 49: 89-97
Prasanna R, Rana A, Chaudhary V, Joshi M, Nain L (2012) Cyanobacteria-PGPR interactions for effective nutrient and pest management strategies in agriculture. In: Satyanarayana T, Johri BN, Prakash A (eds) Microorganisms in sustainable agriculture and biotechnology. Springer, Dordrecht, pp 173–195
Prasanna R, Triveni S, Bidyarani N, Babu S, Yadav K, Adak A, Khetarpal S, Pal M, Shivay YS, Saxena AK (2014) Evaluating the efficacy of cyanobacterial formulations and biofilmed inoculants for leguminous crops. Arch Agron Soil Sci 60:349–366
Prasanna R, Bidyarani N, Babu S, Hossain F, Shivay YS, Nain L (2015a) Cyanobacterial inoculation elicits plant defense response and enhanced Zn mobilization in maize hybrids. Cogent Food Agric 1:995807
Prasanna R, Babu S, Bidyarani N, Kumar A, Triveni S, Monga D, Mukherjee AK, Kranthi S, Gokte-Narkhedhar Adak A, Yadav K, Nain L, Saxena AK (2015b) Prospecting cyanobacteria fortified composts as plant growth promoting and biocontrol agents in cotton. Exp Agric 51:42–65
Prasanna R, Kanchan A, Ramakrishnan B, Ranjan K, Venkatachalam S, Hossain F, Shivay YS, Krishnan P, Nain L (2016) Cyanobacteria-based bioinoculants influence growth and yields by modulating the microbial communities favourably in the rhizospheres of maize hybrids. Eur J Soil Biol 75:15–23
Ramsey PW, Rillig MC, Feris KP, Holben WE, Gannon JE (2006) Choice of methods for soil microbial community analysis : PLFA maximizes power compared to CLPP and PCR-based approaches. Pedobiologia 50:275–280
Ringelberg DB, Stair JO, Almeida J, Norby RJ, O’Neill EG, White DC (1997) Consequences of rising atmospheric carbon dioxide levels for the belowground microbiota associated with white oak. J Environ Quality 26:495–503
Roesti D, Gaur R, Johri BN (2006) Plant growth stage, fertiliser management and bio-inoculation of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria affect the rhizobacterial community structure in rain-fed wheat fields. Soil Biol Biochem 38:1111–1120
Rokhzadi A, Toashih V (2011) Nutrient uptake and yield of chickpea (Cicer arietinum L.) inoculated with plant growth- promoting rhizobacteria. J Plant Nutr 5:44–48
Romdhane SB, Aouani ME, Mhamdi R (2007) Inefficient nodulation of chickpea (Cicer arietinum L.) in the arid and Saharan climates in Tunisia by Sinorhizobium ciceri meliloti biovar medicaginis. Ann Microbiol 57:15–19
Rouhrazi K, Khodakaramian G (2015) Phenotypic and genotypic diversity of root-nodulating bacteria isolated from chickpea (Cicer arietinum L.) in Iran. Ann Microbiol 65:2219–2227
Schwieger F, Tebbe CC (2000) Effect of field inoculation with Sinorhizobium ciceri meliloti L33 on the composition of bacterial communities in rhizospheres of a target plant (Medicago sativa) and a non-target plant (Chenopodium album)—linking of 16S rRNA gene-based single-strand conformation polymorphism community profiles to the diversity of cultivated bacteria. Appl Environ Microbiol 66:3556–3565
Simon HM, Jahn CE, Bergerud LT, Sliwinski K, Weimer PJ, Willis DK, Robert M, Sliwinski MK, Goodman RM (2005) Cultivation of mesophilic soil crenarchaeotes in enrichment cultures from plant roots cultivation of mesophilic soil crenarchaeotes in enrichment cultures from plant roots. Appl Environ Microbiol 71:4751–4760
Singh M, Awasthi A, Soni SK, Singh R, Verma RK, Kalra A (2015) Complementarity among plant growth promoting traits in rhizospheric bacterial communities promotes plant growth. Sci Rep 5:15500
Snell F D and Snell C T (1954) Colorimetric Methods of Analysis, 3rd Edition, 4 Dyan Nostrand Company Inc, pp 512-513 & 516- 518, New York
Subbiah B, Asija G (1956) A rapid procedure for the estimation of available nitrogen in soils. Curr Sci 25: 259-260
Svitlana D (2013) Ecological safe growing of chickpea in the area of steppe of Ukraine. J Life Sci 7:1185–1190
Tagore GS, Namdeo SL, Sharma SK, Kumar N (2013) Effect of Mesorhizobium ciceri and phosphate solubilizing bacterial inoculants on symbiotic traits, nodule leghemoglobin, and yield of chickpea genotypes. Int J Agron Article ID 581627:8 pp
Trabelsi D, Mengoni A, Ben Ammar H, Mhamdi R (2011) Effect of on-field inoculation of Phaseolus vulgaris with rhizobia on soil bacterial communities. FEMS Microbiol Ecol 77:211–222
Van Cauwenberghe J, Michiels J, Honnay O (2015) Effects of local environmental variables and geographical location on the genetic diversity and composition of Mesorhizobium ciceri leguminosarum nodulating Vicia cracca populations. Soil Biol Biochem 90:71–79
Vessey J (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Virtanen AI, Jorma J, Linkola H, Linnasalmi A (1947) Relation between nitrogen fixation and leghaemoglobin. Acta Chem Scand 1:90–111
Yao H, He Z, Wilson MJ, Campbell CD (2000) Microbial biomass and community structure in a sequence of soil with increasing fertility and changing land use. Microb Ecol 40:223–237
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol Fertil Soils 29:111–129
Zhang L, Chen W, Burger M, Yang L, Gong P, Wu Z (2015) Changes in soil carbon and enzyme activity as a result of different long-term fertilization regimes in a greenhouse field. PLoS One 10:1–13
Zhou J, Guan D, Zhou B, Zhao B, Ma M, Qin J, Jiang X, Chen S, Cao F, Shen D, Li J (2015) Influence of 34 years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol Biochem 90:42–51
Acknowledgements
This investigation was partially funded by the funds from the Network Project on Microorganisms ‘Application of Microorganisms in Agricultural and Allied Sectors’ (AMAAS) granted by the Indian Council of Agricultural Research (ICAR), New Delhi, to RP and SERB project, DST, Government of India to BR. The authors gratefully acknowledge the support of Dr. Hegde, Division of Genetics, ICAR-IARI, in providing the chickpea germplasm and the Division of Agronomy, ICAR-IARI, New Delhi, for providing necessary facilities for the analyses of soil samples. The authors are also thankful to the Division of Microbiology, ICAR-IARI, New Delhi, for providing the necessary facilities to undertake this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Chengrong Chen
Electronic supplementary material
Supplementary Fig S1
(PPTX 2351 kb)
Supplementary Fig S2
(PPTX 2351 kb)
Supplementary Fig S3
(PPTX 2351 kb)
Rights and permissions
About this article
Cite this article
Ramakrishnan, B., Kaur, S., Prasanna, R. et al. Microbial inoculation of seeds characteristically shapes the rhizosphere microbiome in desi and kabuli chickpea types. J Soils Sediments 17, 2040–2053 (2017). https://doi.org/10.1007/s11368-017-1685-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1685-5