Abstract
Streptomyces albus J1074 is one of the most popular and convenient hosts for heterologous expression of gene clusters directing the biosynthesis of various natural metabolic products, such as antibiotics. This fuels interest in elucidation of genetic mechanisms that may limit secondary metabolism in J1074. Here, we report the generation and initial study of J1074 mutant, deficient in gene bldA for tRNALeu UAA, the only tRNA capable of decoding rare leucyl TTA codon in Streptomyces. The bldA deletion in J1074 resulted in a highly conditional Bld phenotype, with depleted formation of aerial hyphae on certain solid media. In addition, bldA mutant of J1074 was unable to produce endogenous antibacterial compounds and two heterologous antibiotics, moenomycin and aranciamycin, whose biosynthesis is directed by TTA-containing genes. We have employed a new TTA codon-specific β-galactosidase reporter system to provide genetic evidence that J1074 bldA mutant is impaired in translation of TTA. In addition, we have discussed the possible reasons for differences in the phenotypes of bldA mutants described here and in previous studies, providing knowledge to study bldA-based regulation of antibiotic biosynthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The actinobacterium Streptomyces albus J1074 was isolated in 1980 as a S. albus G derivative deficient in the SalGI restriction-modification system (Chater and Wilde 1980). Later, J1074 served as a model to study phage restriction phenomena in streptomycetes, and in 1990s it has gained prominence as a host for heterologous expression of antibiotic biosynthesis genes (Rodriguez et al. 1993, 2000). Fast, highly dispersed growth and genetic amenability make J1074 a strain of first choice for expression of heterologous gene clusters from actinobacteria (Baltz 2010). Previous studies (Lopatniuk et al. 2014; Iqbal et al. 2016) portray J1074 as an optimal starting point to develop an efficient platform for heterologous expression (metagenomic) purposes. It is expected that such a platform would greatly increase our ability to discover bioactive natural products encrypted in environmental DNA and silent gene clusters of actinobacteria. In this context, it is essential to elucidate genetic mechanisms that control secondary metabolism in J1074 to find limiting factors and the ways to circumvent them. It has to be mentioned that regulation of antibiotic production has been relatively well studied in few models of streptomycetes (Liu et al. 2013), although it is not yet understood to what extent this knowledge can be extrapolated onto new strains, such as J1074.
Transcriptional control of gene expression is arguably the most important regulatory layer in bacteria, and antibiotic production is no exception. In addition, streptomycetes possess a highly unusual regulatory switch based on leucyl (TTA) codon and its cognate tRNALeu UAA, encoded by gene bldA. TTA codons are located exclusively in genes for auxiliary functions such as morphogenesis and antibiotic production, and bldA deficiency is not lethal. There is a considerable temporal gap between occurrence of UUA-containing transcripts and their subsequent translation (Gramajo et al. 1993; Pope et al. 1996; Rebets et al. 2006). Failure to develop aerial mycelium and spores on top of substrate mycelium is a hallmark of all bldA-mutants (the “Bald” phenotype) studied to date. The latter is thought to be the result of blocked translation of TTA-containing gene for master regulator AdpA (Takano et al. 2003; Nguyen et al. 2003). The influence of bldA deficiency on antibiotic biosynthesis remains more controversial, as well as the exact chain of events linking bldA to delayed antibiotic production (Guthrie and Chater 1990; Gramajo et al. 1993; Chater and Chandra 2008; Makitrynskyy et al. 2013).
The effects of bldA deletion on J1074 have not been studied so far, and it remained unknown to what extent colony development and antibiotic production will be affected by the former. Here, we show that on certain (minimal) solid media bldA-deficient mutant J1074 produces aerial hyphae and spores less densely than the parental strain; in the same time, under many culture conditions parental and mutant strains are phenotypically indistinguishable. In line with many other reports, our bldA mutant’s ability to express TTA codon-containing antibiotic biosynthesis gene clusters is strictly dependent on tRNALeu UAA. Finally, we describe a novel reporter system that was used to study TTA codon-based regulatory mechanisms in S. albus.
Materials and methods
Microorganisms, vectors, and culture conditions
Streptomyces albus SAM2, a derivative of J1074 carrying single attB φC31 site (Bilyk and Luzhetskyy 2014), was used throughout the study. Escherichia coli DH5α was used for routine subcloning. E. coli ET12567 (pUB307) was used to perform intergeneric conjugation from E. coli to S. albus. Escherichia coli strains were grown under standard conditions (Sambrook and Russell 2001). Bacillus cereus ATCC19637 and Debaryomyces hansenii VKM Y-9 were used as antibiotic-sensitive test cultures (growth temperature −30 °C). RedET-mediated gene replacements (Datsenko and Wanner 2000) were carried out with the help of REDIRECT system (Gust et al. 2003). All vectors, cosmids, plasmids, and BAC DNAs are listed in Table 1. For intergeneric matings, S. albus strains were grown on soy-flour mannitol (SFM) agar (Kieser et al. 2000) at 30 °C. To reveal endogenous antibacterial and antifungal activities, S. albus strains were grown on SG2 agar (in g/L: glucose 20, yeast extract 5, soytone 10, pH 7.2 prior to autoclaving) and other solid media as detailed in Electronic Supplementary Materials (ESM). For submerged production of moenomycin and aranciamycin S. albus strains were grown in liquid medium SG1 (SG2 supplemented with 2 g/L CaCO3) at 28 °C for 5 days. For phenotypic examinations, S. albus were grown on SFM and SMMS agar (Kieser et al. 2000), as well as other media as described in ESM. Wherever needed, strains were maintained in the presence of apramycin (25 μg/mL); chromogenic substrate X-Gal was added to the media to a final concentration of 50 μg/mL; cumate (50 mM) was used to induce SCO3479 expression from plasmids pRV4 and pRV3.
Plasmids and BACs construction
Bacterial artificial chromosome (BAC) BAC_1N12 was used to prepare bldA (XNR_1995) knockout BAC. For this purpose, apramycin resistance cassette aac(3)IV-oriT flanked with P-GG and B-CC sites was amplified from plasmid patt-saac-oriT (Myronovskyi et al. 2014) with primers Am-bldAdelF and Am-bldAdelR (all primer sequences are given in Table s1, ESM). The resulting 1378-bp PCR product was used to replace bldA gene in BAC_1N12 using recombineering. The resulting BAC was named 1N12d_bldA. This and other constructs generated in course of the work were verified by sequencing. Plasmid pTOSbldA (complementation of the bldA deletion). The bldA gene together with putative promoter region was amplified with primers bldA-Hind-for and bldA-Xba-rev yielding a product 675 bp in length. The PCR product was digested with enzymes HindIII–XbaI and cloned into respective sites of vector pTOS to give pTOSbldA. Plasmid pTESsco3479 (expression of β-galactosidase gene SCO3479 under promoter ermEp*). Gene SCO3479 was amplified with primers Sco3479upXbaI and Sco3479rpMfeIBgl from the chromosome of S. coelicolor. The PCR product, 3016 bp in length, was digested with restriction endonucleases XbaI and BglII, and then ligated to XbaI–BglII sites of vector pTES. Plasmids pOOB109 and pOOB110. The 3.3-kb fragment of S. coelicolor M145 genome that encompassed the entire coding sequence of SCO3479 plus 0.3-kb putative promoter region was amplified with primers Sco3479upBglII and 3479rpMfeIBglII. The resulting amplicon was treated with restriction endonuclease BglII and cloned into BamHI + BglII-digested vector pTES. Two clones were selected, referred to as pOOB109 and pOOB110, which contained SCO3479 in two orientations within vector pTES. In pOOB109, SCO3479 is under control of its own promoter as well as truncated ermEp*; and in pOOB110, SCO3479 is under control of its own promoter. Plasmid pOOB114. 3017-bp fragment containing entire coding sequence of SCO3479 plus 29-bp putative promoter was amplified from plasmid pOOB109 with primers lacZ_XbaI_TGA2 and 3479rpMfeIBglII. In this way, leucyl codon CTG in 19th position of SCO3479 was replaced with stop codon TGA. The resulting amplicon was treated with XbaI and MfeI and cloned into XbaI–EcoRI sites of pTES to give pOOB114. Plasmid pRV3. 3017-bp fragment containing entire coding sequence of SCO3479 plus 29-bp putative promoter was amplified from plasmid pOOB109 with primers lacZ_XbaI_TTA1 and Sco3479_6Hisrp. In this way, leucyl codon CTG in 8th position of SCO3479 was replaced with synonymous codon TTA and six histidine codons CAC were appended upstream of stop codon. The resulting amplicon was treated with XbaI and MfeI and cloned into SpeI–EcoRI sites of pGCymRP21 to give pRV3. In the latter, SCO3479 replaced gusA gene. Plasmid pRV4. 3017-bp fragment containing entire coding sequence of SCO3479 plus 29-bp putative promoter was amplified from plasmid pOOB109 with primers Sco3479upXbaI and Sco3479_6Hisrp. In this way, native sequence of SCO3479 was appended upstream of hexahistidine-stop codon sequence [(CAC)6-TGA]. The resulting amplicon was treated with XbaI and MfeI and cloned into SpeI–EcoRI sites of pGCymRP21 to give pRV4.
Generation and verification of the S. albus recombinant strains
All constructs were transferred into S. albus conjugally, as described elsewhere; gene replacements in S. albus were generated as described in Myronovskyi et al. (2014). Apramycin resistance marker flanked with B-GG and P-GG sites was evicted from the genome of bldA mutant with recombinase Int-phiC31 (plasmid pUWLint31). pTOS vector sequences from bldA complementation strain were evicted with recombinase Dre (plasmid pUWLDre). PCR was employed to confirm the presence of the plasmids, cosmids, and expected gene replacements in the chromosomes of streptomycetes.
Determination of antibiotic production
Moenomycin production was analyzed in S. albus strains carrying cosmid moeno38-6. The latter leads to accumulation of two moenomycins in S. albus—nosokomycin A2 and its precursor lacking terminal galacturonic residue (Ostash et al. 2013; Lopatniuk et al. 2014). Strains were grown in SG1. Quantity of moenomycins was determined by antibiotic disc (Ø 5 mm, Whatman) diffusion assay against spores (107 per plate) of moenomycin-sensitive Bacillus cereus ATCC19637 as described in Makitrynskyy et al. (2010). Productivity index (PI) was calculated according to the equation: PI = (Ø growth inhibition zone, mm/Ø disc, mm) − 1. PI = 0 is the lowest possible value and corresponds to antibiotic production below detection limit of the bioassay (˂2% of production level of the control strain). The PIs were referred back to equal amounts of dry biomass (10 mg) in different strains.
For aranciamycin production, control and pOJ436ara-carrying strains were grown for 24 h in preculture medium (tryptic soy broth, TSB; Himedia) at 30 °C and 1 mL of the preculture was used to inoculate 50 mL (in 300-mL flasks with glass beads) of main medium SG1. After 5 days of growth, biomass was separated from supernatant via centrifugation. Spent medium (10–20 mL) was extracted with equal volume of ethylacetate. Ethylacetate extract was dried in vacuo, then reconstituted in methanol, and subjected to TLC (immobile phase: silicagel plates F60, Merck; mobile phase: chloroform:methanol = 95:5) and disc diffusion assay against B. cereus ATCC19637 spores (107 per plate) on modified minimal agar (g/L: KH2PO4 3, K2HPO4 7, sodium citrate × 4H2O 0.5, MgSO4 × 7H2O 0.1, (NH4)2SO4 1, glucose 2, bacto peptone 0.3, agar 16) (Anagnostopoulos and Spizizen 1961).
Aranciamycin production and native antibiotic activity of S. albus strains were also monitored using agar plug antibiotic diffusion assay. Briefly, strains were grown on SG1 agar for 5 days. Then, agar plugs (Ø 5 mm) were cut off the lawn and stacked on top of TSB agar plates with test culture D. hansenii spread immediately prior to the experiments or B. cereus spores as it is described above. Halos of growth inhibition around the plugs were measured after 18 h of incubation.
Scanning electron microscopy (SEM)
Small pieces of 3-day-old lawns were cut off the OM or SFM agar plate samples, vacuum-dried, and directly analyzed on a Jeol JSM-T220A scanning microscope.
Results
Generation and growth characteristics of S. albus strains carrying bldA knockout
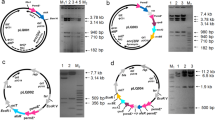
For S. albus J1074 bldA gene knockout, we prepared BAC 1N12_bldA-I, where bldA was replaced with aac(3)IV-oriT cassette flanked with B-GG and P-GG sites for recombinase PhiC31. With this BAC, we successfully generated respective S. albus mutant (∆bldA-I) and then its markerless derivative (upon expression of PhiC31 from plasmid pUWLInt31). The markerless bldA mutant was referred to as OK3. OK3 genotype was confirmed by PCR (see Fig. S1, Electronic Supplementary Materials, ESM). We also introduced S. albus SAM2 bldA gene on vector pTOS back into OK3, and, after pTOS sequences eviction, generated markerless complemented strain OK3 + bldA.
In submerged culture, OK3 and the parent (SAM2) strains exhibited similar dynamics of growth and biomass accumulation (Fig. S2). The strains were also incubated on a number of solid media and analyzed after 3 and 5 days. On most media, OK3 was macroscopically and microscopically indistinguishable from the parent (Figs. S3, S4). Nevertheless, we have found that certain minimal media did result in visible changes in lawn morphology of OK3 (see Fig. S4). The most pronounced morphological differences were seen on SFM and SMMS (Figs. 1 and S5). On these media, OK3 produced sparse aerial hyphae and spore chains without significant delay as compared to the parental strain.
Streptomyces albus J1074 bldA mutant phenotype. Parental (SAM2), mutant and complemented strains were grown for 3 days on SFM (a) and SMMS (b). The same lawns were used to obtain scanning electron microscopic views of colonial surfaces of SFM (c) and SMMS-grown cultures (d). The substrate mycelium of OK3 strain on SFM (c) appeared to be fragmented into spore-like chains (white triangles)
Antibiotic production by OK3 strain
Genomic potential of S. albus J1074 for secondary metabolism is significant (Zaburannyi et al. 2014), yet, to date, its expression required genetic interventions (Olano et al. 2014; Brana et al. 2014). Compounds of paulomycin family are the only known antibacterially active metabolites to be accumulated by J1074, although their production pattern was unstable (Olano et al. 2014; Gonzalez et al. 2016) We tested over a dozen of the most common agar media used for Streptomyces cultivation, and found those that reproducibly elicit the production of antibiotic compounds by J1074 (Figs. S6, S7). Particularly, S. albus accumulated antibacterial and (to a lesser extent) antifungal compounds when growing on SG2 agar. Using SG2, we compared antibiotic activity of SAM2, bldA mutant OK3 and OK3 + bldA. The results are summarized in Fig. 2. OK3 did not accumulate detectable amounts of antibacterial compounds and introduction of bldA restored antibiotic production to the mutant. The antifungal activity of OK3 was not impaired and even slightly increased as compared to SAM2. Using HPLC–MS (as detailed in ESM), we confirmed that production of candicidin-like compounds by OK3 is increased as compared to parent strain, while paulomycins were not found in OK3 (Fig. S8).
The bldA knockout in S. albus abolished the production of antibacterial compound(s), but not antifungal one(s). For the bioassay, agar plugs were cut off 5-day lawns of S. albus strains grown on SG2 agar, and stacked on top of plates with test cultures. Note the small halos of D. hansenii growth inhibition around SAM2 and OK3 + bldA plugs, and increased halo around OK3 plug
Next, we compared the ability of SAM2 and OK3 to heterologously produce antibiotics whose biosynthesis depend on TTA codon-containing genes. These were moenomycin and aranciamycin biosynthesis gene clusters, carried on cosmids moeno38-6 (Lopatniuk et al. 2014) and pOJ436ara (Luzhetskyy et al. 2007), respectively. As can be judged from bioactivity and TLC-based assays, moeno38-6+ and pOJ436ara+ transconjugants of the parental strain abundantly produced respective antibiotics, while OK3 strain did not (Figs. 3, S9–S10).
Aranciamycin (a) and moenomycin (b) production is blocked in bldA mutant of S. albus. Strains were grown in SG1 medium, and productivity indices were calculated from disc diffusion assays as described in “Materials and methods”. SAM2, OK3—strains carrying empty vector pSET152; SAM2/OK3-ara, SAM2/OK3-moe—S. albus strains carrying either cosmid pOJ436ara or cosmid moeno38-6, respectively
The β-galactosidase reporter system to detect and elucidate bldA-based regulation
The above-described results imply that, in line with what is known for the other Streptomyces (Chater and Chandra 2008; Liu et al. 2013), failure to translate codon UUA in transcripts of secondary metabolism genes is the primary reason for the inability of OK3 to produce endogenous and heterologous antibiotics. Yet, with two exceptions (Wang et al. 2009; Makitrinskyy et al. 2013), direct evidence for involvement of bldA in regulation at the level of UUA translation is absent for all studied cases. In S. albus J1074, the bldA mutation and the observed phenotype (lack of antibacterial activity of the strain) are bridged by a complicated cascade of regulatory and structural genes. Codon-swap reporters based on β-galactosidase and luciferase genes are used in enterobacteria and yeast to peer into regulatory events at the level of translation of a certain codon (Urbonavicius et al. 2003; Lamichhane et al. 2013). We set out to develop an analogous reporter system for Streptomyces albus J1074, which would enable straightforward phenotypic detection of translation of UUA. Here, we took advantage of the fact that J1074 is one of the rare streptomycetes having no detectable intra- or extracellular enzymatic activity towards lactose analog, X-Gal (King and Chater 1986). Indeed, our analysis confirmed that none of the seven annotated S. coelicolor β-galactosidases had counterparts within J1074. Using reciprocal best BLAST hit strategy (Kuzniar et al. 2008), S. coelicolor M145 protein Sco3479 (referred hereafter to as LacZsc) was identified as an ortholog of E. coli LacZ. Several lacZ sc -carrying plasmids were prepared (Fig. 4a) and expression of functional β-galactosidase from all of them (except for pOOB114 which carries in-frame stop codon in position 19 of lacZ sc ) was confirmed. Particularly, gene lacZ sc conferred lacZ-deficient E. coli to the ability to hydrolyze X-Gal; in liquid and solid media, different lacZ + sc S. albus strains produced blue color of different intensity (Fig. S11). The latter most likely reflects different strength of transcription of lacZ sc .
Codon-specific β-galactosidase reporter for S. albus visualizes arrested TTA codon translation in bldA mutant OK3. All plasmids (a) carrying S. coelicolor M145 β-galactosidase gene SCO3479 (lacZ sc ) contain actinophage ϕC31 int-attP integration module and apramycin resistance marker aac(3)IV. They differ in the type of promoter (shaded gray) that drives lacZ sc transcription, and presence of distinct codons (shown as ovals) in positions 8 and 19 of lacZ sc ORF (if not shown, then CTC codon is present). In pOOB109 and pOOB110, ermEp is rendered nonfunctional through truncation of its last 30 nts. In pRV3 and pRV4 lacZ sc , transcription is cumate-inducible (Horbal et al. 2014). Four OK3 derivatives (b) were streaked onto TSA X-Gal-containing plates in the absence (c) and in presence (d) of cumate. CTC+ reporter is expressed in OK3, while TTA+ is not. The latter is expressed in wild type (SAM2) strain (see ESM, Fig. S11)
We introduced TTA, CTC, and stop codon-containing lacZ sc reporter plasmids (pRV3, pRV4, pOOB114, respectively) individually into OK3 strain. As expected, the plasmids did not cause the production of blue color (X-Gal hydrolysis) in the absence of inducer (cumate). In the presence of 50 mM cumate only the strain carrying CTC-specific reporter (pRV4) turned blue (Fig. 4). The ability of OK3-pRV3 to hydrolyze X-Gal was restored upon introduction of bldA (Fig. S12). All available data suggest that bldA deletion directly and specifically impaired the translation of TTA codon in lacZ sc reporter gene.
Discussion
In this work, we have carried out a set of experiments aimed to study the effects of bldA deletion on S. albus J1074 and to develop a simple reporter system for further investigation of bldA-mediated regulation in J1074.
Perhaps, the most striking property of Bld phenotype (displayed by bldA mutants of streptomycetes, vide supra) is its conditionality. That is, the degree of morphological and antibiotic synthesis defects strongly depend on growth conditions and level of transcription TTA codon-containing (TTA+) genes (Guthrie and Chater 1990; Leskiw et al. 1993; Gramajo et al. 1993; Pope et al. 1996; Xu et al. 2008). The conditionality of Bld phenotype of S. albus OK3 has extreme manifestation. Indeed, we were able to identify media, where OK3 exhibits impaired morphological development, but under no conditions did we observe complete cessation of aerial mycelium and spore formation. To the best of our knowledge, there is no experimentally validated information on gene networks that govern morphogenesis in S. albus J1074. However, as far as we can judge from in silico analysis of J1074 genome (Zaburannyy et al. 2014), the bld- and whi-cascades are essentially the same in J1074 and other model streptomycetes. Gene XNR4181 encoding ortholog of S. coelicolor AdpA (also known as BldH), a master regulator of colonial differentiation and (often) antibiotic biosynthesis (Chater and Chandra 2008), contains TTA codon, and thus should be under bldA control. Hence, either UUA within adpA mRNA is mistranslated efficiently enough to allow colonial development, or other (as-yet-unknown) genes compensate/bypass the loss of AdpA. In Streptomyces, there are several reports on mistranslation of various UUA+ mRNAs, including adpA, in the absence of bldA-encoded tRNALeu UAA (Leskiw et al. 1991; Makitrinskyy et al. 2013). Although existence of a subset of TTA+ genes capable of translation in bldA mutants appears to be paradoxical, an explanation for this was offered (Trepanier et al. 2002). Briefly, propensity for mistranslation is attributed to the nature of the first nucleotide downstream of the TTA codon. If either A or G follow TTA, then the ribosome is able to mistranslate the codon; otherwise (C/T downstream of TTA) translation is aborted. Thus, in the absence of tRNALeu UAA, only mRNA containing the quadruplets TTAC/T will not be translated, and so regulatory effect will be attained. There is TTAC quadruplet in case of S. albus J1074 adpA; thus, codon context hypothesis does not seem to apply. The position of TTA within the coding sequence could be important. In most streptomycete genes, it is located close to the start codon (Zaburannyy et al. 2009), while in adpA gene family, it is positioned roughly in the middle of the ORF. Also, enhancement of UUA translation in adpA mRNA in the presence of S-adenosylmethionine was reported (Xu et al. 2008). These facts underscore the importance of environmental (rather than genetic) factors in translation of adpA mRNA, and they might play a crucial role in shaping the morphology of OK3 strain.
The bldA knockout abolished production of two heterologous antibiotics, moenomycin and aranciamycin, the biosynthesis of which is controlled by TTA-containing genes. Moreover, OK3 no longer produced endogenous antibacterial compounds. According to all available data, glycosylated antibiotics, paulomycins, are the principal source of antibacterial activity exhibited by S. albus J1074 (Majer and Chater 1987; Olano et al. 2014). We also believe that paulomycins accounted for most (if not all) of the antibacterial activity of SAM2, given the characteristic reddish coloration of S. albus lawns on SG2 agar, and the absence of that color in OK3, although detailed analysis was not pursued. Cessation of their production by OK3 strain is most likely due to presence of TTA codon within regulatory gene SSGH_05342 (plm30) encoding LuxR family regulator essential for paulomycin production (Gonzalez et al. 2016). At the same time, level of antifungal activity of OK3 is not affected as compared to SAM2, and even appears to be elevated. S. albus genome carries several gene clusters for biosynthesis of compounds that might target eukaryotic cells (Zaburannyy et al. 2014). One of these clusters (around gene XNR_RS29020), directing the production of candicidin-like compound, is free of TTA codons, and so could be expressed in bldA-minus background.
Our work has once again highlighted all the challenges associated with interpretation of complex, conditional, and composite phenotypes of bldA mutants. For example, since TTA codons are embedded into genes for pleiotropic and pathway-specific transcriptional factors, any observable defect of bldA mutant would be the net result of deregulated transcription and translation. It is thus difficult to account for and disentangle the relative contribution of each genetic and environmental factor to a final bldA-minus phenotype. This calls for development of a simple reporter system where effects of bldA knockout on translation can be reliably detected and studied in the most direct way. Several such systems (mostly based on antibiotic resistance genes) were employed in the past, and they gathered valuable information about bldA function (Leskiw et al. 1991; Pope et al. 1996). Nevertheless, available reporter genes are not without shortcomings, such as unnatural (for Streptomyces) codon usage biases; poor control of transcription; and absence, in most cases, of TTA-free version of the reporter gene. We developed, for S. albus J1074, a specialized codon reporter system based on S. coelicolor M145 β-galactosidase gene SCO3479 (lacZ sc ). It allows tight transcriptional control over SCO3479 expression, ease of phenotypic detection (in presence of chromogenic substrates), and His-tagged protein tracking. The simplicity of our reporter system lends itself to a rigorous elucidation of the regulatory role of TTA codons at the level of translation, as our initial experiments (vide supra) suggest.
A number of recent studies suggest that it is not the delayed expression of bldA gene but formation of mature (translationally competent) tRNA holds the key to its regulatory function (Leskiw et al. 1991; Xu et al. 2008; Pettersson and Kirsebom 2011). Yet, besides initial in silico predictions (Rokytskyy et al. 2016) nothing is known about the genetics of tRNA maturation in Streptomyces. Likewise, there is a growing body of data on the crucial role of environmental factors in tRNA biology (Laxman et al. 2013; Moukadiri et al. 2014; Sakai et al. 2016), although this area remains unexplored in case of bldA gene. We envision that the above-described reporter system will facilitate the screening of growth conditions and genes that modulate UUA translation efficiency, both in wild type and bldA-minus S. albus backgrounds.
References
Anagnostopoulos C, Spizizen J (1961) Requirements for transformation of Bacillus subtilis. J Bacteriol 81(5):741–746
Baltz RH (2010) Streptomyces and Saccharopolyspora hosts for heterologous expression of secondary metabolite gene clusters. J Ind Microbiol Biotechnol 37(8):759–772. doi:10.1007/s10295-010-0730-9
Bilyk B, Luzhetskyy A (2014) Unusual site-specific DNA integration into the highly active pseudo-attB of the Streptomyces albus J1074 genome. Appl Microbiol Biotechnol 98(11):5095–5104. doi:10.1007/s00253-014-5605-y
Braña AF, Rodríguez M, Pahari P, Rohr J, García LA, Blanco G (2014) Activation and silencing of secondary metabolites in Streptomyces albus and Streptomyces lividans after transformation with cosmids containing the thienamycin gene cluster from Streptomyces cattleya. Arch Microbiol 196:345–355. doi:10.1007/s00203-014-0977-z
Chater K, Chandra G (2008) The use of the rare UUA codon to define “expression space” for genes involved in secondary metabolism, development and environmental adaptation in Streptomyces. J Microbiol 46:1–11
Chater KF, Wilde LC (1980) Streptomyces albus G mutants defective in the SalGI restriction-modification system. J Gen Microbiol 116(2):323–334
Datsenko K, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Fedoryshyn M, Petzke L, Welle E, Bechthold A, Luzhetskyy A (2008) Marker removal from actinomycetes genome using Flp recombinase. Gene 419(1–2):43–47. doi:10.1016/j.gene.2008.04.011
González A, Rodríguez M, Braña AF, Méndez C, Salas JA, Olano C (2016) New insights into paulomycin biosynthesis pathway in Streptomyces albus J1074 and generation of novel derivatives by combinatorial biosynthesis. Microb Cell Fact 21:56. doi:10.1186/s12934-016-0452-4
Gramajo H, Takano E, Bibb M (1993) Stationary-phase production of the antibiotic actinorhodin in Streptomyces coelicolorA3(2) is transcriptionally regulated. Mol Microbiol 7(6):837–845
Gust B, Challis GL, Fowler K, Kieser T, Chater KF (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci USA 100:1541–1546
Guthrie EP, Chater KF (1990) The level of a transcript required for production of a Streptomyces coelicolor antibiotic is conditionally dependent on a tRNA gene. J Bacteriol 172:6189–6193. doi:10.1128/jb.172.11.6189-6193.1990
Herrmann S, Siegl T, Luzhetska M, Petzke L, Jilg C, Welle E, Erb A, Leadlay PF, Bechthold A, Luzhetskyy A (2012) Site-specific recombination strategies for engineering actinomycete genomes. Appl Environ Microbiol 78(6):1804–1812. doi:10.1128/AEM.06054-11
Horbal L, Fedorenko V, Luzhetskyy A (2014) Novel and tightly regulated resorcinol and cumate-inducible expression systems for Streptomyces and other actinobacteria. Appl Microbiol Biotechnol 98(20):8641–8655. doi:10.1007/s00253-014-5918-x
Iqbal HA, Low-Beinart L, Obiajulu JU, Brady SF (2016) Natural product discovery through improved functional metagenomics in Streptomyces. J Am Chem Soc 138(30):9341–9344. doi:10.1021/jacs.6b02921
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces genetics. John Innes Foundation, Norwich
King AA, Chater KF (1986) The expression of the Escherichia coli lacZ gene in Streptomyces. J Gen Microbiol 132(6):1739–1752. doi:10.1099/00221287-132-6-1739
Kuzniar A, van Ham RC, Pongor S, Leunissen JA (2008) The quest for orthologs: finding the corresponding gene across genomes. Trends Genet 24:539–551. doi:10.1016/j.tig.2008.08.009
Lamichhane TN, Blewett NH, Crawford AK, Cherkasova VA, Iben JR, Begley TJ, Farabaugh PJ, Maraia RJ (2013) Lack of tRNA modification isopentenyl-A37 alters mRNA decoding and causes metabolic deficiencies in fission yeast. Mol Cell Biol 33:2918–2929. doi:10.1128/MCB.00278-13
Laxman S, Sutter BM, Wu X, Kumar S, Guo X, Trudgian DC, Mirzaei H, Tu BP (2013) Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell 154:416–429. doi:10.1016/j.cell.2013.06.043
Leskiw BK, Lawlor EJ, Fernandez-Abalos JM, Chater KF (1991) TTA codons in some genes prevent their expression in a class of developmental, antibiotic-negative, Streptomyces mutants. Proc Natl Acad Sci USA 88:2461–2465
Leskiw BK, Mah R, Lawlor EJ, Chater KF (1993) Accumulation of bldA-specified tRNA is temporally regulated in Streptomyces coelicolor A3(2). J Bacteriol 175:1995–2005. doi:10.1128/jb.175.7.1995-2005.1993
Liu G, Chater KF, Chandra G, Niu G, Tan H (2013) Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol Mol Biol Rev 77(1):112–143. doi:10.1128/MMBR.00054-12
Lopatniuk M, Ostash B, Luzhetskyy A, Walker S, Fedorenko V (2014) Generation and study of the strains of streptomycetes—heterologous hosts for production of moenomycin. Russ J Genet 50(4):360–365
Luzhetskyy A, Mayer A, Hoffmann J, Pelzer S, Holzenkämper M, Schmitt B, Wohlert SE, Vente A, Bechthold A (2007) Cloning and heterologous expression of the aranciamycin biosynthetic gene cluster revealed a new flexible glycosyltransferase. ChemBioChem 8(6):599–602
Majer J, Chater KF (1987) Streptomyces albus G produces an antibiotic complex identical to paulomycins A and B. J Gen Microbiol 133:2503–2507. doi:10.1099/00221287-133-9-2503
Makitrynskyy R, Rebets Y, Ostash B, Zaburannyi N, Rabyk M, Walker S, Fedorenko V (2010) Genetic factors that influence moenomycin production in streptomycetes. J Ind Microbiol Biotechnol 37:559–566
Makitrynskyy R, Ostash B, Tsypik O, Rebets Y, Doud E, Meredith T, Luzhetskyy A, Bechthold A, Walker S, Fedorenko V (2013) Pleiotropic regulatory genes bldA, adpA and absB are implicated in production of phosphoglycolipid antibiotic moenomycin. Open Biol 3:130121. doi:10.1098/rsob.130121
Moukadiri I, Garzón MJ, Björk GR, Armengod ME (2014) The output of the tRNA modification pathways controlled by the Escherichia coli MnmEG and MnmC enzymes depends on the growth conditions and the tRNA species. Nucleic Acids Res 42:2602–2623. doi:10.1093/nar/gkt1228
Myronovskyi M, Rosenkränzer B, Luzhetskyy A (2014) Iterative marker excision system. Appl Microbiol Biotechnol 98(10):4557–4570. doi:10.1007/s00253-014-5523-z
Nguyen KT, Tenor J, Stettler H, Nguyen LT, Nguyen LD, Thompson CJ (2003) Colonial differentiation in Streptomyces coelicolor depends on translation of a specific codon within the adpA gene. J Bacteriol 185:7291–7296. doi:10.1128/JB.185.24.7291-7296.2003
Olano C, García I, González A, Rodriguez M, Rozas D, Rubio J, Sánchez-Hidalgo M, Braña AF, Méndez C, Salas JA (2014) Activation and identification of five clusters for secondary metabolites in Streptomyces albus J1074. Microb Biotechnol 7:242–256. doi:10.1111/1751-7915.12116
Ostash B, Campbell J, Luzhetskyy A, Walker S (2013) MoeH5: a natural glycorandomizer from the moenomycin biosynthetic pathway. Mol Microbiol 90:1324–1338. doi:10.1111/mmi.12437
Pettersson BM, Kirsebom LA (2011) tRNA accumulation and suppression of the bldA phenotype during development in Streptomyces coelicolor. Mol Microbiol 79:1602–1614. doi:10.1111/j.1365-2958.2011.07543.x
Pope MK, Green BD, Westpheling J (1996) The bld mutants of Streptomyces coelicolor are defective in the regulation of carbon utilization, morphogenesis and cell–cell signalling. Mol Microbiol 19:747–756. doi:10.1046/j.1365-2958.1996.414933.x
Rebets YV, Ostash BO, Fukuhara M, Nakamura T, Fedorenko VO (2006) Expression of the regulatory protein LndI for landomycin E production in Streptomyces globisporus 1912 is controlled by the availability of tRNA for the rare UUA codon. FEMS Microbiol Lett 256(1):30–37
Rodriguez L, Oelkers C, Aguirrezabalaga I, Braña AF, Rohr J, Méndez C, Salas JA (2000) Generation of hybrid elloramycin analogs by combinatorial biosynthesis using genes from anthracycline-type and macrolide biosynthetic pathways. J Mol Microbiol Biotechnol 2(3):271–276
Rodríguez A, Olano C, Vilches C, Méndez C, Salas JA (1993) Streptomyces antibioticus contains at least three oleandomycin-resistance determinants, one of which shows similarity with proteins of the ABC-transporter superfamily. Mol Microbiol 8(3):571–582
Rokytskyy I, Koshla O, Fedorenko V, Ostash B (2016) Decoding options and accuracy of translation of developmentally regulated UUA codon in Streptomyces: bioinformatics analysis. Springerplus 5:982. doi:10.1186/s40064-016-2683-6
Sakai Y, Miyauchi K, Kimura S, Suzuki T (2016) Biogenesis and growth phase-dependent alteration of 5-methoxycarbonylmethoxyuridine in tRNA anticodons. Nucleic Acids Res 44:509–523. doi:10.1093/nar/gkv1470
Sambrook J, Russell DW (2001) Molecular cloning, a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New-York
Takano E, Tao M, Long F, Bibb MJ, Wang L, Li W, Buttner MJ, Bibb MJ, Deng ZX, Chater KF (2003) A rare leucine codon in adpA is implicated in the morphological defect of bldA mutants of Streptomyces coelicolor. Mol Microbiol 50:475–486
Trepanier N, Jensen S, Alexander D, Leskiw B (2002) The positive activator of cephamycin C and clavulanic acid production in Streptomyces clavuligerus is mistranslated in a bldA mutant. Microbiology 148:643–656
Urbonavicius J, Stahl G, Durand JM, Ben Salem SN, Qian Q, Farabaugh PJ, Björk GR (2003) Transfer RNA modifications that alter +1 frameshifting in general fail to affect −1 frameshifting. RNA 9:760–768. doi:10.1261/rna.5210803
Wang J, Schully KL, Pettis GS (2009) Growth-regulated expression of a bacteriocin, produced by the sweet potato pathogen Streptomyces ipomoeae, that exhibits interstrain inhibition. Appl Environ Microbiol 75:1236–1242. doi:10.1128/AEM.01598-08
Xu D, Kwon HJ, Suh JW (2008) S-Adenosylmethionine induces BldH and activates secondary metabolism by involving the TTA-codon control of bldH expression in Streptomyces lividans. Arch Microbiol 189:419–426. doi:10.1007/s00203-007-0336-4
Zaburannyi N, Rabyk M, Ostash B, Fedorenko V, Luzhetskyy A (2014) Insights into naturally minimised Streptomyces albus J1074 genome. BMC Genom 15:97. doi:10.1186/1471-2164-15-97
Zaburannyy N, Ostash B, Fedorenko V (2009) TTA Lynx: a web-based service for analysis of actinomycete genes containing rare TTA codon. Bioinformatics 25:2432–2443. doi:10.1093/bioinformatics/btp402
Acknowledgements
This work was supported by grant BG-41Nr from the Ministry of Education and Science of Ukraine (to B.O.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koshla, O., Lopatniuk, M., Rokytskyy, I. et al. Properties of Streptomyces albus J1074 mutant deficient in tRNALeu UAA gene bldA . Arch Microbiol 199, 1175–1183 (2017). https://doi.org/10.1007/s00203-017-1389-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-017-1389-7