Abstract
The 16S rDNA PCR-DGGE and rpoB quantitative PCR (RQ-PCR) techniques were used to evaluate the effects of dietary flavomycin and florfenicol on the autochthonous intestinal microbiota of hybrid tilapia. The fish were fed four diets: control, dietary flavomycin, florfenicol and their combination. After 8 weeks of feeding, 6 fish from each cage were randomly chosen for the analysis. The total number of intestinal bacteria was determined by RQ-PCR. The results showed that dietary antibiotics significantly influenced the intestinal microbiota and dramatically reduced the intensity of total intestinal bacterial counts. The intensity of some phylotypes (EU563257, EU563262 and EU563255) were reduced to non-detectable levels by both dietary antibiotics, while supplementation of florfenicol to the diet also reduced the intensity of the phylotypes EU563242 and EU563262, uncultured Mycobacterium sp.-like, uncultured Cyanobacterium-like and uncultured Cyanobacterium (EU563246). Dietary flavomycin only reduced the OTU intensity of one phylotype, identified as a member of the phylum Fusobacteria. The antibiotic combination only reduced the phylotypes EU563242 and EU563262. Based on our results, we conclude that the reduced effect of florfenicol on intestinal microbiota was stronger than that of flavomycin, and when flavomycin and florfenicol were added in combination, the effect of florfenicol overshadowed that of flavomycin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture animals are colonized by trillions of microorganisms that have a symbiotic relationship with their host and are distributed in gill, body surface and gastrointestinal (GI) tract (Frenkiel and Mouëza 1995; Armstrong et al. 2001; Izvekova et al. 2007). The majority of these microbes inhabits the GI tract and plays an important role in nutritional, physiological and pathological events (Denev et al. 2009; Merrifield et al. 2010; Nayak 2010). During the last decade, several studies have showed that the composition of fish intestinal microbiota is highly variable and is affected by the developmental stages, diet and environmental conditions (González et al. 1999; Ringø and Birkbeck 1999; Spanggaard et al. 2000; Huber et al. 2004).
In China, antibiotics as growth promoters are widely used in aquafeeds, especially flavomycin and florfenicol. Flavomycin is a glycolipid antibiotic produced by Streptomyces species and inhibits peptidoglycan polymerases through impairment of the transglycolase activities of penicillin-binding proteins (Butaye et al. 2003). Flavomycin is known to change the equilibrium of gut microbiota and is active primarily against Gram-positive bacteria but also to some extent against certain Gram-negative bacteria, such as Pasteurella and Brucella (Huber and Nesemann 1968). Bacteria species such as Clostridium perfringens and many other clostridia species, several species of Enterococcus, including E. gallinarum, E. casseliflavus, E. faecium, E. mundtii and E. hirae, are reported to be of natural resistance to flavomycin (Devriese 1980; Dutta and Devriese 1980, 1982; Butaye et al. 1998, 2000a, b, 2001). Another important antibiotic is florfenicol—a broad-spectrum bacteriostatic antibiotic binding the 50S ribosomal subunit of susceptible pathogens (Plumb 2004). This antibiotic is reported to be effective against important fish pathogens such as Yersinia ruckeri, Flavobacterium psychrophilum and Aeromonas salmonicida (Fukui et al. 1987; Samuelsen et al. 1998; Bruun et al. 2000), and Pasteurella multocida, Mannheimia haemolytica, Actinobacillus pleuropneumoniae and Streptococcus suis in vitro (Priebe and Schwarz 2003). However, the influence of antibiotics on intestinal microbiota has been determined by traditional culture-based method (Samuelsen et al. 1998; Bruun et al. 2000; Butaye et al. 2001). A large percentage of the intestinal microbiota cannot be cultured, resulting in limited understanding of the impact of antibiotics on the autochthonous intestinal microbiota. Consequently, the objective of the present study was to obtain better knowledge and understanding of the intestinal bacterial community of hybrid tilapia (Oreochromis niloticus ♀ × O. aureus ♂) reared in cages and how supplementation of dietary florfenicol and flavomycin, either singly or in combination, impacts the autochthonous intestinal microbiota.
Materials and methods
Experimental diets
Basal diets containing 26.0% protein and 3.0% lipid (Table 1) were formulated according to Li (2001). In the present study, four different diets were used: basal diet (CK), diet supplemented with 20 mg florfenicol kg−1, diet supplemented with 20 mg flavomycin kg−1 and a diet supplemented with 10 mg florfenicol kg−1 and 10 mg flavomycin kg−1.
Feeding trial
The culture experiment was conducted in a 4,000-m2 earthen pond at a local aquaculture farm, Jiaxing, Zhejiang, China. Juvenile hybrid tilapia (Oreochromis niloticus ♀ × O. aureus ♂) was acclimated in a floating net cage (4.0 m × 2.0 m × 1.5 m) for 2 weeks. Water depth of the pond was approximately 1.5 m. After 2 days of starvation, uniform fish (50.89 ± 0.27 g) were randomly distributed into 12 floating net cages (1.1 m × 1.1 m × 1.1 m). Each dietary group was fed in triplicate cages, and each cage contained 20 fish. The fish were hand-fed 3% of initial body weight three times a day (08:00, 11:30 and 17:30), and the feed ration was adjusted weekly to insure tilapias in each cage consume the diet pellets within 1 h. Each cage was individually aerated, and one tenth of the experimental pond water was exchanged by fully aerated tap water each week. During the feeding period, rearing temperature was 27.0 ± 3.0°C, while dissolved oxygen (DO) >5.0 mg oxygen l−1, pH 7.8, NH4 +-N < 0.50 mg nitrogen l−1 and NO2-N < 0.05 mg nitrogen l−1. The photoperiod was fixed at a natural condition from 5:00 to 19:00.
Sampling of the autochthonous gut microbiota
Six fish from each cage were randomly collected after 8-week feeding for gut bacterial analysis. Sampling of the autochthonous microbiota from the whole intestine was carried out after two days of starvation as previously described (Zhou et al. 2007). Briefly, the digestive tracts were aseptically removed in their entirety, slit open with a sterile scalpel, and the contents and non-adherent bacteria were rinsed three times in phosphate-buffered saline (PBS; 0.1 M, pH 7.2). The surface of each intestine was homogenized using a glass homogenizer as described elsewhere (LeaMaster et al. 1997) and stored in 2-ml Eppendorf tubes at −20°C until analysis (Zhou et al. 2009a).
DGGE analysis
Pooled gut samples from six fish in each cage (~200 mg) were used to avoid erroneous conclusions due to individual variation in gut microbiota as described by Ringø et al. (1995), Spanggaard et al. (2000) and He et al. (2009). The total genomic DNA from the pooled gut samples were extracted using cetyltrimethylammonium bromide (CTAB; Griffiths et al. 2000) and lysozyme methods (Miller et al. 1999) with some modifications. Briefly, the gut samples were mixed with 500 μl 5 mg ml−1 lysozyme solution. After incubation at 37°C for 2 h, 50 μl 10 mg ml−1 proteinase K (Sigma, St. Louis, MO, USA) was added and mixed gently, followed by incubation at 55°C for 20 min. Then 500 μl CTAB lysis buffer (100 mM Tris–HCl, 100 mM Na-EDTA, 1.5 M NaCl, 1% CTAB, 2% SDS, pH 8.0) was added and incubated at 65°C for 2 h. The DNA was recovered by precipitation with isopropanol and purified as described by Liu et al. (2008). The V3 region of the 16S rRNA gene was amplified with primers 338f (5′-ACTCCTACGGGAGGCAGCAG-3′) with a 40 base GC clamp at the 5′ end and 518r (5′-ATTACCGCGGCTGCTGG-3′). The 50-μl PCR reaction system contained 1× PCR buffer (20 mM Tris–HCl (pH 8.4) and 50 mM KCl), 200 μM dNTP, 500 nM each primer, 1.75 mM MgCl2, 670 ng μl−1 bovine serum albumin, 1.25 U Platinum® Taq DNA polymerase (Invitrogen, USA) and 2 μl purified DNA. The PCR conditions were as follows: 5 min of initial denaturation at 94°C, followed by 28 cycles of 30 s of denaturation at 94°C, 30-s annealing at 65°C (decreasing 1°C per cycle until 56°C), and 30-s extension at 72°C and a final extension at 72°C for 10 min. PCR products were examined by 2% agarose gel electrophoresis. DGGE was performed with a D-Code universal mutation system (Bio-Rad, Hercules, CA, USA). PCR products (~800 ng) were loaded onto polyacrylamide gels in 0.5× TAE buffer, with a gradient of 40–60% denaturant. Electrophoresis was performed at 60°C, 65 V for 16 h. After electrophoresis, gels were stained for 20 min in distilled water containing ethidium bromide (0.5 μg ml−1) and visualized under UV light. DGGE bands were excised from the gels, resuspended in 100 μl distilled water and kept at 4°C overnight. The supernatant was used as the template for a second round of PCR under the same conditions. The PCR products were ligated into pGEM-T Easy vector (Promega, Madison, WI, USA) for sequencing (Invitrogen, Shanghai, China). Representative sequences were deposited in the NCBI database under accession numbers EU563242–EU563265.
Total intestinal bacteria analysis
Enumeration of total bacteria was conducted by real-time PCR according to Takahashi et al. (2006) and Silkie and Nelson (2009) with some modifications. Briefly, several cultured bacteria were selected based on the predominant microbiota present in DGGE with an abundance index greater than 5%. In this study, Clostridium thermocellum B108 (Gram-positive bacteria) and Sphingomonas sp. B222 (Gram-negative bacteria) were chosen as standards. Both bacterial species were cultured overnight in Luria–Bertani (LB) medium, and the total number of bacteria was counted using a hemocytometer. Thereafter, each bacterial strain was mixed equally at 0.5 × 108 cells ml−1, and total genomic DNA was extracted from 1 ml of the combined mixture using CTAB (Griffiths et al. 2000) and lysozyme methods (Miller et al. 1999). To increase the concentration of purified intestinal DNA, the genomic DNA was pre-cooled at −70°C for ~2 h, freeze-dried (CHRIST, Osterode, Germany) overnight and solved in TE buffer to the ideal concentration of 50 ng μl−1.
Serial dilutions of standards at 103, 104, 105 and 106 CFU per template reaction were prepared for calibration. The RNA polymerase β-subunit gene (rpoB; one copy in bacteria) was amplified using the primers rpoB1698f (5′-AACATCGGTTTGATCAAC-3′) and rpoB2041r (5′-CGTTGCATGTTGGTACCCAT-3′; Dahllöf et al. 2000). The reaction mixture (20 μl) was prepared according to the manufacturer’s protocol: 7 μl PCR-grade water, 1 μl each primer (5 μmol l−1), 10 μl 2× real-time PCR master mix (SYBR Green; TOYOBO, Shanghai, China) and 1 μl DNA template (50 ng μl−1). The PCR conditions consisted of initial denaturation at 95°C for 5 min, 40 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 30 s, with a final extension step at 72°C for 5 min.
The concentration of each standard (CFU ml−1) was inputted into the LightCycler 2.0 software using the threshold cycle value (CT) to construct a standard for absolute quantification analysis. The number of bacteria present in unknown samples (12 samples) was calculated based on the standard curve. Each sample was analyzed in at least four replicates.
Statistical analysis
The gel images were analyzed using the public domain NIH Image program to calculate relative abundance (RA, %; Simpson et al. 1999). Cluster analysis was performed based on the unweighted pair group method using the arithmetic mean algorithm (UPGMA) by the program NTSYS. In this study, pairwise similarity coefficient (Cs) less than 0.60 is regarded as significant difference; while 0.60 ≤ Cs < 0.85 is marginal difference, and Cs ≥ 0.85 is very similar according to Sun et al. (2004). The Shannon index of bacterial diversity, H, was calculated as Shannon and Weaver (1963) described.
Results are presented as mean ± SD. Data were subjected to one-way ANOVA to test the effect of dietary treatment. When significant differences were detected (P < 0.05), Duncan’s multiple range test was used to compare mean values among dietary treatments. All statistical analysis was carried out using the statistic software SPSS version 10.0.
Results
DGGE profiles of intestinal microbiota in tilapia
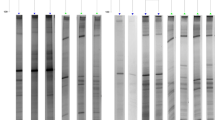
Bacterial DGGE profiles of four different treatments showed significant differences (Fig. 1, Tables 2, 4), and the difference of the interior-group was more significant than that of inter-group. There were 18.67 ± 0.47, 15.00 ± 0.82, 14.33 ± 0.47 and 14.66 ± 0.47 OTUs in CK, flavomycin, florfenicol and antibiotic combination groups, respectively (Table 2). The pairwise similarity coefficients (Cs) matrix for the intestinal bacterial community of hybrid tilapia based on the DGGE fingerprints is shown in Table 3. The bacterial community in flavomycin group was similar to that of CK with a Cs value of 0.84. The bacterial community of CK was marginally different to that of florfenicol and the combination of flavomycin and florfenicol.
DGGE profile generated from the V3-16S rDNA fragments of the bacteria from the intestinal wall of hybrid tilapia O. niloticus ♀ × O. aureus ♂. CK1–3 represent samples taken from the intestine of tilapia fed control diet without antibiotic supplement; Fm1–3 are samples from the intestine of tilapia fed diet supplemented with flavomycin; Fn1–3 are samples from the intestine of tilapia fed diet supplemented with florfenicol; and FF1–3 are samples from the intestine of tilapia fed diet supplemented with flavomycin and florfenicol
Identification of dominant DGGE bands
A total of 19 representative OTUs were retrieved from the bacterial DGGE profiles (Table 4). Proteobacteria (2 OTUs), Actinobacteria (2 OTUs), Cyanobacteria (3 OTUs), Fusobacterium (1 OTU) and Firmicutes (1 OTU) were the predominant autochthonous bacteria in hybrid tilapia intestine. The relative abundance (RA) results showed that OTU 3 (uncultured bacterium, EU418508), 7 (uncultured bacterium, EF532770), 8 (uncultured Cyanobacterium, EF630240), 10 (uncultured prokaryote, AJ867878), 11 (uncultured bacterium clone, DQ675149), 13 (Sphingomonas sp., EU442226) and 18 (uncultured β-Proteobacterium, EF697165) were not affected by dietary antibiotics. In contrast to these results, the intensities of OTU 4 (uncultured bacterium, AB206034), 12 (Streptomyces sp., EU159565) and 19 (uncultured prokaryote, AJ867878) were reduced to non-detectable levels by dietary flavomycin and florfenicol. One interesting observation was that OTU 17, an uncultured bacterium with 100% similarity to accession no. EF599665, was reduced to non-detectable levels by dietary florfenicol and the antibiotic combination, but no difference in RA was observed between the control group (CK) and fish that received flavomycin. Compared with CK, supplementation of dietary florfenicol decreased the intensities of OTU 1 (uncultured bacterium, AJ504589), 2 (uncultured Cyanobacterium, DQ158167), 5 (uncultured Cyanobacterium, EU751409) and 14 (uncultured Mycobacterium sp., EF438322), but the intensities of OTU 9 (uncultured bacterium, EF669487) and 16 (uncultured bacterium, AJ548786) were elevated. An interesting finding was that the intensity of OTU 15 on the DGGE, identified as a member of the phylum Fusobacteria, was reduced only by dietary flavomycin. The combination of the two antibiotics significantly decreased OTU 1 as a RA value of 1.37 ± 0.12 was observed in the combined antibiotics group compared to the RA value of 2.60 ± 0.24 in CK.
Total intestinal bacterial counts
The total intestinal bacterial counts analyzed by RQ-PCR varied from 0.20 ± 0.01 to 1.11 ± 0.10 CFU × 107 g−1 as shown in Table 2. Supplementation of dietary florfenicol and flavomycin significantly reduced (P < 0.05) the intestinal bacteria. Shannon diversity indexes (H) of the antibiotics treatments were significantly lower (P < 0.05) than that of CK. The value of H for florfenicol group was higher than that of flavomycin and antibiotic combination (P < 0.05), but the Shannon equitability indexes (E H) were not affected by dietary antibiotics compared to CK (P > 0.05).
Discussion
In the present study, the autochthonous intestinal microbiota was significantly modulated by flavomycin. This result is consistent with previous reports that flavomycin reduced the incidence of the animal pathogens Salmonella and Clostridium in pre-slaughter broilers (Bolder et al. 1999) and modulated the ruminal gut microbiota (Edwards et al. 2005). In the study of broiler chicks, Gunal et al. (2006) demonstrated that the counts of total bacteria and Gram-negative bacteria were significantly decreased by flavomycin after 21 and 42 days of feeding. Zhou et al. (2009b) observed that dietary flavomycin affected the autochthonous intestinal bacterial community in tilapia. In the present study, several intestinal phylotypes such as Streptomyces sp.-like bacterium, uncultured bacterium (EU563262), uncultured prokaryote-like bacterium and Fusobacteria bacterium (EU563264) were reduced by flavomycin. Fusobacterium species are Gram-negative bacteria (Garcia et al. 1992) and have been reported in bovine rumen, pig, poultry and fish (GU301238; Tan et al. 1996; Anderson et al. 2000; Edwards et al. 2005). F. necrophorum is generally regarded as an opportunistic pathogen (Brazier et al. 2002) but has been reported to be inhibited by flavomycin in a study of sheep (Edwards et al. 2005). More recently, Jeong et al. (2009) showed that Fusobacterium spp. in human gut were susceptible to flavomycin. Previous studies reported that Fusobacterium spp. have a very high rate of deamination by converting excessive dietary amino acids to ammonia (Russell et al. 1991; Attwood et al. 1998), and suppression of Fusobacterium by flavomycin has been reported to have a favorable effect on nitrogen metabolism (Edwards et al. 2005).
The present study showed that supplementation of dietary florfenicol reduced a number of autochthonous intestinal bacteria in tilapia compared to flavomycin. For example, the intensity of Streptomyces sp.-like bacterium, uncultured bacteria (EU563262 and EU563265) and uncultured prokaryote-like bacterium were reduced to non-detectable levels by dietary florfenicol. On the other hand, some gut bacteria including uncultured Mycobacterium sp.-like bacterium, uncultured Cyanobacterium-like bacterium, uncultured Cyanobacterium (EU563246) and uncultured bacterium (EU563242) were partly decreased. Mycobacteria are obligate aerobic, acid-fast, Gram-positive, non-spore forming, non-motile and prevalent in soil and water (Frerichs 1993). In two recent studies (He et al. 2009; Zhou et al. 2009a) using DGGE, Mycobacterium sp.-like bacteria were detected in the tilapia intestine. In the present study, florfenicol affected the RA value of Mycobacterium sp.-like bacteria from 1.40 ± 0.16 to 0.93 ± 0.21; however, dietary flavomycin had no effect.
Three species of Cyanobacteria were detected in the tilapia intestine (Table 4). Cyanobacteria possess the capability to store abundant nutrients, and some species can convert gaseous nitrogen to ammonia via nitrogen fixation (Stewart 1967). Cyanobacteria are well known for their ability to produce a large number of diverse secondary metabolites (Vining 1992), which cause mortality, initiate or promote tumors or deteriorate the health of several cultivated species (nile tilapia, catfish, white shrimp and rainbow trout; Smith et al. 2008). Previous investigations have reported Cyanobacteria in the intestine of filter-feeding fish such as Atlantic menhaden (Brevoortia tyrannus), silver carp (Hypophthalmichtyhs molitrix) or tilapia (Oreochromis niloticus ♀ × O. aureus ♂; Friedland et al. 2005; Kolmakov et al. 2006; He et al. 2009). In the present study, two species of Cyanobacteria were reduced by florfenicol.
Based on the results of Cs matrix and dendrogram (data not shown), the bacterial community in antibiotic combination group was very similar to that of florfenicol group. The effect of antibiotic combination on intestinal bacteria was highly similar to that observed for the florfenicol group, as the intensities of some phylotypes (EU563242, EU563255, EU563257, EU563262, and EU563265) were reduced to non-detectable levels in both the florfenicol and antibiotic combination groups. Finally, the total number of bacteria in the antibiotic combination group was intervenient between both dietary antibiotic groups. Thus, we concluded that the effect of antibiotic combination on intestinal bacteria was not reinforced, but the effect of florfenicol overshadowed that of flavomycin. In the present study, tilapia were fed 20 mg kg−1 of florfenicol or flavomycin, but in combination, the dosage of each was 10 mg kg−1. Previous studies showed that the effects of antibiotic on pathogens are heavily dependent on dosage (Akinbowale et al. 2007; Lai et al. 2009). Florfenicol at a daily dose of 10 mg kg−1 was effective in decreasing mortalities induced by Edwardsiella ictaluri in channel catfish (Ictalurus punctatus; Gaunt 2004), and florfenicol showed high antimicrobial activities (MIC90 ≤ 1 mg ml−1) in vitro against Actinobacillus pleuropnemoniae, Pasteurella ultocida and Mannheimia haemolytica at 0.12–4.00 mg ml−1 (Shin et al. 2005). Future work should aim to enhance our understanding of the effect of dietary florfenicol on gut microbiota in tilapia. In our study, it is interesting to find that some low-abundance bacteria in fish intestine were more easily affected by antibiotics administration (Table 4). Whether this part of bacteria is disadvantaged groups or contributed to the PCR bias still need further confirmation.
In conclusion, dietary florfenicol and flavomycin supplemented alone or in combination could affect the autochthonous intestinal microbiota. The intensity of some bacteria was reduced to non-detectable levels by dietary antibiotics, and the total bacterial diversity was also decreased following the administration of antibiotics. When antibiotics combined together, the effect of florfenicol overshadowed that of flavomycin.
References
Akinbowale OL, Peng HH, Grant P, Barton MD (2007) Antibiotic and heavy metal resistance in motile aeromonads and pseudomonads from rainbow trout (Oncorhynchus mykiss) farms in Australia. Int J Antimicrob Agents 30:177–182
Anderson DB, McCracken VJ, Aminov RI, Simpson JM, Mackie RI, Verstegen MW A, Gaskins HR (2000) Gut microbiology and growth-promoting antibiotics in swine. Nutr Abstr Rev 70:101–108
Armstrong E, Yan LM, Boyd KG, Wright PC, Burgess JG (2001) The symbiotic role of marine microbes on living surfaces. Hydrobiologia 461:37–40
Attwood GT, Klieve AV, Ouwerkerk D, Patel BKC (1998) Ammonia-hyperproducing bacteria from New Zealand ruminants. Appl Environ Microbiol 64:1796–1804
Bolder NM, Wagenar JA, Putirulan FF, Veldman KT, Sommer M (1999) The effect of flavophospholipol (flavomycin) and salinomycin sodium (Sacox) on the excertion of Clostridium perfringens, Salmonella enteritidis, and Campylobacter jejuni in broilers after experimental infection. Poultry Sci 78:1681–1689
Brazier JS, Hall V, Yusuf E, Duerden BI (2002) Fusobacterium necrophorum infections in England and Wales 1990–2000. J Med Microbiol 51:269–272
Bruun MS, Schmidt AS, Madsen L, Dalsgaard I (2000) Antimicrobial resistance patterns in Danish isolates of Flavobacterium psychrophilum. Aquaculture 187:201–212
Butaye P, Devriese LA, Haesebrouck F (1998) Effects of different test conditions on MICs of food animal growth-promoting antibacterial agents for enterococci. J Clin Microbiol 36:1907–1911
Butaye P, Devriese LA, Haesebrouck F (2000a) Effects of different medium supplements on the MICs of the growth-promoting antibiotic bambermycin against enterococci. J Antimicrob Chemother 46:713–716
Butaye P, van Damme K, Devriese LA, van Damme L, Baele M, Lauwers S, Haesebrouck F (2000b) In vitro susceptibility of Enterococcus faecium isolated from food to growth-promoting and therapeutic antibiotics. Int J Food Microbiol 54:181–187
Butaye P, Devriese LA, Haesebrouck F (2001) Differences in antibiotics resistance patterns of Enterococcus faecalis and Enterococcus faecium from farm and pet animals. Antimicrob Agents Chemother 45:1374–1378
Butaye P, Devriese LA, Haesebrouck F (2003) Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin Microbiol Rev 16:175–188
Dahllöf I, Baillie H, Kjelleberg S (2000) rpoB-based microbial community analysis avoids limitations inherent in 16S rRNA gene intraspecies heterogeneity. Appl Environ Microbiol 66:3376–3380
DeBruyn JM, Chewning CS, Sayler GS (2007) Comparative quantitative prevalence of Mycobacteria and functionally abundant nidA, nahAc and nagAc dioxygenase genes in coal tar contaminated sediments. Environment 41:5426–5432
Denev S, Staykov Y, Moutafchieva R, Beev B (2009) Microbial ecology of the gastrointestinal tract of fish and the potential application of probiotics and prebiotics in finfish aquaculture. Int Aquac Res 1:1–29
Devriese LA (1980) Two new types of resistance to lincomycin in pathogenic staphylococci from animals. Ann Microbiol 131:261–266
Dutta GN, Devriese LA (1980) Susceptibility of Clostridium perfringens of animal origin to fifteen antimicrobial agents. J Vet Pharmacol Therp 3:227–236
Dutta GN, Devriese LA (1982) Susceptibility of fecal streptococci of poultry origin to nine growth promoting agents. Appl Environ Microbiol 44:832–837
Edwards JE, McEwan NR, McKain N, Walker N, Wallace RJ (2005) Influence of flavomycin on ruminal fermentation and microbial populations in sheep. Microbiology 151:717–725
Finegold SM, Vaisanen ML, Molitoris DR, Tomzynski TJ, Song Y, Liu CX, Collins MD, Lawson PA (2003) Cetobacterium somerae sp. nov. from human feces and emended description of the genus Cetobacterium. Syst Appl Microbiol 26:177–181
Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104:13780–13785
Frenkiel L, Mouëza M (1995) Gill ultrastructure and symbiotic bacteria in Codakia orbicularis (Bivalvia, Lucinidae). Zoomorphology 115:51–61
Frerichs GN (1993) Mycobacteriosis: nocardiosis. In: Bacterial Diseases of Fish (Inglis V, Roberts RJ, Bromage NR ed.). Blackwell, London, pp 219–235
Friedland KD, Ahrenholz DW, Hass LW (2005) Viable gut passage of cyanobaceria through the filter-feeding fish Atlantic menhaden, Brevoortia tyrannus. J Plantkton Res 27:715–718
Fukui H, Fujihara Y, Kano T (1987) In vitro and in vivo antibacterial activities of florfenicol, a new fluorinated analog of thiamphenicol, against fish pathogens. Fish Pathol 22:201–207
Garcia MM, Becker SA, Brooks BW, Berg JN, Finegold SM (1992) Ultrastructure and molecular characterization of Fusobacterium necrophorum biovars. Can J Vet Res 56:318–325
Gaunt P (2004) Determination of dose rate of florfenicol in feed for control of mortality in channel catfish Ictalurus punctatus (Rafinesque) infected with Edwardsiella ictaluri, etiological agent of Eneteric septicemia. World J Aquacul Soc 35:257–267
González CJ, López-Díaz TM, García-López ML, Prieto M, Otero A (1999) Bacterial microflora of wild brown trout (Salmo trutta), wild pike (Esox lucius), and aquacultured rainbow trout (Oncorhynchus mykiss). J Food Prot 62:1270–1277
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2000) Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66:5488–5491
Gunal M, Yayli G, Kaya O, Karahan N, Sulak O (2006) The effects of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. Int J Poul Sci 5:149–155
He SX, Zhou ZG, Liu YC, Shi PJ, Yao B, Ringø E, Yoon I (2009) Effects of dietary Saccharomyces cerevisiae fermentation product (DVAQUA®) on growth performance, intestinal autochthonous bacterial community and non-specific immunity of hybrid tilapia (Oreochromis niloticus ♀ × O. aureus ♂) cultured in cages. Aquaculture 294:99–107
Huber G, Nesemann G (1968) Moenomycin, an inbibitor of cell wall synthesis. Biophys Res Commun 30:7–13
Huber I, Spanggaard B, Appel KF, Rossen L, Neilson T, Gram L (2004) Phylogenetic analysis and in situ identification of the intestinal microbial community of rainbow trout (Oncorhynchus mykiss Walbaum). J Appl Microbiol 96:117–132
Izvekova GI, Izvekov EI, Plotnikov AO (2007) Symbiotic microflora in fishes of different ecological groups. Biol Bull 34:610–618
Jeong SH, Song YK, Cho JH (2009) Risk assessment of ciprofloxacin, flavomycin, olaquindox and colistin sulfate based on microbiological impact on human gut biota. Regul Toxicol Pharm 53:209–216
Kolmakov VI, Gladyshev MI, Kravchuk ES, Chuprov SM, Anishchenkob OV, Ivanova EA, Trusova MY (2006) Species-specific stimulation of Cyanobacteria by silver carp Hypophthalmichtyhs molitrix (Val.). Dokl Biol Sci 408:283–285
Lai HT, Hou JH, Su CI, Chen CL (2009) Effects of chloramphenicol, florfenicol, and thiamphenicol on growth of algae Chlorella pyrenoidosa, Isochrysis galbana, and Tetraselmis chui. Ecotoxicol Environ Saf 72:329–334
LeaMaster BR, Walsh WA, Brock JA, Fujioka RS (1997) Cold stress-induced changes in the aerobic heterotrophic gastrointestinal tract bacterial flora of red hybrid tilapia. J Fish Biol 50:770–780
Li Q (2001) Analysis of the processing technology for expanded aquafeed. Chin Food Feed Proc 8:18–19 (article in Chinese)
Liu YC, Zhou ZG, Yao B, Shi PJ, He SX, Hølvold LB, Ringø E (2008) Effect of intraperitoneal injection of immunostimulatory substances on allochthonous gut microbiota of Atlantic salmon (Salmo salar L.) determined using denaturing gradient gel electrophoresis. Aquacult Res 39:635–646
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bøgwald J, Castex M, Ringø E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302:1–18
Miller DN, Bryant JE, Madsen EL, Ghirose WC (1999) Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl Environ Microbiol 65:4715–4724
Mohamed NM, Enticknap JJ, Lohr JE, McIntosh SM, Hill RT (2008) Changes in bacterial communities of the marine sponge Mycale laxissima on transfer into aquaculture. Appl Environ Microbiol 74:1209–1222
Nayak SK (2010) Role of gastrointestinal microbiota in fish. Aqual Res 1–21. doi:10.1111/j.1365-2109.2010.02546.x
Nehme B, Letourneau V, Forster RJ, Veillette M, Duchaine C (2008) Culture-independent approach of the bacterial bioaerosol diversity in the standard swine confinement buildings, and assessment of the seasonal effect. Environ Microbiol 10:665–675
Osaka T, Yoshie S, Tsuneda S, Hirata A, Inamori Y (2006) Identification of acetate- or methanol-assimilating bacteria under nitrate-reducing conditions by stable-isotope probing. Microb Eco 52:253–266
Pearce DA, van der Gast CJ, Lawley B, Ellis-Evans JC (2003) Bacterioplankton community diversity in a maritime Antarctic lake determined by culture dependent and culture independent techniques. FEMS Microbiol Ecol 45:59–70
Plumb D (2004) Florfenicol. In: Plumb D (ed) Veterinary drug handbook, 5th edn. Iowa State Press, Ames, IA, pp 335–336
Priebe S, Schwarz S (2003) In vitro activities of florfenicol against bovine and porcine respiratory tract pathogens. Antimicrob Agents Chemother 47:2703–2705
Ringø E, Birkbeck TH (1999) Intestinal microflora of fish larvae and fry. Aquacult Res 30:73–93
Ringø E, Strøm E, Tabachek JA (1995) Intestinal microflora of salmonids: a review. Aquacult Res 26:773–789
Russell JB, Onodera R, Hino T (1991) Ruminal protein fermentation: new perspectives on previous contradictions. In: Tsuda T, Sasaki Y, Kawashima R (eds) Physiological aspects of digestion and metabolism in ruminants. Academic Press, San Diego, pp 681–697
Samuelsen OB, Hjeltnes B, Glette J (1998) Efficacy of orally administered florfenicol in the treatment of furunculosis in Atlantic salmon. J Aquat Anim Health 10:56–61
Shannon CE, Weaver W (1963) The mathematical theory of communication. University Illinois Press, Urbana, p 117
Shin SJ, Kang SG, Nabin R, Kang ML, Yoo HS (2005) Evaluation of the antimicrobial activity of florfenicol against bacteria isolated from bovine and porcine respiratory disease. Vet Microbiol 106:73–77
Silkie SS, Nelson KL (2009) Concentrations of host-specific and generic fecal markers measured by quantitative PCR in raw sewage and fresh animal feces. Water Res 43:4860–4871
Simpson JM, McCracken VJ, White BA, Gaskins HR, Mackie RI (1999) Application of denaturant gradient gel electrophoresis for the analysis of the porcine gastrointestinal microbiota. J Microbiol Meth 36:167–179
Smith JL, Boyer GL, Zimba PV (2008) A review of cyanobacterial odorous and bioactive metabolites: impact and management alternatives in aquaculture. Aquaculture 280:5–20
Spanggaard B, Huber I, Nielson J, Nielson T, Appel KF, Gram L (2000) The microflora of rainbow trout intestine: a comparison of traditional and molecular identification. Aquaculture 182:1–15
Stewart WDP (1967) Nitrogen-fixing plants: the role of biological agents as providers of combined nitrogen is discussed. Science 158:1426–1432
Sun HY, Deng SP, Raun WR (2004) Bacterial community structure and diversity in a century-old manure-treated agroecosystem. Appl Environ Microbiol 70:5868–5874
Takahashi H, Konuma H, Hara-Kudo Y (2006) Development of a quantitative real-Time PCR method to enumerate total bacterial counts in ready-to-eat fruits and vegetables. J Food Prot 69:2504–2508
Tan ZL, Nagaraja TG, Chengappa MM (1996) Fusobacterium necrophorum infections: virulence factors, pathogenic mechanism and control measures. Vet Res Commun 20:113–140
Uz I, Ogram AV (2006) Cellulolytic and fermentative guilds in eutrophic soils of the Florida everglades. FEMS Microbiol Ecol 57:396–408
Vining LC (1992) Secondary metabolites-inventive evolution or biochemical diversity—a review. Gene 115:135–140
Zhou ZG, Ding ZK, Lv HY (2007) Effects of dietary short chain fructo-oligosaccharides on intestinal microflora, mortality and growth performance of juvenile white shrimp, Litopenaeus vannamei. J World Aquacult Soc 38:296–301
Zhou ZG, He SX, Liu YC, Shi PJ, Huang GX, Yao B (2009a) The effects of dietary yeast culture or short-chain fructo-oligosaccharides on the autochthonous intestinal bacterial communities in juvenile hybrid tilapia Oreochromis niloticus ♀ × O. aureus ♂. J World Aquacult Soc 40:450–459
Zhou ZG, Liu YC, He SX, Shi PJ, Gao XH, Yao B, Ringø E (2009b) Effects of dietary potassium diformate (KDF) on growth performance, feed conversion and intestinal bacterial community of hybrid tilapia (Oreochromis niloticus ♀ × O. aureus ♂). Aquaculture 291:89–94
Acknowledgments
This research was supported by the National High Technology Research and Development Program of China (863 Program; grant 2007AA100601) and the Key Program of Transgenic Plant Breeding (2009ZX08003-020B).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Shuang-Jiang Liu.
Rights and permissions
About this article
Cite this article
He, S., Zhou, Z., Liu, Y. et al. Effects of the antibiotic growth promoters flavomycin and florfenicol on the autochthonous intestinal microbiota of hybrid tilapia (Oreochromis niloticus ♀ × O. aureus ♂). Arch Microbiol 192, 985–994 (2010). https://doi.org/10.1007/s00203-010-0627-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-010-0627-z