Abstract
The twin-arginine transport (Tat) system is dedicated to the translocation of folded proteins across the bacterial cytoplasmic membrane. Proteins are targeted to the Tat system by signal peptides containing a twin-arginine motif. In Salmonella enterica serovar Typhimurium and Escherichia coli many Tat substrates are known or predicted to bind a molybdenum cofactor in the cytoplasm prior to export. In the case of N- and S-oxide reductases, co-ordination of molybdenum cofactor insertion with protein export involves a ‘Tat proofreading’ process where chaperones of the TorD family bind the signal peptides, thus preventing premature export. Here, a genetic approach was taken to determine factors required for selenate reductase activity in Salmonella and E. coli. It is reported for both biological systems that an active Tat translocase and a TorD-like chaperone (DmsD) are required for complete in vivo reduction of selenate to elemental red selenium. Further mutagenesis and in vitro biophysical experiments implicate the Salmonella ynfE gene product, and the E. coli YnfE and YnfF proteins, as putative Tat-targeted selenate reductases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The twin-arginine translocation (Tat) apparatus is a protein translocation system found in the cytoplasmic membranes of many bacteria and archaea (Berks et al. 2003). Proteins routed through the Tat pathway are synthesised as precursors with N-terminal signal peptides bearing a conserved SRRxFLK ‘twin-arginine’ amino acid motif (Berks 1996) and transported in a fully folded conformation (DeLisa et al. 2003).
Salmonella enterica serovar Typhimurium (hereafter ‘Salmonella’) and Escherichia coli are genetically related enteric bacteria and members of the γ-Proteobacteria. In these biological systems the majority of known, or predicted, Tat-targeted proteins are complex redox enzymes that must acquire their prosthetic groups in the cytoplasm prior to the export event (Sargent 2007). Furthermore, a high proportion of these are ‘molybdo-enzymes’ that are known, or predicted, to contain a version of the molybdenum cofactor at their active sites (Kisker et al. 1997). In E. coli, Tat-targeted molybdo-enzymes are often subjected to a ‘Tat proofreading’ process prior to export, where a specific binding-protein (or ‘Tat chaperone’) interacts directly with a Tat signal peptide, possibly to prevent premature export until all other biosynthetic processes have been completed (Sargent 2007). The most well-studied examples of Tat proofreading chaperones are the E. coli TorD (Buchanan et al. 2008), DmsD (Chan et al. 2008), and NapD (Maillard et al. 2007) systems, and homologs of these can be identified encoded by a vast number of prokaryotic genomes (Turner et al. 2004).
Molybdo-enzymes are widespread in nature and have been harnessed for the catalysis of two-electron oxidoreductase reactions across all domains of life (Kisker et al. 1997). Selenium is a metalloid trace element, chemically related to sulphur, and is utilised in a number of diverse enzymatic reactions (Stolz et al. 2006). Under aerobic conditions environmental selenium exists as the soluble oxyanions selenate (SeO4 2–) and selenite (SeO3 2–). A number of prokaryotes, including E. coli (Turner et al. 1998), can metabolise these oxyanions to insoluble, red, elemental selenium, which is thought to accumulate as nanoparticles on the surface of the cell (Gerrard et al. 1974; Stolz et al. 2006). The route of selenate reduction to elemental selenium is understood to proceed via a selenite intermediate and thus the action of a selenate reductase in concert with a separate selenite reductase is required for the complete reduction of selenate. Some bacteria can use selenate as a respiratory electron acceptor, the best example being Thauera selenatis (Schröder et al. 1997). The T. selenatis selenate reductase is encoded by the serABDC operon (Krafft et al. 2000) and is a soluble, periplasmic, heterotrimeric enzyme comprising a molybdo-protein subunit (SerA), a Fe–S cluster-containing subunit (SerB), and a b-type cytochrome (SerC). The serD gene product is a member of the TorD family of peptide-binding proteins (Turner et al. 2004) and is therefore probably a Tat-proofreading chaperone that interacts with the Tat signal peptide of the SerA subunit. A related, but membrane-bound, selenate reductase has been identified in Enterobacter cloacae SLD1a-1 where the molybdo-subunit is also believed to be oriented towards the periplasmic side of the membrane by the Tat translocase (Watts et al. 2003; Ridley et al. 2006; Ma et al. 2007).
While it is well established that E. coli can reduce exogenous selenate or selenite to elemental red selenium (Gerrard et al. 1974; Turner et al. 1998), the precise identity of both the selenate and selenite reductase enzymes remains a contentious issue. For example, in contrast to the well-characterised molybdenum-dependent and Tat-targeted selenate reductase from Thauera selenatis, selenate reductase activity in E. coli has been variably assigned to the membrane-bound, but cytoplasmically-oriented, respiratory nitrate reductases (Avazeri et al. 1997) or to a cytoplasmic YgfNM molybdo-protein complex (Bebien et al. 2002).
In this work, we used the complete reduction of selenate to elemental red selenium in vivo as a facile reporter screen to first establish, and then genetically dissect, selenate reductase activity in Salmonella. We constructed a bank of isogenic deletion strains in Salmonella that demonstrated that both the tat operon (STM3973-75) and the dmsD gene (STM1495) were essential for selenate, but not selenite, reduction to elemental red selenium. This observation led us to re-examine the role of the Tat pathway in selenate reductase activity in E. coli. As with Salmonella, we show here that the Tat translocase and the DmsD Tat chaperone are essential for in vivo selenate reduction in E. coli. We go on to identify putative Tat-targeted molybdo-enzymes in both organisms that are required for selenate reduction in vivo.
Materials and methods
Bacterial strains and plasmids
Strains used in this study are listed in Table 1. For Salmonella studies, in-frame deletion mutants were constructed using the method of Datsenko and Wanner (2000), originally developed for E. coli and adapted here for Salmonella. For E. coli studies using the MC4100 parent strain, in-frame deletion mutants were constructed using the method of Datsenko and Wanner (2000). Experiments based on the E. coli BW25113 parent strain utilised the ‘Keio collection’ of non-lethal deletion/insertion mutants (Baba et al. 2006).
For complementation assays, the complete ynfE and ynfF genes from E. coli K12 were amplified by PCR and cloned into the pSU-PROM (KanR) vector (Jack et al. 2004), which enables constitutive expression from the tat promoter. The resultant plasmids were termed pSU-YnfE and pSU-YnfF. Where indicated, the E. coli dmsD gene was expressed in trans from vector pSU-DmsD (Jack et al. 2004).
Assay conditions for in vivo selenate reduction
For standard ‘microaerobic’ growth, sealed Universal tubes (25 ml) containing 5 ml LB broth were inoculated at 1:100 dilution from overnight pre-cultures and incubated for 24 h at 37°C with shaking at 200 rpm. For true ‘aerobic’ growth, 10 ml of LB broth in a 250-ml conical flask, stoppered with a porous foam bung, was used. For true ‘anaerobic’ growth, a tightly sealed Universal was filled to the brim with LB broth and incubated without shaking. Where indicated, sodium selenate (Na2SeO4 2–) or sodium selenite (Na2SeO3 2–) was added to a final concentration of 10 mM, and sodium tungstate was added to a final concentration of 1 mM.
Protein production and purification
The pQE-DmsDST plasmid encodes Salmonella DmsD with a C-terminal hexahistidine tag. The vector was prepared by amplifying the Salmonella dmsD gene with primers 5′-GCGCGCCCATGGCCACTTTTTTACAACGTGATG-3′ and 5′-GCGCGCAGATCTACGGAATAACGGTTTTACAGC-3′ and cloning as an NcoI/BglII fragment into pQE60 (Qiagen). The protein was overproduced in MC4100 cells containing the pREP4 plasmid (Roche) encoding lacI. Recombinant DmsD monomer was prepared by immobilised metal affinity chromatography using a 5-ml HisTrap Sepharose column (GE Healthcare) and ion-exchange chromatography using a 5-ml HiTrap Q Sepharose column (GE Healthcare).
The pQM plasmid encodes a MalE protein with a flexible C-terminal extension punctuated by a hexahistidine tag and, using the unique in-frame XmaI and NcoI sites in this vector, fusions can be generated in which a peptide is located C-terminal to MalE and between the Factor Xa site and the hexahistidine tag (Maillard et al. 2007). DNA encoding the Salmonella DmsA, YnfE and YnfF twin-arginine signal peptides (DmsASP, YnfESP and YnfFSP, respectively) was separately amplified and cloned into pQM to give the pQM-DmsASP-ST, pQM-YnfESP-ST and pQM-YnfFSP-ST vectors, respectively. The MalE-signal peptide fusions were overproduced and purified as described (Maillard et al. 2007).
Isothermal titration calorimetry
ITC was performed at 28°C in a VP-ITC microcalorimeter (MicroCal Inc.) and samples were dialysed overnight into the same buffer (typically 50 mM Tris.HCl pH 7.5). Routinely, 500 μl of 100–150 μM DmsD monomer was loaded into the syringe, while the sample cell contained 1.4 ml of the MalE-signal peptide fusion protein (10–15 μM). Typically, titrations consisted of 25–35 × 8–10 μl injections of the DmsDhis protein. Data analysis was performed with the Origin software supplied by MicroCal Inc.
Results
Anaerobic, Tat-dependent selenate reductase activity in Salmonella and E. coli
To examine whether Salmonella possesses the ability to fully reduce selenate to selenium, the LT2 strain was cultured overnight, with shaking but in a sealed tube, in the presence of exogenous selenate. Formation of elemental red selenium was observed (Fig. 1a). The culture conditions here should be regarded as ‘microaerobic’ at best, however, since bona fide aerobic growth under well-aerated conditions did not lead to selenium formation (Fig. 1a). Conversely, a static anaerobic culture was able to fully reduce selenate to selenium (Fig. 1a). We conclude that at least one anaerobically expressed gene is required for selenate reduction to selenium in Salmonella.
Involvement of the Tat pathway in anaerobic selenate reduction. (a) Salmonella LT2 and a ΔtatABC derivative, DIG100, and (b) E. coli K12 MC4100 and a ΔtatABCD, ΔtatE derivative, DADE, were grown under microaerobic (‘±O2’), aerobic (‘+O2’), and anaerobic (‘–O2’) conditions in the presence of 10 mM selenate. The tat mutants (‘Δtat’) were also grown under microaerobic conditions in the presence of 10 mM selenite (‘Δtat + SeO3 2–’)
Given the periplasmic localization of the best-characterised selenate reductase from T. selenatis (Schröder et al. 1997), and the involvement of the tatC gene in selenate reduction in En. cloacae SLD1a-1 (Ma et al. 2007), we next examined the role of the Salmonella Tat translocase in elemental selenium production from selenate. A strain deleted for tatABC was constructed and found to be unable to reduce selenate to selenium (Fig. 1a). The tatABC lesion specifically affects selenate reductase activity, since growth in the presence of exogenous selenite rescued elemental red selenium production in this strain (Fig. 1a). Transformation of the Salmonella tat mutant (DIG100) with a plasmid encoding the E. coli tatABCD operon restored production of selenium (not shown). These data clearly implicate a Tat-dependent protein in the selenate reduction process in Salmonella.
Salmonella and E. coli often show basic similarities in their overall physiology. We decided, therefore, to reinvestigate the selenate reductase activity of E. coli. Given the previous research in this area (Avazeri et al. 1997; Bebien et al. 2002), we were surprised when an E. coli tat mutant (DADE) proved incapable of the complete reduction of selenate to selenium in vivo (Fig. 1b). Also like Salmonella, this activity was repressed under aerobiosis but induced under microaerobic or anaerobic growth conditions (Fig. 1b). We conclude that at least one Tat-dependent, anaerobically active protein is required for the complete reduction of selenate to selenium in E. coli.
Selenate reduction requires an active dmsD gene
The serABDC operon encoding the T. selenatis periplasmic Tat-targeted selenate reductase codes for a Tat chaperone (SerD) of the TorD family (Krafft et al. 2000; Turner et al. 2004). TorD-like proteins are dedicated to molybdo-enzyme biosynthesis and are involved in regulating transport and co-ordinating cofactor insertion into the catalytic subunit (Sargent 2007). The Salmonella LT2 genome (McClelland et al. 2001) encodes five members of the TorD and DmsD clades of the wider TorD family (Turner et al. 2004), namely STM3821 (equivalent to E. coli torD), STM1495 (dmsD), STM1137 (ycdY), STM0610 (not present in E. coli), and STM4308 (not present in E. coli). In-frame deletion mutants were constructed in each of the five genes. One of the mutants (DIG1495), carrying a lesion in the gene-encoding DmsD (Qiu et al. 2008), was found to be defective in selenate reduction to selenium (Fig. 2a). Inactivation of dmsD led specifically to the loss of selenate reductase activity since the mutant strain retained the ability to reduce selenite to elemental red selenium (not shown).
Involvement of Tat proofreading chaperones in selenate reduction. (a) Salmonella LT2 mutants DIG3821 (‘ΔtorD’), DIG1495 (‘ΔdmsD’), DIG1137 (‘ΔycdY’), DIG610 (‘ΔSTM0610’), DIG4308 (‘ΔSTM4308’), and (b) E. coli K12 strains FTD100 (‘ΔtorD’), FTD102 (‘ΔdmsD’), and MC4100DycdY (‘ΔycdY’), were each grown microaerobically in the presence of 10 mM selenate. Salmonella strain DIG1495 and E. coli strain FTD102 were also transformed with pSU-DmsD encoding E. coli DmsD (Jack et al. 2004) and grown microaerobically in the presence of 10 mM selenate and 25 μg/ml kanamycin. (c) Salmonella LT2 and (d) E. coli K12 MC4100 were grown microaerobically in the presence of 10 mM selenate and either without (‘–W’) or with (‘+W’) additional tungstate at 1 mM final concentration. In addition, (d) E. coli K12 mutant strains DSS401 (‘ΔdmsABC’) and DSR106 (‘ΔygfN’) were grown microaerobically with 10 mM selenate
The equivalent experiment was then carried out with E. coli mutants deleted for Tat chaperones. A single in-frame deletion in the dmsD gene was sufficient to abolish elemental red selenium production from selenate in E. coli (Fig. 2b).
Taking the data presented in Figs. 1 and 2 together, it is clear that a Tat-dependent molybdo-protein has a key role to play in selenate reduction in both Salmonella and E. coli.
The Salmonella DmsD protein interacts with signal peptides from DmsA, YnfE and YnfF
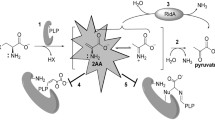
The 3D crystal structure of Salmonella DmsD has been solved (Qiu et al. 2008). By analogy with its E. coli homolog, this chaperone would be expected to be involved in the biosynthesis of the membrane-bound molybdo-enzyme dimethyl sulphoxide (DMSO) reductase (Ray et al. 2003), encoded by the dmsABC operon (STM0964-6 in Salmonella), and perhaps bind directly to the Tat signal peptide of DmsA (Oresnik et al. 2001). The Salmonella DmsD protein was overproduced and purified and its ability to interact directly with the Salmonella DmsA Tat signal peptide was tested by isothermal titration calorimetry (Fig. 3b). A genetic fusion was constructed that allows the production of the DmsA signal peptide as C-terminal fusion to E. coli MalE (maltose binding protein). This MalE-DmsASP fusion also carries a histag at its extreme C-terminus to allow purification of full-length fusion protein only. Titration of the isolated MalE-DmsASP with purified DmsD resulted in a sigmoidal binding curve that reached a clear saturation point (Fig. 3b) resulting in an apparent dissociation constant (K d) of 104 nM (Fig. 3e). As with our previous work using MalE fusion proteins as carriers for peptides in ITC experiments (Maillard et al. 2007; Buchanan et al. 2008), control titrations involving Salmonella DmsD and the MalE protein alone demonstrated no non-specific interactions were detectable (data not shown).
Salmonella DmsD interacts with the DmsA, YnfE, and YnfF signal peptides. (a) Amino acid sequence alignment of the Salmonella DmsA (STM0964), YnfE (STM1499), and YnfF (STM1498) twin-arginine signal peptides. The relative locations of the n-region, h-region, and twin-arginine sequence motif are indicated. (b) ITC analysis of the interaction between Salmonella DmsD and the DmsA signal peptide. Titration of a MalE-DmsASP fusion protein (15 μM) with 150 μM DmsD monomer was carried out in Tris buffer at 28°C. The best fit to these data give ΔH obs = –9.62 kcal/mol and TΔS obs = 0.01 kcal/mol. (c) Titration of a MalE-YnfESP fusion protein (15 μM) with 150 μM DmsD monomer was carried out in Tris buffer at 28°C. The best fit to these data give ΔH obs = –8.80 kcal/mol and TΔS obs = 1.33 kcal/mol. (d) Titration of a MalE-YnfFSP fusion protein (10 μM) with 50 μM DmsD monomer was carried out in Tris buffer at 28°C. The best fit to these data give ΔH obs = –17.8 kcal/mol and TΔS obs = 6.8 kcal/mol. (e) Relative apparent dissociation constants (K d) and binding stoichiometries (n value) associated with the data presented in b, c
Unusually for a Tat proofreading chaperone, the gene encoding DmsD is not genetically linked to the dmsABC operon where its cognate binding partner is expressed. Instead, Salmonella dmsD is part of the ynfEFGHdmsD operon (STM1499-95) located elsewhere on the chromosome. Interestingly, YnfE and YnfF are closely related to DmsA at the amino acid level, and in E. coli YnfF has been shown to have DMSO reductase activity (Lubitz and Weiner 2003). Both YnfE and YnfF bear N-terminal Tat signal peptides (Fig. 3a) and this raises the possibility that, unusually, DmsD may have more than one interaction partner. Titration of Salmonella DmsD against either a MalE-YnfESP chimera (Fig. 3c) or a MalE-YnfFSP fusion protein (Fig. 3d) demonstrated strong interactions in both cases. Indeed, binding affinities between DmsD and YnfESP or YnfFSP were slightly tighter than that observed for DmsASP, with apparent K d’s being calculated as 45 and 10 nM, respectively (Fig. 3e). Clearly, the DmsD protein can recognise three different Tat signal peptides in vitro, which also suggests this chaperone is involved in the biosynthesis of three different enzymes in vivo.
YnfE is involved in selenate reduction by Salmonella and E. coli
In E. coli, the dmsD gene is essential for activity of the DMSO reductase molybdo-enzyme (Oresnik et al. 2001; Ray et al. 2003). However, an E. coli strain devoid of dmsABC (DSS401) retains the ability to reduce selenate to selenium in vivo (Fig. 2d). The membrane-bound DMSO reductase is therefore not involved in this process. In addition, YgfN, the uncharacterized putative molybdo-protein identified in E. coli (Bebien et al. 2002) does not seem to have a clear role either (Fig. 2d). However, growth of either Salmonella or E. coli in the presence of excess tungstate inhibited the selenate reduction activity (Fig. 2c, d), strongly implicating a molybdo-enzyme in this process (Kletzin and Adams 1996). Given the dependence on dmsD for selenate reduction (Fig. 2a, b), the genetic linkage of dmsD to genes encoding two putative Tat-dependent molybdo-enzymes (Lubitz and Weiner 2003), and the interaction between the signal peptides of those enzymes and DmsD (Fig. 3), we decided to explore further the roles of ynfE and ynfF in selenate reduction in vivo.
An in-frame deletion in ynfE (DIG102), an in-frame deletion in ynfF (DIG103), and a ynfEF double deletion (DIG101) were constructed in Salmonella LT2. The ΔynfF strain retained selenate reductase activity (Fig. 4a); however, both the single ΔynfE strain, and the ΔynfEF double mutant, were unable to produce elemental red selenium from selenate (Fig. 4a). All strains retained the ability to reduce exogenous selenite to selenium (not shown), which, therefore, implicates Salmonella YnfE as a potential selenate reductase.
Involvement of YnfE and YnfF in selenate reduction. (a) Salmonella LT2 mutants DIG101 (‘ΔynfEF’), DIG102 (‘ΔynfE’), DIG103 (‘ΔynfF’), and (b) E. coli K12 strains DSR101 (‘ΔynfEF’), DSR104 (‘ΔynfE’), and DSR105 (‘ΔynfF’), were each grown microaerobically in the presence of 10 mM selenate. E. coli strain DSR101 (ΔynfEF) was also transformed separately with plasmids pSU-YnfE, pSU-YnfF, or pSU-DmsD, encoding E. coli ynfE, ynfF or dmsD, and grown microaerobically in the presence of 10 mM selenate and 25 μg/ml kanamycin
The equivalent experiment was then carried out in E. coli (Fig. 4b). In frame deletion mutants in ynfE, ynfF, and a double ynfEF mutant were constructed. In the case of E. coli, only the ΔynfEF double-deletion strain was unable to reduce selenate to elemental red selenium (Fig. 4b). Deletion of ynfEF was specific to the initial selenate reduction process, since growth of the mutant in the presence of selenite led to selenium production (not shown). To exclude any polar effects of the ynfEF deletion on expression of the downstream dmsD gene, the ΔynfEF strain was transformed with plasmid pSU-DmsD, which constitutively expresses dmsD (Jack et al. 2004). Expression of dmsD in trans was unable to complement the ynfEF phenotype (Fig. 4b). Conversely, production of either YnfE or YnfF from a plasmid restored the ability of the E. coli ynfEF double mutant to completely reduce selenate to selenium in vivo (Fig. 4b). Thus in E. coli not only YnfE, but also YnfF, is implicated strongly as Tat-dependent selenate reductase isoenzymes.
Additional factors required for in vivo selenate reduction in E. coli
In E. coli, the in vivo reduction of selenate to elemental red selenium appeared to be inhibited in cells grown under aerobic conditions (Fig. 1). Further genetic analysis established that the genes encoding the global regulators FNR and ArcA are both essential for selenate reduction under the conditions tested here (Fig. 5a). Although loss of both regulators is likely to have wide-ranging pleiotropic effects, a putative FNR recognition sequence could be identified in the promoter region of the ynfEFGHdmsD operon implicated here as encoding the selenate reductase (Fig. 5b).
Other genes required for selenate reduction by E. coli K12. a E. coli K12 strains BW25113 (parent strain of the Keio collection (Baba et al. 2006), together with ΔFNR (RM102), ΔarcA, ΔubiC, ΔubiE, and ΔmenA strains were each grown microaerobically in the presence of 10 mM selenate. b The DNA sequence of the E. coli ynfEFGHdmsD operon promoter region. Nucleotides are numbered in reverse from the translation start site of the ynfE gene. A putative FNR recognition sequence is shown in blue, and a putative ModE recognition sequence is highlighted in red. The location of a putative ribosome binding site upstream of ynfE is boxed (colour figure online)
The molybdo-enzyme encoded by the E. coli ynfEFGHdmsD operon is predicted to be closely related in both structure and function to the membrane-bound DMSO reductase (Lubitz and Weiner 2003). The E. coli DMSO reductase uses electrons supplied by menaquinol (Geijer and Weiner 2004) to reduce DMSO to dimethyl sulphide. Further genetic analysis established that the ubiC gene product, which is essential for production of ubiquinone (Wu et al. 1993), was not involved in the selenate reduction process (Fig. 5a). However, genetic inactivation of ubiE, required for both ubiquinone and menaquinone biosynthesis (Lee et al. 1997), or menA, required for menaquinone biosynthesis (Suvarna et al. 1998), blocked the complete reduction of selenate to elemental red selenium in vivo (Fig. 5a). Moreover, both the ubiE amd menA mutants retain the ability to reduce selenite to selenium in vivo (not shown). Taken altogether, these data suggest that menaquinone is an essential player in the initial reduction of selenate to selenite.
Discussion
A broad spectrum of bacteria and archaea are able to reduce selenate and selenite. These oxyanions are water soluble but also highly toxic at low concentrations, and by contrast elemental selenium is essentially insoluble and non-toxic. The ability to harness microbial transformation of selenate and selenite, therefore, has great potential for bioremediation projects. Apart from the case of T. selenatis, however, unequivocal identification of specific selenate and selenite reductase enzymes has proved less than straightforward. In this work we provide qualitative, but compelling, evidence for the identity of the selenate reductases in the related γ-Proteobacteria Salmonella LT2 and E. coli K12. In both organisms our data point to similar operons encoding molybdenum-dependent oxidoreductases as being central to the initial selenate reduction process. The simplest interpretation of our data is that the selenate reductase itself is YnfE. In E. coli, the YnfE homolog YnfF also contributes some activity in vivo. These proteins are molybdenum cofactor-containing enzymes and by analogy with the related DMSO reductase from E. coli, are probably tightly associated with the periplasmic face of the cytoplasmic membrane where they couple menaquinol oxidation to selenate reduction. Our own efforts to quantify in vitro the selenate reductase activity afforded by YnfE and YnfF using the library of mutants strains constructed here and classical redox-dye based enzyme assays were not successful. This was most likely due to a combination of low levels of gene expression (Lubitz and Weiner 2003), low specific activities, and lability of the enzymes.
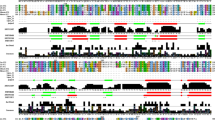
Sequence analysis suggests YnfE and YnfF are more closely related in overall structure to the membrane-bound DMSO reductase, DmsA, than the T. selenatis soluble selenate reductase or the non-exported nitrate reductase, NarG (Fig. 6). Both YnfE and YnfF are predicted to contain an ‘F0’ FeS cluster (Fig. 6a); however, neither appear to contain a conserved active-site aspartic acid residue similar to that known to be a molybdenum ligand in the NarG nitrate reductase (Bertero et al. 2003; Jormakka et al. 2004) and subsequently implicated for the T. selenatis enzyme (Stolz et al. 2006). Instead, YnfE and YnfF contain a similarly conserved serine residue (Fig. 6b) that has been shown to be a direct molybdenum ligand in E. coli DmsA (Trieber et al. 1996; George et al. 2007). We have shown here, however, that DmsA does not contribute to E. coli selenate reduction in vivo, and it seems more likely that differences in the size, shape, or chemical composition of the active site pocket are contributing to substrate specificity. One obvious point of similarity between YnfE and T. selenatis SerA is the presence of a second conserved serine near the active site (Fig. 6b), the role of which in determining substrate specificity remains to be tested experimentally.
Amino acids predicted to be at the YnfE and YnfF active sites. The complete primary sequences of E. coli YnfE (‘Ec_YnfE’), E. coli YnfF (‘Ec_YnfF’), Salmonella YnfE (‘St_YnfE’), Salmonella YnfF (‘St_YnfF’), T. selenatis SerA (‘Ts_SerA’), E. coli DmsA (‘Ec_DmsA’), and E. coli NarG (‘Ec_NarG’) were aligned using ClustalW2 (Larkin et al. 2007. http://www.ebi.ac.uk/Tools/clustalw2/index.html) and highlighted using Boxshade (Hofmann K, Baron M unpublished. http://www.ch.embnet.org/software/BOX_form.html). Sections of the alignment covering (a) the putative FeS cluster binding residues, and (b) the molybdenum active site, were then extracted and annotated. In (a) the orange arrows point to the ligands of the FeS cluster. In (b) the red arrow points to the NarG Aspartate-223 residue, termed Aspartate-222 in structure papers (Bertero et al. 2003; Jormakka et al. 2004), which is a direct ligand to the molybdenum in this enzyme. The purple arrow points to DmsA Serine-205, termed Serine-176 in biophysical studies (George et al. 2007), thought to be a direct molybdenum ligand in DMSO reductase. The blue arrow points to a conserved serine side chain found in enzymes that display selenate reductase activity (colour figure online)
A more obvious point of similarity between YnfE, YnfF and T. selenatis SerA is the presence of N-terminal twin-arginine signal peptides on each reductase and co-expression of the structural genes with that encoding a Tat chaperone. In E. coli, the YnfE and YnfF signal peptides are known to be active in protein targeting (Tullman-Ercek et al. 2007) and have been shown very recently to interact with the DmsD chaperone from that organism (Chan et al. 2009). While the other well-characterised Tat chaperones appear to have strict substrate specificity (Hatzixanthis et al. 2005; Maillard et al. 2007), the calorimetric analysis described here of the interaction between Salmonella DmsD and the YnfE, YnfF and DmsA signal peptides (Fig. 3), as well as the new role for dmsD in selenate reduction in vivo (Fig. 2), clearly shows that a single chaperone can be involved in the biosynthesis of more than one enzyme in the cell. Indeed, by using the Salmonella DmsD system as a model it is possible to consider the three different peptide ligands of this chaperone in a ‘natural mutagenesis’ binding study. The primary sequences of Salmonella DmsASP and YnfESP, for example, have very different n-regions (Fig. 3), which immediately suggests that the n-regions are not critical to the peptide recognition process. This would bring the recognition mechanism closer to that of the related E. coli TorD protein, which interacts primarily with the h-region of the TorA signal peptide (Buchanan et al. 2008). Moreover, the h-regions of DmsASP and YnfFSP share a high degree of identity and similarity, yet the apparent dissociation constants appear quite different (Fig. 3). This demonstrates, essentially through natural selection, what range of side-chain modifications can be tolerated in a given peptide ligand, and that even very subtle changes to the chemistry of a peptide can modify its binding efficacy without disrupting its physiological role.
It is established in this work that the Tat translocase, the global regulator FNR, and the menaquinone biosynthetic machinery, are all required for the complete reduction of selenate to elemental red selenium in E. coli. This brings our knowledge of this area sharply into line with what has been recently described for this process in En. cloacae SLD1a-1 (Ma et al. 2007; Yee et al. 2007; Ma et al. 2009). Moreover, the relatedness of the products of the ynfEFGHdmsD operon to the membrane-bound Tat-dependent DMSO reductase of E. coli (Lubitz and Weiner 2003) are consistent with recent biochemical studies of the En. cloacae SLD1a-1 system, which suggested the selenate reductase was also a membrane-bound, but periplasmically oriented, molybdo-enzyme in that organism (Watts et al. 2003; Ridley et al. 2006).
Finally, it should be considered that there is perhaps a more complicated explanation for the data presented here. Selenate metabolism in enteric bacteria has been heavily studied over a number of years (Turner et al. 1998; Stolz et al. 2006) and a great many different mutant strains have been isolated that are defective in various aspects of this process. Clearly, aspects of selenium metabolism must occur inside the cell (Turner et al. 1998) and yet somehow the final elemental selenium must be deposited outside the cell (Gerrard et al. 1974; Bebien et al. 2002). Given that our observations are based on the final production of elemental selenium, it should be considered that YnfE may be involved in a late extracytoplasmic step in the complex metabolism of selenate. One metabolite in the metabolism of selenate is volatile dimethyl selenide, the production of which occurs naturally (Turner et al. 1998) and can also be artificially boosted in E. coli (Swearingen et al. 2006a; Swearingen et al. 2006b). Dimethyl selenide can react chemically to generate dimethyl selenoxide (Rael et al. 1996), a compound with a similar structure to DMSO (Filatov et al. 2005). Moreover, dimethyl selenoxide can also be produced by enzymatic oxidation of dimethyl selenide in some biological systems (Goeger and Ganther 1994). It is possible that selenate metabolism in Salmonella and E. coli is leading to the production of dimethyl selenoxide, which in turn may be accepted as a substrate by the DMSO reductase homolog YnfE. Exactly how dimethyl selenoxide reduction might be linked to production of elemental red selenium, however, and why growth with selenite leads to similar selenium deposits in the absence of any Tat-dependent molybdo-enzymes, suggests this alternative hypothesis deserves further rigorous testing.
Abbreviations
- TMAO:
-
Trimethylamine N-oxide
- Tat:
-
Twin-arginine translocation
References
Avazeri C, Turner RJ, Pommier J, Weiner JH, Giordano G, Vermeglio A (1997) Tellurite reductase activity of nitrate reductase is responsible for the basal resistance of Escherichia coli to tellurite. Microbiology 143:1181–1189
Baba T et al (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008
Bebien M, Kirsch J, Mejean V, Vermeglio A (2002) Involvement of a putative molybdenum enzyme in the reduction of selenate by Escherichia coli. Microbiology 148:3865–3872
Berks BC (1996) A common export pathway for proteins binding complex redox cofactors? Mol Microbiol 22:393–404
Berks BC, Palmer T, Sargent F (2003) The Tat protein translocation pathway and its role in microbial physiology. Adv Microb Physiol 47:187–254
Bertero MG et al (2003) Insights into the respiratory electron transfer pathway from the structure of nitrate reductase A. Nat Struct Biol 10:681–687
Birkmann A, Sawers RG, Böck A (1987) Involvement of the ntrA gene product in the anaerobic metabolism of Escherichia coli. Mol Gen Genet 210:535–542
Buchanan G, Maillard J, Nabuurs SB, Richardson DJ, Palmer T, Sargent F (2008) Features of a twin-arginine signal peptide required for recognition by a Tat proofreading chaperone. FEBS Lett 582:3979–3984
Casadaban MJ, Cohen SN (1979) Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci USA 76:4530–4533
Chan CS et al (2008) Identification of residues in DmsD for twin-arginine leader peptide binding, defined through random and bioinformatics-directed mutagenesis. Biochemistry 47:2749–2759
Chan CS, Chang L, Rommens KL, Turner RJ (2009) Differential interactions between Tat-specific redox enzyme peptides and their chaperones. J Bacteriol 191:2091–2101
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
DeLisa MP, Tullman D, Georgiou G (2003) Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway. Proc Natl Acad Sci USA 100:6115–6120
Filatov AS, Block E, Petrukhina MA (2005) Dimethyl selenoxide. Acta Crystallogr C 61:o596–598
Geijer P, Weiner JH (2004) Glutamate 87 is important for menaquinol binding in DmsC of the DMSO reductase (DmsABC) from Escherichia coli. Biochim Biophys Acta 1660:66–74
George GN, Doonan CJ, Rothery RA, Boroumand N, Weiner JH (2007) X-ray absorption spectroscopic characterization of the molybdenum site of Escherichia coli dimethyl sulfoxide reductase. Inorg Chem 46:2–4
Gerrard TL, Telford JN, Williams HH (1974) Detection of selenium deposits in Escherichia coli by electron microscopy. J Bacteriol 119:1057–1060
Goeger DE, Ganther HE (1994) Oxidation of dimethylselenide to dimethylselenoxide by microsomes from rat liver and lung and by flavin-containing monooxygenase from pig liver. Arch Biochem Biophys 310:448–451
Hatzixanthis K, Clarke TA, Oubrie A, Richardson DJ, Turner RJ, Sargent F (2005) Signal peptide-chaperone interactions on the twin-arginine protein transport pathway. Proc Natl Acad Sci USA 102:8460–8465
Jack RL, Buchanan G, Dubini A, Hatzixanthis K, Palmer T, Sargent F (2004) Coordinating assembly and export of complex bacterial proteins. EMBO J 23:3962–3972
Jormakka M, Richardson D, Byrne B, Iwata S (2004) Architecture of NarGH reveals a structural classification of Mo-bisMGD enzymes. Structure 12:95–104
Kisker C, Schindelin H, Rees DC (1997) Molybdenum-cofactor-containing enzymes: structure and mechanism. Annu Rev Biochem 66:233–267
Kletzin A, Adams MW (1996) Tungsten in biological systems. FEMS Microbiol Rev 18:5–63
Krafft T, Bowen A, Theis F, Macy JM (2000) Cloning and sequencing of the genes encoding the periplasmic-cytochrome B-containing selenate reductase of Thauera selenatis. DNA Seq 10:365–377
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) ClustalW and ClustalX version 2. Bioinformatics 23:2947–2948
Lee PT, Hsu AY, Ha HT, Clarke CF (1997) A C-methyltransferase involved in both ubiquinone and menaquinone biosynthesis: isolation and identification of the Escherichia coli ubiE gene. J Bacteriol 179:1748–1754
Lubitz SP, Weiner JH (2003) The Escherichia coli ynfEFGHI operon encodes polypeptides which are paralogues of dimethyl sulfoxide reductase (DmsABC). Arch Biochem Biophys 418:205–216
Ma J, Kobayashi DY, Yee N (2007) Chemical kinetic and molecular genetic study of selenium oxyanion reduction by Enterobacter cloacae SLD1a-1. Environ Sci Technol 41:7795–7801
Ma J, Kobayashi DY, Yee N (2009) Role of menaquinone biosynthesis genes in selenate reduction by Enterobacter cloacae SLD1a-1 and Escherichia coli K12. Environ Microbiol 11:149–158
Maillard J et al (2007) Structural diversity in twin-arginine signal peptide-binding proteins. Proc Natl Acad Sci USA 104:15641–15646
McClelland M et al (2001) Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852–856
Oresnik IJ, Ladner CL, Turner RJ (2001) Identification of a twin-arginine leader-binding protein. Mol Microbiol 40:323–331
Qiu Y, Zhang R, Binkowski TA, Tereshko V, Joachimiak A, Kossiakoff A (2008) The 1.38 A crystal structure of DmsD protein from Salmonella typhimurium, a proofreading chaperone on the Tat pathway. Proteins 71:525–533
Rael RM, Tuazon EC, Frankenberger WT (1996) Gas-phase reactions of dimethyl selenide with ozone and the hydroxyl and nitrate radicals. Atmospheric Environ 30:1221–1232
Ray N, Oates J, Turner RJ, Robinson C (2003) DmsD is required for the biogenesis of DMSO reductase in Escherichia coli but not for the interaction of the DmsA signal peptide with the Tat apparatus. FEBS Lett 534:156–160
Ridley H, Watts CA, Richardson DJ, Butler CS (2006) Resolution of distinct membrane-bound enzymes from Enterobacter cloacae SLD1a-1 that are responsible for selective reduction of nitrate and selenate oxyanions. Appl Environ Microbiol 72:5173–5180
Sambasivarao D, Weiner JH (1991) Dimethyl sulfoxide reductase of Escherichia coli: an investigation of function and assembly by use of in vivo complementation. J Bacteriol 173:5935–5943
Sargent F (2007) Constructing the wonders of the bacterial world: biosynthesis of complex enzymes. Microbiology 153:633–651
Schröder I, Rech S, Krafft T, Macy JM (1997) Purification and characterization of the selenate reductase from Thauera selenatis. J Biol Chem 272:23765–23768
Stolz JF, Basu P, Santini JM, Oremland RS (2006) Arsenic and selenium in microbial metabolism. Annu Rev Microbiol 60:107–130
Suvarna K, Stevenson D, Meganathan R, Hudspeth ME (1998) Menaquinone (vitamin K2) biosynthesis: localization and characterization of the menA gene from Escherichia coli. J Bacteriol 180:2782–2787
Swearingen JW Jr, Frankel DP, Fuentes DE, Saavedra CP, Vasquez CC, Chasteen TG (2006a) Identification of biogenic dimethyl selenodisulfide in the headspace gases above genetically modified Escherichia coli. Anal Biochem 348:115–122
Swearingen JW Jr et al (2006b) Expression of the ubiE gene of Geobacillus stearothermophilus V in Escherichia coli K-12 mediates the evolution of selenium compounds into the headspace of selenite- and selenate-amended cultures. Appl Environ Microbiol 72:963–967
Trieber CA, Rothery RA, Weiner JH (1996) Consequences of removal of a molybdenum ligand (DmsA-Ser-176) of Escherichia coli dimethyl sulfoxide reductase. J Biol Chem 271:27339–27345
Tullman-Ercek D et al (2007) Export pathway selectivity of Escherichia coli twin arginine translocation signal peptides. J Biol Chem 282:8309–8316
Turner RJ, Weiner JH, Taylor DE (1998) Selenium metabolism in Escherichia coli. Biometals 11:223–227
Turner RJ, Papish AL, Sargent F (2004) Sequence analysis of bacterial redox enzyme maturation proteins (REMPs). Can J Microbiol 50:225–238
Watts CA, Ridley H, Condie KL, Leaver JT, Richardson DJ, Butler CS (2003) Selenate reduction by Enterobacter cloacae SLD1a-1 is catalysed by a molybdenum-dependent membrane-bound enzyme that is distinct from the membrane-bound nitrate reductase. FEMS Microbiol Lett 228:273–279
Wexler M et al (2000) TatD is a cytoplasmic protein with DNase activity. No requirement for TatD family proteins in sec-independent protein export. J Biol Chem 275:16717–16722
Wu G, Williams HD, Gibson F, Poole RK (1993) Mutants of Escherichia coli affected in respiration: the cloning and nucleotide sequence of ubiA, encoding the membrane-bound p-hydroxybenzoate:octaprenyltransferase. J Gen Microbiol 139:1795–1805
Yee N, Ma J, Dalia A, Boonfueng T, Kobayashi DY (2007) Se(VI) reduction and the precipitation of Se(0) by the facultative bacterium Enterobacter cloacae SLD1a-1 are regulated by FNR. Appl Environ Microbiol 73:1914–1920
Acknowledgments
We thank Prof. T. Palmer (Dundee) for helpful comments and discussion. This work was funded by a BBSRC Doctoral Training Grant awarded to the College of Life Sciences, University of Dundee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by John Helmann.
Rights and permissions
About this article
Cite this article
Guymer, D., Maillard, J. & Sargent, F. A genetic analysis of in vivo selenate reduction by Salmonella enterica serovar Typhimurium LT2 and Escherichia coli K12. Arch Microbiol 191, 519–528 (2009). https://doi.org/10.1007/s00203-009-0478-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-009-0478-7