Abstract
Summary
Sarcopenia was reported to be significantly associated with osteoporosis. In this study, we reported for the first time that sarcopenia was an independent risk predictor of osteoporotic vertebral compression refractures (OVCRFs). Other risk factors of OVCRFs are low bone mass density T-scores, female sex, and advanced age.
Introduction
The purpose of this study was to investigate the association between osteoporotic vertebral compression refractures (OVCRFs) and sarcopenia, and to identify other risk factors of OVCRFs.
Methods
We evaluated 237 patients with osteoporotic vertebral compression fracture who underwent percutaneous kyphoplasty (PKP) in our hospital from August 2016 to December 2017. To diagnose sarcopenia, a cross-sectional computed tomography (CT) image at the inferior aspect of the third lumbar vertebra (L3) was selected for estimating muscle mass. Grip strength was used to assess muscle strength. Possible risk factors, such as age, sex, body mass index (BMI), bone mineral density (BMD), location of the treated vertebra, anterior-posterior ratio (AP ratio) of the fractured vertebra, cement leakage, and vacuum clefts, were assessed. The multivariable analysis was used to determine the risk factors of OVCRFs.
Results
During the follow-up period, OVCRFs occurred in 64 (27.0%) patients. Sarcopenia was present in 48 patients (20.3%), including 21 OVCRFs and 27 non-OVCRFs patients. Sarcopenia was significantly correlated with advanced age, lower BMI, lower BMD, and hypoalbuminemia. Compared with non-sarcopenic patients, sarcopenic patients had higher OVCRFs risk. In univariate analysis, sarcopenia (p = 0.003), female (p = 0.024), advanced age (≥ 75 years; p < 0.001), lower BMD (p < 0.001), lower BMI (p = 0.01), TL junction (vertebral levels at the thoracolumbar junction) (p = 0.01), cardiopulmonary comorbidity (p = 0.042), and hypoalbuminemia (p = 0.003) were associated with OVCRFs. Multivariable analysis revealed that sarcopenia (OR 2.271; 95% CI 1.069–4.824, p = 0.033), lower BMD (OR 1.968; 95% CI 1.350–2.868, p < 0.001), advanced age (≥ 75 years; OR 2.431; 95% CI 1.246–4.744, p = 0.009), and female sex (OR 4.666; 95% CI 1.400–15.552, p = 0.012) were independent risk predictors of OVCRFs.
Conclusions
Sarcopenia is an independent risk predictor of osteoporotic vertebral compression refractures. Other factors affecting OVCRFs are low BMD T-scores, female sex, and advanced age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the increase of the aging population, osteoporotic vertebral compression fracture (OVCF) has become the main cause of low back pain and of being bedridden for a long time, and it seriously affects patients’ quality of life because of its high morbidity and mortality risk [1]. In the recent decades, percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP) have been widely used in the treatment of OVCF for being minimally invasive and having a short operative time, high safety, and fast pain relief [2]. Although PKP and PVP have been used worldwide, the real effect of osteoporotic fractures still remains controversial. A Cochrane systematic review conducted by Buchbinder concluded that conventional vertebroplasty for osteoporotic vertebral fractures is not recommended. However, clinicians found that PKP and PVP did indeed play a role in pain relief, thus it is necessary to grasp their surgical indications more strictly and to select the most appropriate cases [3]. Some patients still suffer from back pain even after undergoing primary surgery and formal anti-osteoporosis treatment, and they were diagnosed with another new vertebral fracture through magnetic resonance imaging (MRI, Sagittal STIR MRI shows high signal intensity within the vertebral bodies). Previous studies indicated that the risk factors of vertebral refracture included steroid use [4], osteoporosis, cement leakage into disks [5], vertebral levels at the thoracolumbar junction (TL junction), and vacuum clefts [6].
Sarcopenia is a syndrome characterized by reduced muscle volume, decreased muscle strength, and reduced muscle activity, and has begun attracting attention in various medical fields. Using the Asian Working Group for Sarcopenia (AWGS) criteria, the estimated prevalence of sarcopenia among the general older population ranged from 4.1 to 11.5% [7]. Sarcopenia also brings a heavy economic burden on society. It has been reported that, in the USA, sarcopenia resulted in additional healthcare costs of more than $18.5 billion in 2001 [8].
It has conclusively been shown that sarcopenia is significantly associated with osteoporosis [9,10,11]. Bone and muscle are interconnected not only because of their adjacent surfaces but also because of the chemical and metabolic properties, and thus, they give it a new name “osteosarcopenia” [12]. The decrease in muscle content, strength, and function will significantly increase the risk of osteoporosis. The skeletal and the muscular organ systems are tightly intertwined: the strongest mechanical forces applied to bones are, indeed, those created by muscle contractions that condition bone density, strength, and microarchitecture. The decrease of BMD will significantly increase the prevalence of sarcopenia. These two health problems often occur concurrently and lead to an increased risk of fragility fracture in aging populations [13]. In a cross-sectional study, Hida et al. found higher prevalence of sarcopenia and lower leg muscle mass among patients with fresh osteoporotic vertebral fracture [14]. In 2013, Iolascon et al. confirmed that the rate of sarcopenia in women with multiple vertebral fragility fractures was two times more than that in women with a single vertebral fracture [15]. Although previous small sample size studies have confirmed sarcopenia was associated with osteoporotic vertebral fractures in women, there was no further study of recurrent fractures. At present, the association between sarcopenia and OVCRFs has not been studied in patients. We hypothesized that sarcopenia was a high-risk factor for OVCRFs. The purpose of this article was to confirm the relationship between sarcopenia and OVCRFs and to investigate other risk factors of vertebral refractures.
Methods
Subjects
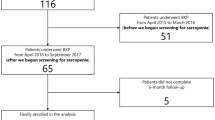
From August 2016 to December 2017, consecutive patients who underwent PKP at the Spine Surgical Department, The First Affiliated Hospital of Wenzhou Medical University, were included in this retrospective study. The inclusion criteria were as follows: (i) of Chinese ethnicity; (ii) underwent preoperative plain film radiography, computed tomography (CT), and magnetic resonance imaging; (iii) first-ever acute or subacute single-level vertebral compression fracture was treated with PKP; (iv) had no complication after surgery, including anaphylactic shock, leakage of cement into the spinal canal, or postoperative neurologic deficit; (v) without additional serious trauma after surgery; and (iv) with regular radiologic studies and osteoporotic medications throughout the 1-year follow-up. The exclusion criteria included (i) pathological fracture, including fractures related to malignancy, infection, or other medical conditions; (ii) burst fracture with retro-pulsed bony fragment into the spinal canal and accompanied by neurologic signs before surgery; (iii) multiple vertebral fractures; (iv) refracture of cemented vertebra; (v) patients concomitant with severe physical disease who could not adhere to the follow-up visits; and (vi) use of steroids or medications for severe liver or kidney diseases. A total of 479 patients were eligible in our study. Applying the inclusion and exclusion criteria, 242 patients were excluded and 237 patients were finally enrolled (Fig. 1).
Measurements and methods
Follow-up strategy
Clinical follow-up after kyphoplasty lasted for 1 year. All the patients were prescribed 1-alpha-OH vitamin D3 (0.5 mcg/day), an active vitamin D supplement and encouraged to take the medication for 1 year. The patients were routinely followed up monthly in the outpatient department for the first 3 months after the operation and subsequently at 3-month intervals, or at any point when there was recurrent back pain. Whenever new vertebral fractures were suspected, an MRI examination was performed. The new vertebral fractures were shown as marrow edema on MRI (Fig. 2), and the patients were grouped into the “OVCRFs” group. The others, who did not experience further OVCRFs, were considered as the control group.
Evaluated factors
Age, sex, body mass index (BMI), preoperative T-score in bone mineral density(BMD) measured by dual-energy X-ray absorptiometry (DXA, Hologic Inc., Bedford, MA, USA) at the lumbar (vertebrae L1–L4), cardiopulmonary comorbidity, serum albumin, hemoglobin (XE-2100, Sysmex Corporation, Hyogo, Japan.), level of education, living alone, and higher Nutritional Risk Screening (NRS) 2002 score were evaluated. Parameters related to vertebral body treatment were considered, including the vertebral levels treated, cement leakage into the disk, preoperative anterior-to-posterior body height ratio (AP ratio), and vacuum clefts. The World Health Organization (WHO) classification system was applied when defining osteoporosis (T-score ≤ − 2.5) and osteopenia (− 2.5 < T-score < − 1). Vertebrae were categorized into the following groups: those at the thoracolumbar junction (T10–L2), those not at the thoracolumbar junction.
Sarcopenia diagnosis
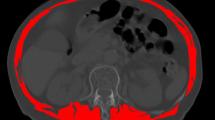
Sarcopenia is diagnosed when patients meet 2 of the following 3 criteria: low skeletal muscle mass, inadequate muscle strength, and inadequate physical performance according to the European Working Group on Sarcopenia (EWGSOP) [16] and the Asian Working Group for Sarcopenia (AWGS) [17]. However, owing to the retrospective nature of other studies, the muscle function was not evaluated, which might have affected the diagnostic accuracy of sarcopenia. At present, the main method for diagnosing sarcopenia is to evaluate the appendicular muscle mass by DXA. The DXA in our hospital did not report the value of the appendicular muscle mass. So we tried to assess muscle mass in other ways. In 2015, Professor Iritani did a research on the prognosis of patients with liver cancer associated with sarcopenia and gave the cutoff points, diagnosed by measure third lumbar vertebra (L3) skeletal muscle index (SMI). We used this measurement and the cutoff points. A cross-sectional CT image at the inferior aspect of the L3 was selected for estimating muscle mass, containing psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis, as described previously [18, 19]. Muscle areas computed from each image were normalized for height (m2) to obtain the L3 SMI (cm2/m2). L3 SMI ≤ 36.0 cm2/m2 in men and ≤ 29.0 cm2/m2 in women were considered as low muscle mass (Fig. 3). Grip strength was used to assess muscle strength, which was measured three times on the dominant hand using an electronic hand dynamometer (EH101; Camry, Guangdong Province, China). The mean of these three measures was used for the calculations [20]. Low handgrip strength was defined as ≤ 26 kg for men and ≤ 18 kg for women. Physical performance was evaluated using the 6-m usual gait speed test. Low gait speed was defined as ≤ 0.8 m/s. This test was not performed in patients with walking limitations. According to the above diagnostic criteria, patients were divided into two groups—sarcopenia and non-sarcopenia.
Statistical analysis
The continuous data that were subjected to normal distribution were presented as mean and standard deviation. Two sample independent t tests (Mann–Whitney U test for non-normally distributed data) and χ2 tests were used to test for differences in continuous and categorical variables, respectively. Binary logistic regression including all factors significantly different in the univariate analysis was performed to determine significant risk factors for the development of subsequent fractures. Results were presented as odds ratio (OR) with 95% confidence intervals (CI). All statistical tests were performed with SPSS for Windows (Release 21.0; SPSS, Chicago, IL, USA). p < 0.05 was considered statistically significant.
Results
Initially, 237 patients undergoing PKP for osteoporotic vertebral compression fracture met the inclusion criteria. OVCRFs occurred in 64 (27.0%) of 237 patients. Sarcopenia was present in 48 of 237 patients (20.3%), including 21 OVCRFs and 27 non-OVCRFs patients. There were 189 non-sarcopenic patients (79.7%), including 43 OVCRFs and 146 non-OVCRFs patients.
Preoperative characteristics are presented in Table 1. Mean patient age was 70.61 ± 8.87 years, and 36 (15.2%) patients were male and 201 (84.8%) were female. Given our predefined criteria of sarcopenia, 48 patients (20.3%) were sarcopenic (10 men and 38 women). Among the preoperative factors analyzed, sex, cardiopulmonary comorbidity, diabetes, level of education, living alone, NRS 2002 score, and hemoglobin did not differ significantly between sarcopenic and non-sarcopenic patients. However, sarcopenic patients were older (p < 0.001) and had lower BMI (p < 0.001), lower BMD (p = 0.025), and lower preoperative serum albumin (p = 0.001). The sarcopenia features, including L3 SMI (p < 0.001) and handgrip strength (p < 0.001), were significantly lower in sarcopenic patients than in non-sarcopenic patients.
The association between the risk factors and the incidences of OVCRFs are listed in Table 2. In univariate analysis, several factors were associated with OVCRFs, including sarcopenia (p = 0.003), female sex (p = 0.024), advanced age (≥ 75 years, p < 0.001), lower BMD (p < 0.001), TL junction (p = 0.01), lower BMI (p = 0.01), cardiopulmonary comorbidity (p = 0.042), and hypoalbuminemia (p = 0.03). Significant differences were not observed in other factors, including diabetes, hemoglobin, vacuum clefts, intradiscal cement leakage, and AP ratio. In multivariate model, after controlling for potential confounders, sarcopenia (OR 2.271; 95% CI 1.069–4.824, p = 0.033), lower BMD (OR 1.968; 95% CI 1.350–2.868, p < 0.001), advanced age (≥ 75 years; OR 2.431; 95% CI 1.246–4.744, p = 0.009), and female sex (OR 4.666; 95% CI 1.400–15.552, p = 0.012) remained as independent predictors for OVCRFs (Table 3).
Discussion
In this study, we confirmed the relationship between sarcopenia and OVCRFs and investigated other risk factors of vertebral refractures. Our findings revealed that among the 237 enrolled patients, 64 (27.0%) developed OVCRFs. Moreover, sarcopenia was present in 48 (20.3%) patients, including 21 OVCRFs and 27 non-OVCRFs patients. The percentage of OVCRFs was 43.8% (21/48) in sarcopenic patients and 22.8% (43/189) in non-sarcopenic patients (p = 0.003). Moreover, the multivariate analysis confirmed that the incidence of sarcopenia in the OVCRF group is twice that in the non-OVCRF group.
Osteoporosis and sarcopenia increase the risk of fragility fracture in the elderly. Interestingly, some studies found synergistic effects between osteoporosis and sarcopenia. Combined effects of osteoporosis and sarcopenia are disastrous to the elderly, which leads to reduced mobility, the risk for falls, and fragility fractures [10, 12]. In a study of 316 community-dwelling Chinese elderly in 2015, Wang et al. found that sarco-osteoporosis defined by the AWGS/WHO criteria was present in 10.4% of men and 15.1% of women aged more than 65 years, and its prevalence rate was higher in people aged 80 years and older [21]. Ilich suggested that women with osteosarcopenic obesity have increased the risk of bone fractures and immobility because of the combined decline in bone and muscle mass and increased fat mass [22]. Once the fracture is diagnosed, patients are advised for strict bed rest for a long time, and complications associated with prolonged bed rest increase, including bedsore, stroke, deep venous thrombosis, and pneumonia. Clinically, it is not common to encounter patients with vertebral fractures who underwent operation or conservative treatment to be hospitalized because of a new vertebral fracture. It has been said that the refracture rate of cemented vertebra and other vertebrae is 12–52%, which brings about a heavy economic burden not only to the family but also to the society [23]. In Europe, the estimated direct cost of all osteoporotic fractures in 2010 was calculated to be €29 billion in the five largest European Union (EU) countries (France, Germany, Italy, Spain, and the UK) and €38.7 billion in the other 27 EU countries [24].
Previous studies have proposed the possible mechanisms of vertebral fracture recurrence. Some authors suggested that the cement augmentation and increased physical activities after vertebroplasty played a role [5, 25], whereas Lindsay et al. explained the occurrence of a vertebral refracture as a progression of the underlying disease [26], Ahn et al. stated that the mechanisms of refracture at adjacent and nonadjacent vertebrae were different. A strength gradient caused by bone cement augmentation might cause an adjacent vertebral refracture (direct pillar effect), whereas a mobility gradient between neighboring segments might provoke a nonadjacent vertebral refracture (dynamic hammer effect) [27].
In our study, 27.0% of our patients developed OVCRFs, which was similar to that reported in previous studies [23, 28]. In 2013, Iolascon et al. evaluated the prevalence of sarcopenia in osteoporotic women with vertebral fractures and obtained results similar to our data [15]. In their study, 22 of the 67 women (32.8%) had sarcopenia, which was higher than that in our study. Most clinicians diagnose sarcopenia by evaluating the appendicular muscle mass via DXA directly. On the basis of muscle mass measurement, we added handgrip strength measure to assess muscle strength, which might have resulted in a lower proportion of patients with sarcopenia than that reported in other studies similar.
In our study, when comparing the control and OVCRFs groups, sarcopenia, age, sex, BMI, cardiopulmonary comorbidity, BMD, albumin, and TL junction were found to be the significant factors. However, after adjusting for other factors, some of them ceased to be significant predictors. As mentioned earlier, the statistical data in Table 1 showed that low BMI and albumin levels were related to sarcopenia, but the results of the multivariate analysis in Table 3 showed that they were not related to vertebral refractures. In 2005, De Laet et al. concluded in their meta-analysis that low BMI was a high-risk factor of all fractures, which was largely independent of age and gender, but dependent on BMD [29]. We speculated that the influence of sarcopenia was more than that of low BMI and low albumin levels.
In 2015, Hida et al. found that higher prevalence of sarcopenia patients with acute OVF compared with patients who did not have an OVF by a cross-sectional study [14]. They found no association between sarcopenia and BMD among patients with acute OVF; however, there was a positive association in patients who did not have an OVF. Another cross-sectional study of 600 community residents demonstrated that sarcopenia can be regarded as an independent risk factor for low BMD of the femoral neck and lumbar spine [9]. Because muscle cells and osteoblasts derive from a common mesenchymal precursor, certain genes may regulate bone and muscle via endocrine and cytokine [30]. In our study, we found associations between sarcopenia and OCVRFs independent of BMD, possible related confounders were not fully adjusted, or our sample size was not large enough. We think that sarcopenia is a potential independent risk factor for OVCFs, a study with normal BMD and low L3 SMI should be taken into account for preventing fractures in the future.
With increasing age, the weakening of muscle contraction will result in a decrease in bone marrow longitudinal stress. Subsequently, the number and quality of bone trabecula can also decrease. Meanwhile, the absorption of calcium in the digestive system decreases, which then leads to the loss of bone mass in elderly patients, especially postmenopausal women [10]. Consequently, elderly women were more likely to develop vertebral refractures. In this study, there was a statistically significant difference in age and sex between the OVCRFs group and non-OVCRFs group. Moreover, multivariate analysis revealed that age (≥ 75 years; OR 2.431; 95% CI 1.246–4.744, p = 0.009) and sex (OR 4.666; 95% CI 1.400–15.552, p = 0.012) were related to OVCRFs after PKP surgery.
Previous investigators reported osteoporosis itself played an important role in new vertebral fractures and were one of the most important risk factors [28, 31, 32]. The result of our study showed that patients with lower BMD were more likely to have a new OVCF than those with normal BMD. In our study, the average T-score was − 3.32 ± 0.82 in the OVCRFs group and − 2.62 ± 0.98 in the non-OVCRFs group (p < 0.001). As it is known, BMD tends to decrease with increasing age because of progressive bone resorption. In a cross-sectional study of 600 community residents, Wu et al. demonstrated that sarcopenia can be regarded as an independent risk factor for low BMD [9]. After adjusting for sarcopenia and age, multivariate logistic regression showed that BMD still remained a significant factor between the two groups (OR 1.968; 95% CI 1.350–2.868, p < 0.001). Our findings are comparable to those of previous studies. We postulate that the most important risk factor for refracture is the osteoporosis itself.
It is well known that the TL junction inherently carries the highest risk for fracture with maximum flexion and spine extension. Kim et al. found the risk of new vertebral compression fractures in adjacent vertebrae at the TL junction was 2.7 times higher than those at the non-TL junction [25]. In 2014, Sun et al. reported, in their retrospective study of 175 patients, that the percentage of OVCRFs was 13.9% (10/72) for treated vertebrae located in the non-TL junction and 26.2% (27/103) in the TL junction [28]. They reported that vertebral fracture in the TL junction has a higher incidence of OVCRFs, especially in osteoporotic patients, which was inconsistent with the reports of some authors who suggested no relationship between OVCRFs and treated vertebral level [31, 33]. From our results, the percentage of OVCRFs was 33.1% (47/142) for treated vertebrae located in the TL junction and 17.9% (17/95) for those treated in the non-TL junction (p = 0.01). However, the results of the multivariate analysis indicated no relationship between OVCRFs risk and treated vertebral level after PKP (p = 0.437).
Other risk factors like vacuum clefts, intradiscal cement leakage, and AP ratio showed no difference between the two groups. According to previous studies, intervertebral discal cement leakage and vacuum clefts may increase the occurrence of OVCRFs [5, 6, 31], which were not proven in our study. We found that the percentage of OVCRFs was 33.3% (19/57) in the vertebrae with vacuum clefts, whereas that for normal vertebrae was 25.0% (45/180) (p = 0.217). Ahn et al. reported that intradiscal cement leakage might increase the stress to the adjacent vertebra and cause a new fracture. Moreover, they stated that the mechanisms of refracture at adjacent and nonadjacent vertebrae were different [27]. In our series, the intradiscal cement leakage was not significantly related to a vertebral refracture. Lee found a difference in OVCRFs incidence between the new fracture group (mean 0.79) and the control group (mean 0.69, p < 0.01) and concluded that decreasing preoperative wedging deformity increases the risk of developing new vertebral compression fracture after PVP [33]. However, interestingly, this is contrary to the study conducted by Rho et al. who retrospectively analyzed the occurrence of new OVCFs in 147 patients and found that the AP ratio of the fractured vertebra did not influence the occurrence of the new vertebral fractures [31], which was comparable to our findings (0.79 vs 0.73, p = 0.242).
There are several limitations to this retrospective study. First, we used a new measurement method and cutoff points for evaluating the muscle mass. Moreover, we are aware that patients with different ethnicities, those from different countries, or those with other diseases might have different cutoff points. Second, because of the high cost of MRI, we routinely reviewed the patient’s X-rays at every 3 months of follow-up visit. However, we could not exclude a small minority of patients who had vertebral refractures with slight pain. This might indeed lead to sample misclassification. Third, we did not obtain details of other correlative medications taken by patients, which might have caused a high heterogeneity and led to a bias. Therefore, the results should be interpreted with caution, and further research is required to confirm our findings.
Conclusion
In our study, OVCRFs occurred in 27% of the patients during the 1-year follow-up after PKP. Sarcopenia is a highly independent risk factor of OVCRFs. Other factors affecting OVCRFs include low BMD T-scores, female sex, and advanced age. For those patients with sarcopenia, physicians should strengthen preoperative and postoperative education, anti-osteoporosis and nutritional comprehensive treatment, guide postoperative functional exercise, and prevent falls, to minimize the occurrence of OVCRFs. A further prospective study involving a larger number of patients with long-term follow-up is necessary to confirm the results of our study.
References
Kim DH, Vaccaro AR (2006) Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J 6:479–487
Garfin SR, Yuan HA, Reiley MA (2001) New technologies in spine - Kyphoplasty and vertebrosplasty for the treatment of painful osteoporotic compression fractures. Spine 26:1511–1515
Buchbinder R, Johnston RV, Rischin KJ, Homik J, Jones CA, Golmohammadi K, Kallmes DF (2018) Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database Syst Rev 4:CD006349
Syed MI, Patel NA, Jan S, Shaikh A, Grunden B, Morar K (2006) Symptomatic refractures after vertebroplasty in patients with steroid-induced osteoporosis. Am J Neuroradiol 27:1938–1943
Lin EP, Ekholm S, Hiwatashi A, Westesson PL (2004) Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. Am J Neuroradiol 25:175–180
Lin CC, Chen IH, Yu TC, Chen A, Yen PS (2007) New symptomatic compression fracture after percutaneous vertebroplasty at the thoracolumbar junction. AJNR Am J Neuroradiol 28:1042–1045
Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M, Asian Working Group for S (2016) Recent advances in sarcopenia research in Asia: 2016 update from the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 17:–767 e761–767
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R (2004) The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52:80–85
Wu CH, Yang KC, Chang HH, Yen JF, Tsai KS, Huang KC (2013) Sarcopenia is related to increased risk for low bone mineral density. J Clin Densitom 16:98–103
Edwards MH, Dennison EM, Aihie Sayer A, Fielding R, Cooper C (2015) Osteoporosis and sarcopenia in older age. Bone 80:126–130
Binkley N, Krueger D, Buehring B (2013) What’s in a name revisited: should osteoporosis and sarcopenia be considered components of “dysmobility syndrome?”. Osteoporos Int 24:2955–2959
Hirschfeld HP, Kinsella R, Duque G (2017) Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 28:2781–2790
Walsh MC, Hunter GR, Livingstone MB (2006) Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos Int 17:61–67
Hida T, Shimokata H, Sakai Y, Ito S, Matsui Y, Takemura M, Kasai T, Ishiguro N, Harada A (2015) Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J 25:3424–3431
Iolascon G, Giamattei MT, Moretti A, Di Pietro G, Gimigliano F, Gimigliano R (2013) Sarcopenia in women with vertebral fragility fractures. Aging Clin Exp Res 25(Suppl 1):S129–S131
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JSW, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101
Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, Suetsugu A, Shiraki M, Shimizu M, Moriwaki H (2015) Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol 50:323–332
Boutin RD, Yao L, Canter RJ, Lenchik L (2015) Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol 205:W255–W266
Szulc P, Feyt C, Chapurlat R (2016) High risk of fall, poor physical function, and low grip strength in men with fracture-the STRAMBO study. J Cachexia Sarcopenia Muscle 7:299–311
Wang YJ, Wang Y, Zhan JK, Tang ZY, He JY, Tan P, Deng HQ, Huang W, Liu YS (2015) Sarco-osteoporosis: prevalence and association with frailty in Chinese community-dwelling older adults. Int J Endocrinol 2015:482940
Ilich JZ, Inglis JE, Kelly OJ, McGee DL (2015) Osteosarcopenic obesity is associated with reduced handgrip strength, walking abilities, and balance in postmenopausal women. Osteoporos Int 26:2587–2595
Kang SK, Lee CW, Park NK, Kang TW, Lim JW, Cha KY, Kim JH (2011) Predictive risk factors for refracture after percutaneous vertebroplasty. Ann Rehabil Med 35:844–851
Strom O, Borgstrom F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jonsson B (2011) Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155
Kim SH, Kang HS, Choi JA, Ahn JM (2016) Risk factors of new compression fractures in adjacent vertebrae after percutaneous vertebroplasty. Acta Radiol 45:440–445
Lindsay R, Burge RT, Strauss DM (2005) One year outcomes and costs following a vertebral fracture. Osteoporos Int 16:78–85
Ahn Y, Lee JH, Lee HY, Lee SH, Keem SH (2008) Predictive factors for subsequent vertebral fracture after percutaneous vertebroplasty. J Neurosurg Spine 9:129–136
Sun G, Tang H, Li M, Liu X, Jin P, Li L (2014) Analysis of risk factors of subsequent fractures after vertebroplasty. Eur Spine J 23:1339–1345
De Laet C, Kanis JA, Oden A et al (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16:1330–1338
Karasik D, Kiel DP (2008) Genetics of the musculoskeletal system: a pleiotropic approach. J Bone Miner Res 23:788–802
Rho YJ, Choe WJ, Chun YI (2012) Risk factors predicting the new symptomatic vertebral compression fractures after percutaneous vertebroplasty or kyphoplasty. Eur Spine J 21:905–911
Uppin AA, Hirsch JA, Centenera LV, Pfiefer BA, Pazianos AG, Choi IS (2003) Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology 226:119–124
Lee WS, Sung KH, Jeong HT, Sung YS, Hyun YI, Choi JY, Lee KS, Ok CS, Choi YW (2006) Risk factors of developing new symptomatic vertebral compression fractures after percutaneous vertebroplasty in osteoporotic patients. Eur Spine J 15:1777–1783
Funding
This work was supported by the National Natural Science Foundation of China (81571190).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 83 kb)
Rights and permissions
About this article
Cite this article
Wang, WF., Lin, CW., Xie, CN. et al. The association between sarcopenia and osteoporotic vertebral compression refractures. Osteoporos Int 30, 2459–2467 (2019). https://doi.org/10.1007/s00198-019-05144-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-019-05144-x