Abstract
Summary
Osteogenesis imperfecta (OI) is a disease causing bone fragility; however, it potentially affects all organs with a high content of collagen, including ears, teeth, and eyes. The study is cross-sectional and compares non-skeletal characteristics in adults with OI that clinicians should be aware of when caring for patients with OI.
Introduction
Osteogenesis imperfecta (OI) is a hereditary connective tissue disorder. The skeletal fragility is pronounced; however, OI leads to a number of extra-skeletal symptoms related to the ubiquity of collagen type 1 throughout the human body. The vast majority of knowledge is derived from studies performed in the pediatric population. Thus, we aimed to investigate the nature and prevalence of ophthalmologic, odontologic, and otologic phenotypes in an adult population with OI.
Methods
The study population comprises 85 Danish OI patients (age 44.9 ± 15.9 years). Fifty-eight patients had OI type I, 12 OI type III, and 15 OI type IV according to the classification by Sillence. Audiometric evaluations and dental examinations were performed in 62 and 73 patients, respectively. Ophthalmologic investigations were performed in 64 patients, including measurements of the central corneal thickness.
Results
All patients, except two, had corneal thickness below the normal reference value. Patients with OI type I and patients with a quantitative collagen defect had thinner corneas compared to patients with OI type III and other patients with a qualitative collagen defect. One patient in this cohort was diagnosed with and treated for acute glaucoma. Dentinogenesis imperfecta was diagnosed in one fourth of the patients, based on clinical and radiographic findings. This condition was predominately seen in patients with moderate to severe OI. Hearing loss requiring treatment was found in 15 of 62 patients, of whom three were untreated. The most prevalent type of hearing loss (HL) was sensorineural hearing loss, whereas conductive HL was solely seen in patients with OI type III. The patients with the most severe degrees of HL were patients with mild forms of OI. Age was associated with increased HL.
Conclusion
Although significant health problems outside the skeleton are frequent in adult patients with OI, the patients are not consistently monitored and treated for their symptoms. Clinicians treating adult patients with OI should be aware of non-skeletal health issues and consider including regular interdisciplinary check-ups in the management plan for adult OI patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteogenesis imperfecta (OI) is a hereditary disease with a generalized involvement of the connective tissue. It is mostly, but not exclusively, caused by mutations in the genes encoding the α1 and α2 chains of collagen type 1 [1,2,3]. OI is caused either by a normal amount of structurally defective collagen (qualitative defect) or by a decreased amount of structurally normal collagen (quantitative defect). Collagen type 1 is the most abundant protein in the human body [3]. The genes encoding collagen type 1 are COL1A1 and COL1A2 [4]. Skeletal symptoms such as fractures, bone deformities, and pain are dominant features in OI [5], but patients may also suffer from disease in other organs or organ systems with a high content of collagen type 1 [1, 6, 7].
In the eye, collagen type 1 is an important component of the scleral stroma, the layers of the cornea, and the uveal tissues [8,9,10]. In OI, conditions like myopia and astigmatism are common [11] and previous studies show that OI patients in general have thinner corneas than healthy controls [12]. Case reports have described traumatic scleral rupture, retinal detachment, and hemorrhages in OI [13,14,15]. It is, however, not known whether corneal thickness differs between OI types or the type of mutation.
In addition, a considerable proportion of OI patients suffer from dysplasia of the dentine, dentinogenesis imperfecta (DI) [16,17,18]. The organic part of the dentine is primarily composed of collagen type 1 [19] and therefore, collagen type 1 mutations may result in dysplastic dentine and teeth that are susceptible to fracture. In DI, the enamel appears structurally normal but it is vulnerable [20], and the teeth show signs of obliterated pulp chambers, short and thin roots, and discoloration of the crown [20, 21]. The diagnosis of DI is based on clinical and radiographic findings. Only few studies have correlated OI severity and biochemical findings with DI [20, 21] and no studies have shown a correlation to the underlying genetic aberration. DI has been linked to the collagen abnormality (whether qualitative or quantitative), but the reason why some collagen type 1 mutations cause DI and others do not is not yet fully understood [22].
Finally, many OI patients suffer from hearing loss (HL). This is often due to a condition resembling otosclerosis [23]. Abnormalities of the labyrinth and the surrounding temporal bone, and fractures or deformities of the ossicles in the middle ear, can result in sensorineural HL (SNHL) or conductive HL, respectively, or a combination of both termed mixed HL [23, 24]. SNHL commonly occurs in OI from the fourth decade of life, whereas conductive HL usually presents two decades earlier or even in childhood [25]. Adult patients thus often suffer from mixed HL, which has been found in up to 50% [25, 26]. However, the relationship between the prevalence of HL and OI severity or molecular-genetic findings is not well-described.
Thus, to improve knowledge of relationship between OI type and type of mutation and corneal thickness, DI, and hearing loss, we conducted a cross-sectional study in a well characterized population of 85 Danish adults with genetically confirmed OI.
Experimental subjects
Study population
We conducted a cross-sectional study including 85 Danish adult OI patients, aged 19–78 years [27]. Inclusion criteria were diagnosis of OI, and age ≥ 18 years. Exclusion criteria were cancer, treatment with glucocorticoids equivalent to 5 mg prednisolone or more within the last 3 months, other metabolic bone diseases, renal disease, and hepatic disease. The recruitment of patients took place from 2011 to 2013 through databases at university hospitals and regional hospitals in Denmark and by advertisements in the journal of the Danish OI Patient Society, DFOI. An invitation was sent to 126 patients with OI who were in contact with DFOI or cared for at the departments of endocrinology at Aarhus University Hospital (AUH), Odense University Hospital (OUH), or Hvidovre Hospital. We received 95 positive replies, of which a total of 85 patients with OI were included in the study. All participants who were able to travel (n = 83) were examined at AUH and referred to the Department of Ophthalmology, Aarhus University Hospital for an ophthalmological examination, Section for Pediatric Dentistry, Department of Dentistry and Oral Health, Aarhus University for clinical and radiographic dental examination, and to the Department of Otorhinolaryngology, AUH or the Department of Otorhinolaryngology, OUH for audiometric evaluation and tympanometry. Patients unable to travel (n = 2) were examined at their local hospital. All patients provided written informed consent. The studies were approved by the Central Danish Regional Committee on Health Research Ethics (ref-number M-20100108).
Materials and methods
Medical history and anthropometry
In all patients, medical history was obtained and a clinical examination performed. If OI diagnosis was considered likely, we invited the patient to take part in the study. Medical history was obtained by structured medical interviews and clinical characteristics reported, including family disposition, medical history, fracture history, previous and ongoing medication known to affect bone including anti-osteoporotic therapy, and bone deformities including scoliosis by physical examination, scleral hue, and self-reported hypermobility. We systematically obtained anthropometric measures including body height (without shoes, by wall mounted stadiometer when possible, alternatively measuring tape), weight (lightly clothed without shoes), sitting height (1 m ruler), arm span (by wall mounted scale), and head circumference (measuring tape). Finally, we asked all patients about symptoms of visual impairment, DI or hearing loss.
Ophthalmologic evaluation
Scleral color was noted in each patient, and central corneal thickness (CCT) was measured in 64 patients by corneal tomography and pachymetry by rotating Scheimpflug imaging using Pentacam HR© (Oculus, Wetzlar, Germany). We compared the CCT measures in OI patients to 123 myopic individuals evaluated using the same equipment. Anterior chamber depth (ACD) was measured using the Lenstar LS900 laser biometer (Haag-Streit AG, Koeniz, Switzerland).
Clinical and radiographic dental examination
Information on tooth color, tooth wear, tooth fractures, previous dental treatment, and dentures was obtained. As a part of the investigation, clinical photos and alginate impressions of the dental arches were obtained (Aroma fine fast, GC Europe). In individuals with teeth present, a full-mouth periapical survey with digital intraoral radiographs using GX 1000 dental Xray© (Gendex, Des Plaines, IL, USA), as well as a digital panoramic radiograph using the digital radiographic equipment Planmeca Promax© (Planmeca Oy, Helsinki, Finland) was performed. The evaluation of the dental hard tissues included an assessment of the radiographic signs of obliterated pulp chamber, pulp stone, short root, and cervical constriction. The final diagnosis of DI depended on consensus among four examinators (HG, DH, MS, and JH) after assessing clinical recordings, radiolographic signs and evaluation of clinical photos. In two individuals, the diagnosis could not be confirmed due to loss of teeth.
Otologic evaluation
Audiometric evaluation was performed in 62 patients: 47 patients at AUH and 15 patients at OUH. The evaluation included otomicroscopy to ensure passage of the external ear canal.
Pure-tone and speech audiometry were performed in a sound-attenuated room applying conventional audiometric techniques. Air conduction thresholds (AC) and bone conduction thresholds (BC) were determined bilaterally for octave frequencies (AC: 0.25 to 8 kHz and BC: 0.5,1,2, and 4 kHz), and a threshold average calculated for 0.5, 1.0, 2.0, and 3.0 kHz for both AC and BC bilaterally to determine a pure-tone average (PTA). Air-bone gap (ABG) was calculated based on an average for AC and BC frequencies 0.5, 1, 2, and 3.0 kHz [28].
Hearing loss (HL) was classified using the criteria applied by Swinnen et al.: (A) conductive HL: PTA (BC) < 15 dB HL and ABG ≥ 15 dB, (B) sensorineural HL (SNHL): PTA (AC) ≥ 15 dB HL and ABG < 15 dB, (C) high-frequency sensorineural HL: an average of AC thresholds for 4.0, 6.0, and 8.0 kHz > 30 dB, and (D) mixed HL: PTA (BC) ≥ 15 dB HL and ABG ≥ 15 dB [29]. Cases of SNHL and cases of high-frequency SNHL are combined as SNHL in the analysis. Severity of HL was determined on PTA (AC) at the frequencies 0.5, 1.0, 2.0, and 3.0 kHz as normal, mild, moderate, moderate-severe, severe, or profound [30]. Values are measured and reported as uni-or bilateral hearing loss.
Speech reception thresholds were determined with an application of monosyllabic Danish number words, and discrimination score using a standard list of 25 Danish words. The percentage of perceived words was calculated. Contralateral masking was applied appropriately. Information on previously performed ear surgery and hearing aid was collected and confirmed from patient records.
Collagen assessments
Chemical analysis of the collagen type 1 protein was performed using cultured fibroblasts from dermal biopsies. The analysis of the collagen structure and production was done using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of (pro) collagen I as reported previously [27, 31]. Based on the protein analyses, collagen production was characterized as normal, quantitatively defective (normal collagen protein, but in reduced amount), or qualitatively defective (altered collagen protein structure). The clinical diagnosis of OI in the included patients was confirmed genetically, and the mutations have previously been presented [27].
Statistics
All measures, and within group measures, were checked for normal distribution using QQ plots, and where possible, parametric tests were used. One sample T test was used when comparing results to values in the general population. To examine differences between OI types, we used ANOVA or Wilcoxon matched-pairs signed-rank test as appropriate.
We tested paired measurements as left and right by paired sample T test or Wilcoxon, as appropriate. Corneal thickness did not differ significantly between left eye and right eye, and we therefore calculated mean CCT to test for differences between groups. The frequency of thin corneas was evaluated in a myopic population for comparison. We calculated sensitivity and specificity to compare CCT in patients with mild OI to this control population. As measures of hearing level differed between ears in some individuals, we reported a combination of HL type in each individual. The chi-square test was used to test for frequencies and the Kruskal-Wallis test was applied to test severity of HL between groups. Logistic regression or simple and subsequently multiple regression analyses were used to investigate which variables predicted non-skeletal phenotypes in OI. OI type was coded as I = 0, IV = 1, and III = 2; collagen type was coded as quantitative = 1 and qualitative = 2. We assessed assumptions by inspecting variables and examining residuals. R2 and p values were reported accordingly. All statistical analyses were performed using SPSS version 20.0 for Windows (IBM SPSS, IL).
Results
Medical history and anthropometry
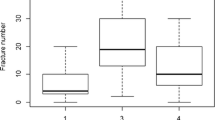
Table 1 displays medical history and anthropometric data. The study comprised 38 men and 47 women, with a mean age of 45 [18–78] years. According to the original Sillence classification, 58 patients had OI type I, 12 patients had OI type III, and 15 patients had OI type IV [6]. This distribution corresponded to non-deforming OI with blue sclerae, progressively deforming OI, and common variable OI with normal sclerae using the more recent classification by Dijk et al. [2]. Age and gender did not differ between the three OI types. On average, patients were shorter with a mean height ± SD of 155.2 ± 21.4 cm (p < 0.001) compared to the normal Danish population [32]. Within the OI population, height differed significantly (p < 0.001) between groups. As expected, post hoc tests revealed that the patients with OI type III were significantly shorter than the other OI groups, with an average height of 105.9 ± 17.2 cm (p < 0.001). Furthermore, type III patients had shorter sitting height and arm span, and lower weight. Head circumference did not differ between the groups.
At inclusion, the patients had suffered a mean of 22 [0–150] fractures. Hypermobility was reported by 65% of the patients with no differences between the groups. Fifty-two percent of the cohort reported an increased tendency of bruising, including 68% of patients with OI type I, and 18 and 21% of patients with OI type III and OI type IV, respectively (p = 0.001).
Collagen structure and production was evaluated by SDS PAGE of collagen type 1 in 67 patients: 64 patients had a qualitatively or quantitatively defective collagen, and in 3 patients, the analysis was normal (OI type I). Of the 43 patients with OI type I, 36 patients had quantitatively defective collagen I and 4 had qualitatively defective collagen I. All patients with OI type III had qualitatively defective collagen I. In OI type IV, one patient had quantitatively and 14 patients had qualitatively defective collagen I.
Ophthalmology
Sixty-four patients underwent an ophthalmologic examination (Table 2). The prevalence of bluish colored sclerae was 90%. CCT was measured in both eyes in 62 patients. In one patient, only the right eye scan was of sufficient quality, and in one patient, the examination was unsuccessful due to technical difficulties. No significant differences were found between CCT measures in the left and in the right eye (data not shown). The mean CCT ± SD was 473 ± 38 μm. With the exception of two patients (both OI type IV), CCT was below the normal reference value, (550 ± 30 μm, mean ± SD) (p < 0.001) [33]. Patients with OI type I had a mean CCT of 461 ± 32 μm. In patients with OI type III and OI type IV, CCT was 510 ± 29 μm and 500 ± 40 μm, respectively (p < 0.001). OI patients with blue sclerae had thinner CCT measures compared to OI patients with white sclerae, 470 ± 32 μm vs 497 ± 46 μm; however, this was not statistically significant (p = 0.69).
Because of the marked difference in CCT between patients with OI type I and healthy controls, we investigated the ability of CCT to discriminate these two populations. Based on sensitivity and specificity, we constructed a receiver operating characteristics (ROC) curve (Fig. 1), and the area under the curve was 0.98 suggesting that CCT is a very strong diagnostic test. By inspecting the coordinates, we found that the optimal threshold was a CCT of 505 μm giving a sensitivity for diagnosing OI type I of 97.6% with a specificity of 94.3%.
Mean anterior chamber depth was 2.73 ± 0.36 mm in the cohort, without significant differences between the three groups, or between patients with quantitative and qualitative collagen defects. One patient (OI type I) was diagnosed with acute glaucoma. Two patients were referred to follow-up for secondary glaucoma.
Dentinogenesis imperfecta
Seventy-three patients with mild to severe OI underwent a full clinical and radiographic dental examination (86% of the cohort). In 18 of 73 patients (25%), DI was verified (Table 1). This comprised 1 patient (2%) with OI type I, 6 patients (86%) with OI type III, and 11 patients (85%) with OI type IV (p < 0.001). The diagnosis was solely based on radiographic examination in 1 patient with DI. In 17 patients, both clinical and radiographic characteristics provided the basis for the diagnosis. Among the 18 patients with confirmed DI, 17 (94%) had a moderate-severe clinical phenotype; one patient had mild OI. The genetic analysis of the patient with mild OI and DI showed a pre-mature stop-codon indicating a quantitative abnormal collagen defect. However, the patients with diagnosed DI who also underwent collagen analyses all had qualitatively defect collagen type 1. Logistic regression analysis revealed that disease severity (type of OI) was associated with an increased risk of DI (R2 = 0.788, p < 0.001), and decreased CCT was associated with a decreased risk of DI (R2 = 0.296, p = 0.002).
Hearing impairment
Sixty-two patients underwent audiometric evaluation categorizing type and severity of hearing difficulties (73% of the cohort) (Table 3). No HL was found in 23 patients (37%), of whom 16 patients had OI type I, none had OI type III, and nine patients suffered from OI type IV. Seventeen (39%) of the patients without HL had a quantitative defective collagen type 1 whereas six (33%) had a qualitative defective collagen type 1. Thirty-nine of the 62 patients (63%) presented with some type of HL, and of these, 28 patients (64%) had OI type I, six patients (100%) had OI type III, and five patients had OI type IV (42%). Twenty-five of the 44 patients (57%) with a quantitative defective collagen type 1 presented with some type of HL, whereas this was the case in 14 of the 18 patients (78%) with a qualitative defective collagen type 1.
Among OI type I patients with mild to profound HL, 18 patients (41%) suffered from SNHL or high frequency SNHL uni- or bilaterally, and five patients (11%) suffered from mixed HL uni- or bilaterally. Five patients (11%) presented with a combination of mixed HL in one ear and SNHL in the other ear. No patients with OI type I suffered from pure conductive HL. Fourteen OI type I patients (32%) had undergone uni- or bilateral middle ear surgery at least once, and 12 patients (27%) used either conventional hearing aids (ten patients) or a bone-anchored hearing device (two patients).
All six patients with OI type III presented with HL. One patient (17%) presented with SNHL bilaterally, two (33%) suffered from mixed HL uni- or bilaterally, conductive HL was found in two patients (33%), and, finally, a combination of mixed HL in one ear and SNHL in the other in one patient. Two OI type III patients (33%) had undergone surgery of the middle ear uni- or bilaterally, and three patients (50%) used hearing aid.
Mild to profound HL was found in three patients with OI type IV (25%) of which one suffered from bilateral SNHL and two (17%) from a combination of mixed HL in one ear and SNHL in the other. In OI type IV patients, there were no cases of only conductive or mixed HL. One patient with OI type IV (8%) had a history of bilateral middle ear surgery, and one patient (8%) used hearing aids.
HL requiring treatment (AC PTA thresholds ≥ 40 dB) was found in 15 OI patients (24%). In 12 of these patients (80%), treatment was provided, leaving HL untreated in 20% of the patients with significantly elevated thresholds.
The prevalence of the various types of HL differed between OI subtypes, as shown in Table 3 (p < 0.001). No difference was found when comparing frequencies of HL types between patients with quantitative or qualitative collagen defects, and also the two groups were comparable with regard to the prevalence of middle ear surgery and the use of hearing aids. SNHL was the most frequent type of HL, both in patients with a qualitative defective collagen type 1 and in patients with a quantitative defective collagen type 1. Patients with mixed HL and mixed/SNHL had more severe HL than patients with solitary SNHL (p < 0.001) (Table 4). Logistic regression analysis demonstrated that increased age (R2 = 0.281, p = 0.001) was independently associated with increased risk of impaired hearing and predicted 72% of cases of impaired hearing.
Genetic mutations found in this study are listed with the corresponding corneal thickness, dental presentation, and type and severity of hearing loss and audiologic phenotype in patients who underwent one or more of these investigations (Suppl. Table 4).
Discussion
The present study comprises the largest cohort of ophthalmological examined adult patients with OI. Overall, the patients have decreased central corneal thicknesses in accordance with reports from previous studies [12]. A correlation has been described between CCT and scleral color; however, estimation of scleral color is subjective and thus unreliable [9, 12]. The color may fade over time from blue in childhood to white or gray in adulthood [12]. It has previously been debated, whether there is an association between blue sclerae and corneal thickness. We found thinner corneas among OI patients with blue sclera compared to OI patients with white sclerae. However, our data showed more significant differences depending on OI types, with a markedly decreased CCT in the mild phenotype, OI type I compared with OI type III and IV indicating that the colored sclerae are caused by changes in the scleral stroma based on collagen type 1 abnormality, and not only based on the fact that the corneas are thin. Moreover, we found that measurement of CCT discriminates type I OI from healthy controls very well with a sensitivity and specificity of 97.6 and 94.3%, respectively.
This difference between OI types and between mild OI and non-OI has not been highlighted previously, but correlates well with the underlying collagen pathology: CCT is markedly lower in patients with OI type I, that have a quantitative collagen defect, whereas patients with a qualitative defect (OI type III and IV) have CCT values below individuals without OI, but not as low as the CCT levels in patients with an isolated quantitative defect. Diagnostic challenges may occur in mild OI. Based on the results obtained in the present study, we suggest using an ophthalmologic examination with CCT measurement in the case of an uncertain diagnosis of OI type I. Thus, a very low CCT may turn out to be an important sign of attenuated OI. This finding, however, needs to be replicated in another and preferably larger population.
The frequency of DI in this cohort (25%) was in concordance with some previous reports [21, 34], but below reported frequencies in other studies [35, 36], and above the frequency of DI in a previous Scandinavian study [20]. The variation in prevalence may reflect inconsistencies in the clinical DI diagnosis, which is based on a subjective visual assessment of a number of clinical and radiographic signs, as well as differences in frequencies of the different OI types between populations. One study describes all cases of DI in OI to be associated with a qualitatively defective collagen type 1 production [21]. Our study indeed supports this since > 80% of patients with moderate-severe OI and a qualitatively defective collagen type 1 had DI whereas only one patient with mild OI also suffered from DI. This suggests that all patients with moderate-severe OI and qualitative collagen deficiencies need referral to an examination in a specialized dental-care unit whereas in patients with mild OI and quantitative collagen deficiencies need referral on an individual basis.
In concordance with a study by Kuurila et al., 52% of the patients in the present cohort suffer from hearing loss [23]. Previous studies conclude that patients with OI type I are more likely to suffer from HL than patients with OI type III and IV. This finding is partially confirmed in the present study, as we found HL to be more common in patients with OI type I than in patients with type IV OI [23]. However, in our study, HL was found to occur in all patients with OI type III. The most severe HL was found among patients with OI type I, indicating the importance of also referring mildly affected patients to a hearing evaluation. Studies in children with HL report conductive HL in up to 64% of cases; however, many reports include cases with otitis media with effusion (fluid in the middle ear) (OME), a condition strongly related to conductive hearing loss [25, 26]. If cases with OME are excluded from the mentioned studies, the prevalence of conductive HL drops to 9%, which is in line with our findings (3%). A prevalence of SNHL of 17% was also reported in the studies on HL in pediatric OI, which would be expected to be lower than in the present study on adult patients, as age is a risk factor for the development of SNHL, as in the general population [37, 38]. Although HL is a known complication to OI, 20% of patients with a HL requiring treatment in the form of hearing aids were untreated. Age was a predictor of hearing loss in our patients; however, we did not find other clinical characteristics that independently predicted impairment of hearing. Patients suffering from OI often endure physical disabilities which may limit social activities. Thus, optimization of treatment with hearing aids or bone-anchored hearing devices should be a priority to prevent social isolation and preserve quality of life. Patients with OI have reduced physical health-related quality of life; however, mental health-related quality of life is found to be preserved [39]. It is important to seek to improve the physical health in these patients in order to improve the physical health-related quality of life.
In line with previous studies, this cohort reported an increased tendency of bruising, two thirds of OI type 1 patients, and significantly fewer in the more severely affected OI patients. This may be related to vulnerable vessels or defect platelet plug formation due to reduced amounts of type 1 collagen [40].
The present study presents well characterized and genetically confirmed cases of OI, which lends strength to our findings. Investigations of adult OI are few, and the current study represents a relatively large study.
However, there are limitations, such as the cross-sectional design. Data are collected at a single time point, and the cohort may not be representative for the OI population in general. As the presented study is a descriptive study, it does not include a matched control group. We can therefore only compare our results to investigations performed previously and under different conditions.
Many adults with OI are routinely monitored and treated for fragile bones. However, these patients endure a number of other physical disabilities due to the general involvement of connective tissue. Although patients with OI are at increased risk of HL and glaucoma, our impression is that many patients are not routinely screened for these conditions, and treatment is inadequate in a considerable proportion of patients. There may be several explanations to this. One possibility is that the patients do not report the symptoms. An example is hearing loss that may develop slowly over several years, and become significant without the patient recognizing its presence. Another explanation may be that bone specialists, who are often responsible for the medical management of these patients, may not be fully aware of these complications or assume that the general practitioners will take care of the non-skeletal symptoms. We speculate whether individuals suffering from a chronic lifelong disease like OI tend to accept greater physical disadvantages than individuals without the same medical history. In OI patients, fracture risk in adulthood is lower than in childhood, but some non-skeletal complications are found with the same or an even higher frequency in adults [41]. Therefore, it is essential that the clinical management of adult OI patients focus on both skeletal and non-skeletal symptoms. Based on these results, a systematic bone-specific follow-up must be complemented by a non-skeletal follow-up as a gold standard in the management of patients with OI. A measure of corneal thickness may be considered in the diagnostic process of an individual with suspected mild OI. Odontologic investigations should be considered in all adults with OI, especially the patients with a moderate-severe phenotype. Our investigations also suggest that all adult patients with OI should be informed about the risk of hearing loss and offered a routine examination.
Grant supporters
This study received financial support from the Clinical Institute of Health, AUH, Denmark; Central Region of Denmark; Osteoporoseforeningen, Denmark; The Danish Association for Public Dentists (TNL/DOFT/ATO); and Grosserer L.F. Foghts Fond.
References
Rauch F, Glorieux FH (2004) Osteogenesis imperfecta. Lancet 363:1377–1385
van Dijk FS, Sillence DO (2014) Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet A 164A:1470–1481
Forlino A, Cabral WA, Barnes AM, Marini JC (2011) New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol 7:540–557
Byers PH, Steiner RD (1992) Osteogenesis imperfecta. Annu Rev Med 43:269–282
Folkestad L, Hald JD, Ersboll AK, Gram J, Hermann AP, Langdahl B, Abrahamsen B, Brixen K (2017) Fracture rates and fracture sites in patients with osteogenesis imperfecta: a nationwide register-based cohort study. J Bone Miner Res 32:125–134
Sillence DO, Senn A, Danks DM (1979) Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 16:101–116
Cundy T (2012) Recent advances in osteogenesis imperfecta. Calcif Tissue Int 90:439–449
Schmut O (1978) The organization of tissues of the eye by different collagen types. Albrecht Von Graefes Arch Klin Exp Ophthalmol 207:189–199
Evereklioglu C, Madenci E, Bayazit YA, Yilmaz K, Balat A, Bekir NA (2002) Central corneal thickness is lower in osteogenesis imperfecta and negatively correlates with the presence of blue sclera. Ophthalmic Physiol Opt 22:511–515
Ehlers N, Hjortdal J (2004) Corneal thickness: measurement and implications. Exp Eye Res 78:543–548
Scott A, Kashani S, Towler HM (2005) Progressive myopia due to posterior staphyloma in type I osteogenesis imperfecta. Int Ophthalmol 26:167–169
Pedersen U, Bramsen T (1984) Central corneal thickness in osteogenesis imperfecta and otosclerosis. ORL J Otorhinolaryngol Relat Spec 46:38–41
Rosbach J, Vossmerbaeumer U, Renieri G, Pfeiffer N, Thieme H (2012) Osteogenesis imperfecta and glaucoma. A case report. Ophthalmologe 109:479–482
Pirouzian A, O'Halloran H, Scher C, Jockin Y, Yaghmai R (2007) Traumatic and spontaneous scleral rupture and uveal prolapse in osteogenesis imperfecta. J Pediatr Ophthalmol Strabismus 44:315–317
Ganesh A, Jenny C, Geyer J, Shouldice M, Levin AV (2004) Retinal hemorrhages in type I osteogenesis imperfecta after minor trauma. Ophthalmology 111:1428–1431
Barron MMD (2008) Hereditary dentine disorders: dentinogenesis imperfecta and dentine dysplasia. Orphanet J Rare Dis 3:31
Biria M, Abbas FM, Mozaffar S, Ahmadi R (2012) Dentinogenesis imperfecta associated with osteogenesis imperfecta. Dent Res J 9:489–494
Chetty M, Roberts T, Stephen LX, Beighton P (2016) Hereditary dentine dysplasias: terminology in the context of osteogenesis imperfecta. Br Dent J 221:727–730
O’Connell AC, Marini JC (1999) Evaluation of oral problems in an osteogenesis imperfecta population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 87:189–196
Saeves R, Lande WL, Ambjornsen E, Axelsson S, Nordgarden H, Storhaug K (2009) Oral findings in adults with osteogenesis imperfecta. Spec Care Dentist 29:102–108
Lund AM, Jensen BL, Nielsen LA, Skovby F (1998) Dental manifestations of osteogenesis imperfecta and abnormalities of collagen I metabolism. J Craniofac Genet Dev Biol 18:30–37
Ben AI, Glorieux FH, Rauch F (2011) Genotype-phenotype correlations in autosomal dominant osteogenesis imperfecta. J Osteoporos 2011:540178
Kuurila K, Kaitila I, Johansson R, Grenman R (2002) Hearing loss in Finnish adults with osteogenesis imperfecta: a nationwide survey. Ann Otol Rhinol Laryngol 111:939–946
Pedersen U (1984) Hearing loss in patients with osteogenesis imperfecta. A clinical and audiological study of 201 patients. Scand Audiol 13:67–74
Pillion JP, Shapiro J (2008) Audiological findings in osteogenesis imperfecta. J Am Acad Audiol 19:595–601
Paterson CR, Monk EA, McAllion SJ (2001) How common is hearing impairment in osteogenesis imperfecta? J Laryngol Otol 115:280–282
Hald JD, Folkestad L, Harslof T, Lund AM, Duno M, Jensen JB, Neghabat S, Brixen K, Langdahl B (2016) Skeletal phenotypes in adult patients with osteogenesis imperfecta-correlations with COL1A1/COL1A2 genotype and collagen structure. Osteoporos Int 27:3331–3341
Jerger J (1970) Clinical experience with impedance audiometry. Arch Otolaryngol 92:311–324
(1995) Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. Otolaryngeal Head Neck Surg 113(3):186–187. https://doi.org/10.1016/S0194-5998(95)70103-6
Swinnen FK, Dhooge IJ, Coucke PJ, D'Eufemia P, Zardo F, Garretsen TJ, Cremers CW, De Leenheer EM (2012) Audiologic phenotype of osteogenesis imperfecta: use in clinical differentiation. Otol Neurotol 33:115–122
Lund AM, Skovby F, Schwartz M (1997) (G586V) substitutions in the alpha 1 and alpha 2 chains of collagen I: effect of alpha-chain stoichiometry on the phenotype of osteogenesis imperfecta? Hum Mutat 9:431–436
Christensen AI, Davidsen M, Ekholm O, Pedersen PV, Juel K (2014) Danskernes Sundhed - Den Nationale Sundhedsprofil 2013. Sundhedsstyrelsen, København
Pedersen IB, Bak-Nielsen S, Vestergaard AH, Ivarsen A, Hjortdal J (2014) Corneal biomechanical properties after LASIK, ReLEx flex, and ReLEx smile by Scheimpflug-based dynamic tonometry. Graefes Arch Clin Exp Ophthalmol 252:1329–1335
Lukinmaa PL, Ranta H, Ranta K, Kaitila I (1987) Dental findings in osteogenesis imperfecta: I. Occurrence and expression of type I dentinogenesis imperfecta. J Craniofac Genet Dev Biol 7:115–125
Malmgren B, Norgren S (2002) Dental aberrations in children and adolescents with osteogenesis imperfecta. Acta Odontol Scand 60:65–71
Schwartz S, Tsipouras P (1984) Oral findings in osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol 57:161–167
Hannula S, Maki-Torkko E, Majamaa K, Sorri M (2010) Hearing in a 54- to 66-year-old population in northern Finland. Int J Audiol 49:920–927
Engdahl B, Tambs K, Borchgrevink HM, Hoffman HJ (2005) Screened and unscreened hearing threshold levels for the adult population: results from the Nord-Trondelag hearing loss study. Int J Audiol 44:213–230
Hald JD, Folkestad L, Harslof T, Brixen K, Langdahl B (2017) Health-related quality of life in adults with osteogenesis imperfecta. Calcif Tissue Int 101(5):473–478
Evensen SA, Myhre L, Stormorken H (1984) Haemostatic studies in osteogenesis imperfecta. Scand J Haematol 33:177–179
Imani P, Vijayasekaran S, Lannigan F (2003) Is it necessary to screen for hearing loss in the paediatric population with osteogenesis imperfecta? Clin Otolaryngol Allied Sci 28:199–202
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All patients provided written informed consent. The studies were approved by the Central Danish Regional Committee on Health Research Ethics (ref-number M-20100108).
Conflicts of interest
Jannie Dahl Hald: none.
Lars Folkestad: received speaker fee from Genzymes.
Christer Zøylner Swan: none.
Jens Wanscher: none.
Malene Schmidt: none.
Hans Gjørup: none.
Dorte Haubek: none.
Christian-Heinrich Leonhard: none.
Dorte Ancher Larsen: none.
Jesper Østergaard Hjortdal: none.
Torben Harsløf: received speaker fee from Amgen, Eli Lilly, and Astra-Zeneca.
Morten Duno: none.
Allan M. Lund: none.
Jens-Erik Beck Jensen: advisory board membership and speaking fees from Amgen, Eli Lilly, and MSD.
Kim Brixen: none.
Bente Langdahl: advisory board membership and speaking fees from Amgen, Eli Lilly, Merck, TEVA, and UCB; received research support from Novo Nordisk, Amgen, and Orkla Health.
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Hald, J., Folkestad, L., Swan, C. et al. Osteogenesis imperfecta and the teeth, eyes, and ears—a study of non-skeletal phenotypes in adults. Osteoporos Int 29, 2781–2789 (2018). https://doi.org/10.1007/s00198-018-4663-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4663-x