Abstract
Summary
The effects of higher than recommended vitamin D doses on bone mineral density (BMD) and quality are not known. In this study, higher intakes, in postmenopausal women undergoing weight control over 1 year, had no effect on areal or volumetric BMD but prevented the deterioration in cortical bone geometry.
Introduction
Studies examining how bone responds to a standard dose of vitamin D supplementation have been inconsistent. In addition, the effects of higher doses on BMD and quality are not known. Postmenopausal women undergoing weight control to improve health outcomes are particularly at risk for bone loss and might benefit from supplemental vitamin D intake above the recommended allowance.
Methods
This 1-year-long, randomized, double-blind controlled study addresses whether vitamin D supplementation, in healthy overweight/obese older women, affects BMD and bone structural parameters. In addition, bone turnover and serum total, free, and bioavailable 25-hydroxyvitamin D (25OHD) responses to one of three daily levels of vitamin D3 (600, 2000, 4000 IU) with 1.2 Ca g/day during weight control were examined.
Results
Fifty-eight women (age, 58 ± 6 years; body mass index, 30.2 ± 3.8 kg/m2, serum 25OHD, 27.3 ± 4.4 ng/mL) were randomized to treatment. After 1 year, serum 25OHD concentrations increased to 26.5 ± 4.4, 35.9 ± 4.5, and 41.5 ± 6.9 ng/mL, in groups 600, 2000, and 4000 IU, respectively, and differed between groups (p < 0.01). Weight change was similar between groups (−3.0 ± 4.1 %). Cortical (Ct) thickness of the tibia changed by −1.5 ± 5.1 %, +0.6 ± 3.2 %, and +2.0 ± 4.5 % in groups 600, 2000, and 4000 IU, respectively, and each group was significantly different from each other (p < 0.05).
Conclusion
The decline in Ct thickness was prevented with higher vitamin D3 supplementation, but there were no other significant changes due to treatment over 1 year. Whether these findings translate to changes in biomechanical properties leading to reduced fracture risk should be addressed in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D maintains calcium and phosphate homeostasis and is responsible for bone health throughout life [1, 2]. However, the optimum vitamin D dose and serum 25-hydroxyvitamin D (25OHD) concentration for best skeletal outcomes are less clear. The controversy has been fueled by an inconsistent relationship between vitamin D and bone mineral density (BMD) in cross-sectional studies, as well as a lack of association between vitamin D supplementation and fracture prevention [3]. Insufficient vitamin D dosing necessary to increase serum 25OHD concentrations to healthy ranges or high baseline serum 25OHD has been suggested as a reason for the non-significant effects of vitamin D supplementation on BMD in some studies [4, 5]. In addition, it has been argued that the bioavailable 25OHD, rather than total 25OHD, concentrations are a better assessment of vitamin D status and therefore should be more strongly associated with BMD [6, 7].
A negative relationship between body weight and serum 25OHD has been previously reported [8–10], and it has been attributed to factors such as adipose tissue acting as a depot for the steroid and impaired vitamin D bioavailability in the obese, inadequate sunlight exposure, true vitamin D deficiency, genetic polymorphisms, or other unknown factors [7, 10–12]. Moreover, serum 25OHD response to vitamin D supplementation is attenuated with excess adiposity in the obese compared to lean individuals [11–13] and could be further influenced by age, ethnicity, baseline serum 25OHD, type, and level of vitamin D intake [14].
Older individuals and, in particular, postmenopausal women are at high risk of bone loss and structural deterioration that lead to loss of bone strength and increased fracture risk [15]. In addition, because postmenopausal women often experience weight gain, many undergo weight control plans designed to reduce obesity-associated comorbidities. Although beneficial, weight loss decreases calcium absorption and promotes bone loss [16]. It has been shown that even a modest loss of 3–4 % body weight can reduce BMD in women [17, 18]. The beneficial effects of a standard vitamin D supplementation in combination with calcium on BMD and bone quality measures have been shown previously, but studies examining higher doses of vitamin D have not been conducted [19].
Whether bone health further improves with higher doses of vitamin D above the recommended levels has been questioned [3]. Moreover, vitamin D interventions on bone quality are not available. The goal in this study is to determine whether vitamin D supplementation, in healthy overweight/obese postmenopausal women (≤70 years of age), attenuates bone changes accompanying weight control over 1 year. We addressed whether there is a dose-dependent effect of vitamin D3 supplementation on BMD, geometry, strength, and bone microstructure over 12 months.
Methods
Subjects
The three doses of vitamin D (3DD) study is a randomized controlled double-blind trial to determine the effects of vitamin D3 supplementation on BMD and bone microarchitecture in older women. We recruited healthy, postmenopausal women (age 50–70 years old; BMI 25–40 kg/m2) through advertising in local newspapers and email list servers. Subject’s enrollment and randomization were conducted during the winter months (January and February) over a 3-year period, to minimize seasonal fluctuations in serum 25OHD concentration.
Participants passing an initial telephone screening questionnaire, followed by laboratory and physical screening, were considered eligible. Subjects were not eligible if they had serum 25OHD ≥30 ng/mL, were <2 years postmenopausal, or were experiencing more than 5 % body weight loss or gain within 3 months prior to recruitment. Subjects diagnosed with diseases known to influence Ca metabolism or undertreatment known to influence Ca or bone metabolism were excluded.
During the first visit to the Nutrition and Bone laboratory, the subjects signed an informed consent approved by the Rutgers University Institutional Review Board and an external data safety monitoring advisory board. This trial was registered at clinicaltrials.gov as NCT01631292. The protocol met the ethical standards in accordance with the Helsinki Declaration.
Study design
This was a 1-year, randomized, double-blind, controlled trial conducted at Rutgers University, New Brunswick, NJ. One month prior to treatment assignments, the subjects underwent a stabilization period during which they were asked to maintain body weight and were given a multivitamin/mineral (Nature Made Multi 50+) and calcium supplement to total 600 IU/day vitamin D and 1.2 g Ca/day (diet and supplement) that continued throughout the study duration. When daily calcium intake was <1.2 g from the diet and the daily multivitamin/mineral supplement combined, an extra calcium supplement without added vitamin D was provided to those subjects (200 mg Ca/tablet; Citracal; Bayer HealthCare LLC). At the end of the stabilization period, the participants were randomly assigned using SAS v9.3 (SAS Institute, Cary, NC, USA) in a double-blind manner to one of three vitamin D treatment groups (habitual dietary intake + supplements) estimated at 600, 2000, and 4000 IU per day.
Vitamin D3 capsules and identical-looking placebos were purchased from Bio Tech Pharmacal, and manufacturer’s stated D3 content was 5000 IU vitamin D3/capsule. Vitamin D3 capsules were analyzed by reversed-phase high-performance liquid chromatography (C18 column) and UV detection in our lab after the study ended and indicated a vitamin D3 content/capsule of 4898 IU. The participants were asked to consume vitamin D3 capsules or placebo during five consecutive weekdays with their largest meal of the day. The 600-IU group received five placebo capsules/week; the 2000-IU group received two vitamin D3 capsules + three placebo/week, and the 4000-IU group received five vitamin D3 capsules/week. All subjects received 400 IU from the multivitamin/mineral supplement. Total vitamin D intake (diet + supplements) is reported in Supplemental Table 1.
Behavior modification/nutrition education
Subjects followed a standard behavior modification nutrition education/weight control program and adhered to individualized moderate energy-restricted diets with modest weight loss goals. Participants recorded their caloric intake with food diaries on a weekly basis to enhance compliance with the nutrition education plan. Food diaries were analyzed from two non-consecutive weekdays and one weekend day at baseline and at regular intervals three times during the intervention using nutrient analysis software (FoodWorks, v17, Long Valley, NJ). A registered dietitian reviewed these diaries and discussed them at weekly visits during the stabilization period and monthly thereafter during group and individualized counseling sessions.
The volunteers were asked to follow their physical activity routine throughout the study, with no specific instructions. Physical activity level was recorded on their food diaries. A numerical score was calculated using a range from 0 to 3 (0—inactivity, 1—low activity, 2—moderate activity, and 3—high activity) that estimated weekly metabolic equivalents [20].
Measurements
Assessment of sun exposure
Quantification of skin color changes with season was performed by using a reflectance spectrophotometer (model CS-100, Konica Minolta Optics Inc.) at two different skin areas: mid outer forearm (exposed) and waist (unexposed). Measurements are reported using Yxy color-indexing system, where Y measures reflectance ranging from 0 (black) to 100 (pure white) [21].
Anthropometric measurements
Weight and height were measured with a balance beam scale and stadiometer, respectively (Detecto, Webb City, MO). Women, wearing minimal clothing, were weighed in the morning at the monthly sessions.
Bone and body composition measurements
Baseline and 12-month measurements of BMD and BMC at total hip, lumbar spine, femoral neck, trochanter, and total body were performed using a dual-energy X-ray absorptiometry (DXA) total body scanner (Lunar Prodigy Advanced; GE-Lunar, CV ≤1 % for all sites). In addition, soft tissues were evaluated including total tissue, fat, and lean tissue compartments.
Volumetric (v) total BMD, trabecular, cortical BMD and BMC, geometry (cortical thickness, Ct.Th), and strength indices (polar moment of inertia (IP), stress-strain index (SSI)) at the distal tibia were measured at specific sites using peripheral quantitative computed tomography (pQCT) (Stratec XCT 3000, Orthometrix). The scans were analyzed using STRATEC XCT-3000 software, v5.4. A scout view allowed the positioning of the cross-sectional measurements along the tibia. The voxel size for all the scans was 0.5 mm, and the slice thickness was 2.4 mm. The precision error (coefficient of variation) was <1.7 % for all the measurements.
A custom software package, pQCT OsteoQ (Inglis Software Solutions Inc., Hamilton, ON), combining threshold-based and region-growing algorithms was used to measure apparent trabecular microstructure (trabecular separation (Tb.Sp), bone volume fraction (BV/TV), trabecular number (Tb.N), and trabecular thickness (Tb.Th)). Short-term validity of bone microstructure measurements indicated a precision error of less than 5 % [22].
Laboratory methods
Fasting serum and urine samples were collected at baseline and months 6 and 12 and were analyzed in batch analysis. Serum was analyzed for the following: 25OHD (radioimmunoassay; DiaSorin) (CV <12.5 %), intact PTH (immunoradioassay; Scantibodies) (CV <6.8 %), and ultrasensitive estradiol (radioimmunoassay; DSL) (CV <8.9 %). The performance of our 25OHD assay has been monitored and issued a proficiency certificate by the vitamin D External Quality Assessment Scheme.
Concentrations of serum vitamin D-binding protein (DBP) were determined by ELISA using two monoclonal antibodies in a sandwich format (ALPCO) (CV <12.7 %). Serum concentrations of free and bioavailable 25OHD were calculated by using a validated algorithm [23]. Colorimetric methods ware used to measure calcium and albumin concentrations.
Bone turnover markers were measured at baseline and after 1 year. Propeptides of type 1 collagen (P1NP) and C-telopeptide of type 1 collagen (CTX) were measured by ELISA (MyBiosource) (CV <15 %).
Compliance
Adherence to treatment protocol was assessed by pill count at each counseling session. Dietary intake of vitamin D and Ca was estimated from food records.
Safety
Study outcomes and adverse events were reviewed periodically by an internal Data Safety Monitoring Committee and Rutgers University IRB. A serious adverse event was defined as resulting in death; it is life-threatening, requires inpatient hospitalization, or results in persistent or significant disability/incapacity. Adverse events such as pain in the legs, swelling of the legs, pain or heaviness in the chest, headaches, dizziness, nausea, fatigue, muscle weakness, muscle aches, abnormal urinary frequency, and abdominal pain were categorized as non-serious adverse events and were recorded during the study. Adverse event forms were filled in at baseline and during monthly counseling sessions by all the subjects. Serum and urinary calcium were measured at baseline and after 12 months of treatment.
Sample size
In a previous study conducted in overweight postmenopausal women, mean age of 61 years, it was found that a moderate weight loss resulted in significantly more BMD loss at the trochanter (−0.031 ± 0.029 g/cm2) in the group supplemented with normal compared to that with high Ca intake (−0.010 ± 0.034 g/cm2) [24]. To be able to detect a similar difference at the trochanter BMD between the three levels of vitamin D3 intake and to allow for two covariates, a sample size of 18 per group (power of 80 % and α set at 0.05) was calculated. Based on previous trials conducted in our lab, an additional four subjects per group were recruited to account for dropouts. We used single-block randomization for vitamin D group assignments generated by the statistical consultant of the study, and the principal investigator (PI) assigned the participants in randomly mixed block sizes until the required sample size was met. The PI, clinical personnel, and subjects remained blinded to the treatment assignments throughout the study.
Statistical analysis
Statistical analyses were conducted with SAS, v9.3. An intention-to-treat (ITT) analysis, including all randomly assigned participants, was used to examine the effect of vitamin D3 supplementation on bone outcomes. The last observation carried forward was used for the subjects with missing data. The groups were compared for baseline characteristics by using one-way ANOVA. Variables considered clinically important, even if they did not reach statistical significance (i.e., body weight) and other factors such as age and years since menopause, were used as covariates in the analyses. Mixed-model analysis of covariance (ANCOVA) was used to compare differences in bone parameters, fat, fat-free soft tissue, hormones, and bone turnover markers over time between treatment groups. Two fixed factors were examined: group (with doses of intervention) and time (baseline and 12 months). When the interaction (group-by-time) was significant, post hoc analysis was conducted with Bonferroni correction for multiple comparisons. Mixed-model ANCOVA has the advantage of adjusting for dependencies between repeated responses and allows for missing data without introducing bias. Pearson correlation coefficients were used to assess the relationship between changes in independent and outcome variables. Stepwise multiple linear regression analysis (forward selection) was performed to select the explanatory variables that would be considered important predictors for bone changes. Calcium intake was controlled and adjusted to meet the requirements of the participants throughout the intervention; therefore, we did not include this as a covariate in the analysis. The recruitment and study startup procedures were conducted during winter months to minimize the effect of sunlight on serum 25OHD. Hence, we did not include season as a covariate in the analysis.
In addition, an as-treated analysis for subjects who completed the study and who were at least 80 % compliant with vitamin D supplementation was conducted for all the outcomes. Values are reported as mean ± SD and graphs with SEM. Categorical values are expressed in percentages. Values ≤0.05 were considered significant.
Results
Participants
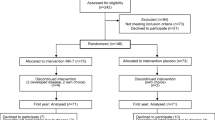
The study participant’s flowchart is shown in Fig. 1. Of the 210 women assessed for eligibility, 102 met the inclusion criteria and were recruited for the study. Twenty-one women withdrew before treatment initiation due to time commitment (n = 10), lack of interest in the study (n = 7), and lack of adherence to the stabilization period intervention (n = 4). Eighty-one women were randomized to one of the three vitamin D3 groups: 600 IU/day (n = 24), 2000 IU/day (n = 28), or 4000 IU/day (n = 29). During the intervention, 23 women in all groups discontinued the study due to time commitment (n = 10), moving away (n = 5), or were lost to follow-up (n = 7). One volunteer, randomized to 600-IU group, lost a dramatic amount of weight (>30 % body weight) and therefore did not meet the criteria for as-treated analysis and was dropped from the study. Final analysis includes 58 women (age, 57.6 ± 5.6 years; BMI, 30.2 ± 3.7 kg/m2), predominantly Caucasian (85 %). At screening, 68 % of the subjects were taking multivitamins and 18 % of them were taking additional vitamin D supplements. Calcium supplementation was used by 63 % of the subjects. Reported habitual intake of vitamin D and Ca (diet and supplements) in the volunteers prior to stabilization month ranged from 64 to 1085 IU/day and from 510 to 1600 mg, respectively. During the stabilization month, vitamin D and Ca intake from diet and supplements averaged 565 ± 14 IU and 1186 ± 71 mg/day, respectively. At screening, serum 25OHD concentration was 22.8 ± 6.4 ng/mL and increased to 27.2 ± 4.3 ng/mL after 1 month of stabilization with a multivitamin/mineral pill.
Skin reflectance (Y) values at the beginning of the study (winter) averaged 47 ± 16 cd/m2 at the exposed area and 67 ± 14 cd/m2 at the unexposed area (p < 0.01), and there were no significant differences between groups. Similarly, after the summer months, skin reflectance was lower (42 ± 11 cd/m2) at the exposed than unexposed area (58 ± 21 cd/m2, p < 0.01) and the groups did not differ significantly. The difference between skin reflectance at the unexposed areas compared to exposed areas remained significant in all subjects throughout the study (p < 0.01).
Nutrient intake and physical activity
Nutrient intake at baseline was similar between groups (Supplemental Table 1). Across all groups, there was a decrease in energy, fat, phosphorus, magnesium, sodium, and vitamin K intake associated with caloric restriction. Vitamin D intake was significantly different between treatment groups (p < 0.001) (Supplemental Table 1). Supplement compliance, as calculated by monthly pill counts, was similar between groups and averaged 83 ± 10 % for the vitamin D capsules/multivitamin tablets and 89 ± 11 % for the Ca tablets. Compliance with vitamin D treatment was ≥80 % in 46 out of 58 subjects. Subjects maintained a moderate level of physical activity over the 12-month study (2.1 ± 0.8), with no significant change in habitual level of activity over time or between groups (data not shown).
Weight, body composition, and BMD
Baseline and 12-month measurements for weight, body composition, and BMD at multiple sites are presented in Table 1. There were no significant differences for baseline characteristics between groups. After 12 months, subjects lost 3.0 ± 4.1 % body weight and 3.1 ± 8.5 % fat mass with no significant difference between groups and the findings were similar for both ITT and as-treated analyses (p value for time <0.02). ITT analysis showed no difference over time between groups for fat-free soft tissue, total body BMC, and BMD with intervention. When using the as-treated analysis of the completers, there were also no significant differences between groups over 12 months of vitamin D treatment (Table 1). No differences between groups with vitamin D3 treatment were found for femoral neck BMD, radius, lumbar spine, total hip, and total body BMD with the as-treated analysis (Table 1).
Trabecular and cortical bone at the tibia
The ITT analysis yielded a statistically significant interaction between treatment and time for cortical thickness (p < 0.03). There was also a loss of total and trabecular BMC over time in all the groups (p < 0.05). Similarly, the completer’s analysis showed that cortical thickness changed differently between groups over time: −1.5 ± 5.1, +0.6 ± 3.2, and +2.0 ± 4.5 % in the 600, 2000, and 4000 IU, respectively (p < 0.05), and each group differed significantly from all others (Bonferroni-corrected p value < 0.05) (Table 2, Fig. 2). In addition, in the as-treated analysis, cortical volumetric BMD (vBMD) did not differ significantly over time between groups (p < 0.1). No other changes in cortical variables between groups or over time were observed (Table 2). Both ITT and as-treated analyses showed a decrease over time in trabecular volumetric BMD and BMC in all women (p < 0.05), and the change was not significantly different between groups. There was a significant time effect for all the trabecular parameters, except Tb.Th, but the change over time between treatment groups was not significantly different (Table 2, Fig. 2). No other parameters were found to differ significantly between groups.
The change (%) in cortical and trabecular parameters at the tibia over 12 months at three doses of vitamin D3. Comparison between the groups was examined by mixed-model ANCOVA analysis with time (0, 12 months) and group (600, 2000, and 4000 IU) as independent variables. *p < 0.05, for interaction effect, groups are significantly different from each other. Cortical parameters: volumetric bone mineral density (BMD), area, and cortical thickness (Ct.Th). Trabecular parameters: volumetric BMD, number (Tb.N), separation (Tb.Sp), and BV/TV (bone volume/total volume)
Bone-regulating hormones and bone turnover markers
Bone-regulating hormones and bone turnover markers are shown in Table 3. The increase in serum 25OHD in response to 600, 2000, or 4000 IU vitamin D3/day in this overweight/obese population was 3.8 ± 4.1, 7.4 ± 6.5, and 14.1 ± 8.1 ng/mL, respectively (p value for interaction between time and treatment <0.01) (Table 3, Fig. 3). No subject had a serum 25OHD level below 20 ng/mL after any of the interventions. The change in free 25OHD and bioavailable 25OHD differed with treatment dose (p value for interaction <0.02) (Table 3). Serum DBP concentration decreased over time in all groups (p < 0.05), but there was no significant difference due to vitamin D3 dose. After 12 months of treatment, PTH decreased by 10.6 ± 16.6 % (p < 0.01) and the change did not differ significantly between groups (Table 3, Fig. 3). The bone turnover markers, CTX and P1NP, increased by 10.0 ± 15.9 and 17.6 ± 20.2 %, respectively, from baseline to 12 months (p < 0.05), and the rise did not differ significantly between groups (Table 3). A trend for serum estradiol to decrease by 4.5 ± 22.6 % in all the groups was observed (p < 0.07).
Changes in 25OHD and PTH over 12 months at three doses of vitamin D3. Comparison between the groups was examined by mixed-model ANCOVA analysis with time (0, 12 months) and group (600, 2000, and 4000 IU) as independent variables. Values with different superscripts are significantly different from each other over 12 months of intervention (p < 0.05 for interaction effect); *p < 0.05 for time effect, the groups are not significantly different from each other
Pearson correlation and stepwise regression
The relationship between the changes in body weight, body composition and bone parameters, hormones, and bone turnover markers was analyzed within each treatment group. In the 600-IU group, trochanter BMD loss correlated with weight loss (r = 0.475, p < 0.03) and total body fat loss (r = 0.531, p < 0.02). Furthermore, in the 600-IU group, there was a trend for bone loss at the femoral neck to correlate with weight loss (r = 0.435, p < 0.07). There was no significant relationship between any variables at higher levels of vitamin D3 intake (2000 and 4000 IU). An inverse relationship between changes in serum CTX, but not P1NP, and body weight (r = −0.640, p < 0.01) and fat mass (r = −0.659, p < 0.01) was found in the 600-IU group, but not in the other treatment groups.
Stepwise regression
Using bone outcomes as dependent variables and changes in body composition and hormone levels as independent variables, a stepwise regression was performed. Only the independent variables that reached significance were included in the final model. Analysis indicated that during weight loss, at 600-IU vitamin D intake, changes in trochanter BMD were explained by changes in body weight (R 2 = 23.5 %, p < 0.05). At higher levels of vitamin D intake, changes in body weight were not significantly associated with changes in trochanter BMD. In addition, weight loss was the major predictor for the increase in CTX (R 2 = 46 %, p = 0.002) in the 600-IU group, but not at higher levels of intake.
Safety
There were no significant differences for serum calcium concentrations between groups at baseline or after the intervention, as measured. No subject presented with hypercalcemia (>10.5 mg/dL) at 12 months. The urinary calcium excretion (corrected for creatinine) and creatinine clearance were similar between groups (Table 3). No serious adverse events were reported during the study. The cumulative frequency of non-serious events was not significantly different between groups at any time point during the study (Supplemental Table 2).
Discussion
Vitamin D is essential for bone health and prevents rickets and osteomalacia by promoting calcium absorption required for bone mineralization. However, to what extent vitamin D may prevent bone loss during moderate caloric restriction and attenuates bone quality is largely unknown. In this 1-year-long trial, we examined the effect of three vitamin D doses on areal BMD (aBMD) and bone quality during modest weight loss. In this overweight/obese population of postmenopausal women, the increase in serum 25OHD in response to 600, 2000, or 4000 IU vitamin D3/day occurred in a dose-response manner. After 12 months of intervention and 3 % weight reduction, there was a decrease in cortical thickness in the 600-IU group but not in the higher vitamin D groups, whereas no other parameters (bone geometry, strength, and microstructure) differed due to the dose of vitamin D3 intake.
It is well established that weight loss (3–10 %) decreases BMD at various bone sites as compared to weight-stable women especially at older ages [24–26]. This decrease is attributed to at least a few factors including a decrease in nutrient intake [27, 28] or a decrease in calcium absorption [29, 30], reduced mechanical loading [25], and reduced estradiol or insulin-like growth factor levels accompanying weight reduction [16]. In this 1-year trial, there was no bone loss over time, which may be attributed to the small amount of weight loss while consuming the recommended intake of Ca and vitamin D (or higher intakes) in all groups of women. However, there was an increase in bone turnover possibly due to aging or the slight energy restriction, but this did not differ between groups.
In weight-stable women, there are many studies examining the effect of supplemental vitamin D intake on bone [31–38]. Ooms et al. showed that 400 IU/day compared to placebo did not prevent bone loss that occurs with aging over 1 year [33]. In some trials, BMD loss was attenuated with 700–800 IU/day [31, 32] and calcium supplements (500–1200 mg), while others, using similar doses, found no effect of vitamin D on BMD [35, 36]. Higher doses of vitamin D on BMD also yielded inconsistent results. While daily doses of 1428 IU D2 and 1000 mg calcium compared to calcium alone did not influence bone [34], others reported higher BMD at the lumbar spine and hip with 5000 IU D3/day and 320 mg calcium/day compared to placebo [39]. Nevertheless, none of these studies included bone quality measures or used a model of energy restriction. In addition, it is possible that the inclusion of supplemental micronutrients in the current study may have influenced the effect on bone in our study compared to other trials.
To date, most interventions with vitamin D supplementation have used areal BMD derived from DXA as a surrogate measure of fracture risk [40]. However, the two-dimensional measurement cannot adequately assess bone geometry and, consequently, bone strength that are independent of changes in areal BMD. Peripheral quantitative computerized tomography captures geometrical changes at the cortical and trabecular bone compartments, which respond differently to physiological and pathological factors [41]. In this study, supplemental vitamin D3 did not affect aBMD, but the higher intakes influenced bone geometry, specifically an increase in cortical thickness, that may translate to improved biomechanical strength.
A positive association between 25OHD levels and bone quality at the radius and tibia was found in older Caucasian men [42], and some reported a catabolic effect of PTH on the cortical bone in both obese and non-obese individuals [43]. On the other hand, excess PTH has been shown to maintain or even have anabolic effects on trabecular bone [44], while high levels of PTH can result in an increased endocortical resorption, cortical thinning, and cortical porosity [45]. Few studies have examined the relationship between 25OHD and the cortical and trabecular bones of the tibia in older women, but one reported a positive association between 25OHD and cortical vBMD (that was negative with PTH) but no relationship of either 25OHD or PTH with trabecular vBMD [46]. It is thus hypothesized that suppression of PTH that can be achieved by vitamin D supplementation may improve cortical vBMD in obese women, independently of modest body weight changes. Weight loss, on the other hand, has been shown to either decrease total vBMD with trend to decrease cortical vBMD in postmenopausal women [47] but not in premenopausal women [26]. Reports of energy restriction in rodent models with more dramatic weight loss show a decrease in cortical bone parameters, such as vBMD, BMC, and Ct.Th [48], and another study indicated that trabecular parameters were also affected (BV/TV and Tb.Th) [48, 49]. The current study suggests that weight loss (time effect) causes a decrease in trabecular vBMD, BMC, and BV/TV and increase in separation, and the addition of vitamin D had no effect in preventing the trabecular decline with modest weight loss over 1 year. Also, even at high intakes of vitamin D3, cortical vBMD was unaffected, but there was a positive effect on cortical thickness.
Determining an optimum level of vitamin D supplementation and serum 25OHD concentration may be important in the older population to minimize bone loss or changes in quality during energy-controlled diets. In addition, the overweight or obese population typically has lower serum 25OHD than normal weight persons and is at risk of bone loss during energy restriction [16]. The rise in serum 25OHD was 0.19 and 0.27 ng/mL per microgram vitamin D intake when consuming 2000 or 4000 IU/day, respectively. A slightly lower rise in 25OHD (0.16 ng/mL/μg/day) was found in a previous study when given similar vitamin D3 doses and may be due to their higher BMI (>35 kg/m2) [50] compared to 30 kg/m2 in the current study. Free and bioavailable 25OHD also increased with increasing doses of vitamin D intake and remained at a steady 0.02 and 7 % of total 25OHD concentration, respectively, throughout the study and in all groups. Furthermore, although there are seasonal influences on 25OHD levels, measurements of skin reflectance, as a marker of sun exposure, indicated no differences between groups or from baseline to final measurements in this 1-year study.
After 1 year of intervention, bone turnover markers increased in all subjects with no significant differences between groups. Studies with vitamin D supplementation on bone turnover in postmenopausal women are scarce and have yielded mixed results. In a previous study in women (50–80 years) with similar baseline serum 25OHD concentrations, after 1 year (no weight loss), vitamin D supplementation reduced bone turnover, but the reduction was greater in their standard dose (800 IU/day) compared to the high dose (6500 IU) [38]. In another study, there was no effect of vitamin D treatment at any dose (400, 800, or 1000 IU) on bone turnover [51]. In the current study, the rise in bone turnover was likely due to the combined effect of aging [52] and weight loss [24, 28].
There are several limitations in this study including the sample population consisting of healthy postmenopausal women. The results may not be applicable to other populations with low serum 25OHD concentrations or with chronic conditions. However, the intention of this study was to examine whether higher than recommended vitamin D doses in individuals have additional beneficial effects on bone. In addition, a transient increase in BMD remodeling with vitamin D and calcium supplementation is possible in a 1-year study, and bone changes would ideally be examined in a longer intervention consisting of more remodeling cycles [53]. Another limitation in this study is the analysis of multiple endpoints raising the possibility of chance results. Therefore, we used Bonferroni correction for multiple comparison to account for this. Although errors associated with DXA measurements are possible, especially in overweight/obese subjects with excess body fat surrounding the bone or due to changes in total body fat in these individuals [54, 55], the modest weight loss achieved in this study was unlikely to influence the precision errors of DXA measurements. Total fat loss did not exceed 2 kg of fat representing significantly less than the 6-kg excess fat surrounding the bone shown to produce measurement errors by DXA [55]. Moreover, pQCT measurements of vBMD avoid the errors associated with DXA and provide additional information about bone microarchitecture. These measurements are especially important in studies of the obese who show evidence of compromised bone quality [16]. Other advantages include study design, with subject recruitment conducted in the same season (winter), so the seasonal influence on 25OHD concentrations over 1 year was similar between individuals or groups. Also, we were able to measure DBP concentrations and free and bioavailable 25OHD metabolites and to determine whether DBP modulates the relationship between 25OHD and bone outcome.
In conclusion, higher doses of vitamin D had an insignificant effect on bone parameters except for preventing a decline in cortical thickness over 1 year in healthy postmenopausal women. Whether this vitamin D effect on bone geometry remains sustained over time and indicates greater strength and fracture reduction in older individuals remains to be determined.
References
DeLuca HF (2008) The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J 2:224–36
Reichel H, Norman AW (1989) Systemic effects of vitamin D. Annu Rev Med 40:71–8
Reid IR, Bolland MJ, Grey A (2014) Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet 383:146–55
Lappe JM, Heaney RP (2012) Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinol 4:95–100
Jorde R, Grimnes G (2015) Vitamin D and health: the need for more randomized controlled trials. J Steroid Biochem Mol Biol 148:269–74
Powe CE, Ricciardi C, Berg AH et al (2011) Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res 26:1609–16
Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M (2014) Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol 144(Pt A):132–7
Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH (1988) Low circulating vitamin D in obesity. Calcif Tissue Int 43:199–201
Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA (2004) The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab 89:1196–9
Jungert A, Roth HJ, Neuhäuser-Berthold M (2012) Serum 25-hydroxyvitamin D3 and body composition in an elderly cohort from Germany: a cross-sectional study. Nutr Metab (Lond) 9:42
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–3
Drincic AT, Armas LA, Van Diest EE, Heaney RP (2012) Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 20:1444–8
Gallagher JC, Yalamanchili V, Smith LM (2013) The effect of vitamin D supplementation on serum 25(OH)D in thin and obese women. J Steroid Biochem Mol Biol 136:195–200
Zittermann A, Ernst JB, Gummert JF, Börgermann J (2014) Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: a systematic review. Eur J Nutr 53:367–74
Andreopoulou P, Bockman RS (2015) Management of postmenopausal osteoporosis. Annu Rev Med 66:329–42
Shapses SA, Sukumar D (2012) Bone metabolism in obesity and weight loss. Annu Rev Nutr 32:287–309
Salamone LM, Cauley JA, Black DM et al (1999) Effect of a lifestyle intervention on bone mineral density in premenopausal women: a randomized trial. Am J Clin Nutr 70:97–103
Uusi-Rasi K, Sievänen H, Pasanen M, Oja P, Vuori I (2001) Maintenance of body weight, physical activity and calcium intake helps preserve bone mass in elderly women. Osteoporos Int 12:373–9
Inst. Med (2011) Dietary reference intakes for calcium and vitamin D. Washington, DC: Natl. Acad. Press
Ainsworth BE, Haskell WL, Herrmann SD et al (1998) Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 43:1575–81
Takiwaki H (1998) Measurement of skin color: practical application and theoretical considerations. J Med Invest 44:121–6
Wong AK, Beattie KA, Min KK, Webber CE, Gordon CL, Papaioannou A et al (2014) A trimodality comparison of volumetric bone imaging technologies. Part I: short-term precision and validity. J Clin Densitom 18:124–35
Vermeulen A, Verdonck L, Kaufman JM (1999) A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672
Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA (2005) Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res 20:455–63
Villareal DT, Fontana L, Weiss EP et al (2006) Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med 166:2502–10
Uusi-Rasi K, Sievanen H, Kannus P, Pasanen M, Kukkonen-Harjula K, Fogelholm M (2009) Influence of weight reduction on muscle performance and bone mass, structure and metabolism in obese premenopausal women. J Musculoskelet Neuronal Interact 9:72–80
Jensen LB, Kollerup G, Quaade F, Sorensen OH (2001) Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res 16:141–147
Ricci TA, Heymsfield SB, Pierson RN Jr, Stahl T, Chowdhury HA, Shapses SA (2001) Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr 73:347–352
Cifuentes M, Riedt CS, Brolin RE, Field MP, Sherrell RM, Shapses SA (2004) Weight loss and calcium intake influence calcium absorption in overweight postmenopausal women. Am J Clin Nutr 80:123–30
Shapses SA, Sukumar D, Schneider SH, Schlussel Y, Sherrell RM, Field MP, Ambia-Sobhan H (2013) Vitamin D supplementation and calcium absorption during caloric restriction: a randomized double-blind trial. Am J Clin Nutr 97:637–45
Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ (1992) Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327:1637–42
Dawson-Hughes B, Harris SS, Krall EA, Dallal GE, Falconer G, Green CL (1995) Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamin D. Am J Clin Nutr 61:1140–5
Ooms ME, Roos JC, Bezemer PD, Vandervijgh WJF, Bouter LM, Lips P (1995) Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab 80:1052–58
Cooper L, Clifton-Bligh PB, Nery ML et al (2003) Vitamin D supplementation and bone mineral density in early postmenopausal women. Am J Clin Nutr 77:1324–29
Meier C, Woitge HW, Witte K, Lemmer B, Seibel MJ (2004) Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: a randomized controlled open-label prospective trial. J Bone Miner Res 19:1221–30
Aloia JF, Talwar SA, Pollack S, Yeh J (2005) A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med 165:1618–23
Jorde R, Sneve M, Torjesen PA, Figenschau Y, Hansen JB, Grimnes G (2010) No significant effect on bone mineral density by high doses of vitamin D3 given to overweight subjects for one year. Nutr J 9:1
Grimnes G, Joakimsen R, Figenschau Y, Torjesen PA, Almås B, Jorde R (2012) The effect of high-dose vitamin D on bone mineral density and bone turnover markers in postmenopausal women with low bone mass—a randomized controlled 1-year trial. Osteoporos Int 23:201–11
Mocanu V, Stitt PA, Costan AR, Voroniuc O, Zbranca E, Luca V, Vieth R (2009) Long-term effects of giving nursing home residents bread fortified with 125 μg [5000 IU] vitamin D3 per daily serving. Am J Clin Nutr 89:1132–1137
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–97
Griffith JF, Genant HK (2008) Bone mass and architecture determination: state of the art. Best Pract Res Clin Endocrinol Metab 22:737–64
Barbour KE, Zmuda JM, Horwitz MJ, Strotmeyer ES, Boudreau R, Evans RW, Ensrud KE, Gordon CL, Petit MA, Patrick AL, Cauley JA (2011) Osteoporotic Fractures in Men (MrOS) Research Group. The association of serum 25-hydroxyvitamin D with indicators of bone quality in men of Caucasian and African ancestry. Osteoporos Int 22:2475–85
Charopoulos I, Tournis S, Trovas G, Raptou P, Kaldrymides P, Skarandavos G, Katsalira K, Lyritis GP (2006) Effect of primary hyperparathyroidism on volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in postmenopausal women. J Clin Endocrinol Metab 91:1748–53
Duan Y, De Luca V, Seeman E (1999) Parathyroid hormone deficiency and excess: similar effects on trabecular bone but differing effects on cortical bone. J Clin Endocrinol Metab 84:718–22
Dempster DW, Müller R, Zhou H, Kohler T, Shane E, Parisien M, Silverberg SJ, Bilezikian JP (2007) Preserved three-dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone 41:19–24
Lauretani F, Bandinelli S, Griswold ME, Maggio M, Semba R, Guralnik JM, Ferrucci L (2008) Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res 23:400–8
Sukumar D, Ambia-Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, Shapses SA (2011) Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Res 26:1339–48
Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM (2008) Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res 23:870–8
Mardon J, Zangarelli A, Walrand S, Davicco MJ, Lebecque P, Demigné C, Horcajada MN, Boirie Y, Coxam V (2008) Impact of energy and casein or whey protein intake on bone status in a rat model of age-related bone loss. Br J Nutr 99:764–72
Dhaliwal R, Mikhail M, Feuerman M, Aloia JF (2014) The vitamin D dose response in obesity. Endocr Pract 6:1–25
Macdonald HM, Wood AD, Aucott LS et al (2013) Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: a 1-year double-blind RCT in postmenopausal women. J Bone Miner Res 28:2202–13
Ensrud KE, Palermo L, Black DM et al (1995) Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res 10:1778–87
Aloia JF, Arunabh-Talwar S, Pollack S, Yeh JK (2008) The remodeling transient and the calcium economy. Osteoporos Int 19:1001–9
Knapp KM, Welsman JR, Hopkins SJ, Shallcross A, Fogelman I, Blake GM (2015) Obesity increases precision errors in total body dual-energy x-ray absorptiometry measurements. J Clin Densitom 18:209–16
Yu EW, Thomas BJ, Brown JK, Finkelstein JS (2012) Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res 27:119–24
Acknowledgments
We thank Robert Zurfluh, RD, and the other clinical staff for their invaluable clinical assistance and Lihong Hao, PhD, for her laboratory assistance with vitamin D3 analysis of the supplements. We are also grateful for the commitment of the volunteers. This study was supported by NIH-AG12161 and Busch Biomedical Award (BBGP2010695157).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
During the first visit to the Nutrition and Bone laboratory, the subjects signed an informed consent approved by the Rutgers University Institutional Review Board. This trial was registered at clinicaltrials.gov as NCT01631292. The protocol met the ethical standards in accordance with the Helsinki Declaration.
Funding
This study is funded by NIH-AG12161, NJAES (0153866) and a Busch Biomedical Award (BBGP2010695157).
Trial number and date: NCT01631292.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Pop, L.C., Sukumar, D., Schneider, S.H. et al. Three doses of vitamin D, bone mineral density, and geometry in older women during modest weight control in a 1-year randomized controlled trial. Osteoporos Int 28, 377–388 (2017). https://doi.org/10.1007/s00198-016-3735-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3735-z