Abstract
Mastocytosis is a rare condition characterized by abnormal mast cell proliferation and a broad spectrum of manifestations, including various organs and tissues. Osteoporosis is one of the most frequent manifestations of systemic mastocytosis, particularly in adults. Osteoporosis secondary to systemic mastocytosis is a cause of unexplained low bone mineral density that should be investigated when accompanied by suspicious clinical elements. Bone involvement is often complicated by a high recurrence of fragility fractures, mainly vertebral, leading to severe disability. The mechanism of bone loss is the result of different pathways, not yet fully discovered. The main actor is the osteoclast with a relative or absolute predominance of bone resorption. Among the stimuli that drive osteoclast activity, the most important one seems to be the RANK-RANKL signaling, but also histamine and other cytokines play a significant role in the process. The central role of osteoclasts made bisphosphonates, as anti-resorptive drugs, the most rational treatment for bone involvement in systemic mastocytosis. There are a few small studies supporting this approach, with large heterogeneity of drug and administration scheme. Currently, zoledronate has the best evidence in terms of gain in bone mineral density and bone turnover suppression, two surrogate markers of anti-fracture efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mastocytosis comprehends different expressions of an uncommon disease caused by an abnormal proliferation and invasion of neoplastic mast cells (MCs) in many tissues with a predominant involvement of bone marrow and skin [1, 2]. This abnormal proliferation is driven by many mediators, of which the stem cell factor (SCF) that acts through KIT, a transmembrane receptor with tyrosin kinase activity, is of utmost importance [3, 4]. Almost 80 % of the cases present the D816V mutation of KIT receptor. Cutaneous mastocytosis which is the most common form seen in childhood usually has an auto-resolving pattern with few cases of persistence into adulthood [5].
Conversely, the adult onset tends to be characterized by a systemic and chronic variant; in fact, many adult patients are affected by systemic mastocytosis (SM) [6]. It is defined by the involvement of at least one extracutaneous organ with the most common ones being bone, gastrointestinal tract, lymph nodes, and spleen; in this form, cutaneous localization could not be present [7, 8]. Skin involvement could cause urticaria (urticaria pigmentosa), pruritus, flushing, and angioedema. The release of mediators can also provoke diarrhea, nausea, vomiting, bone loss, and anaphylaxis. In the most severe form, tissue invasion could lead to hypersplenism, hepatopathy and ascites, malabsorption, focal bone lesions, and cytopenia [6–14]. The most common course of SM is indolent (ISM) and skin features are often present; fortunately, the most severe forms with poor prognosis like aggressive SM, MC leukemia, and MC sarcoma are rare (Table 1) [15]. Clinical suspicion of mastocytosis should reasonably start with the presence of varied signs and symptoms that relate to MC mediator release and/or after recognition of the characteristic skin lesions. However, its diagnosis cannot be based on symptoms alone. Firstly, serum tryptase levels should be determined (normal value <11.4 ng mL). In most patients with SM, they correlate with the total burden of MCs and their activation. However, an increased tryptase level alone is not pathognomonic of SM, as elevated levels can be found in other diseases such as chronic urticaria, renal insufficiency, other hematological diseases, onchocercosis, and ischemic myocardial disease or in the presence of heterophile antibodies. Furthermore, tryptase levels can transiently increase during an anaphylactic reaction. On the other hand, the observation that about 5 % of patients with ISM had normal serum tryptase level suggests that this assay can be confidently used for screening in selected cases of unexplained osteoporosis, but serum tryptase level in the normal range cannot exclude SM diagnosis. In patients with suspected mastocytosis but with serum tryptase level <11.4 ng/ml, analysis of urinary histamine mediators can be useful in differential diagnosis. Suspicion of bone marrow mastocytosis, a variant of ISM without skin lesions, as a possible cause of osteoporosis is one of the indications for a bone biopsy in the presence of high histamine metabolite excretion, also with serum tryptase level in the normal range. Also, the absence of urticaria or angioedema in severe reactions to hymenoptera stings with hypotension might represent the most relevant factor in identifying patients with mastocytosis, regardless of their serum tryptase levels [16]. However, SM can only be verified by histological and/or molecular findings on biopsy material other than the skin. As bone marrow is almost always involved, the histopathological evaluation of bone marrow biopsy specimens is crucial to establish the diagnosis of SM, to assess tissue burden of MCs, and to rule out the presence of other hematological disease (Fig. 1). In accordance with the WHO diagnostic criteria, one major and four minor criteria have been defined for the diagnosis of SM [15]. SM is diagnosed when the major and one minor or when three minor diagnostic criteria are fulfilled. The major diagnostic criterion is the presence of multifocal compact MC infiltrates in aggregates (≥15 MCs per cluster). Immunohistochemical staining using antibodies against CD117 (KIT) and against tryptase is strongly recommended. Minor criteria include various morphological, immunohistochemical, molecular, and serological findings. Abnormal MC morphology (>25 % of MCs), including spindle-shaped MCs, abnormal granulation, or cytoplasmic projections, is one of the minor criteria. The aberrant expression of CD25 and/or CD2 is another important diagnostic marker as these antigens are expressed only on neoplastic MCs in SM and not on normal/reactive MCs. Flow cytometry is also a sensitive and reliable method to identify the expression of aberrant MC markers. Furthermore, an activating point mutation at codon 816, especially KIT D816V, is detectable in majority of the patients with SM. Finally, the serological detection of a persistently raised serum tryptase level (>20 ng mL) is used as a minor criterion of SM.
Histopathology of systemic mastocytosis. a Low power view showing several pale mast cells aggregates, localized to perivascular or paratrabecular areas (H&E, 40×); b intermediate power view showing a mast cell aggregate in a perivascular location (H&E, 100×); the aggregate is composed of round and spindly mast cells accompanied by small lymphocytes and sparse eosinophilic granulocytes; c high power view of Giemsa stain, showing visible although reduced basophilic granulation in round and spindly mast cells (Giemsa, 400×); d mast cells are positive for CD117 by immunohistochemistry (anti-CD117 antibody revealed by peroxidase-DAB method, 200×)
Prevalence of mastocytosis-related osteoporosis
Bone manifestations are one of the most frequent symptoms of SM, particularly in adults. Patients may present with poorly localized bone pain, diffuse osteopenia, or osteoporosis with fragility or pathologic fractures, diffuse osteosclerosis, or both focal osteolytic and osteosclerotic bone lesions [14].
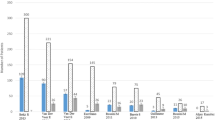
According to the traditional WHO criteria (bone mineral density, BMD, T-score < −2.5) [17], the reported prevalence of osteoporosis in patients with ISM ranges from 18 to 31 % (Fig. 2) [14, 18–21]. However, in these studies, also elderly patients were included: thus, the real incidence of mastocytosis-related osteoporosis remains unclear. According to guidelines of the International Society for Clinical Bone Densitometry [22], using Z-score (SD below the age and gender-matched mean BMD reference value) with a threshold value of <−2.0, mastocytosis-related low BMD was diagnosed more accurately in 12 % of women and in 33 % of men with ISM [20] (Fig. 3). The higher prevalence of osteoporosis in men compared to women was recently confirmed by other studies [21]. Mastocytosis-related low BMD defined by Z-score < 2.0 was found more frequently at the lumbar site than at the femur in men (33 % versus 5 %, respectively) [14] (Fig. 3).
Prevalence of mastocytosis-related low bone mineral density (BMD) (Z-score BMD < −2.0) in 199 patients (81 women and 118 men) with ISM [14]
One of the clinical hallmarks of mastocytosis-related osteoporosis is bone pain, which is often debilitating, particularly in case of extensive bone marrow involvement [23]. This clinical feature should raise suspicion for this secondary cause of osteoporosis.
Often, the osteoporotic bone involvement in MS is characterized by the presence of concomitant focal osteolytic or osteosclerotic bone lesions [14]; but the prevalence is not well known because in most of the studies, investigators did not make a full skeletal radiographic examination in all patients.
Available data about the prevalence of osteoporosis in SM with or without skin lesions are currently inconsistent. Barete et al. [19] observed that patients with SM without skin involvement had the same risk of developing osteoporosis as patients with skin lesions. Also, in our experience [20], there was not any difference in the prevalence of low BMD between patients with or without skin involvement in ISM patients. In contrast with another experience in ISM patients [21], absence of urticaria pigmentosa was an independent predictor of fragility fractures.
A higher prevalence of low BMD has been reported in the subgroup of ISM patients without history of anaphylaxis [20]. So it should be considered that osteoporosis in the absence of typical skin involvement or trigger factors for anaphylaxis might be the only sign of a latent ISM. In consequence, prevalence of SM-related osteoporosis is possibly underestimated. Osteoporosis without a certain etiological cause should lead to suspect the presence of bone marrow mastocytosis. Currently, incidence of SM in patients with idiopathic osteoporosis is not known and only indirectly estimable from the limited data about the prevalence of SM in the general population, which is probably underestimated: 0.5–1 per 10,000 [7, 24].
Consistent with the higher prevalence of mastocytosis-related low BMD in men and vertebral site, we observed that the prevalence of at least one vertebral fracture was 20 % in men and 14 % in women [14]. Not infrequently vertebral fractures were multiple. In our experience, they are often biconcave (more than 70 %) at morphometry (Fig. 4) [14]. In a recent study [21] conducted on 157 patients (92 women and 65 men, mean age 54 years), 83 of them experienced a total of 235 fractures: 140 were low-energy trauma ones; 87 were localized at the vertebral column, and 53 were at non-vertebral ones, including 2 hip fractures. Older age, male gender, and urinary methyl histamine levels turn out to be independent predictors of developing osteoporotic manifestations [21]. However, in a longitudinal study [25], only urinary methylimidazole acetic acid levels had a significant positive association with new osteoporotic fracture in univariate, but not in multivariate analysis, perhaps because urinary histamine metabolite reflect mast cell burden to only a minor extent, or because the local exposure from the mast cell mediators is of greater importance than the effect of circulating mediators.
Male sex, high serum levels of bone resorption marker type 1 collagen C-telopeptide (CTX, Z-score ≥ +1), low hip BMD (T-score ≤ −1), absence of urticaria pigmentosa, and alcohol intake were independent predictors of future fractures in patients presenting with ISM [25]. In our experience, serum tryptase levels cannot be used as a predictor of osteoporosis involvement in patients with SM [14].
Pathogenesis
The most frequent site of involvement is the lumbar spine than the hip, reflecting a major loss of trabecular than cortical bone. This is confirmed also by the fact that the majority of fractures occur at the vertebral bodies. The explanation probably lies in the higher propensity of clonal MCs to colonize the bone marrow, with a prevalent involvement of the most metabolically active bone tissue.
Focusing on bone histomorphometric analysis, in a few patients with osteoporosis in whom a bone biopsy was obtained, an increased [26–29] or normal [30] number of osteoclasts was reported. Recently, histomorphometric analysis revealed that ISM is characterized by deterioration of bone structure (decrease in bone trabeculae) and increased osteoid and bone cell (both osteoclasts and osteoblasts) number [28]. Some morphologic and histologic features, such as reduction in trabeculae number and thickness, are similar to those observed in glucocorticoid-induced osteoporosis. This could explain the similar morphometric characteristics of the vertebral fractures. However, osteoblasts are typically decreased by glucocorticoids. ISM could be associated with high bone turnover and the extent of bone cell activity depends on mast cell number.
Osteoporosis in SM has been attributed either to neoplastic infiltration or probably more to the local release of mediators (histamine, heparin, tryptase, lipid mediators, and cytokines) (Fig. 5). The complete activation of MC takes place in three subsequent phases. The first one happens in few seconds with degranulation of prestored mediators: histamine, tryptase and other proteases, proteoglycans (heparin, chondroitin sulfates), and cytokines (TNF-alpha, IL-4, SCF, and FGF) [31]. In a second phase, MCs synthesize and release within minutes lipid mediators derived from membranes, namely prostaglandins, leukotrienes, and platelet-activating factor [32, 33]. In a third phase, occurring within minutes to hours after the first phase, MCs synthesize large amounts of various pro-inflammatory cytokines (again TNF-alpha, IL-1, IL-6, IL-8, IL-5, and IL-13). In particular, cytokines such as TNF-alpha, IL-1, and IL-6, promoting osteoclast activity or inhibiting osteoblast function, have been suggested to play a role [34–36]. However, in the SM, the manifestations of the disease are predominantly due to the chronic production of the same chemical mediators without trigger, which lead to the clinical-pathologic features of the disorder.
Pathogenesis of mastocytosis-related osteoporosis. Osteoporosis in SM has been attributed to the local release of mediators by mast cells. In particular, cytokines such as tumor necrosis factor alpha, IL-1, IL-6, and IL-17 have been suggested to play a role, both in promoting osteoclast activity and inhibiting osteoblast function. Osteoclast activity is also directly stimulated by the local release of other mediators such as histamine, heparin, tryptase, and RANKL. Also, WNT/β-catenin pathway with its inhibitors (DKK1 and sclerostin) might be affected in patients with SM, with consequent inadequate osteoblast bone formation. Paradoxically very high concentrations of tryptase appear to stimulate osteoblast activity
Histamine is a key mediator and the most abundant one; it exerts stimulatory effects on osteoclasts and their precursors by autocrine and paracrine pathways [37, 38]. Further proven histamine role in bone remodeling comes from a study on murine model knockout for histamine decarboxylase, an enzyme necessary in its production. Histological specimen obtained from these animals showed a reduced osteoclast number and an increased bone formation [39]. A positive correlation between histamine metabolites and risk of osteoporotic manifestation has been reported [21]. Interestingly, osteoclast precursors have been discovered to be the main source of histamine. The effect on resorption is thus related to direct influence on osteoclasts and osteoclast precursors, but there is also an indirect action mediated through an enhanced expression of receptor activator of nuclear factor kappa-B ligand (RANKL) by osteoblasts [36] . This data lead us to hypothesize the possible bone-protective effect of H1 and H2 receptor antagonist demonstrated in some in vivo studies [40–46]. RANKL is secreted not only by osteoblasts but also by other cells including bone marrow stromal cells. These cells also express osteoprotegerin (OPG), a decoy receptor for RANKL, and thus a physiological inhibitor of RANK-RANKL signaling. Some authors reported an increase in OPG and RANKL levels in patients affected by mastocytosis, suggesting the involvement of the RANKL/RANK/OPG pathway in mastocytosis-related osteoporosis [47, 48]. Interestingly, MCs itself produce RANKL and OPG [48, 49].
In addition, cytokines related to tissue remodeling may be synthesized by MCs (for example, TGF-beta, FGF, and VEGF) [37]: some of these cytokines might be clinically relevant in patients with SM, by causing liver and bone marrow fibrosis and contributing to bone turnover.
Systemic bone remodeling activity can be quantified using biochemical bone turnover markers (BTMs), such as bone-specific alkaline phosphatase (bALP) or CTX. As previously reported [20], in patients with osteoporotic manifestations, serum BTMs were often found to be normal, sometimes above (and in such cases, they were associated with an increased uptake in bone scintiscan), or even below the normal range. Indeed, in our [20] and other cross-sectional experiences [28] none of the investigated BTMs were predictive of vertebral fractures in patients with ISM and to have BTM in the normal range was no guarantee for not having vertebral fractures. Considering the intrinsic limitation of the evaluation of these serum BTMs, they cannot be indicative of focal bone lesions or of the activity and balance of local bone multicellular unit, but they can only be indicative of generalized increase in bone turnover and remodeling. We speculate that in condition of BTM in the normal range, a generalized increase of bone turnover could be ruled out but not a local unbalance between osteoblast and osteoclast activity. An alternative explanation of the fractures in patients without alteration of serum BTM levels might be the local exposure from the MC mediators, without systemic effects. These results might suggest that mastocytosis-related osteoporosis could be characterized not only by absolute but also by relative or local prevalence of osteoclastic activity on osteoblastic activity.
However, in a recent longitudinal study [25], high level of serum CTX appeared to be an independent predictor of future fragility fractures in patients with ISM. CTX levels were higher in patients with aggressive systemic mastocytosis compared to cutaneous or indolent systemic forms [47], and they significantly correlated with serum tryptase level. In our experience in ISM patients, serum tryptase levels also significantly correlated with bALP serum levels [14], a marker of bone formation, and very high serum tryptase levels were associated with diffuse osteosclerosis [20]. The correlations between serum tryptase and BTM levels, as well as among these last ones and disease aggressiveness, support the existence of a link between bone remodeling process and number of mast cells.
Osteoblast differentiation is predominantly regulated by the canonical WNT pathway [50], which acts as the master regulator of osteogenesis together with bone morphogenetic proteins. WNT pathway plays a key role in determining the fate of mesenchymal stem cells. In the absence of β-catenin, these cells do not differentiate into mature osteocalcin-expressing osteoblasts but only into chondrocytes. It also promotes osteoblastogenesis by suppressing adipogenesis, through PPARγ inhibition (peroxisome proliferator-activated receptor gamma). In some circumstances, decreased WNT signaling might also result in an increased osteoclastogenesis and bone resorption, by decreasing the osteoblast expression of OPG. The regulation of WNT pathway in the bone is predominantly driven by the production of receptor inhibitors such as DKK1 and sclerostin. Sclerostin is a regulator of late osteoblast/pre-osteocyte differentiation; DKK1 expression is exclusively confined to osteoblasts and maturing osteocytes. Increased levels of sclerostin or DKK1 are associated with osteopenia or osteolytic lesions [50]. Enhanced levels of sclerostin [48] or DKK1 [51] were recently reported, even if inconsistently, in patients with ISM. These results suggest that also WNT/β-catenin pathway might be affected in patients with SM, with consequent inadequate bone formation, contributing to the presence of low bone mass. Interestingly, the serum levels of sclerostin and 25-hydroxyvitamin D were negatively correlated, and this might suggest that vitamin D levels are determinants of sclerostin levels in ISM patients [52].
Treatment options
What we learned from pathophysiology is that in SM with bone involvement there, is a relative or absolute prevalence of bone resorption. The key actor of bone resorption is the osteoclast. In fact, we have seen in the paragraph above that many pathways active in SM converge to activate this cell. This rationale brought the use of the most used and known anti-resorptive drug, bisphosphonates, for the treatment of bone manifestation in SM.
Even if in small studies, bisphosphonate therapy has proved to be effective in increasing vertebral BMD, but to a lesser extent in increasing femoral neck BMD as we can see in Table 2 [19, 53–59]. The first report was from Cundy et al. in 1987 [53] that described a reduction of bone turnover markers using oral clodronate. Ten years later, Marshall et al. [54] published a small case series of three patients treated with yearly intravenous (iv) pamidronate showing a positive effect on lumbar BMD but a slight decrease at femoral neck site. A similar conclusion was reported in a case report by Brumsen et al. [55] with pamidronate iv induction and subsequent oral maintenance therapy. There are also anecdotal reports of the use of risedronate and alendronate in SM-related osteoporosis but without any clear data on follow-up [19]. It has been reported that in many SM patients with unexplained bone pain accompanied by osteopenia, pain resolves rapidly after initiation of a bisphosphonate [41].
The problem of poor compliance with oral bisphosphonates is well known and documented, and the option of a less frequent administration would be very attractive to patients. Recently, we reported the results of a study on 25 patients with ISM treated with one 5-mg iv infusion of zoledronate [59]. Zoledronate is an aminobisphosphonate with a well known positive effect on BMD and fracture occurrence in patients with postmenopausal and glucocorticoid-induced osteoporosis to whom it is given once a year. It is also used for the treatment of bone involvement in the oncologic field for bone solid malignancy metastasis and multiple myeloma with a more intense regimen of 4-mg monthly infusions. The most frequent adverse event was represented by a flu-like reaction, generally following only the first infusion. In patients with ISM, one iv infusion of zoledronate guaranteed a positive effect on BMD and BTM, surrogates of anti-fracture efficacy, for at least 1 year [59]. Mean BMD gain was greater than any bisphosphonates ever tried before, both in lumbar and femoral sites. However, the anti-fracture efficacy of bisphosphonates is yet to be confirmed by long-term randomized study. Interesting, 1 year after only ZOL infusion the BTM continued to remain suppressed [59]. In terms of BMD or BTM, recognized surrogate markers of anti-fracture efficacy, it is known in postmenopausal osteoporosis that the effects of a single 5-mg dose of zoledronate are sustained for at least 5 years [60] and that fracture risk appears to be reduced for more than 1 year after a single infusion of zoledronate [61]. We think that mastocytosis-related osteoporosis without fragility fractures (not severe according to 1994 WHO definition, [17]) might be comparable to postmenopausal osteoporosis. So, in our opinion, the standard postmenopausal dosage might be enough to keep the risk of fracture low in patients with non-severe forms of osteoporosis associated to ISM. It is well known that major and most feared side effects of zoledronate (osteonecrosis of the jaw, atypical fractures, and arrhythmias) are dose-dependent [62]. Therefore, we think it is reasonable to shorten the interval between infusions (for example 4 mg iv every 4 weeks as commonly used in multiple myeloma) only in the most severe osteoporosis secondary to SM, when the benefits largely outweigh the risks of treatment.
In the condition of severe osteoporosis, it is also reasonable to add another treatment such as interferon alpha that has proven efficacy in previous studies [58, 63–65] and to give additional advantage when administered with bisphosphonates. Indeed, some studies reported the additional positive effects of interferon treatment on the BMD: the group of Laroche in 2007 [57] and 2011 [58] documented the additional positive effect of a combination therapy with monthly iv pamidronate and interferon, mainly at the lumbar site (mean annual increase = 12.6 %) and much less at femoral neck (mean annual increase = 1.93 %). In case of very severe osteoporosis, new major fractures, or depending also on important systemic extraskeletal manifestations of SM, the possible additional use of interferon alpha for the management of mastocytosis-related osteoporosis should be considered.
Discussion and conclusions
SM should be included as a potential diagnosis in the screening of all premenopausal women and men presenting with an unexplained fragility fracture or low BMD and postmenopausal women with suspicion of secondary osteoporosis. In most cases, diagnosis of mastocytosis is fairly straightforward due to its typical cutaneous lesions (urticaria pigmentosa, characterized by symmetrically distributed 0.5-cm red brown macules and papules, which induce erythema, wheal, and pruritus when scratched). However, it can be challenging in cases without skin manifestations (nearly 50 % in our cohort). Mastocytosis should be suspected in patients, regardless of characteristic skin manifestations, with recurrent anaphylaxis, either spontaneously or in response to stimuli (hymenoptera stings, drugs, alcohol, foods, cold, heat, physical exertion, emotional stress….), or other clinical symptoms related to the release of mast cell mediators (facial flushing, pruritus, palpitations, dizziness, episodes of hypotension, syncope, angioedema, breathing difficulties, abdominal pain, nausea, vomiting, diarrhea, headache, sweating, fatigue, arthralgia, myalgia, anxiety and depression) [66]. The presence of idiopathic osteoporosis or unexplained fragility fracture, in the absence of trigger factors for anaphylaxis, might be the only sign of a latent ISM. Serum tryptase level is recommended as the biomarker of choice to screen patients with unexplained osteoporosis and/or suspected mastocytosis, because it is a fairly specific mediator of mast cells and is widely available (Fig. 6). However, an increased tryptase level alone is not pathognomonic of SM and, on the other hand, level in the normal range cannot exclude SM diagnosis (Fig. 6). Establishing a diagnosis of SM requires one major and one minor, or three minor, criteria using the WHO classification, and therefore bone marrow should be screened with tryptase immunohistochemical, KIT molecular biology and cytometric analysis (Fig. 6).
Bone involvement in SM appears an intriguing clinical model, ranging from osteoporosis to osteosclerosis, due to the proximity of the mast cell to bone remodeling surfaces and the production of a large number of chemical mediators and cytokines capable of modulating bone turnover by this cell. Following diagnosis of SM, a number of staging investigations should be recommended, including BTM and BMD measurements with vertebral morphometry, skeletal X-rays, and eventually bone scan and other radiological imaging technologies. The role of BTM in clinical practice remains uncertain. However, high serum levels of BTM are suspicious for mastocytosis bone involvement, and in this condition a bone scan is recommended. Increased uptake at the scintiscan can detect focal and asymptomatic bone lesions, to be subjected to targeted radiographic assessment, whereas a diffuse uptake has a prognostic value [67]. Sensitivity and specificity of BMD assessment to predict vertebral fracture is limited in SM [14], likely because of the patchy distribution of neoplastic MCs, or because there are other bone features affected in addition to BMD. Follow-up should be scheduled at different intervals on a case-by-case basis according to the needs of individual patients; BMD measurements may be performed every 18 months in osteoporotic condition or every third year if only osteopenia.
The present evidences suggest that bisphosphonates represent the first-line treatment for mastocytosis-related osteoporosis, even if anti-fracture efficacy is yet to be confirmed by long-term randomized studies. Reviewing published literature on the treatment of mastocytosis-related osteoporosis, it is highlighted the general poor level of evidence of the efficacy of various therapeutic options due to paucity of data, particularly from randomized controlled trials, and lack of data from long-term studies. However, in clinical practice, calcium and vitamin D are recommended for osteopenia and bisphosphonates, per os or iv, in the case of osteoporosis, on the basis of some efficiency on BMD gain and BTM reduction. These drugs are not always well tolerated in SM, both per os, due to worsening of digestive symptoms, and iv with frequently and severe acute phase response [59, 62]. Additionally the residual long-term effect of bisphosphonate raises some concerns especially in young patients, particularly if women are of childbearing age.
Future approaches rely on the use of other bone target therapy. In the last years, a biologic anti-resorptive drug, denosumab, has been developed for the treatment of postmenopausal osteoporosis. This drug uses monoclonal antibodies directed against RANKL in order to disrupt the RANK-RANKL activation pathway of the osteoclast. We have seen in the pathogenesis section how this pathway is primarily involved in the induction of osteoporosis in mastocytosis, as MCs were shown to produce RANKL [48, 49]. So this would be the rationale for the treatment of mastocytosis relates osteoporosis with anti-RANKL, and denosumab may be a particularly viable alternative in case of bisphosphonate intolerance, but no clinical experience is reported until now, and there are some safety concerns about the risk of anaphylaxis. A rational approach, especially in the presence of low bone formation markers, might be the osteoblast stimulation with teriparatide, the 1–34 active fragment of parathyroid hormone. However, no experiences with the use of this anabolic treatment have been reported until now, and the description of an increase in MCs in parathyroid bone disease [68] suggests some safety concerns in SM. Based on current knowledge in our opinion, the use of teriparatide should not be proposed as an alternative approach in the management of mastocytosis-related osteoporosis as this treatment might further enhance the growth and proliferation of abnormal MCs and induce more aggressive forms of SM. Another possible therapeutic option with bone-forming agents could be the Wnt pathway, targeting, for example, DKK-1 or sclerostin, but their exact role in SM is yet to be completely clarified.
Currently, there is no evidence for an effect of anti-osteoporosis drugs on mast cell load. Cytoreductive drugs are currently indicated only in advanced/aggressive form of SM, but these agents may also be considered for patients with ISM affected by severe osteoporosis with new fractures despite bisphosphonate treatment, after careful risk/benefit analysis. IFN-α is considered the first-line cytoreductive agent: it has been shown to improve symptoms related to mast cell mediators, decrease bone marrow mast cell infiltration, and reduce mastocytosis-related osteoporosis, ascites/hepatosplenomegaly, cytopenia, and skin lesions, despite the fact that its beneficial effects are restricted by significant toxicities [69]. Cladribine can be used in SM patients who are refractory to IFN-α [70]. Tyrosine kinase inhibitors are a promising group of drugs indicated in aggressive forms of SM as they target KIT [71]; currently, there are no data on their effects on BTM, BMD, and fracture risk. Early institution of antimediator therapy may reverse some disease-related bone changes [41, 72]; however, there are no definitive studies in this regard.
References
Krishnaswamy G, Ajitawi O, Chi DS (2006) The human mast cell: an overview. Methods Mol Biol 315:13–34
Arock M, Valent P (2010) Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol 3:497–516
Valent P (1994) The riddle of the mast cell: kit(CD117)-ligand as the missing link? Immunol Today 15:111–114
Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD (1999) Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13). Blood 94:2333–2342
Fried AJ, Akin C (2013) Primary mast cell disorders in children. Curr Allergy Asthma Rep 13:693–701
Sperr WR, Valent P (2012) Diagnosis, progression patterns and prognostication in mastocytosis. Expert Rev Hematol 5:261–274
Valent P (2013) Mastocytosis: a paradigmatic example of a rare disease with complex biology and pathology. Am J Cancer Res 3:159–172
Lee JK, Whittaker SJ, Enns RA, Zetler P (2008) Gastrointestinal manifestations of systemic mastocytosis. World J Gastroenterol 14:7005–7008
Pardanani A (2013) Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol 88:612–624
Stoecker MM, Wang E (2012) Systemic mastocytosis with associated clonal hematologic nonmast cell lineage disease: a clinicopathologic review. Arch Pathol Lab Med 136:832–838
Valent P, Sperr WR, Akin C (2010) How I treat patients with advanced systemic mastocytosis. Blood 116:5812–5817
George TI, Horny HP (2011) Systemic mastocytosis. Hematol Oncol Clin North Am 25:1067–1083
Sokol H, Georgin-Lavialle S, Canioni D et al (2013) Gastrointestinal manifestations in mastocytosis: a study of 83 patients. J Allergy Clin Immunol 132:866–873 e1–3
Rossini M, Zanotti R, Viapiana O, Tripi G, Orsolini G, Idolazzi L, Bonadonna P, Schena D, Escribano L, Adami S, Gatti D (2014) Bone involvement and osteoporosis in mastocytosis. Immunol Allergy Clin North Am 34:383–396
Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, Castells M, Sperr WR, Kluin-Nelemans HC, Hamdy NA, Lortholary O, Robyn J, van Doormaal J, Sotlar K, Hauswirth AW, Arock M, Hermine O, Hellmann A, Triggiani M, Niedoszytko M, Schwartz LB, Orfao A, Horny HP, Metcalfe DD (2007) Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest 37:435–453
Zanotti R, Lombardo C, Passalacqua G, Caimmi C, Bonifacio M, De Matteis G, Perbellini O, Rossini M, Schena D, Busa M, Marcotulli MC, Bilò MB, Franchini M, Marchi G, Simioni L, Bonadonna P (2015) Clonal mast cell disorders in patients with severe Hymenoptera venom allergy and normal serum tryptase levels. J Allergy Clin Immunol 136:135–139
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO study Group. Osteoporos Int 4:368–381
Escribano L, Alvarez-Twose I, Sánchez-Muñoz L, Garcia-Montero A, Núñez R, Almeida J, Jara-Acevedo M, Teodósio C, García-Cosío M, Bellas C, Orfao A (2009) Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol 124:514–521
Barete S, Assous N, de Gennes C, Granpeix C, Feger F, Palmerini F et al (2010) Systemic mastocytosis and bone involvement in a cohort of 75 patients. Ann Rheum Dis 69:1838–1841
Rossini M, Zanotti R, Bonadonna P, Artuso A, Caruso B, Schena D, Vecchiato D, Bonifacio M, Viapiana O, Gatti D, Senna G, Riccio A, Passalacqua G, Pizzolo G, Adami S (2011) Bone mineral density, bone turnover markers and fractures in patients with indolent systemic mastocytosis. Bone 49:880–885
van der Veer E, van der Goot W, de Monchy JG, Kluin-Nelemans HC, van Doormaal JJ (2012) High prevalence of fractures and osteoporosis in patients with indolent systemic mastocytosis. Allergy 67:431–438
Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML (2008) International society for clinical densitometry 2007 adult and pediatric official positions. Bone 43:1115–1121
Hermine O, Lortholary O, Leventhal PS, Catteau A, Soppelsa F, Baude C, Cohen-Akenine A, Palmérini F, Hanssens K, Yang Y, Sobol H, Fraytag S, Ghez D, Suarez F, Barete S, Casassus P, Sans B, Arock M, Kinet JP, Dubreuil P, Moussy A (2008) Case–control cohort study of patients’ perceptions of disability in mastocytosis. PLoS One 3(5):e2266
Cohen SS, Skovbo S, Vestergaard H, Kristensen T, Møller M, Bindslev-Jensen C, Fryzek JP, Broesby-Olsen S (2014) Epidemiology of systemic mastocytosis in Denmark. Br J Haematol 166:521–528
van der Veer E, Arends S, van der Hoek S, Versluijs JB, de Monchy JG, Oude Elberink JN, van Doormaal JJ (2014) Predictors of new fragility fractures after diagnosis of indolent systemic mastocytosis. J Allergy Clin Immunol 134:1413–1421
Fallon MD, Whyte MP, Teitelbaum SL (1981) Systemic mastocytosis associated with generalized osteopenia. Histopathological characterization of the skeletal lesion using undecalcified bone from two patients. Hum Pathol 12:813–820
Chines A, Pacifici R, Avioli LV, Teitelbaum SL, Korenblat PE (1991) Systemic mastocytosis presenting as osteoporosis: a clinical and histomorphometric study. J Clin Endocrinol Metab 72:140–144
Seitz S, Barvencik F, Koehne T, Priemel M, Pogoda P, Semler J, Minne H, Pfeiffer M, Zustin J, Püschel K, Eulenburg C, Schinke T, Amling M (2013) Increased osteoblast and osteoclast indices in individuals with systemic mastocytosis. Osteoporos Int 24:2325–2334
De Gennes C, Kuntz D, de Vernejoul MC (1992) Bone mastocytosis. A report of nine cases with a bone histomorphometric study. Clin Orthop Relat Res 279:281–291
Delling G, Ritzel H, Werner M (2001) Histological characteristics and prevalence of secondary osteoporosis in systemic mastocytosis. A retrospective analysis of 158 cases. Pathologe 22:132–140
Metcalfe DD (2008) Mast cells and mastocytosis. Blood 112:946–955
Schwartz LB (1987) Mediators of human mast cells and human mast cell subsets. Ann Allergy 58:226–235
Macpherson JL, Kemp A, Rogers M, Mallet AI, Toia RF, Spur B, Earl JW, Chesterman CN, Krilis SA (1989) Occurrence of platelet-activating factor (PAF) and an endogenous inhibitor of platelet aggregation in diffuse cutaneous mastocytosis. Clin Exp Immunol 77:391–396
Theoharides TC, Boucher W, Spear K (2002) Serum interleukin-6 reflects disease severity and osteoporosis in mastocytosis patients. Int Arch Allergy Immunol 128:344–350
Brockow K, Akin C, Huber M, Metcalfe DD (2005) IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin Immunol 115:216–223
Chiappetta N, Gruber B (2006) The role of mast cells in osteoporosis. Semin Arthritis Rheum 36:32–36
Galli SJ, Tsai M (2008) Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci 49:7–19
Dobigny C, Saffar JL (1997) H1 and H2 histamine receptors modulate osteoclastic resorption by different pathways: evidence obtained by using receptor antagonists in a rat synchronized resorption model. J Cell Physiol 173:10–18
Biosse-Duplan M, Baroukh B, Dy M, de Vernejoul MC, Saffar JL (2009) Histamine promotes osteoclastogenesis through the differential expression of histamine receptors on osteoclasts and osteoblasts. Am J Pathol 174:1426–1434
Fitzpatrick LA, Buzas E, Gagne TJ, Nagy A, Horvath C, Ferencz V et al (2003) Targeted deletion of histamine decarboxylase gene in mice increases bone formation and protects against ovariectomy-induced bone loss. Proc Natl Acad Sci U S A 100:6027–6032
Escribano L, Akin C, Castells M, Schwartz LB (2006) Current options in the treatment of mast cell mediator-related symptoms in mastocytosis. Inflamm Allergy Drug Targets 5:61–77
Worobec AS, Metcalfe DD (2002) Mastocytosis: current treatment concepts. Int Arch Allergy Immunol 127:153–155
Lytinas M, Kempuraj D, Huang M et al (2002) Azelastine’s inhibition of histamine and tryptase release from human umbilical cord blood-derived cultured mast cells as well as rat skin mast cell-induced vascular permeability: comparison with olopatadine. Allergy Asthma Proc 23:45–51
Hadzijusufovic E, Peter B, Gleixner KV, Schuch K, Pickl WF, Thaiwong T, Yuzbasiyan-Gurkan V, Mirkina I, Willmann M, Valent P (2010) H1-receptor antagonists terfenadine and loratadine inhibit spontaneous growth of neoplastic mast cells. Exp Hematol 38:896–907
Turner PJ, Kemp AS, Rogers M, Mehr S (2012) Refractory symptoms successfully treated with leukotriene inhibition in a child with systemic mastocytosis. Pediatr Dermatol 29:222–223
Mullol J, Bousquet J, Bachert C, Canonica GW, GimenezArnau A, Kowalski ML, Simons FE, Maurer M, Ryan D, Scadding G (2015) Update on rupatadine in the management of allergic disorders. Allergy 70(suppl 100):1–24
Guillaume N, Desoutter J, Chandesris O, Merlusca L, Henry I, Georgin-Lavialle S, Barete S, Hirsch I, Bouredji D, Royer B, Gruson B, Lok C, Sevestre H, Mentaverri R, Brazier M, Meynier J, Hermine O, Marolleau JP, Kamel S, Damaj G (2013) Bone complications of mastocytosis: a link between clinical and biological characteristics. Am J Med 126:75.e1
Rabenhorst A, Christopeit B, Leja S, Gerbaulet A, Kleiner S, Förster A, Raap U, Wickenhauser C, Hartmann K (2013) Serum levels of bone cytokines are increased in indolent systemic mastocytosis associated with osteopenia or osteoporosis. J Allergy Clin Immunol 132:1234–1237
Ali AS, Lax AS, Liljestrom M, Paakkari I, Ashammakhi N, Kovanen PT, Konttinen YT (2006) Mast cells in atherosclerosis as a source of the cytokine RANKL. Clin Chem Lab Med 44:672–674
Rossini M, Gatti D, Adami S (2013) Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int 93:121–132
Rossini M, Viapiana O, Zanotti R, Tripi G, Perbellini O, Idolazzi L, Bonifacio M, Adami S, Gatti D (2015) Dickkopf-1 and sclerostin serum levels in patients with systemic mastocytosis. Calcif Tissue Int 96:410–416
Rossini M, Adami S, Zanotti R, Viapiana O, Idolazzi L, Biondan M, Gatti D (2014) Serum levels of bone cytokines in indolent systemic mastocytosis associated with osteopenia or osteoporosis. J Allergy Clin Immunol 133:933–935
Cundy T, Beneton MN, Darby AJ, Marshall WJ, Kanis JA (1987) Osteopenia in systemic mastocytosis: natural history and responses to treatment with inhibitors of bone resorption. Bone 8:149–155
Marshall A, Kavanagh RT, Crisp AJ (1997) The effect of pamidronate on lumbar spine bone density and pain in osteoporosis secondary to systemic mastocytosis. Br J Rheumatol 36:393–396
Brumsen C, Hamdy NA, Papapoulos SE (2002) Osteoporosis and bone marrow mastocytosis: dissociation of skeletal responses and mast cell activity during long-term bisphosphonate therapy. J Bone Miner Res 17:567–569
Lim AY, Ostor AJ, Love S, Crisp AJ (2005) Systemic mastocytosis: a rare cause of osteoporosis and its response to bisphosphonate treatment. Ann Rheum Dis 64:965–966
Laroche M, Bret J, Brouchet A, Maziéres B (2007) Clinical and densitometric efficacy of the association of interferon alpha and pamidronate in the treatment of osteoporosis in patients with systemic mastocytosis. Clin Rheumatol 26:242–243
Laroche M, Livideanu C, Paul C, Cantagrel A (2011) Interferon alpha and pamidronate in osteoporosis with fracture secondary to mastocytosis. Am J Med 124:776–778
Rossini M, Zanotti R, Viapiana O, Tripi G, Idolazzi L, Biondan M, Orsolini G, Bonadonna P, Adami S, Gatti D (2014) Zoledronic acid in osteoporosis secondary to mastocytosis. Am J Med 127:1127.e1–4
Grey A, Bolland MJ, Horne A, Wattie D, House M, Gamble G, Reid IR (2012) Five years of anti-resorptive activity after a single dose of zoledronate—results from a randomized double-blind placebo-controlled trial. Bone 50:1389–1393
Reid IR, Black DM, Eastell R, Bucci-Rechtweg C, Su G, Hue TF, Mesenbrink P, Lyles KW, Boonen S (2013) Reduction in the risk of clinical fractures after a single dose of zoledronic acid 5 milligrams. J Clin Endocrinol Metab 98:557–563
Rossini M, Adami G, Adami S, Viapiana O, Gatti D (2016) Safety issues and adverse reactions with osteoporosis management. Expert Opin Drug Saf 14:1–12
Weide R, Ehlenz K, Lorenz W, Walthers E, Klausmann M, Pfluger KH (1996) Successful treatment of osteoporosis in systemic mastocytosis with interferon alpha-2b. Ann Hematol 72:41–43
Lehmann T, Beyeler C, Lämmle B et al (1996) Severe osteoporosis due to systemic mast cell disease: successful treatment with interferon alpha-2B. Br J Rheumatol 35:898–900
Butterfield JH (2005) Interferon treatment for hypereosinophilic syndromes and systemic mastocytosis. Acta Haematol 114:26–40
Valent P, Escribano L, Broesby-Olsen S, Hartmann K, Grattan C, Brockow K, Niedoszytko M, Nedoszytko B, Oude Elberink JN, Kristensen T, Butterfield JH, Triggiani M, Alvarez-Twose I, Reiter A, Sperr WR, Sotlar K, Yavuz S, Kluin-Nelemans HC, Hermine O, Radia D, van Doormaal JJ, Gotlib J, Orfao A, Siebenhaar F, Schwartz LB, Castells M, Maurer M, Horny HP, Akin C, Metcalfe DD, Arock M (2014) Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy 69:1267–1274
Chen CC, Andrich MP, Mican JM, Metcalfe DD (1994) A retrospective analysis of bone scan abnormalities in mastocytosis: correlation with disease category and prognosis. J Nucl Med 35:1471–1475
Turner RT, Iwaniec UT, Marley K, Sibonga JD (2010) The role of mast cells in parathyroid bone disease. J Bone Miner Res 25:1637–1649
Pardanani A (2013) How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage). Blood 121:3085–3094
Tefferi A, Li CY, Butterfield JH, Hoagland HC (2001) Treatment of systemic mast-cell disease with cladribine. N Engl J Med 344:307–309
Verstovsek S (2013) Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression. Eur J Haematol 90:89–98
Graves L 3rd, Stechschulte DJ, Morris DC, Lukert BP (1990) Inhibition of mediator release in systemic mastocytosis is associated with reversal of bone changes. J Bone Miner Res 5:1113–1119
Acknowledgments
The authors thank Sara Rossini who provided editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Rossini, M., Zanotti, R., Orsolini, G. et al. Prevalence, pathogenesis, and treatment options for mastocytosis-related osteoporosis. Osteoporos Int 27, 2411–2421 (2016). https://doi.org/10.1007/s00198-016-3539-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3539-1