Abstract
Summary
The efficacy and safety of weekly oral odanacatib (ODN) 50 mg for up to 8 years were assessed in postmenopausal women with low bone mineral density (BMD). Treatment with ODN for up to 8 years resulted in continued or maintained increases in BMD at multiple sites and was well tolerated.
Introduction
ODN is a selective inhibitor of cathepsin K. In a 2-year phase 2b study (3/10/25/50 mg ODN once weekly [QW] or placebo) and extensions (50 mg ODN QW or placebo), ODN treatment for 5 years progressively increased BMD and decreased bone resorption markers in postmenopausal women with low BMD (ClinicalTrials.gov NCT00112437).
Methods
In this prespecified interim analysis at year 8 of an additional 5-year extension (years 6 to 10), patients (n = 117) received open-label ODN 50 mg QW plus weekly vitamin D3 (5600 IU) and calcium supplementation as needed. Primary end points were lumbar spine BMD and safety. Patients were grouped by ODN exposure duration.
Results
Mean (95 % confidence interval [CI]) lumbar spine BMD changes from baseline were 4.6 % (2.4, 6.7; 3-year continuous ODN exposure), 12.9 % (8.1, 17.7; 5 years), 12.8 % (10.0, 15.7; 6 years), and 14.8 % (11.0, 18.6; 8 years). Similar patterns of results were observed for BMD of trochanter, femoral neck, and total hip versus baseline. Geometric mean changes from baseline to year 8 for bone resorption markers were approximately −50 % (uNTx/Cr) and −45 % (sCTx), respectively (all groups); bone formation markers remained near baseline levels. No osteonecrosis of the jaw, delayed fracture union, or morphea-like skin reactions were reported.
Conclusions
Treatment with ODN for up to 8 years resulted in gains in BMD at multiple sites. Bone resorption markers remained reduced, with no significant change observed in bone formation markers. Treatment with ODN for up to 8 years was well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postmenopausal osteoporosis is characterized by a loss of bone mass, which results from bone resorption exceeding bone formation leading to microstructural bone defects and increased fracture risk [1]. As populations age, osteoporotic fractures are an increasing problem and represent a substantial economic and social burden [2–4].
Currently available therapeutic agents for treating patients with postmenopausal osteoporosis and preventing fractures include anti-resorptive agents, such as bisphosphonates and the human monoclonal antibody to the receptor activator of nuclear factor-kappaB ligand (RANKL), denosumab, and the anabolic agent teriparatide, which has been shown to prevent vertebral fractures [5]. The US Food and Drug Administration (FDA), however, has recommended restricting the use of bisphosphonates to 3–5-year duration, except in patients still at increased risk of fracture [6]. The FDA has recommended that teriparatide not be used for more than 2 years over a patient’s lifetime [7]. There is, therefore, a need for long-term treatment for osteoporosis with a new mechanism of action to provide greater choices to physicians and patients.
Odanacatib (ODN) has a novel mode of action, selectively inhibiting cathepsin K (CatK), a lysosomal cysteine protease highly expressed in osteoclasts, which degrades the collagen matrix components of bone [8]. Unlike anti-resorptive agents that reduce the number of osteoclasts to decrease bone resorption and secondarily reduce osteoblast number, ODN specifically inhibits osteoclast bone resorption activity, while maintaining osteoclast number and viability [9–11].
ODN was studied in a placebo-controlled, double-blind, randomized, 2-year phase 2b dose-ranging study in postmenopausal women with low bone mineral density (BMD) [12]. Patients completing the first 2 years of this study could be eligible for extension studies, first for 1 year and then for a further 2-year period, providing up to 5-year exposure to ODN [13, 14]. These studies showed that treatment with ODN progressively increased BMD and decreased bone resorption markers, whereas bone formation markers remained near baseline. Treatment with ODN for up to 5 years was generally well tolerated. A further 5-year extension (years 6 to 10) was planned to evaluate the long-term efficacy and safety of weekly oral ODN 50 mg. Here, we present results from the prespecified interim year 8 analysis of this 5-year extension.
Methods
Study design
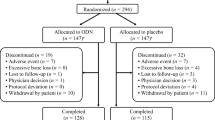
This was a prespecified interim analysis of an ongoing 5-year extension (years 6 to 10) to a phase 2b clinical study investigating the efficacy and safety of ODN for the treatment of osteoporosis in postmenopausal women (ClinicalTrials.gov NCT00112437; protocol number 004) (Fig. 1). Study design and results for the initial 2-year study [12] and subsequent 1-year [13] and 2-year extension [14] phases have been reported in detail previously.
Schematic of study design. Group A received ODN 50 mg for 8 years or ODN 25 mg for 2 years followed by 6 years of ODN 50 mg; group B received ODN 50 mg for the last 6 years and placebo or ODN 3 or 10 mg during the first 2 years; group C received ODN 50 mg for the last 5 years and placebo or ODN 3 mg during the first 2 years and placebo in year 3; and group D received ODN 50 mg for the last 3 years, placebo during the previous 3 years, and doses of ODN between 10 and 50 mg during the first 2 years. aPatient groups included in the year 8 interim analysis had been receiving ODN 25 or 50 mg once weekly for between three and eight consecutive years at the time of this analysis, bBMD was measured using dual-energy X-ray absorptiometry, c n = 24 for lumbar spine and n = 22 for other sites, and d n = 22 for lumbar spine and n = 21 for other sites. BMD bone mineral density, ODN odanacatib in milligram; dosing was once weekly
In the initial 2-year, randomized, double-blind, multicenter, dose-finding study, eligible postmenopausal women with low BMD were assigned, using a computer-generated randomized allocation schedule, to receive placebo or oral ODN, taken without regard to food intake, at 3, 10, 25, or 50 mg once weekly (Fig. 1). For year 3, all women in each of the five original dose groups were re-randomized to receive ODN 50 mg once weekly or placebo in a 1:1 ratio. For years 4 and 5, all women who had received ODN 3 mg once weekly or placebo in years 1 and 2 were switched to ODN 50 mg once weekly; other patients remained on the same regimen as in year 3. For the present extension, all women received open-label ODN 50 mg once weekly, attended clinic visits every 6 months, and were followed up by telephone every 3 months.
Ten discrete patient subgroups were defined based upon the treatments received since the initial randomization into the study (Fig. 1). An interim analysis was conducted at year 8, with a focus on the long-term BMD effects of ODN. For this reason, and in order to describe the results with greater clarity, patient subgroups were combined in four different treatment groups based on their exposure to effective ODN doses. The treatment groups are described below and presented in greater detail in Fig. 1.
-
Group A (n = 28) included patients treated with ODN 50 mg continuously for 8 years or with ODN 25 mg during the first 2 years followed by 6 years of ODN 50 mg.
-
Group B (n = 34) included patients who were treated with ODN 50 mg for the last 6 years and who had received placebo or lower doses of ODN (3 or 10 mg) during the first 2 years.
-
Group C (n = 23) included patients treated with ODN 50 mg for the last 5 years and who had received placebo or ODN 3 mg during the first 2 years and placebo in year 3.
-
Group D (n = 32) included patients treated with ODN 50 mg for the last 3 years and who had received placebo during the previous 3 years and doses of ODN between 10 and 50 mg during the first 2 years.
All participants were also provided with supplemental vitamin D3 (5600 IU once weekly), and those with a calcium intake <1000 mg/day (assessed via a dietary calcium questionnaire) were given a daily supplement of calcium carbonate (500 mg/day). The study was conducted in accordance with Good Clinical Practice guidelines and was approved by local institutional review boards.
Participants
The initial 2-year phase 2b study enrolled postmenopausal women, aged 45–85 years, with no previous history of fragility fractures and a BMD T-score ≤ −2.0 at the lumbar spine, trochanter, femoral neck, or total hip but not <−3.5 at any site. Women completing year 5 of the extension study were eligible for enrollment in this additional 5-year extension study, unless they demonstrated significant clinical or laboratory abnormalities that were judged to cause additional risks or to complicate interpretation of the study results. BMD at the year 5 visit was not an inclusion/exclusion criterion for this extension study. Patients gave written informed consent prior to enrollment at the initiation of the study and at the beginning of each subsequent extension phase.
End points
Details of study procedures have been described previously [12–14]. Briefly, BMD was measured by dual-energy X-ray absorptiometry scans (using lunar and hologic instruments at the various sites) at the hip (total, femoral neck, and trochanter), lumbar spine, and 1/3 radius at months 72, 84, and 96. All BMD scans were centrally evaluated. Biochemical markers of bone turnover (serum bone-specific alkaline phosphatase [sBSAP], serum N-terminal propeptide of type I collagen [sP1NP], urinary N-telopeptide of type I collagen corrected for creatinine [uNTx/Cr], serum C-telopeptide of type I collagen [sCTx], serum cross-linked carboxy-terminal telopeptide of type I collagen [s1CTP], and serum tartrate-resistant acid phosphatase isoform 5b [sTRAP 5b]) were measured in the fasting state (at months 72, 84, and 96 for sP1NP and uNTx and at month 96 for the other biomarkers). Individual biomarker assays (Synarc, Lyon, France) were as described previously [14].
The primary efficacy end point of this 5-year extension is percent change from baseline in BMD at the lumbar spine at years 8 and 10. Secondary efficacy end points include percent change from baseline in BMD for trochanter, femoral neck, total hip, and 1/3 radius and change from baseline in biochemical markers of bone resorption (uNTx/Cr and sCTx), sTRAP 5b (a biomarker of osteoclast numbers), s1CTP (a C-terminal fragment of collagen produced by matrix metalloproteinases which is further digested by CatK to generate CTx and is, therefore, a target engagement marker for CatK inhibition) [15], and biochemical markers of bone formation (sBSAP and sP1NP), analyzed as a log-transformed fraction from baseline. Baseline was defined as the first study visit in the initial phase 2b study for all end points.
Safety was evaluated based on the incidence of adverse events (AEs), which were recorded throughout, and clinical and laboratory evaluations performed at each 6-monthly visit. All patients who received at least one dose of study medication during the years 6 to 8 extension period were included in the safety analysis. Special attention was given to skin, respiratory, dental, and delayed fracture union events.
Statistical analysis
All results were summarized as descriptive analyses using summary statistics. BMD end points were evaluated for the full analysis set (FAS) as percent change from baseline in raw arithmetic means with 95 % confidence intervals (CIs). Biochemical markers were evaluated for the per protocol (PP) population as log-transformed fraction from baseline and transformed back to a geometric mean percent change from baseline with 95 % CIs. Missing data were not imputed or estimated. Safety and tolerability were evaluated in the all-patients-as-treated (APaT) population. One interim analysis was to be performed at year 8.
Results
Patients
A total of 117 women entered the years 6 to 10 extension of the initial study. At the time of this year 8 analysis, 28 patients had received ODN 25 or 50 mg once weekly for eight consecutive years (group A), while 34 patients had taken ODN 50 mg for six consecutive years (group B), 23 patients for five consecutive years (group C), and 32 patients for three consecutive years (group D). At year 8, the FAS population evaluable for lumbar spine BMD comprised 87 patients, and 57 women were included in the PP population evaluable for biomarkers (Fig. 1). Baseline demographics and characteristics at study entry of the women entering the years 6 to 10 extension are shown in Table 1.
Bone mineral density
Lumbar spine BMD at year 8 increased from baseline after treatment with ODN 50 mg for all groups (Fig. 2). Mean increases in lumbar spine BMD (95 % CI) were 14.8 % (11.0, 18.6) for patients in group A, 12.8 % (10.0, 15.7) for group B, 12.9 % (8.1, 17.7) for group C, and 4.6 % (2.4, 6.7) for group D. Similar patterns of results were observed for BMD for trochanter, femoral neck, and total hip compared with baseline (Fig. 2). Results for BMD at 1/3 radius showed a slight decrease compared with baseline. Time-effect profiles for patients who received ODN 25 or 50 mg once weekly for eight consecutive years are shown in Fig. 3 for the FAS population and for the “completer” population with complete data at months 60 to 96.
Percent change from baseline in BMD at year 8 (FAS population, ODN 25a or 50 mg once weekly). Group A received ODN 50 mg for 8 years or ODN 25 mg for 2 years followed by 6 years of ODN 50 mg, group B received ODN 50 mg for the last 6 years and placebo or ODN 3 or 10 mg during the first 2 years, group C received ODN 50 mg for the last 5 years and placebo or ODN 3 mg during the first 2 years and placebo in year 3, and group D received ODN 50 mg for the last 3 years, placebo during the previous 3 years, and doses of ODN between 10 and 50 mg during the first 2 years. aFourteen patients in group A received ODN 25 mg for 2 years before receiving ODN 50 mg (see Fig. 1), b n = 24 for lumbar spine and n = 22 for other sites, and c n = 22 for lumbar spine and n = 21 for other sites. BMD bone mineral density, FAS full analysis set, ODN odanacatib, SE standard error
Percent change from baseline in BMD over time for patients receiving up to 8 years of continuous treatment with ODN 25a or 50 mg once weekly (group A) for a FAS population and b “completer” population (each time point corresponds to the same patients followed over time). aFourteen patients in group A received ODN 25 mg for 2 years before receiving ODN 50 mg (see Fig. 1). BMD bone mineral density, FAS full analysis set, ODN odanacatib, SE standard error
Bone turnover biomarkers
Approximately 50 % of participants were included in the PP analysis of the biochemical markers of bone turnover. Table 2 shows the geometric mean percent changes from baseline in these markers at year 8. At year 8, biomarkers of bone resorption were substantially inhibited, with uNTx/Cr and sCTx values reduced compared with baseline. There was no appreciable variation in uNTx/Cr or sCTx values between the groups who had received continuous ODN treatment for between 3 and 8 years. Levels of sTRAP 5b and s1CTP increased in all groups compared with baseline. There was no significant change in the bone formation markers sBSAP and sP1NP.
Safety and tolerability
All 117 patients who entered and received treatment in this 5-year extension were included in the extension safety analysis (APaT population). A summary of AEs is reported in Table 3. Overall, 114 (97.4 %) patients reported at least one AE, and 12 (10.3 %) patients had AEs that were thought by the local investigator to be related to treatment with ODN. There was no clinically important difference among the four treatment groups in the proportions of patients with a drug-related AE or who discontinued study participation because of an AE. Of the 31 (26.5 %) patients who recorded a serious AE, two experienced events that were thought by the local investigator to be related to treatment with ODN; one patient in group A discontinued her participation because of a basal cell carcinoma thought by the investigator to be drug related and a second patient in group A reported nausea which was also assessed by the investigator to be drug related but continued treatment. Two additional patients discontinued participation in the study because of serious AEs; one patient in group D was diagnosed with gastric cancer that was considered unrelated to study medication, and one patient in group B developed pancreatic cancer that was considered unrelated to study medication. This latter patient died during the study period (secondary to metastatic pancreatic carcinoma), 2 months after discontinuing the study.
Because of reports of adverse skin effects with another CatK inhibitor, balicatib [16, 17], skin-related AEs were evaluated with particular interest. In total, 39 (33.3 %) patients reported at least one dermatologic AE and 4 (3.4 %) patients experienced AEs that were considered by the local investigator to be related to ODN (Table 3); one patient in group A was reported to have basal cell carcinoma, one patient in group B was reported to have hyperkeratosis, and two patients in group C were reported to have rash (not otherwise characterized) and alopecia areata, respectively. No cases of morphea-like skin lesions were reported.
Also of particular interest, because of reports of adverse experiences with approved osteoporosis treatments or other CatK inhibitors, were any cases of osteonecrosis of the jaw (ONJ), serious respiratory AEs, or delayed fracture union events. Ultimately, there were no reports of ONJ and no episodes of delayed fracture union. The only serious respiratory AEs were a case of infectious exacerbation of chronic obstructive pulmonary disease and a pneumothorax resulting from a broken rib sustained in a fall. In addition, a diaphyseal fracture of the femur was reported as a serious AE in a patient with a history of fracture of the same femur 20 years prior.
Discussion
The results presented here demonstrate that extended treatment with ODN 25 or 50 mg once weekly for up to 8 years resulted in continuation or maintenance of increases in BMD at multiple sites compared with baseline. In those women who had received continuous ODN treatment for 8 years (group A “completer population”, wherein patients received ODN 25 or 50 mg for 2 years before receiving ODN 50 mg for 6 years), lumbar spine and trochanter BMD continued to increase progressively up to year 6, after which the rate of increase slowed or the increases were maintained up to year 8. At the femoral neck and total hip, BMD gains during the first 5 years of continuous ODN treatment were maintained for the following 3 years of continuous ODN treatment. Between years 6 and 8, 1/3 radius BMD remained stable at a level within 5 % below baseline. As there was no placebo group beyond 5 years, the changes in BMD at a particular site relative to untreated women cannot be determined. Results from the earlier phases of the study indicate a protective effect of ODN on 1/3 radius BMD; during the initial 2 years of the study, 1/3 radius BMD steadily decreased in the placebo group, whereas it was maintained in the ODN 25- and 50-mg groups [12]. Similarly, after 5-year continuous ODN treatment, 1/3 radius BMD was maintained near baseline or above in women treated continuously with ODN 50 mg compared with women who switched to placebo after 2 years of treatment with ODN 50 mg [14]. In comparison, using a high-resolution peripheral quantitative computed tomography technique, which measures volumetric BMD (vBMD) rather than areal BMD as in our study, and a different region of interest, a study in postmenopausal women who were not receiving bone-active drugs has shown site-specific changes at the distal radius, with a change in total density of −1.7 % within 1 year [18]. With this technique, alendronate has been shown to prevent decline of, and denosumab to increase, distal radius BMD in postmenopausal women over 1 year [19]. Another study, measuring areal BMD, found that alendronate reduced the decline of, and denosumab increased, distal radius BMD in postmenopausal women over 1 year [20]. In the case of ODN, vBMD assessment within a randomized, placebo-controlled, 2-year trial has shown a significant treatment difference from placebo (3.84 %) at the distal radius [21]. Significant differences from placebo were also found in trabecular vBMD, cortical vBMD, cortical thickness, cortical area, and strength (failure load), estimated using finite element analysis of HR-pQCT scans (treatment difference at radius, 2.64 %). At the distal radius, ODN significantly increased trabecular thickness and bone volume/total volume versus placebo. At a more proximal radial site, ODN attenuated the increase in cortical porosity found with placebo (treatment difference −7.7 %, p = 0.066). In the current study, improvements in BMD were generally greatest in those patients who had received continuous ODN 50 mg for the longest period of time. It is unknown whether the increases or maintenance of BMD with ODN are a consequence of increased mineralization, as observed in animal models of fracture healing [22], or secondary to modeling effects, with reduced remodeling coupled with increased modeling-based bone formation, as observed in ODN-treated animals [23]. One study in ODN-treated animals reported an increase in the bone formation rate at the periosteal level, thus likely a modeling process, while mineralization apposition rate was not modified at sites with decreased remodeling [9]. Conservation of modeling-based formation has also been seen in denosumab-treated animals [24].
The analysis of biochemical markers indicates that bone resorption is reduced by ODN and that this effect is sustained with long-term ODN treatment. The reductions from baseline in the bone resorption markers uNTx/Cr and sCTx were similar for each treatment group receiving continuous ODN for between 3 and 8 years. The bone formation markers sBSAP and sP1NP remained near baseline levels for all of the treatment groups. Previously published data from this study showed an initial decrease in sBSAP and sP1NP for the first 6 months of ODN treatment, with gradual increases thereafter back to baseline levels by the end of year 2 [12]. Another 2-year, double-blind, randomized study of ODN 50 mg or placebo once weekly in postmenopausal women with low BMD also found that sP1NP initially decreased during the first 6 months but did not differ from placebo by 2 years [25]. Together, these results indicate that bone formation is only moderately and transiently affected by ODN therapy. Levels of sTRAP 5b, an indicator of osteoclast cell number [26], were higher than baseline across the treatment groups. This is consistent with findings from preclinical studies suggesting that osteoclast numbers are unaffected or tend to increase with ODN [10, 27]. Since s1CTP is a substrate for CatK [15], it would be expected to accumulate in the setting of a CatK inhibitor. Indeed, levels of s1CTP were elevated, with the highest levels in the treatment groups exposed to continuous ODN for the longest period of time. Unlike the currently available anti-resorptive agents, bisphosphonates and denosumab, which substantially reduce both markers of bone resorption and bone formation, ODN appears to reduce the levels of bone formation markers only transiently and modestly. A possible explanation for this differential effect on bone resorption and formation is that although ODN inhibits CatK, thereby preventing the breakdown of bone collagen by osteoclasts, it does not reduce the number of osteoclasts, as do bisphosphonates and denosumab [28]. With ODN treatment, osteoclasts are still present; therefore, the signaling between the bone-resorbing osteoclasts and bone-forming osteoblasts in bone-remodeling units may be maintained, allowing the preservation of bone formation [29, 30]. Although bone remodeling at the tissue level is reduced by ODN, continued modeling could account for the maintenance of bone formation markers.
Overall, ODN appears to be well tolerated with prolonged use. Special attention was given to skin, respiratory, dental, and delayed fracture union events because of reports of related pathology with approved osteoporosis therapies or other CatK inhibitors previously in development [16, 17, 31, 32]. After up to 8 years of treatment with ODN, no cases of morphea-like skin reactions, ONJ, or delayed fracture union were reported in this study.
A limitation of this years 6 to 10 extension of the ODN phase 2b study is the low patient number remaining in each group and potential bias from patients who discontinued from the study over time. The lack of randomization (patients were carried over from their originally assigned treatment) and the open-label nature of this extension may cause additional bias. Although there was no placebo group, the study can be used to detect AEs that may emerge after long-term treatment. This study extension was performed in order to obtain information about long-term treatment effects of ODN, even though it was understood that the extension would be underpowered for hypothesis testing.
In conclusion, ODN 50 mg once weekly effectively increased BMD in women with osteoporosis and was well tolerated during long-term use. Furthermore, efficacy was maintained up to 8 years of treatment. Preliminary results of the phase 3 fracture outcome trial (Long-Term Odanacatib Fracture Trial [LOFT]), involving over 16,000 postmenopausal women with osteoporosis, have shown that ODN significantly reduces the risk of new morphometric vertebral, clinical hip, and clinical non-vertebral fractures compared with placebo [33].
References
NIH Consensus Development Panel (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57
Rizzoli R, Branco J, Brandi ML et al (2014) Management of osteoporosis of the oldest old. Osteoporos Int 25:2507–2529
Whitaker M, Guo J, Kehoe T, Benson G (2012) Bisphosphonates for osteoporosis—where do we go from here? N Engl J Med 366:2048–2051
Eli Lilly and Company (2012) FORTEO prescribing information. www.forteo.com. Accessed 26 Jan 2016
Gauthier JY, Chauret N, Cromlish W et al (2008) The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett 18:923–928
Cusick T, Chen CM, Pennypacker BL et al (2012) Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. J Bone Miner Res 27:524–537
Masarachia PJ, Pennypacker BL, Pickarski M et al (2012) Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. J Bone Miner Res 27:509–523
Stoch SA, Zajic S, Stone J et al (2009) Effect of the cathepsin K inhibitor odanacatib on bone resorption biomarkers in healthy postmenopausal women: two double-blind, randomized, placebo-controlled phase I studies. Clin Pharmacol Ther 86:175–182
Bone HG, McClung MR, Roux C et al (2010) Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res 25:937–947
Eisman JA, Bone HG, Hosking DJ et al (2011) Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 26:242–251
Langdahl B, Binkley N, Bone H et al (2012) Odanacatib in the treatment of postmenopausal women with low bone mineral density: five years of continued therapy in a phase 2 study. J Bone Miner Res 27:2251–2258
Sassi ML, Eriksen H, Risteli L et al (2000) Immunochemical characterization of assay for carboxyterminal telopeptide of human type I collagen: loss of antigenicity by treatment with cathepsin K. Bone 26:367–373
Rünger TM, Adami S, Benhamou CL et al (2012) Morphea-like skin reactions in patients treated with the cathepsin K inhibitor balicatib. J Am Acad Dermatol 66:e89–e96
Peroni A, Zini A, Braga V, Colato C, Adami S, Girolomoni G (2008) Drug-induced morphea: report of a case induced by balicatib and review of the literature. J Am Acad Dermatol 59:125–129
Kawalilak CE, Johnston JD, Olszynski WP, Kontulainen SA (2014) Characterizing microarchitectural changes at the distal radius and tibia in postmenopausal women using HR-pQCT. Osteoporos Int 25:2057–2066
Seeman E, Delmas PD, Hanley DA et al (2010) Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 25:1886–1894
McClung MR, Lewiecki EM, Cohen SB et al (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354:821–831
Cheung AM, Majumbdar S, Brixen K et al (2014) Effects of odanacatib on the radius and tibia of postmenopausal women: improvements in bone geometry, microarchitecture, and estimated bone strength. J Bone Miner Res 29:1786–1794
Pennypacker BL, Gilberto D, Gatto NT et al (2016) Odanacatib increases mineralized callus during fracture healing in a rabbit ulnar osteotomy model. J Orthop Res 34:72–80
Pennypacker BL, Chen CM, Zheng H et al (2014) Inhibition of cathepsin K increases modeling-based bone formation, and improves cortical dimension and strength in adult ovariectomized monkeys. J Bone Miner Res 29:1847–1858
Ominsky MS, Libanati C, Niu QT et al (2015) Sustained modeling-based bone formation during adulthood in cynomolgus monkeys may contribute to continuous BMD gains with denosumab. J Bone Miner Res 30:1280–1289
Brixen K, Chapurlat R, Cheung AM et al (2013) Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab 98:571–580
Halleen JM, Tiitinen SL, Ylipahkala H, Fagerlund KM, Vaananen HK (2006) Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin Lab 52:499–509
Pennypacker B, Shea M, Liu Q et al (2009) Bone density, strength, and formation in adult cathepsin K (−/−) mice. Bone 44:199–207
Baron R, Ferrari S, Russell RG (2011) Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48:677–692
Jensen PR, Andersen TL, Pennypacker BL, le Duong T, Delaissé JM (2014) The bone resorption inhibitors odanacatib and alendronate affect post-osteoclastic events differently in ovariectomized rabbits. Calcif Tissue Int 94:212–222
Sims NA, Ng KW (2014) Implications of osteoblast-osteoclast interactions in the management of osteoporosis by antiresorptive agents denosumab and odanacatib. Curr Osteoporos Rep 12:98–106
McClung M, Harris ST, Miller PD et al (2013) Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 126:13–20
Sutton EE, Riche DM (2012) Denosumab, a RANK ligand inhibitor, for postmenopausal women with osteoporosis. Ann Pharmacother 46:1000–1009
McClung MR, Langdahl B, Papapoulos S et al (2015) Odanacatib anti-fracture efficacy and safety in postmenopausal women with osteoporosis: results from the phase III Long-Term Odanacatib Fracture Trial (LOFT). IBMS BoneKEy 13:Article number:677. Abstract OC4.4
Acknowledgments
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ. Medical writing support was provided by Annette Smith, PhD, of Complete Medical Communications, funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ. Authors’ roles: study design CD, NV, and AL; study conduct CD, AL, and DG; data collection JH, IR, JARP, CD, RK, and DG; data analysis RR, PDM, RK, NV, AL, and DG; data interpretation RR, JH, PDM, CD, RK, NV, AL, and DG; statistical expertise NV and RK; revising manuscript content RR, C-LB, JH, PDM, IR, JARP, CD, RK, NV, AL, and DG; approved final version RR, C-LB, JH, PDM, IR, JARP, CD, RK, NV, AL, and DG; and RK, NV, AL, and DG take responsibility for the integrity of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
R. Rizzoli has received fees for board membership from Amgen, Danone, Merck, Servier, and Takeda and lecture fees from Amgen, Danone, GSK, Merck, and Servier. C.-L. Benhamou has received fees for board membership from Rottapharm, Pierre Fabre, Amgen, Novartis, and MSD and grants from Amgen and Servier. J. Halse has received a grant and travel expenses to an investigator meeting from MSD Norway AS for this study and consultancy fees from MSD Norway AS, Eli Lilly Norway AS, grants from Amgen AB Norway, and lecture fees from GSK Norway AS and Amgen AB Norway. P. D. Miller has participated in scientific advisory boards for Alexion, Amgen, AgNovos, Lilly, Merck, Radius Pharma, and Roche and in speaker bureaus for Alexion Pharmaceuticals, Amgen, and Radius Health. He has received research grants from Alexion, Amgen, Boehringer Ingelheim, Immunodiagnostics, Lilly, Merck, Merck Serono, NBHA, Novartis, Novo Nordisk, Radius Pharma, Roche Diagnostics, and Takeda. I.R. Reid has received fees from Merck for consultancy, lectures, and board membership, and his institution has received a grant from Merck for this study. J. A. Rodríguez Portales has received travel expenses to attend an investigator meeting, and his institution has received grants from Merck for this and other studies. C. DaSilva, N. Verbruggen, and D. Gurner are employees of and hold stock/stock options in Merck & Co., Inc. R. Kroon was an employee of MSD at the time of the study. A.T. Leung was an employee of Merck & Co., Inc. at the time of the study and currently holds stocks of the company.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material
(PDF 975 kb)
Rights and permissions
About this article
Cite this article
Rizzoli, R., Benhamou, CL., Halse, J. et al. Continuous treatment with odanacatib for up to 8 years in postmenopausal women with low bone mineral density: a phase 2 study. Osteoporos Int 27, 2099–2107 (2016). https://doi.org/10.1007/s00198-016-3503-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3503-0