Abstract
Summary
Osteoporosis (weak bones) is a disorder that has high morbidity, mortality, and healthcare utilization. Effective treatment is available for this disorder, but many patients choose not to start therapy. This is the first study showing an intervention that increases the initiation rates to medications for osteoporosis.
Introduction
One out of six patients prescribed an oral bisphosphonate does not initiate therapy, a phenomenon known as primary non-adherence. Reasons for bisphosphonate primary non-adherence have been identified, but not interventions that positively impact primary adherence rates. The purpose of this study is to determine the effectiveness of interactive voice response technology to improve oral bisphosphonate primary adherence.
Methods
This was a prospective, randomized controlled trial conducted in January–December 2014 at Kaiser Permanente Colorado, an integrated healthcare system. Adults with a new oral bisphosphonate prescription for osteoporosis or osteopenia which was not purchased within 14–20 days of being ordered were included. There were 127 and 118 patients in the intervention group and control groups, respectively. The intervention group received an interactive voice response phone call followed by a letter 1 week later if primary non-adherence continued, whereas the control group did not receive any outreach. The primary outcome was the proportion of patients who purchased their oral bisphosphonate within 25 days of randomization.
Results
There were 62/127 (48.8 %) intervention patients and 36/118 (30.5 %) control patients who purchased their bisphosphonate prescription within 25 days of randomization (OR = 2.17, 95 % CI 1.29–3.67). When adjusted for age, sex, history of bone mineral density scan and fracture, the odds ratio for intervention versus control group was 2.3 (95 % CI 1.34–3.94).
Conclusion
An interactive voice response phone call and follow-up letter significantly improved primary adherence to oral bisphosphonate therapy. Such an intervention could be considered for improving primary adherence rates to other medication classes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A total of 54 million Americans aged 50 and older have osteoporosis or osteopenia. The National Osteoporosis Foundation reports that, in the USA, osteoporosis is responsible for 2 million fractures and $19 billion in related costs each year. Due to the growing elderly population, those numbers are expected to increase to 3 million fractures and $25.3 billion each year by 2025 [1]. Bisphosphonates, the preferred treatment in most patients with osteoporosis, reduce fracture risk by 50 % [2], yet they are widely underutilized [3], leading to higher fracture rates, resource utilization, and costs [4].
A key contributor to bisphosphonate underutilization is non-adherence, both primary and secondary. Secondary non-adherence (SNA), which is well-described in the literature, is failure to consistently refill a medication after the first fill, while primary non-adherence (PNA) is defined as failure to purchase a newly prescribed medication.
The utilization of electronic prescribing has facilitated the study of PNA by making it possible to systematically identify medications that are prescribed but never purchased. Bisphosphonate PNA has been reported to be as high as 37.5 % [5]. Several studies have examined patient-reported concerns that contribute to bisphosphonate PNA, which include belief in medication effectiveness, distrust of medications, lack of knowledge of the disease state, fear of side effects and risks, lack of awareness of the prescription, and concern with the number of medications a patient is taking [4, 6]. Interventions that address these concerns and improve adherence will play a key role in improving clinical and cost-related outcomes.
Studies of interventions that impact bisphosphonate PNA are lacking. We hypothesize that an intervention aimed at addressing patient concerns using an interactive voice response (IVR) system, follow-up letter, and clinical pharmacy specialists will improve PNA in patients newly prescribed an oral bisphosphonate.
Methods
Study design and setting
This prospective, randomized controlled trial was conducted at Kaiser Permanente Colorado (KPCO), an integrated health plan that serves more than 600,000 members at 28 medical office buildings. Members’ prescriptions are typically filled internally at Kaiser Permanente pharmacies. The study was conducted from January through December 2014 and was approved by the KPCO institutional review board.
Study participants
Adults, aged 18 years and older, with a new oral bisphosphonate prescription for osteoporosis or osteopenia, which was not purchased within 14–20 days of being ordered, were included. A prescription was defined as “new” if the patient had not received an oral or intravenous bisphosphonate within the previous 12 months. Patients with prescriptions sent to a non-KPCO pharmacy, residing in a skilled nursing facility, or without continuous KPCO membership 12 months before and 25 days after enrollment were excluded.
Patients were identified administratively via KPCO electronic pharmacy and membership databases, and inclusion and exclusion criteria were verified manually via chart review.
Intervention
Intervention patients were contacted on the day of enrollment by an automated interactive voice response (IVR) phone call. If no one answered, a voicemail message instructed the patient to contact a toll-free number to retrieve the message. The phone call focused on known reasons why patients do not initiate therapy (Online supplement – IVR Phone Call Script). It highlighted the benefits and risks of bisphosphonate therapy, including an option to hear additional medication information that explained and quantified the benefits and risks in more detail. Patients could choose to be transferred to the mail order pharmacy to fill the prescription and could also indicate if the prescription had already been purchased at a non-KPCO pharmacy by pressing a number on their phone. The IVR phone call was scripted in English, and the average phone call duration was 145 s for patients who opted to listen to the optional additional medication information, and 109 s if that portion was not included.
If the medication was still not purchased 7 days after receiving the IVR phone call, then a letter reminding the patient of their prescription and explaining benefits and risks of bisphosphonate therapy was mailed (Online Supplement – Letter). Based on delivery of test letters to study investigators, the expected arrival date was 4 days after being placed in the mail room at the ambulatory clinic. The letter was written in English or Spanish, and the version corresponding to the patient’s preferred language documented within the electronic medical record was sent. Patients were followed for 14 days after expected letter arrival to determine if the prescription was purchased.
Usual care patients (control group) did not receive the phone call or letter.
For patients purchasing at least one prescription and with at least 6 months continuous membership following enrollment, secondary adherence was measured by calculating the medication possession ratio (MPR) and reporting it as both a continuous variable and categorized as greater than or equal to 0.8 versus less than 0.8. The MPR was calculated by dividing the total medication days’ supply purchased within 180 days of the initial prescription purchase date by 180 days, “trimming the end” of purchases made near the end of the 180-day period when indicated to avoid overestimating adherence. For example, if a 60-day supply were purchased on day 170 of the 180-day observation period, only 10 days supply from this purchase were counted instead of the full 60.
Enrollment and randomization
Enrollment occurred every Monday according to the diagram depicted in Fig. 1. For example, patients with prescriptions written between Monday, January 6th and Sunday, January 12th, but not purchased by Sunday, January 26th, were categorized as primary non-adherent. These patients were randomized to intervention or control groups and enrolled on Monday, January 27th.
Patients were randomized 1:1 via random number generator into the intervention or usual care group.
Study outcomes
The primary outcome was the proportion of patients who purchased their prescription within 25 days of study enrollment. The 25 days was determined as follows: enrollment and IVR phone call made on day 0; letter sent on day 7, if indicated; letter to be delivered by day 11; and up to 14 days follow-up after receiving the letter, which was day 25. Secondary outcomes were (1) the proportion of intervention patients meeting the primary outcome who purchased their prescription before and after the expected letter arrival date, (2) identifying factors other than the intervention that were independently associated with primary adherence, and (3) the MPR for 6 months in patients in both groups who met the primary outcome.
Sample size calculation
The usual care group was expected to have a 74 % rate of primary non-adherence, based upon rates reported in the literature [7]. To detect an absolute improvement of 15 % with 80 % power and significance level of 0.05, each group needed 121 patients.
Statistical analysis
Primary and secondary outcomes data pertaining to primary adherence were analyzed based on intention-to-treat analysis using the chi-squared test, regardless of whether patients were successfully contacted. Mean MPR data was analyzed using the Student’s t test, and the proportion of patients with an MPR >80 % was analyzed using the chi-squared test. A p value <0.05 indicated statistical significance.
Logistic regression analysis was conducted to control for potentially confounding effects of age, sex, history of BMD test in the past year, and history of fracture status, and to identify whether any of these were independently associated with primary medication adherence. Odds ratios and adjusted odds ratios were calculated using PROC LOGISTIC. Statistical analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC).
Results
As shown in Fig. 2, a total of 1338 patients were identified as meeting screening criteria, 1092 patients were excluded, and 1 patient was lost to follow-up due to death. Of the 245 remaining patients included in the statistical analysis, there were 127 and 118 patients randomized to the intervention and control groups, respectively. The demographic and baseline characteristics of the two groups were similar (Table 1). Across both groups, the mean age was 71.5 years and 93 % of patients were female. Alendronate was the most commonly prescribed bisphosphonate, and 86 % of all study patients had a diagnosis of osteoporosis.
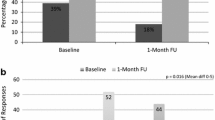
The percent of patients who purchased their prescription within 25 days of study enrollment was significantly greater in the intervention group compared to the control group (62/127, 48.8 % vs. 36/118, 30.5 %, unadjusted OR = 2.17, 95 % CI = 1.29–3.67). After adjusting for age, sex, history of bone mineral density (BMD) test, and history of a fracture, the OR for primary adherence in the intervention versus control group was 2.30 (95 % CI 1.34–3.94). Figure 3 displays the percent of patients who became primary adherent from day 0 through 25 after the IVR phone call was made to intervention patients.
Of the intervention patients with primary adherence to bisphosphonate therapy (n = 62), the majority purchased the bisphosphonate before the letter arrival date (11 days after randomization) compared to after (76 vs. 24 %, respectively). The control group showed a similar pattern with 64 % purchasing their prescription before day 11 versus 36 % after (p = 0.208 for difference between the two groups). None of the letters that were sent to intervention patients were returned to KPCO for unsuccessful delivery. Neither sex nor age or history of a BMD test were significantly associated with primary adherence, but patients with a history of fracture were significantly more likely to have primary adherence to bisphosphonate therapy (OR = 2.21, 95 % CI 1.11–4.40). Indeed, 27/50 (54 %) patients in both groups with a history of fracture had primary adherence, compared to 71/195 (36 %) of those with no prior fracture (p = 0.024).
The mean MPR for intervention patients was 69 % (95 % CI 61–77) versus 60 % (95 % CI 49–71) in control patients (p < 0.001). The percent of patients in the intervention and control groups who had a MPR ≥80 % was 43.6 and 41.7 %, respectively (p = 0.856).
Discussion
Given the protection bisphosphonates confer against the devastating outcomes of osteoporotic fractures, it is imperative to find an intervention that improves adherence. Efforts have traditionally focused on improving secondary adherence, which misses patients who are prescribed a bisphosphonate but never start it. To our knowledge, this is the first study of an intervention that significantly improved primary adherence rates to oral bisphosphonate therapy, which is consistent with our hypothesis. It is not clear whether the improvement is attributable to the intervention’s function as a reminder that a prescription was ordered or whether the knowledge gained about the benefits and risks of bisphosphonate therapy prompted patients to purchase their prescription, but it is likely both these elements contributed to the intervention’s success.
The intervention utilized in this study is feasible and easily reproducible for other medication classes and has the potential to reach large patient populations using minimal resources. Our results, with 48.8 and 30.5 % of intervention and control patients purchasing their new bisphosphonate during the study period, were comparable to those reported with a similar intervention in patients with primary non-adherence to statin therapy, where 42.3 % of intervention patients purchased their new statin prescription compared to 26.0 % in the control group [7].
Although we did not reach 121 patients per group according to the power calculation, the primary outcome was still statistically significant so this did not interfere with our results.
Our study did not focus on an intervention to improve secondary adherence, but the mean MPR for intervention patients was still statistically significantly better. This is promising considering that 40 to 74 % of patients who initiate bisphosphonate therapy discontinue it within the first year [8, 9]. However, data suggests an MPR ≥80 % is needed to provide adequate fracture protection benefits [10], and our study did not show a difference in the proportion of patients reaching this threshold. Combining interventions proven to successfully improve bisphosphonate secondary adherence [11–15] with the IVR phone call may be a useful strategy.
A strength of our study was the logistical regression analysis that ruled out potential confounders (age, sex, BMD test, and history of fracture) that may affect primary adherence rates. It is not surprising that patients in both the intervention and control arms who experienced a previous fracture were more likely to purchase their bisphosphonate compared to patients who did not have a history of fracture.
One of the challenges with studying primary non-adherence is lack of standardized definition that includes a timeframe after which a patient is considered primary non-adherent. We chose the range of 14–20 days from the date of prescription because previous studies have demonstrated that up to 95 % of patients fill their medication within the first 2 weeks of being prescribed [16, 17].
A limitation to our study is that the IVR phone call was only available in English. We attempted to address this by supplying a Spanish version of the follow-up letter when Spanish was documented as the preferred language in the medical record. However, we identified no patients who required the Spanish letter. Incomplete documentation of language preference or the fact that Hispanic population has a lower risk of osteoporosis [18] may have been factors in this observation.
An IVR phone call plus a follow-up letter represents a practical intervention to improve primary adherence to bisphosphonates effectively and efficiently while also positively impacting secondary adherence. Application of this process may be useful to improve primary adherence to other medications, especially those for chronic disease states.
References
National Osteoporosis Foundation (2013) What is osteoporosis? http://www.nof.org/articles/7. Accessed 9 Sep 2013
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348(9041):1535–1541
Andrade SE, Majumdar SR, Chan KA, Buist DS, Go AS, Goodman M, Smith DH, Platt R, Gurwitz JH (2003) Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med 163:2052–2057
Yood RA, Mazar KM, Andrade SE, Emani S, Chan W, Kahler KH (2008) Patient decision to initiate therapy for osteoporosis: the influence of knowledge and beliefs. J Gen Intern Med 23(11):1815–1821
Gadkari AS, McHorney CA (2010) Medication nonfulfillment rates and reasons: narrative systematic review. Curr Med Res Opin 26:683–705
Hogan KN, Milchak JL, Heilmann RMF, Billups SJ, Delate T (2013) Evaluation of primary nonadherence to oral bisphosphonate therapy. J Am Geriatr Soc 61(11):2046–2047
Derose SF, Green K, Marrett E, Tunceli K, Cheetham TC, Chiu VY, Harrison TN, Reynolds K, Vansomphone SS, Scott RD (2013) Automated outreach to increase primary adherence to cholesterol-lowering medications. JAMA Intern Med 173(1):38–43
Sale JE, Gignac MA, Hawker G, Frankel L, Beaton D, Bogoch E, Elliot-Gibson V (2011) Decision to take osteoporosis medication in patients who have had a fracture and are ‘high’ risk for future fracture: a qualitative study. BMC Musculoskelet Disord 12:92
Schousboe JT, Dowd BE, Davison ML, Kane RL (2010) Association of medication attitudes with non-persistence and non-compliance with medication to prevent fractures. Osteoporos Int 21:1899–1909
Penning-van Beest FJA, Erkens JA, Olson M, Herings RMC (2008) Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int 19:511–517
Fenerty SD, West C, Davis SA, Kaplan SG, Feldman SR (2012) The effect of reminder systems on patients’ adherence to treatment. Patient Prefer Adherence 6:127–135
Delmas PD, Vrijens B, Eastell R, Roux C, Pols HA, Ringe JD, Grauer A, Cahall D, Watts NB, Improving Measurements of Persistence on Actonel Treatment (IMPACT) Investigators (2007) Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 92(4):1296–1304
Clowes JA, Peel NF, Eastell R (2004) The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 89(3):1117–1123
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21(9):1453–1460
Recker RR, Gallagher R, MacCosbe PE (2005) Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc 80(7):856–861
Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV (2009) New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Serv Res 44(5):1640–1661
Liberman JN, Hutchins DS, Popiel RG, Patel MH, Jan SA, Berger JE (2010) Determinants of primary nonadherence in asthma-controller and dyslipidemia pharmacotherapy. Am J Pharm Benefits 2(2):111–118
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29(11):2520–2526
Acknowledgments
This study was funded by Kaiser Permanente Colorado. The institution had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 144 kb)
Rights and permissions
About this article
Cite this article
Cizmic, A.D., Heilmann, R.M.F., Milchak, J.L. et al. Impact of interactive voice response technology on primary adherence to bisphosphonate therapy: a randomized controlled trial. Osteoporos Int 26, 2131–2136 (2015). https://doi.org/10.1007/s00198-015-3116-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3116-z