Abstract
Summary
This study was designed to compare the effects of alendronate (ALN), strontium ranelate (SR), and zoledronic acid (ZOL) on bone-implant osseointegration in ovariectomized rats. Histological examination and biomechanical tests show that ZOL, ALN, and SR enhance bone-implant osseointegration; ALN and SR have similar effects, while ZOL enhances bone-implant osseointegration more than ALN and SR
Introduction
This study aims to compare the effects of ALN, SR, and ZOL on bone-implant osseointegration in ovariectomized rats.
Methods
Sixty female Sprague–Dawley rats were included in this study. Of them, 48 rats were ovariectomized (OVX) and assigned to four groups: OVX (OVX + Veh), ALN (OVX + ALN), SR (OVX + SR), and ZOL (OVX + ZOL). And another 12 rats were sham-operated as a control group (Sham). Four weeks after ovariectomy, HA-coated titanium implants were inserted into the tibias bilaterally in all rats. Then the rats in groups ALN, SR, and ZOL were systemically administrated with alendronate (7 mg/kg/week, orally), strontium ranelate (500 mg/kg/day, orally), or a single injection of zoledronic acid (0.1 mg/kg, iv), respectively. Twelve weeks after implantation, all rats were sacrificed to get the femurs and tibias. Histological examination and biomechanical tests were used to evaluate bone-implant osseointegration in all groups.
Results
ALN, SR, and ZOL significantly increased distal femoral BMD when compared with group OVX; ZOL increased BMD significantly more than ALN and SR (P < 0.05). Significant increase of bone-to-implant contact and peri-implant bone fraction were observed in groups ALN, SR, and ZOL when compared with group OVX (P < 0.05). Groups ALN and SR were inferior to groups ZOL and Sham (P < 0.05) in bone-to-implant contact and peri-implant bone fraction. Similar results were found in biomechanical testing (max pushout force).
Conclusions
In rats losing bone rapidly after ovariectomy, systemic administration of ZOL, ALN, and SR causes better bone-implant osseointegration when compared to OVX; ALN and SR have similar positive effects on osseointegration, while ZOL, that was given in a dose with more positive BMD effect than that of ALN or SR, causes better osseointegration than either ALN or SR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postmenopausal osteoporosis is a disease of abnormal bone metabolism induced by estrogen deficiency and characterized by low bone mass and microarchitectural deterioration [1]. Although same clinical studies suggest that osteoporosis is not a contraindication for implant placement [2, 3], animal experiments have shown that bone-loss condition affects osseointegration of implant [4, 5]. Anti-osteoporosis drugs have been proved to promoting osseointegration and implant fixation, including bisphosphonate agents [6–8], calcitonin [8], estrogen [9], strontium ranelate [10], and parathyroid hormone [11].
Alendronate and zoledronic acid are nitrogen-containing bisphosphonate agents, that suppress farnesyl diphosphate synthase, and subsequently inhibit bone resorption and bone remodeling activity [12]. Alendronate is an excellent drug which is used most widely clinically; nearly 20 years of experiences have accumulated plenty of clinical evidences in treating osteoporosis [13, 14]. A few preclinical studies have been conducted to prove the effectiveness of alendronate on bone-implant osseointegration and implant fixation after surgery [6, 15]. Our previous study has also proved that systemic administration of alendronate could enhance bone-implant osseointegration in ovariectomized rats [16]. Zoledronic acid is also an agent of bisphosphonate. With a single dose of injection, it could significantly increase bone mineral density and biomechanical properties of the bone [17, 18]. Clinical studies have showed pronounced efficacy of yearly 5-mg infusions of zoledronic acid in improving osteoporosis and decreasing hip, vertebral, and nonvertebral fractures [19, 20]. Preclinical studies have also demonstrated the efficacy of zoledronic acid on bone-implant osseointegration and implant fixation in animal models of osteoporosis [7, 21]. Furthermore, some clinical studies have also suggested potential benefits of bisphosphonates (including alendronate and zoledronic acid) treatment on implant initial fixation and long-term survival after total arthroplasty of the knee or hip [22–24].
Strontium ranelate is another drug to treat osteoporosis. A lot of clinical studies have demonstrated its efficacy in increasing BMD and decreasing vertebral and hip fractures [25–28]. At present, there are still controversies on the mechanism of strontium ranelate in treating osteoporosis. Some authors attribute the main effect of strontium ranelate to the incorporation of strontium into the bone [29]. However, more authors hold that it has a dual action in preventing osteoporosis: it can stimulate bone formation through enhancing preosteoblastic cells replication and increasing collagen and noncollagenic proteins synthesis by mature osteoblast-enriched cells in vitro [30, 31]. Meanwhile, it can also inhibit bone resorption due to direct and/or matrix-mediated inhibition of osteoclast activity [31]. Some animal experiments have also proved the efficacy of strontium ranelate on bone-implant osseointegration and implant fixation [10, 32].
It seems that alendronate, zoledronic acid, and strontium ranelate enhance bone-implant osseointegration in osteoporotic bone, but concerns still exist on which one is more effective. ZOL is an intrinsically more potent agent than ALN in inhibiting bone resorption [33], meaning that less of it is needed to achieve the same effect as ALN. In customary dose, zoledronic acid might be stronger than alendronate in promoting bone-implant osseointegration. While strontium ranelate may have dual activities (increasing bone formation and decreasing bone resorption) to prevent osteoporosis, it should be more effective than alendronate to enhance bone-implant osseointegration. However, there is still no direct comparative study on these three drugs. Our current study was designed to compare the effects of these three drugs on bone-implant osseointegration in bone loss rats.
Methods
Animals
Sixty 3-month-old female Sprague–Dawley rats (obtained from Medical Experimental Animal Center of Guangdong Province, weighing 229.4 ± 11.5 g) were used in this study. Animals were housed and acclimatized at a standard laboratory diet and tap water, under climate-controlled conditions (25 °C, 55 % humidity, 12-h light/12-h darkness). This study was approved by the Animal Care Committee of Sun Yat-Sen University (NO. IACUC-2010-1001) and was in compliance with the guiding principles in the Guide for the Care and Use of Laboratory Animals [34].
Implants
The implants, which were the same to our previous study [35], were supplied by Engineering Research Center in Biomaterials of Sichuan University. It was a cylinder titanium rod with 1 mm in diameter and 10 mm in length and was plasma-sprayed with hydroxyapatite (HA, 100 μm in thickness) under a Metco MN Plasma System and an AR-2000 Thermal Spray Robot (Metco, USA).
Surgical procedure
After 7 days of acclimatization, 48 rats were underwent bilateral ovariectomization (OVX), and the surgical procedure was reported in our previous study [35]. Another 12 rats underwent sham operation as a control group (Sham).
Four weeks after ovariectomization, HA-coated titanium implants were inserted into proximal tibiae bilaterally, and the surgical procedure was also reported in our published paper [35]. Briefly, under general anesthesia by 10 % chloral hydrate, an incision to expose the knee was made, and a channel (about 1.5 mm in diameter) was created by an electric drill (1 mm in diameter) from the intercondylar eminence to the medullary canal. The channel was about 15 mm in depth, and the implant was then inserted into this channel.
Treatment
After implantation, the ovariectomized rats were randomly assigned into four groups (12 rats in each group): OVX, ALN, SR, and ZOL. One week after implantation, the rats in group ALN were administrated with alendronate sodium 7 mg/kg/week orally; the rats in group SR were administrated with strontium ranelate 500 mg/kg/day orally; while the rats in group ZOL received a single dose of zoledronic acid (0.1 mg/kg, iv). The rats in group Sham did not receive any drug treatments. The observation time was 12 weeks. Twelve weeks after implantation, all rats were sacrificed to get the femurs and tibias.
Bone mineral density
The BMD of the distal femur was determined by Dual Energy X-ray Absorptiometry (DXA, GE LUNAR-Prodigy), with the regional high resolution and small-animal scan mode. And the measurement precision of this DXA technique was ±2.0 %. The region of interest to be examined was at the metaphysis of the femur (0.5 mm × 0.5 mm), which was 0.5 mm close to the epiphyseal plate.
Histological examination
The right tibia of each rat was taken to perform histological examination. The method was similar to our previous study [35]. First, the proximal 1/2 of the tibiae with implants were fixed in 4 % paraformaldehyde for 24 h and dehydrated with increasing concentrations of alcohol (70, 95, and 100 %, 2 days each concentrate). Then the specimens were embedded in methyl methacrylate without decalcification. Transverse cutting of 70-μm sections were performed at the proximal 1/2 of the implant (at metaphysis of the tibia) by using a SP1600 microtome (Leica Microsystems, Wetzlar, Germany); three sections were cut in each tibia. Histomorphometric analysis was performed with a semiautomated digitizing image analyzer system, consisting of an OLYMPUS BX51 microscope, a computer-coupled QImaging Retiga EXi Camera and BioQuant Osteo 2009 software. Bone-to-implant contact was calculated as a length percentage of the direct bone-implant interface to total implant surface; and peri-implant bone fraction was defined as the area percentage of the bone within a circle of 100 μm around the implant.
This histological examination was finished at The Center for New Drug Function Research, School of Life Science and Biopharmacology in Guangdong Pharmaceutical University. The tester of these two parameters was an engineer in this laboratory, and we did not tell him the information of grouping and treatment. Three sections from each implant were evaluated.
Biomechanical testing
The left tibia of each rat was taken to perform biomechanical testing, which has been also described in our previous experiment [35]. In brief, a quadrate mold with a specially designed cavity was made by polymethyl methacrylate. A rongeur was used to remove the bone at the proximal and distal of the tibia, so as to expose the proximal 2 mm and the distal surface of the implant, and then the tibia was placed in the cavity with the exposed implant upward. Then polymethyl methacrylate was used again to fix the tibia. The quadrate molds with tibiae were then fixed on a commercial material testing system (MTS 858 bionix II machine, MTS System Inc., Minneapolis, MN, USA). Compression loads were applied vertically to the proximal end of the implant at a speed of 5 mm/min, and the force and displacement data were recorded at 100 Hz. Maximum pushout force was calculated.
The biomechanical test was performed at the Orthopedic Research Center of the First Affiliated Hospital of Sun Yat-sen University. An engineer in this center helped us to finish the biomechanical test; he did not know the grouping information and treatment of the specimen. Each specimen was chosen randomly to the test machine.
Statistical analysis
All results were recorded as mean ± SD, and statistical analyses were performed using the statistics package SPSS 11.0 (SPSS, Chicago, IL, USA). One-way ANOVA was conducted to evaluate the difference among groups, and LSD method was applied for multiple comparisons. All statistical analysis was considered significant when P < 0.05.
Results
Two rats died in groups OVX and ALN in 1 week after implantation, and these two rats were excluded in statistical analysis. One tibia in group ALN was lost during histological examination. Biomechanical test failed in one tibia in group ZOL, and these data were invalid. Thus, these two rats were also excluded in statistical analysis.
Bone mineral density
The BMD of groups ZOL, ALN, and SR significantly increased when compared with that of group OVX (P < 0.05), and mean increases of percentage were 22.8, 11.4, and 12.4 %, respectively. ZOL increased BMD significantly more than ALN and SR (P < 0.05). The results of BMD were showed in Table 1.
Histological examination
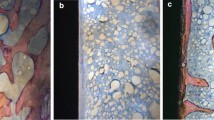
Significant increase of bone-to-implant contact was observed in groups ALN, SR, and ZOL when compared with group OVX (1.6-fold, 1.7-fold, and 2.1-fold of OVX, respectively). The bone-to-implant contact rate of group SR was higher than that of ALN, but there was no significant difference (P > 0.05). The bone-to-implant contact of groups ALN and SR were significantly inferior to that of groups ZOL and Sham (P < 0.05). Similar results were found in peri-implant bone fraction (Table 1 and Fig. 1).
Biomechanical testing
The maximum pushout force of groups ALN, SR, and ZOL was increased by16.7, 23.3, and 59.4 % when compared with group OVX, respectively (P < 0.05). The maximum pushout force of group ZOLs and Sham was significantly higher than that of groups ALN and SR; no significant difference was found between groups ALN and SR (Table 1).
Discussion
In this study, we confirmed the results of previous studies that zoledronic acid, alendronate, and strontium ranelate caused better bone-implant osseointegration in ovariectomized rats (Fig. 1). As it showed, the bone-to-implant contact of groups ALN, SR, and ZOL were 1.6-fold, 1.7-fold, and 2.1-fold of OVX. Similar results were observed in biomechanical testing, as the max pushout force of groups ALN, SR, and ZOL was greater by16.7, 23.3, and 59.4 %, respectively, when compared with that of group OVX.
Many animal experiments have been conducted to evaluate the effect of systemic administration of alendronate [6, 8, 15, 16], zoledronic acid [7, 21, 36], and strontium ranelate [10, 32] on bone-implant osseointegration in bone loss in animals. In a recent study [16], we established osteoporosis in rat models by ovariectomy, and implants were inserted into the proximal tibias, and then the rats were treated with alendronate orally every week for 12 weeks; histomorphometry results showed that alendronate improved bone-implant osseointegration rate of hydroxyapatite implants when compared with OVX control group. In another study [35], we also confirmed the efficacy of alendronate on bone-implant osseointegration in ovariectomized rats. Similar results were also reported by other researchers [6, 8, 15]. Except for alendronate, studies have also demonstrated the efficacy of strontium ranelate in enhancing bone-implant osseointegration. In the study of Maïmoun et al. [10], they inserted titanium implants into the proximal tibias of rats and then treated the animals with strontium ranelate orally 5 days a week (625 mg/kg/day). Eight weeks after implantation, micro-CT and biomechanical testing indicated that strontium ranelate significantly improved bone-to-implant contact, pullout strength, and bone microarchitecture around the implant. Similarly, Li et al. [32] also found that systemic treatment with strontium ranelate could dose-dependently improve HA-coated screw fixation in OVX rats and facilitate the stability of the implant in the osteoporotic bone. However, in a recent study, Linderbäck et al. [37] reported no significant improvements of pullout force, and the microarchitecture of the cancellous bone was observed around the implants after treatment with strontium ranelate. Zoledronic acid is another bisphosphonate agent to treat osteoporosis. Recently, it becomes to be a considerable interest in investigations looking to improve implant osseointegration. In a study of Carvas et al. [21], glucocorticoid-induced osteoporosis models were made, and implants were inserted into the tibias, and then the rats received a single-dose injection of zoledronic acid (0.1 mg/kg, iv). They demonstrated that a single dose of zoledronic acid could reverse the detrimental effects of glucocorticoids on the bone-implant osseointegration of titanium implants. Similar results were also reported by Yildiz et al. [36]; in their experiment, rabbits were ovariectomized to induce bone-loss models and then treated with a single injection of zoledronic acid. As a result, histomorphometric, resonance frequency, and radiodensitometric analyses showed significant improvement in osseointegration of implants in the zoledronic acid-treated group when compared with OVX control group. Thus, it can be seen that our experiment and other studies demonstrated that alendronate, strontium ranelate, and zoledronic acid improved bone-implant osseointegration in ovariectomized rats.
Currently, few studies have been performed to compare the effect of alendronate, strontium ranelate, and zoledronic acid on bone-implant osseointegration. Our results indicated that alendronate was comparable to strontium ranelate in promoting bone-implant osseointegration in ovariectomized rats (Fig. 1), which was different from our initial speculation. No significant differences were observed between groups ALN and SR in bone-to-implant contact, peri-implant bone fraction, and max pushout force. Some studies have been designed to compare the effect of alendronate and strontium ranelate on bone-implant osseointegration. In the study of Maïmoun et al. [10], titanium rods were implanted into the proximal tibias of 6-month-old female rats, and then animals were treated with alendronate or strontium ranelate. Their results showed that both alendronate and strontium ranelate induced a similar pullout force, but only strontium ranelate significantly improved bone formation and the intrinsic bone material quality around the implant. However, in the study of Linderbäck et al. [37], they found no significant improvements of pullout force in strontium ranelate-treated rats, but animals treated with alendronate showed a doubled pullout force. They concluded that strontium ranelate was weaker than alendronate in enhancing bone-implant osseointegration and implant fixation. Nevertheless, no osteoporosis models were created in their experiments, and more studies are needed to compare their effect in bone loss or osteoporosis condition. We are the first to compare the effects of alendronate and strontium ranelate on bone-implant osseointegration in bone-loss animals (ovariectomized rats) and found no significant differences between them.
Alendronate is a bisphosphonate agent, which mainly inhibits bone resorption and bone turnover rate and subsequently results in an increase in bone formation and bone mineral density [38, 39]; while strontium ranelate is considered to be a dual-acting agent (although controversies are still existed) with both anti-resorptive and anabolic benefits on the skeleton [40]. However, our present study failed to show significant difference in increasing bone-implant osseointegration between them. The pharmacological properties of strontium ranelate may be of great help for us to understand it [31]. Although strontium ranelate inhibits bone resorption [41], the inhibitory effect is relatively low, which is close to those of salmon calcitonin [31]. It has been proved that the inhibitory effects of alendronate are significantly stronger than salmon calcitonin [42]. Furthermore, postmenopausal osteoporosis is a disease characterized by a significant increase in bone resorption due to estrogen deficiency; with a stronger activity of anti-resorption than strontium ranelate, alendronate could compensate for its insufficient ability in promoting bone formation. And this may be the causes why strontium ranelate was comparable to alendronate in enhancing bone-implant osseointegration.
Our present study also revealed that zoledronic acid enhanced bone-implant osseointegration more than alendronate and strontium ranelate in ovariectomized rats (Fig. 1). The bone-to-implant contact of group ZOL was 1.3-fold and 1.2-fold that of groups ALN and SR, respectively (P < 0.05). Currently, no study was reported to compare zoledronic acid with other drugs in enhancing bone-implant osseointegration, so we are the first to perform similar experiment. Some studies have been conducted to compare the anti-osteoporosis effect of zoledronic acid with alendronate, and this may be of assistance for us to understand why zoledronic acid appeared stronger than alendronate and strontium ranelate in promoting bone-implant osseointegration. Of these studies, most of them conclude that zoledronic acid was superior to alendronate in the prevention of osteoporosis. In a study of Gasser et al. [17], they treated the female Wistar rats with a single dose of zoledronic acid (0.8, 4, 20, 100, or 500 μg/kg) or alendronate (200 μg/kg) prior to bilateral ovariectomy. Their results showed a tenfold higher potency of zoledronic acid than alendronate in preventing ovariectomy-induced bone loss. Hadji et al. [43] compared the effect of a once-yearly intravenous dose of zoledronic acid with a once-weekly oral dose of alendronate in postmenopausal women. In their study, serum levels of N-telopeptide of collagen type I (NTx) and procollagen 1 C-terminal extension peptide (P1NP) were assessed. Twelve months after treatment, zoledronic acid exhibited a greater and faster reduction of serum bone turnover marks (NTx and P1NP) than alendronate. Saag et al. [44] also reported that zoledronic acid reduced bone resorption markers (urine NTx and serum β-C-telopeptide of type I collagen, β-CTX) more rapidly than alendronate in postmenopausal women with low bone mineral density. Studies have demonstrated that zoledronic acid is the most potent bisphosphonate agent to inhibit bone resorption [33], meaning that less of it is needed to achieve the same effect as ALN. It has been demonstrated that ZOL fully blocks bone loss in OVX rats is about 0.008 mg/kg/month [17], while ALN is 0.120 mg/kg/month [45]. Thus, ZOL is 15 times more potent than ALN in blocking OVX-induced bone loss in rats. In the current study, a regular dose of 0.1 mg/kg of ZOL and 84 mg/kg of alendronate were given in 12 weeks. Because alendronate was administrated orally, the absorption rate was about 1 %, so the absorbed ALN was 0.84 mg/kg, which was 8.4 times than ZOL. Since ALN is 15 times less potent than ZOL, but only 8.4 times of ZOL was used, it would be predicted that ALN was less effective than ZOL. In a word, more pronounced anti-resorption potent may contribute zoledronic acid to be more efficient in enhancing bone-implant osseointegration than alendronate and strontium ranelate in our study.
It should make clear that, in our current study, bone-loss models were induced by ovariectomization, which lead to a rapid loss of bone. But this circumstance rarely occurs in humans, even in postmenopausal women. And this might influence the effects of these drugs on osseointegration. The implants were inserted to the rats 4 weeks after ovariectomization. At this time, the rats were barely in a state of bone loss, which was most likely not very osteopenic. In this study, the doses of alendronate, zoledronic acid, and strontium ranelate administrated to the rats were 7 mg/kg/week, 0.1 mg/kg, and 500 mg/kg/day, respectively. Under this condition, we found that zoledronic acid appeared to have the strongest effects on bone-implant osseointegration, but it is still unclear whether increasing the dose of one drug (e.g., ALN) would affect the results of this experiment. And this study was an animal experiment, which might be different from human conditions. Although alendronate and zoledronic acid have been used clinically to improve initial fixation and long-term survival of an implant [22–24], it is still questioned by some authors [46].
In summary, in ovariectomized rat, systemic administration of ZOL, ALN, and SR enhances bone-implant osseointegration. Orally administration of ALN (7 mg/kg/week) and SR (500 mg/kg/day) have similar effect in promoting bone-implant osseointegration, while a single-dose injection of ZOL (0.1 mg/kg) might enhance bone-implant osseointegration more than oral administration of ALN(7 mg/kg/week) and SR (500 mg/kg/day) in ovariectomized rats.
References
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Becker W, Hujoel PP, Becker BE et al (2000) Osteoporosis and implant failure: an exploratory case-control study. J Periodontol 71(4):625–31
Holahan CM, Koka S, Kennel KA et al (2008) Effect of osteoporotic status on the survival of titanium dental implants. Int J Oral Maxillofac Implants 23(5):905–910
Keller JC, Stewart M, Roehm M et al (2004) Osteoporosis-like bone conditions affect osseointegration of implants. Int J Oral Maxillofac Implants 19(5):687–694
Duarte PM, César Neto JB, Gonçalves PF et al (2003) Estrogen deficiency affects bone healing around titanium implants: a histometric study in rats. Implant Dent 12(4):340–346
Jensen TB, Bechtold JE, Chen X et al (2007) Systemic alendronate treatment improves fixation of press-fit implants: a canine study using nonloaded implants. J Orthop Res 25:772–778
Qi M, Hu J, Li J et al (2012) Effect of zoledronate acid treatment on osseointegration and fixation of implants in autologous iliac bone grafts in ovariectomized rabbits. Bone 50(1):119–127
Duarte PM, de Vasconcelos Gurgel BC, Sallum AW et al (2005) Alendronate therapy may be effective in the prevention of bone loss around titanium implants inserted in estrogen-deficient rats. J Periodontol 76(1):107–114
Qi MC, Zhou XQ, Hu J et al (2004) Oestrogen replacement therapy promotes bone healing around dental implants in osteoporotic rats. Int J Oral Maxillofac Surg 33:279–285
Maïmoun L, Brennan TC, Badoud I et al (2010) Strontium ranelate improves implant osseointegration. Bone 46(5):1436–1441
Shirota T, Tashiro M, Ohno K et al (2003) Effect of intermittent parathyroid hormone (1-34) treatment on the bone response after placement of titanium implants into the tibia of ovariectomized rats. J Oral Maxillofac Surg 61:471–480
Kimmel DB (2007) Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res 86(11):1022–1033
Bone HG, Hosking D, Devogelaer JP et al (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350(12):1189–1199
Rosen CJ, Hochberg MC, Bonnick SL et al (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20(1):141–151
Viera-Negrón YE, Ruan WH, Winger JN et al (2008) Effect of ovariectomy and alendronate on implant osseointegration in rat maxillary bone. J Oral Implantol 34:76–82
Chen BL, Xie DH, Zheng ZM et al (2011) Comparison of the effects of alendronate sodium and calcitonin on bone-prosthesis osseointegration in osteoporotic rats. Osteoporos Int 22(1):265–270
Gasser JA, Ingold P, Venturiere A et al (2008) Long-term protective effects of zoledronic acid on cancellous and cortical bone in the ovariectomized rat. J Bone Miner Res 23(4):544–551
Little DG, Smith NC, Williams PR et al (2003) Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J Bone Miner Res 18(7):1300–1307
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356(18):1809–1822
Eastell R, Black DM, Boonen S et al (2009) Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab 94(9):3215–3225
Carvas JS, Pereira RM, Caparbo VF (2010) A single dose of zoledronic acid reverses the deleterious effects of glucocorticoids on titanium implant osseointegration. Osteoporos Int 21(10):1723–1729
Prieto-Alhambra D, Javaid MK, Judge A et al (2011) Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: population based retrospective cohort study. BMJ 343:d7222. doi:10.1136/bmj.d7222
Hilding M, Aspenberg P (2006) Postoperative clodronate decreases prosthetic migration: 4-year follow-up of a randomized radiostereometric study of 50 total knee patients. Acta Orthop 77:912–916
Friedl G, Radl R, Stihsen C et al (2009) The effect of a single infusion of zoledronic acid on early implant migration in total hip arthroplasty. A randomized, double-blind, controlled trial. J Bone Joint Surg Am 91:274–281
Reginster JY, Seeman E, De Vernejoul MC et al (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis, treatment of peripheral osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90(5):2816–22
Kanis JA, Johansson H, Oden A et al (2011) A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX(®). Osteoporos Int 22(8):2347–2355
Roux C, Fechtenbaum J, Kolta S et al (2008) Strontium ranelate reduces the risk of vertebral fracture in young postmenopausal women with severe osteoporosis. Ann Rheum Dis 67(12):1736–1738
Liu JM, Wai-Chee Kung A, Pheng CS et al (2009) Efficacy and safety of 2 g day of strontium ranelate in Asian women with postmenopausal osteoporosis. Bone 45(3):460–465
Blake GM, Compston JE, Fogelman I (2009) Could strontium ranelate have a synergistic role in the treatment of osteoporosis? J Bone Miner Res 24(8):1354–1357
Marie PJ, Hott M, Modrowski D et al (1993) An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J Bone Miner Res 8:607–615
Reginster JY (2002) Strontium ranelate in osteoporosis. Curr Pharm Des 8(21):1907–1916
Li Y, Feng G, Gao Y et al (2010) Strontium ranelate treatment enhances hydroxyapatite-coated titanium screws fixation inosteoporotic rats. J Orthop Res 28(5):578–582
Widler L, Jaeggi KA, Glatt M et al (2002) Highly potent geminal bisphosphonates. From pamidronate disodium (Aredia) to zoledronic acid (Zometa). J Med Chem 45(17):3721–3738
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals: Eighth Edition. National Academies, Washington (DC), p 248
Chen BL, Li YQ, Xie DH et al (2012) Low-magnitude high-frequency loading via whole body vibration enhances bone-implant osseointegration in ovariectomized rats. J Orthop Res 30(5):733–739
Yildiz A, Esen E, Kürkçü M et al (2010) Effect of zoledronic acid on osseointegration of titanium implants: an experimental study in an ovariectomized rabbit model. J Oral Maxillofac Surg 68(3):515–23
Linderbäck P, Agholme F, Wermelin K et al (2012) Weak effect of strontium on early implant fixation in rat tibia. Bone 50(1):350–356
Yaffe A, Kollerman R, Bahar H et al (2003) The influence of alendronate on bone formation and resorption in a rat ectopic bone development model. J Periodontol 74(1):44–50
Nijenhuis T, van der Eerden BC, Hoenderop JG et al (2008) Bone resorption inhibitor alendronate normalizes the reduced bone thickness of TRPV5(-/-) mice. J Bone Miner Res 23(11):1815–1824
Bonnelye E, Chabadel A, Saltel F et al (2008) Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 42(1):129–138
Su Y, Bonet J, Deloffre P et al (1992) The strontium salt S12911 inhibits bone resorption in mouse calvaria and isolated rat osteoclast cultures. J Bone Miner Res 17(Sl):188
Licata AA (1997) Bisphosphonate therapy. Am JMed Sci 313(1):17–22
Hadji P, Gamerdinger D, Spieler W et al (2012) Rapid Onset and Sustained Efficacy (ROSE) study: results of a randomised, multicentre trial comparing the effect of zoledronic acid or alendronate on bone metabolism in postmenopausal women with low bone mass. Osteoporos Int 23(2):625–633
Saag K, Lindsay R, Kriegman A et al (2007) A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone 40(5):1238–1243
Seedor JG, Quartuccio HA, Thompson DD (1991) The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J Bone Miner Res 6(4):339–46
Hansson U, Toksvig-Larsen S, Ryd L et al (2009) Once-weekly oral medication with alendronate does not prevent migration of knee prostheses: A double-blind randomized RSA study. Acta Orthop 80(1):41–45
Acknowledgments
This study was funded by the Guangdong Provincial Science and Technology Foundation in 2007 (NO2007B312004). The histological examination of this study was finished in The Center for New Drug Function Research, School of Life Science and Biopharmacology, Guangdong Pharmaceutical University. Thanks to Prof. QingNan Li for the help of histological examination. The biomechanical testing was finished in the Orthopedic Research Center of the First Affiliated Hospital of Sun Yat-sen University, and we thank JianWei Chen for the help of biomechanical testing.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Both BaiLing Chen and YiQiang Li are first co-authors.
Rights and permissions
About this article
Cite this article
Chen, B., Li, Y., Yang, X. et al. Zoledronic acid enhances bone-implant osseointegration more than alendronate and strontium ranelate in ovariectomized rats. Osteoporos Int 24, 2115–2121 (2013). https://doi.org/10.1007/s00198-013-2288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2288-7