Abstract
Summary
Vertebral fracture assessment (VFA) scanning is a useful tool to aid vertebral fracture identification. In this evaluation, we show that introduction of a comprehensive fracture risk assessment pathway incorporating VFA has enhanced diagnosis of vertebral fractures and improved targeting of investigations and treatment.
Introduction
Vertebral fractures are a common manifestation of osteoporosis and are associated with an increased risk of future vertebral and non-vertebral fractures. VFA is a method of imaging the thoraco-lumbar spine and a useful tool to aid vertebral fracture identification. In August 2008, a new one-stop pathway was introduced incorporating VFA and laboratory investigations at the time of bone mineral density assessment. The aims of this evaluation were to evaluate the clinical utility of VFA in identifying vertebral fractures which had not presented clinically and to evaluate the impact of this on management.
Methods
We performed a retrospective 6-month review of the new pathway focussing on those patients undergoing VFA who were suspected to have a vertebral fracture. The outcomes of VFA, spinal X-rays and investigations were evaluated.
Results
Three thousand five hundred twenty-six individuals underwent fracture risk assessment over a 6-month period, of which1,833 underwent VFA. Previously undiagnosed vertebral fractures were found in 202 individuals (36 were in retrospect apparent on prior imaging, and 29 were new vertebral fractures in patients with pre-existing vertebral fractures). Diagnosis of a vertebral fracture led to further investigation in all individuals and altered management in 59 (29 %) individuals. A potentially modifiable underlying cause was found in 42 (21 %).
Conclusions
Introduction of a fracture risk assessment service incorporating VFA and a one-stop pathway has enhanced vertebral fracture identification and targeting of treatment and management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vertebral fractures (VFX) are a common manifestation of osteoporosis with a prevalence as high as 25 % in European woman over 50 years [1]. The risk of sustaining a VFX increases with age, and they are strong independent predictors of future vertebral and non-vertebral fractures [2–6]. VFX are associated with significant morbidity including back pain [7], increasing kyphosis [8] and reduced mobility [9] and are also associated with increased mortality [10]. Initiation of treatment significantly reduces the risk of further fracture in osteopenic as well as osteoporotic individuals with a prevalent VFX [11–13]. Treatment has also been shown to improve quality of life and reduce pain [14].
Identification of VFX is important to optimise risk stratification. The incidence of a further VFX within the first year is almost 20 % [2], and the risk of hip fracture is doubled [15]. There is an increased risk of future fracture even in the case of asymptomatic VFX which may be included as a clinical risk factor in the estimation of absolute fracture risk using FRAX™ [16]. However, it is estimated that only approximately a third of VFX come to clinical attention [17], and strategies to improve case-finding of VFX are therefore worthwhile. One such strategy involves use of vertebral fracture assessment (VFA) scanning.
VFA is a method of imaging the thoraco-lumbar spine using low-dose ionising radiation by means of dual-energy X-ray absorptiometry (DXA). VFA is a sensitive and specific technique in the diagnosis of VFX [18], and as it involves exposure to a much lower dose of ionising radiation than spine radiographs, it can be used with a lower index of suspicion for fracture. Furthermore, it can be performed at the same time as measuring bone mineral density (BMD) [19]. VFA is increasingly being used in clinical practice, and the International Society for Clinical Densitometry (ISCD) has published recommendations for the clinical use of VFA [20]. This position statement, based on available evidence, advises a targeted approach to the use of VFA. The generic recommendations may not provide the optimal approach within all healthcare settings, and a few studies have examined the clinical efficacy of targeted and non-targeted implementation [21–23]. In a recent evaluation, a large cohort of individuals referred for DXA also underwent VFA using a non-targeted approach. In one of six patients, the VFX diagnosed were previously unidentified leading to a significant change in management, further supporting the use of VFA in routine clinical practice [24].
VFA was introduced into the clinical service in Sheffield, UK, in August 2008. Prior to this, patients referred for fracture risk assessment would complete a risk factor questionnaire and undergo measurement of spine and hip BMD using DXA. Any patient known to have VFX, or found to have either low BMD for age (Z-score ≤ −2) or unexplained bone loss, was recalled for further investigation in the metabolic bone clinic. Approximately 15 % of all referrals required recall for these reasons. The main drivers for change to this pathway were (1) a desire to incorporate VFA scanning to improve case-finding of VFX; (2) a recognition that many recalls to clinic could have been avoided because the underlying reasons for fractures were obvious and management could have been implemented in primary care and (3) a preference expressed by patients to minimise visits to the hospital. No additional funding was available for this initiative, and so, it was necessary to redesign the whole patient pathway. We estimated that the additional cost of an enhanced diagnostic assessment to the commissioners of the service could be offset by reducing the recall rate to clinic by 50 %.
Sheffield fracture risk assessment service (FRAS)
Referrals for fracture risk assessment are received from primary and secondary care in accordance with locally agreed guidelines which, at the time of this evaluation, were broadly in line with the Royal College of Physicians guidelines [25] but also including individuals with prior fragility fractures. The new pathway is summarised in Fig. 1. All patients are required to complete a risk factor questionnaire to provide clinical and lifestyle information, and prospective height loss (height loss since previous DXA scan) and historical height loss (height loss since recollected height at age 25 years) are assessed.
VFA is undertaken in patients identified as being at higher risk of VFX immediately following BMD scanning. The main criterion for VFA is based on age, and so in the majority of cases, the need for VFA is apparent from the initial referral enabling efficient scheduling. Three scanners in the department are used for the clinical service (two Hologic Discovery A models with a movable C-arm, and a Hologic Discovery C model with a fixed scanner arm (Hologic Inc., Bedford, MA)). Additional time is scheduled for patients requiring VFA to allow for the imaging scan to be acquired, analysed and interpreted. There is sufficient flexibility in the scheduling to allow VFA to be undertaken in the small proportion of patients in whom the indications are not apparent until the time of their appointment. Similarly, VFA may be undertaken in the lateral supine position for patients booked onto the scanner without a C-arm which has comparable ability to identify VFX [26].
Interpretation of the VFA image involves a visual read approach to identify the presence of VFX, using the algorithm-based qualitative (ABQ) technique. This is a modified visual approach focussing on evidence of vertebral endplate depression as the primary indicator of fracture deformity [27]. If a VFX is suspected, the patient is referred for spine radiographs to confirm or refute the diagnosis. Spine radiographs are obtained in the adjacent radiology department, and images are accessed electronically for interpretation using ABQ at the time of the appointment. The scanning technicians are trained to make the clinical decisions within the patient pathway using a triage approach to determine which patients require VFA, spinal X-rays and laboratory tests. This includes undertaking the visual read of the VFA scans and spine radiographs. The triage process is supervised by the chief scan technician with additional adjudication from metabolic bone physicians when required [28].
Laboratory investigations are undertaken in any patients found to have previously unknown VFX, low BMD for age or unexplained bone loss (Table 1). The completed fracture risk assessment is reported by one of four experienced metabolic bone physicians to the referring physician. Each individualised report includes a summary of the BMD, VFA, X-ray and laboratory results; a comment on the technical validity of the measurements; an estimate of absolute fracture risk (taking into account the BMD results and clinical risk profile) and advice on management, further investigations and follow up. All spine radiographs are reported independently by the musculoskeletal radiologists also utilising the ABQ methodology. Although pathway decisions during the patient’s appointment are based on un-reported X-rays, the formal report is available to us by the time the report is issued. Any discrepancies in X-ray interpretation between the metabolic bone physicians and the radiologist are reviewed in regular X-ray conferences to reach a consensus.
The aims of this evaluation were (1) to evaluate the clinical utility of VFA within the new pathway in identifying previously unidentified VFX, (2) to examine the utility of the one-stop pathway in identifying underlying causes of VFX and (3) to evaluate the impact on management resulting from the diagnosis of VFX.
Methods
For this evaluation, we initially identified all patients attending for fracture risk assessment over a 6-month period between October 2008 and March 2009 at the Metabolic Bone Centre, Northern General Hospital, Sheffield, UK. Patients are routinely coded according to which components of the pathway they require, and this information is entered into a departmental database. This allowed identification of all patients in whom a previously undiagnosed vertebral fracture was suspected from VFA (i.e. those who underwent VFA and spine radiographs). We performed a detailed retrospective review of this group of patients. Information was collated by a single investigator from the medical records, DXA scans and completed questionnaires. In this evaluation, we focussed on the utility of VFA in identifying VFX. We have not included the evaluation of patients in whom the VFX had already been identified prior to referral.
Results
A total of 3,526 patients (2,930, 83 % women and 596, 17 % men) were assessed in the FRAS pathway over this 6-month period. One thousand eight hundred thirty-three (52 %) underwent VFA, of whom 360 subsequently had spine radiographs because a previously undiagnosed VFX was suspected. VFX was confirmed on spine radiographs in 202 patients (11 % of those having a VFA scan, 56 % of those in whom radiographs were obtained). Demographics of patients undergoing FRAS are shown in Table 2. The most common indication for undergoing VFA was age (81 %), followed by historical height loss and corticosteroid use (data not shown).
Characteristics of the 360 patients who underwent VFA and spine radiographs are shown in Table 3. Patients confirmed as having one or more VFX were more likely to have a history of a prior non-VFX and had significantly lower BMD at both lumbar spine and hip (p = <0.001). There was a greater difference in spine than hip BMD between those with and without VFX. This is consistent with the known site specificity of BMD measurement. WHO classification was made on the basis of the lower of the two readings between hip and lumbar spine BMD. Individuals with VFX confirmed on spine radiographs were more likely to have a clinical risk factor (e.g. current smoker, excess alcohol consumption or first-degree relative with hip fracture) than individuals without VFX.
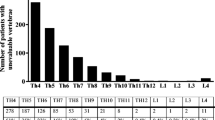
In total, 438 VFX were confirmed in 202 patients. The numbers of VFX identified ranged from 1 to 13, with 44 % of patients having two or more VFX. The distribution of VFX was bimodal with peaks at T12 and T8. In retrospect, VFX were apparent on prior imaging in 36 individuals. These fractures were not known to us at the time of referral either because they had not been reported as VFX and were therefore missed, or because X-rays had been obtained in another hospital and we were unaware and hence unable to review these until after the patient’s appointment. Of the remaining 158 individuals suspected of having VFX, non-VFX deformity was identified on radiographs in the majority with normal vertebrae in only 28 (Table 4).
A total of 195 individuals with VFX underwent investigations. Seven of the 202 VFX cases did not undergo investigations within the department because they declined or they had insufficient time. In these cases, further investigation was recommended to the referring physician. Finding of a VFX led to a change in management in 59 patients:
-
Initiation of treatment in 53 patients, who would not otherwise have been treated on the basis of BMD and clinical risk factors
-
Change in treatment in six patients found to have a new VFX despite treatment.
Furthermore, the suspicion of a VFX led to:
-
Thirty-four individuals undergoing investigations who would not have done so without knowledge of a VFX
-
Identification of a previously unsuspected underlying cause in 42 individuals (Table 5).
Table 5 Unknown underlying cause identifieda
Identification of VFX did not alter the treatment recommendation in 143 patients as osteoporosis treatment was indicated on the basis of their BMD and other risk factors. However, under the old pathway, all 202 patients identified as having a VFX would have been recalled to clinic. Under the new pathway, only 53 individuals (26 %) with VFX were recalled to clinic for more detailed evaluation. Reasons for recall to clinic included the occurrence of new fractures despite treatment, very severe or unexplained osteoporosis and abnormalities on the initial investigations requiring further evaluation.
Discussion
We report the results of a systematic evaluation of a new fracture risk assessment service. The new service was introduced to incorporate VFA scans and laboratory investigations in a ‘one-stop’ pathway, and our objective was to examine the impact of VFA scanning. Over a 6-month period, 202 patients were found to have VFX which had not presented clinically. This represents 11 % of the patients having VFA scans. Investigation of these individuals revealed modifiable underlying causes in 42, and osteoporosis treatment was introduced or altered in 59 on the basis of the finding of VFX. A management plan for implementation in primary care was made in the majority of these patients, with only 26 % requiring further evaluation in the metabolic bone clinics. The total recall to clinic for all patients undergoing fracture risk assessment was <5 % [29], compared with 15 % prior to introduction of the new pathway. Previously, all 202 individuals with VFX would have required recall to clinic for further investigation.
This evaluation has confirmed that VFA used as a screening tool in higher risk individuals has enhanced our identification of VFX within a fracture risk assessment pathway. Although spine radiographs are considered the standard investigation for detection of VFX, they involve exposure to a significant dose of ionising radiation and should not be performed without a reasonable clinical suspicion of VFX. The relatively low dose of ionising radiation used in VFA scanning (3 μSv versus 600 μSv for spine radiographs) [30] enables its use as a screening investigation, and we have shown that, when targeted to individuals at moderate risk of VFX, the yield of previously undiagnosed VFX is worthwhile.
Several other studies have investigated the clinical utility of VFA in identifying VFX and its subsequent impact on management. In one study, a fifth of the population undergoing VFA were found to have a VFX which was unknown in 69 % [24]. In comparison to our clinical population, individuals undergoing VFA were much younger (mean age 53 versus 62 years) and unselected (the first 2,500 individuals referred from within secondary care for BMD scanning). Nonetheless, a higher prevalence of VFX compared with our evaluation was reported. There are two explanations for this apparent difference. Firstly, the number of VFX identified in our evaluation does not represent the total prevalence of VFX in the population attending for fracture risk assessment as we focussed only on those previously unidentified VFX diagnosed through VFA scans. Secondly, different methodology was used to identify VFX. The ABQ method we use depends on evidence of vertebral end plate changes as an indicator of VFX and has been previously shown to identify fewer VFX compared with other morphometric techniques where there is a greater focus on vertebral height reduction [27, 31].
Currently, there remains uncertainty about the optimal target group for VFA [19, 21, 23]. One study evaluated the use of VFA in women comparing two approaches: routine versus targeted VFA [21]. Routine VFA was indicated for all women aged 65 years or older. Indications for targeted VFA included the following: height loss of >2.5 cm, dowager hump, suspected fracture on spine DXA and known VFX. Routine VFA was more effective with one in five individuals being identified with a VFX, but 96 % of the population required VFA. In comparison, targeted VFA was less effective and detected 9.6 % of VFX, missing a significant proportion, but considerably fewer (5 %) underwent VFA.
Middleton et al. explored the use of vertebral fracture scores to select women for screening [23]. A large population of women who had already undergone routine VFA were examined retrospectively, and risk factors for VFX were identified using multivariate logistic regression to create vertebral fracture scores. Different thresholds of vertebral fracture scores were explored and compared with BMD thresholds (osteopenic, osteoporotic or both). Clinical risk factors significantly associated with VFX on VFA included: age, femoral neck BMD, prior clinical fracture, height loss and kyphosis. Use of vertebral fracture scores reduced numbers undergoing VFA by 30 % whilst still identifying >90 % of women with VFX when compared with routine VFA.
The aim of an effective strategy would be to identify all VFX using the minimum number of VFA scans. It is particularly important to identify VFX in those patients who would not otherwise be considered for further investigation or treatment. This is the rationale for the ISCD recommendations which focus on assessment of osteopenic individuals. However, we chose not to utilise the ISCD indications in full. In order to schedule appointments efficiently, it is impractical for the decision to undertake VFA to depend on the BMD result. By using age thresholds as the main indication for VFA, the decision to perform VFA may be made in the majority of individuals prior to appointment and additional time can be allocated to allow acquisition, analysis and interpretation of VFA and a decision to be made regarding the need for additional investigations. However, there is enough flexibility within the appointment system to accommodate VFA in those who require it on the day of appointment.
Use of the ISCD recommendations in our service would have meant that 19 individuals we identified with a VFX would not have undergone VFA. Of these, 14 would not have otherwise received treatment, and in the remaining five, the presence of a VFX would not have altered management. We do not know if additional VFX would have been identified using the ISCD indications. We chose a simple approach to select individuals for VFA using an age threshold as the main criterion in addition to simple clinical risk factors. Our approach is pragmatic and works well within our clinical service. We therefore suggest it could be applicable to other service models in clinical practice.
Another difference in our pathway from ISCD recommendations is in the use of spine radiographs. The ISCD advise that radiographs are not required if the appearances on VFA clearly indicate the presence of VFX. We elected to perform spine radiographs in all patients suspected of having VFX on VFA. The presence of vertebral fracture was confirmed in 56 % of patients having radiographs. In the remaining 44 %, other spine pathology causing the appearance of vertebral deformity was present in the majority, with only 8 % having no significant abnormality on radiographs. We believe the use of radiographs is important for two reasons. Firstly, the resolution on VFA images is inferior to that on radiographs and may be insufficient to differentiate osteoporotic from other pathological fractures due to causes such as malignancy or Paget’s disease. Secondly, within our pathway, the VFA images are interpreted by trained technicians and metabolic bone physicians who are not radiologists. We believe it is important to have formal radiological confirmation of all VFX, and all spine radiographs are reviewed by the musculoskeletal radiologists. The ISCD also advise that all non-evaluable vertebrae should be recorded to ensure accurate interpretation of VFA and to avoid over-diagnosis of VFX. It is therefore important to consider the quality of VFA image when interpreting scans. Previous studies have shown 97 % of the thoracic vertebrae below T7 are visualised adequately on VFA compared with 70–42 % from T6–4 [32, 33]. Although we do not systematically record which vertebrae are non-evaluable (as we wish to provide a comprehensive report to the referring physician primarily to aid clinical decision making), it is our practice to make a comment in the report about the technical reliability of BMD and VFA. If there is a high suspicion of VFX or VFA quality is poor, patients are referred on an individual basis for spinal X-ray.
Although most patients with VFX have low BMD, we identified a proportion of patients with VFX with normal BMD. Whilst treatment of patients with VFX who are osteoporotic or osteopenic is known to reduce future fracture risk [34], we currently do not know if this is also true for individuals with normal BMD. These VFX may have resulted from significant trauma and may be old. However, investigation for an underlying cause of VFX is particularly important in those with normal BMD in view of the possibility of a pathological fracture. Interestingly, when compared with other studies, we identified a lower proportion of individuals with VFX who were osteopenic or had normal BMD [21, 24]. This difference may be explained by the different methods used to define VFX. It has been shown that a VFX identified using the ABQ method is more likely to be associated with osteoporosis compared with other morphometric techniques [31].
We also evaluated the incorporation of laboratory investigations within the one-stop assessment to identify underlying causes of VFX. Potentially modifiable causes were identified in a clinically important proportion of patients with vitamin D deficiency being the most common contributory cause, present in two thirds of our patients with VFX. Furthermore, in 34 individuals, investigations would not have been performed without knowledge of a VFX as they did not fulfil any of our other indications for undergoing investigations.
We acknowledge there are several limitations to this evaluation:
-
It is a retrospective evaluation of our clinical service. As a consequence, patient-reported data could not be validated, and data were incomplete for some patients.
-
Resolution of VFA is inferior to that of spine radiographs, particularly in the upper thoracic spine and VFX may have been missed. It was not possible to identify what proportion of vertebrae was unreadable on VFA within this evaluation because this information was not recorded systematically. However, if VFA quality was felt to be poor but there was a high index of suspicion of a VFX, a decision was made on an individual basis to perform spinal radiographs for further clarification.
-
We could not assess how many VFX were missed—either in patients who had VFA and were not referred for X-ray or in those who did not fulfil the criteria for VFA. However, in those individuals who underwent VFA but did not proceed to spinal X-ray, it is unlikely many VFX have been missed as there is known to be good agreement between VFA and spinal X-ray interpretation using the ABQ method [31]. Those individuals who did not fulfil the criteria for VFA did not have any clinical risk factors associated with increased risk of VFX, and again, it is unlikely that many VFX have been missed.
In conclusion, introduction of a comprehensive fracture risk assessment pathway incorporating VFA has enhanced our diagnosis of VFX and improved targeting of investigations and treatment. Treatment recommendations were made in a proportion of people who would not have otherwise received treatment. As a result of the new pathway, we have identified modifiable underlying causes and reduced the need to bring patients back for clinic review. VFA scans had the greatest value in terms of potential to change management, in those with osteopenia and no other clinical risk factors. In these patients, identification of VFX has an important impact on the estimation of future fracture risk and clinical management. Furthermore, by preventing future fractures, there are potential long-term health economic benefits.
References
Melton LJ 3rd, Lane AW, Cooper C, Eastell R, O’Fallon WM, Riggs BL (1993) Prevalence and incidence of vertebral deformities. Osteoporos Int 3:113–119
Lindsay R, Silverman SL, Cooper C et al (2001) Risk of new vertebral fracture in the year following a fracture. JAMA 285:320–323
van Geel TA, Huntjens KM, van den Bergh JP, Dinant GJ, Geusens PP (2010) Timing of subsequent fractures after an initial fracture. Curr Osteoporos Rep 8:118–122
Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, Adachi JD (2003) Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 33:522–532
Cauley JA, Hochberg MC, Lui LY, Palermo L, Ensrud KE, Hillier TA, Nevitt MC, Cummings SR (2007) Long-term risk of incident vertebral fractures. JAMA 298:2761–2767
Black DM, Arden NK, Palermo L, Pearson J, Cummings SR (1999) Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res 14:821–828
Nevitt MC, Ettinger B, Black DM, Stone K, Jamal SA, Ensrud K, Segal M, Genant HK, Cummings SR (1998) The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med 128:793–800
Kado D, Huang M, Karlamangla A, Cawthon P, Katzman W, Hillier T, Ensrud K, Cummings S (2012) Factors associated with kyphosis progression in older women: 15 years experience in the Study of Osteoporotic Fractures. J Bone Miner Res. doi:10.1002/jbmr.1728
Shiraki M, Kuroda T, Shiraki Y, Aoki C, Sasaki K, Tanaka S (2010) Effects of bone mineral density of the lumbar spine and prevalent vertebral fractures on the risk of immobility. Osteoporos Int 21:1545–1551
Hasserius R, Karlsson MK, Nilsson BE, Redlund-Johnell I, Johnell O (2003) Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int 14:61–68
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Cummings SR, San Martin J, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Chen P, Miller PD, Delmas PD, Misurski DA, Krege JH (2006) Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res 21:1785–1790
Lau AN, Ali SH, Sawka AM, Thabane L, Papaioannou A, Gafni A, Adachi JD (2008) Improvement in health-related quality of life in osteoporosis patients treated with teriparatide. BMC Musculoskelet Disord 9:151
Melton LJ 3rd, Atkinson EJ, Cooper C, O’Fallon WM, Riggs BL (1999) Vertebral fractures predict subsequent fractures. Osteoporos Int 10:214–221
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ 3rd (1992) Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 7:221–227
Schousboe JT, Debold CR (2006) Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int 17:281–289
Lewiecki EM, Laster AJ (2006) Clinical review: clinical applications of vertebral fracture assessment by dual-energy X-ray absorptiometry. J Clin Endocrinol Metab 91:4215–4222
Schousboe JT, Vokes T, Broy SB, Ferrar L, McKiernan F, Roux C, Binkley N (2008) Vertebral fracture assessment: the 2007 ISCD Official Positions. J Clin Densitom 11:92–108
Middleton ET, Steel SA (2008) Routine versus targeted vertebral fracture assessment for the detection of vertebral fractures. Osteoporos Int 19:1167–1173
Vokes TJ, Gillen DL (2010) Using clinical risk factors and bone mineral density to determine who among patients undergoing bone densitometry should have vertebral fracture assessment. Osteoporos Int 21:2083–2091
Middleton ET, Gardiner ED, Steel SA (2009) Which women should be selected for vertebral fracture assessment? Comparing different methods of targeting VFA. Calcif Tissue Int 85:203–210
Jager PL, Jonkman S, Koolhaas W, Stiekema A, Wolffenbuttel BH, Slart RH (2011) Combined vertebral fracture assessment and bone mineral density measurement: a new standard in the diagnosis of osteoporosis in academic populations. Osteoporos Int 22:1059–1068
Royal College of Physicians (1999) Osteoporosis: clinical guidelines for prevention and treatment. http://bookshop.rcplondon.ac.uk/details.aspx?e=136
Paggiosi MA, Finigan J, Peel N, Eastell R, Ferrar L (2012) Supine vs decubitus lateral patient positioning in vertebral fracture assessment. J Clin Densitom 15:454-60
Jiang G, Eastell R, Barrington NA, Ferrar L (2004) Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int 15:887–896
McCloskey EV, Vasireddy S, Threlkeld J, Eastaugh J, Parry A, Bonnet N, Beneton M, Kanis JA, Charlesworth D (2008) Vertebral fracture assessment (VFA) with a densitometer predicts future fractures in elderly women unselected for osteoporosis. J Bone Miner Res 23:1561–1568
Diacon C, Peel NF (2010) Evaluation of a one-stop fracture risk assessment pathway. Osteoporos Int. p S521
Vokes T, Bachman D, Baim S, Binkley N, Broy S, Ferrar L, Lewiecki EM, Richmond B, Schousboe J (2006) Vertebral fracture assessment: the 2005 ISCD Official Positions. J Clin Densitom 9:37–46
Ferrar L, Jiang G, Schousboe JT, DeBold CR, Eastell R (2008) Algorithm-based qualitative and semiquantitative identification of prevalent vertebral fracture: agreement between different readers, imaging modalities, and diagnostic approaches. J Bone Miner Res 23:417–424
Rea JA, Steiger P, Blake GM, Fogelman I (1998) Optimizing data acquisition and analysis of morphometric X-ray absorptiometry. Osteoporos Int 8:177–183
Ferrar L, Jiang G, Barrington NA, Eastell R (2000) Identification of vertebral deformities in women: comparison of radiological assessment and quantitative morphometry using morphometric radiography and morphometric X-ray absorptiometry. J Bone Miner Res 15:575–585
Kanis JA, Johnell O, Black DM, Downs RW Jr, Sarkar S, Fuerst T, Secrest RJ, Pavo I (2003) Effect of raloxifene on the risk of new vertebral fracture in postmenopausal women with osteopenia or osteoporosis: a reanalysis of the Multiple Outcomes of Raloxifene Evaluation trial. Bone 33:293–300
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuet, KP., Charlesworth, D. & Peel, N.F.A. Vertebral fracture assessment scans enhance targeting of investigations and treatment within a fracture risk assessment pathway. Osteoporos Int 24, 1007–1014 (2013). https://doi.org/10.1007/s00198-012-2255-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-2255-8