Abstract
Summary
Many osteoporotic women prescribed strontium ranelate have previously received bisphosphonates. Prior bisphosphonate use blunted the spinal bone mineral density (BMD) response for 6 months. Hip BMD was blunted to a degree for 2 years, although there was an overall increase in hip BMD in contrast to the heel where BMD did not increase.
Introduction
Many osteoporotic women commenced on strontium ranelate have already received treatment with bisphosphonates. This study investigates whether prior bisphosphonate use impairs the subsequent therapeutic response to strontium ranelate.
Methods
Women were recruited who were either bisphosphonate naïve or currently receiving a bisphosphonate. All women received strontium ranelate and were followed up for 2 years.
Results
One hundred and twenty women were recruited. After 2 years, the bisphosphonate-naïve group had significant BMD increases of 8.9%, 6.0% and 6.4% at the spine, hip and heel, respectively. In the prior bisphosphonate group, BMD increased significantly at the spine (4.0%) and hip (2.5%) but not at the heel. At all time points at all sites, the BMD increase was greater in the bisphosphonate-naïve group. BMD at the spine did not increase during the first 6 months in the prior bisphosphonate group but then increased in parallel with the bisphosphonate-naïve group. In contrast, the difference between the two groups in hip BMD continued to increase throughout the 2 years. P1NP was suppressed in the prior bisphosphonate group for the first 6 months.
Conclusions
After bisphosphonate exposure, the BMD response to strontium ranelate is blunted for only 6 months at the spine. At the hip, a degree of blunting was observed over 2 years, although there was an overall increase in hip BMD in contrast to the heel where no increase in BMD was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Strontium ranelate has been demonstrated to reduce the incidence of vertebral and non-vertebral fractures in postmenopausal women [1, 2]. These fracture prevention trials involved treatment-naïve women; however, in clinical practice, many women who commence strontium ranelate have already received treatment with bisphosphonates. This is certainly the case in the UK where bisphosphonates are the recommended first-line treatment for osteoporosis unless contraindicated [3]. Even after their discontinuation, bisphosphonates continue to suppress bone turnover [4], and this may have consequences on the response to subsequent treatments for osteoporosis.
We have previously reported that women with prior bisphosphonate exposure have a blunted bone mineral density (BMD) response to strontium ranelate for 6 months at the spine and for 1 year at the hip and heel [5]. We believe that this is mostly due to reduced strontium uptake into the skeleton as strontium is almost exclusively deposited in newly formed bone tissue [6] and prior bisphosphonate use reduces bone turnover leading to reduced new bone formation. Furthermore, it has been suggested that strontium ranelate alters the balance of bone turnover in favour of bone formation and therefore may have anabolic properties leading to increased amounts of bone tissue [7]. This potentially provides a second mechanism by which prior bisphosphonate use may blunt the BMD response to strontium ranelate as has previously been demonstrated with anabolic therapy [8, 9].
As we have reported a blunting of the BMD response to strontium ranelate in women with prior bisphosphonate use, it is important to know for how long this blunting persists. This is particularly important at the hip and heel where blunting was seen throughout the first year of treatment with strontium ranelate. In this article, we report the results of a second year extension to our original study which aimed to investigate the long-term effects of prior bisphosphonate use on the subsequent therapeutic response to strontium ranelate.
Materials and methods
Year 1
A detailed description of the design of the first year of this study has already been reported [5]. To summarise, postmenopausal women aged 50–80 with either a T-score of less than −2.5 at the hip/spine or a T-score of −2.0 and one other risk factor for fracture were recruited if they were either bisphosphonate naive or currently being treated with a bisphosphonate for more than 1 year. Women were excluded if they had received prior treatment with strontium ranelate, were unable to give informed consent or could not be reliably assessed by dual-energy X-ray absorptiometry (DXA), or if they had medical conditions associated with bone disease. All women gave written informed consent. All women received strontium ranelate 2 g once a day after a 2-h fast and 1.2 g calcium and 800 IU vitamin D daily (Adcal D3). Women were followed up at 3, 6 and 12 months. BMD was measured at the spine (L2–L4) and hip (total hip) by DXA (Lunar Prodigy, GE Lunar, Madison, WI, USA). Heel (right os calcis) BMD was also measured (Lunar Pixi, GE Lunar, Madison, WI, USA). Bone turnover was assessed using procollagen type 1 amino terminal propeptide (P1NP; Elecsys 2010, Roche diagnostics, IN, USA). A DXA-based vertebral fracture assessment (VFA) was performed at baseline and 12 months to look for vertebral fractures. As described previously, a vertebra was considered to be fractured if the VFA deformity grade was moderate or severe [10].
Year 2
All women completing the first year were invited to enter into the second year extension phase of this study. At the 12-month visit, those women wishing to enter into the extension phase were enrolled after given written informed consent. They then continued treatment with strontium ranelate 2 g once a day and Adcal D3 and were followed up at 18 and 24 months using BMD at the spine, hip and heel and P1NP. After 24 months, a further VFA was performed to detect any incident vertebral fractures occurring in the second year. Compliance was calculated at each visit based on returned medications.
As in the first year of the study, the primary end points were the between-group and the within-group changes in axial BMD over the 24 months of the study. Lumbar spine BMD (L2–L4) was used for analysis; however, if there was a prevalent fracture at baseline or an incident fracture during the 2 years of the study in one of these vertebrae, then the fractured vertebra was excluded from the analysis. At the hip, total hip BMD was used for the analysis as this region of interest demonstrates the greatest increase in hip BMD in response to strontium ranelate [1, 2] and is the recommended region of interest for assessing treatment response at the hip [11]. Precision at our centre, as percent coefficient of variation, has been determined using repeat measures in 36 postmenopausal women as 0.9% at spine and 1.0 for the total hip region [12]. The secondary end points were change in heel BMD, the change in P1NP and fracture incidence.
Data analysis
All 108 women who made up the study population from the first year were included in the second year analysis. Initially, the Kolmogorov–Smirnov test for normality was used to assess the distribution of the data. Baseline characteristics were compared between the groups using either a two-sample t test or Mann–Whitney U test depending on the distribution of the data. Fisher's exact test was used for categorical baseline data.
Changes from baseline for BMD spine, hip and heel and absolute P1NP values at all time points were analysed using mixed modelling to allow for missing data. In the mixed modelling, measurements were assumed to have an autoregressive correlation structure, in which the correlation between measurements on the same patient decreases as the time interval increases. All p values reported from the mixed modelling for within-group and between-group comparisons were obtained by applying t tests to the estimated means or mean changes at each time point. The between-group comparisons were subsequently repeated after adjusting for baseline differences between the groups. The significance level chosen was 0.05. The program package used was SPSS for Windows (version 16.0 SPSS, Inc., Chicago, IL).
Study approval, conflict of interest and funding
For both the first year and the extension phase, ethical approval was obtained from the Hull and East Riding Local Research Ethics Committee. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Clinical trial authorisation was obtained from the Medicines and Healthcare products Regulatory Agency, UK (EudraCT number 2005-003138-16). Servier Laboratories provided the strontium ranelate and a grant to fund the study. ProStrakan provided Adcal D3 as the calcium supplement but no financial support. Servier has paid speaker fees to Dr E Middleton (<£1,000) and Dr M Aye (<£2,000). All authors have no other conflicts of interest. The study design, the data collection, analysis and interpretation, and the manuscript were all carried out by the authors independent of Servier and ProStrakan. All authors had full access to the data and were involved in the manuscript preparation.
Results
Subjects and baseline demographics
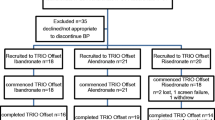
One hundred and twenty women were recruited, 60 to each group, of which 12 discontinued prior to the first visit. Therefore, 108 women (52 prior bisphosphonate and 56 bisphosphonate naive) had follow-up data, and these women made up the study population. One hundred and five women completed the first year of which 97 entered into the second year. Three women discontinued at the 18-month visit resulting in 94 women completing the whole 2 years of the study (45 prior bisphosphonate and 49 bisphosphonate naïve).
The prior bisphosphonate group was older (66.9 vs. 62.5 years, p = 0.001) and had a lower baseline BMD at the spine (0.799 vs. 0.835 g/cm2, p = 0.02) than the bisphosphonate-naïve group. The prior bisphosphonate group also had a lower baseline P1NP consistent with recent antiresorptive therapy. There were no other significant differences between the groups. The baseline demographics of the 108 women in the study population are summarised in Table 1. In the prior bisphosphonate group, the mean (SD) duration of bisphosphonate use was 64.3 (38.5) months. Details of prior bisphosphonate usage are contained in Table 2.
Compliance was similar in both groups. The mean level of compliance with strontium ranelate in the bisphosphonate-naïve and prior bisphosphonate groups, respectively, was 95.6% and 95.0% in the first year, and 93.2% and 94.5% in the second year.
Change in spine BMD with strontium ranelate
Over the 2 years of the study, spine BMD increased both in bisphosphonate-naïve women (+8.9%, 0.074 g/cm2, p < 0.001) and in women with prior bisphosphonate exposure (+4.0%, 0.032 g/cm2, p < 0.001). Within the bisphosphonate-naive group, there was a significant increase in spine BMD after 6-month treatment with strontium ranelate (6-month change: 0.019 g/cm2, p = 0.002). In contrast, the prior bisphosphonate group experienced no significant increase in BMD by 6 months, and a statistically significant increase in spine BMD was only achieved after 12 months of therapy (12-month change: 0.019 g/cm2, p = 0.008).
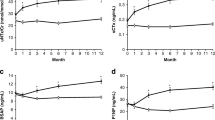
At all time points, the change in spine BMD from baseline was significantly greater in the bisphosphonate-naive group than the prior bisphosphonate group, and the difference persisted after adjusting for baseline differences in age and BMD. The difference between the two groups in terms of change in spine BMD was 0.022 g/cm2 at 6 months, and by 24 months, the difference had increased to 0.042 g/cm2. However, this increase in difference between the groups with time was not significant (p = 0.168). The change from baseline in spine BMD in each group is reported in Fig. 1a.
Change in hip BMD with strontium ranelate
After 2 years of treatment with strontium ranelate, total hip BMD had increased significantly in both the bisphosphonate-naive group (0.047 g/cm2, 6.0%, p < 0.001) and the prior bisphosphonate group (0.019 g/cm2, 2.5%, p < 0.001). Within the bisphosphonate-naive group, the change from baseline in total hip BMD was significant after 6 months (6-month change: 0.014 g/cm2, p < 0.001). In the prior bisphosphonate group, there was no significant change in total hip BMD at 6 months, and by 12 months, the increase in hip BMD from baseline was only just significant (12-month change: 0.006 g/cm2, p = 0.048).
At all time points, the change in total hip BMD from baseline was significantly greater in the bisphosphonate-naive group than the prior bisphosphonate group, and the difference persisted after adjusting for baseline differences in age and BMD. Over the 2 years of the study, the difference between the two groups in terms of change in total hip BMD increased from 0.013 g/cm2 at 6 months to 0.028 g/cm2 at 24 months. This progressive increase in difference between the groups with time was statistically significant (p = 0.036). The change from baseline in total hip BMD in each group is reported in Fig. 1b.
Change in heel BMD with strontium ranelate
Within the bisphosphonate-naive group, there was a significant increase in heel BMD after 6 months of treatment with strontium ranelate (6-month change: 0.011 g/cm2, p < 0.001), and after 2 years, heel BMD had increased by a total of 6.4% (0.025 g/cm2, p < 0.001). There was no significant increase in heel BMD after 2 years of treatment with strontium ranelate in the prior bisphosphonate group. The change from baseline in heel BMD in each group is reported in Fig. 1c.
Change in P1NP in response to strontium ranelate
At baseline, P1NP was significantly lower in the prior bisphosphonate group consistent with recent antiresorptive therapy. In the prior bisphosphonate group, P1NP increased by 43% over the 24 months (30 μg/l to 43 μg/l, p < 0.001). In the bisphosphonate-naive group, P1NP reduced by 17% (54 μg/l to 46 μg/l, p = 0.003). The difference in P1NP between the groups was still significant at 3 months (49 vs. 39 μg/l, p = 0.004), but by 6 months, P1NP was similar in both groups (48 vs. 42 μg/l, p = 0.132) and remained similar for the rest of the 2-year period. Adjusting P1NP for the baseline difference in age did not alter the results. Change in P1NP in response to strontium ranelate is demonstrated in Fig. 2.
Fracture incidence during therapy with strontium ranelate
During the second year of the study, eight women in the prior bisphosphonate group suffered a total of nine incident vertebral fractures between them (four graded as severe on VFA and five as moderate) compared to two women in the bisphosphonate-naïve group (two severe). Non-vertebral fractures occurred in two women in the bisphosphonate-naive group (wrist and metacarpal) and two women in the prior bisphosphonate group (wrist and rib). Fracture incidence over the 2 years is summarised in Table 3.
Discussion
This study provides data on the effect of prior bisphosphonate use on the subsequent bone response to treatment with strontium ranelate. The extension phase of this study demonstrates that the difference in BMD gain between the two groups reported in the first year of the study persists during the second year with no evidence of “catch up” by the prior bisphosphonate group. In this study, the gains in BMD in the bisphosphonate-naïve group were similar to those observed in the SOTI study [1], suggesting that the difference between the groups was due to a reduction in BMD gain in the prior bisphosphonate group.
During treatment with strontium ranelate, strontium is only deposited into new bone packets formed during the treatment period [6, 13]. This induces large increases in BMD as strontium's high atomic number leads to greater X-ray attenuation than calcium. Biopsy studies suggest that the average bone strontium content after 2 years of treatment is around 1% [13]. A bone strontium content of 1% causes a 10% increase in BMD [14] which would be consistent with the 9% increase in BMD observed in the bisphosphonate-naive group in this study. The blunted BMD response observed in the prior bisphosphonate group is therefore likely to result from reduced strontium uptake in to the bone due to a reduced number of new bone packets caused by the prolonged suppression of bone turnover following bisphosphonate therapy [4]. A second potential mechanism for the blunting of the BMD response is provided by evidence that strontium ranelate may alter the balance of bone turnover in favour of bone formation which in turn may lead to an increase in the overall amount of bone tissue [7, 15, 16]. If this is correct, then prior bisphosphonate therapy may also diminish these anabolic actions as has been previously reported for teriparatide [8, 9].
At the spine, there was no increase in BMD in the prior bisphosphonate group during the first 6 months of therapy which led to a significant difference between the two groups at 6 months. Although this difference in BMD persisted for the remainder of the study, there was no further significant increase in the difference, suggesting that after 6 months, the two groups gained BMD at a similar rate. It would therefore appear that the blunting of the BMD response at the spine only lasts for approximately 6 months after the discontinuation of bisphosphonate therapy. This would be consistent with the observation that bone turnover, as measured by P1NP, was suppressed in the prior bisphosphonate group for 3 to 6 months after which bone turnover remained similar between the two groups.
The second year extension of this study demonstrated that hip BMD had increased significantly by 2 years. This confirms that the hip is capable of responding to strontium ranelate after the discontinuation of bisphosphonates. However, in contrast to the spine, the magnitude of the difference between the two groups in terms of hip BMD continued to increase at each visit, and this continued divergence was statistically significant. This suggests that in the prior bisphosphonate group, BMD is gained at a slower rate at the hip throughout the 2 years. The more prolonged blunting at the hip may arise as the hip, unlike the spine, is predominantly cortical bone. Cortical bone is less metabolically active than trabecular bone [17] and as such may take longer to overcome the bisphosphonate-induced suppression of bone turnover. Furthermore, it has been suggested in animal studies that the preferential incorporation of strontium ions into newly formed bone packets, as opposed to older bone packets, is greater in cortical than trabecular bone [18]. Therefore, any reduction in the rate of bone turnover would lead to a greater impedance of strontium uptake into cortical bone than trabecular bone.
At the heel, the blunting of the BMD response to strontium ranelate seems even greater. In the prior bisphosphonate group, 2 years of treatment failed to increase BMD at the heel significantly compared to an increase of 6.4% in the bisphosphonate-naïve group. Like the spine, the heel is predominantly a trabecular bone site so it is interesting that prior bisphosphonate exposure results in the two sites responding so differently to the strontium ranelate. As we have previously hypothesised [5], the difference in duration of blunting may be due to the heel containing predominantly yellow bone marrow, while the spine is a red bone marrow site [19]. Osteoclasts, osteoblasts and various key cytokines involved in bone turnover are derived from the red bone marrow [20], and the relative lack of these at yellow bone marrow sites may prolong the bisphosphonate-induced suppression of bone turnover and thus the blunting of the BMD response to strontium ranelate.
Overall, it appears that the effect of prior bisphosphonate exposure on the response to strontium ranelate depends on the skeletal site studied with only a short duration of blunting at the spine, more prolonged blunting at the hip and the most profound blunting occurring at the heel. If the blunting of the BMD response is predominantly due to reduced strontium uptake into new bone packets [6] resulting from suppressed bone turnover, then this study may provide interesting insights into how different areas of the skeleton recover after the withdrawal of bisphosphonates. To date, studies looking at the withdrawal of bisphosphonates use bone markers to assess the recovery of bone turnover [4]. However, bone markers only provide information on the rate of bone turnover in the skeleton as a whole with areas of greater bone turnover, such as the spine, contributing to the majority of the total level of bone marker.
In a recently published study, Busse et al. [21] reported the effects of strontium ranelate on 15 paired bone biopsy specimens obtained from the iliac crest of women with prior bisphosphonate exposure. This study is unique as the use of paired bone biopsies enables longitudinal assessment of the effects of strontium ranelate on bone tissue. This study demonstrated that bone strontium content increased after both 6 and 12 months of therapy. However, while there was an increase in bone strontium content, the lack of a bisphosphonate-naïve control group makes it unclear whether the increase observed was diminished at all by the prior bisphosphonate use. Busse et al. also reported that, in women with prior bisphosphonate exposure, bone volume and trabecular thickness did not increase during the first 6 months of therapy with strontium ranelate, but significant increases in these parameters occurred after 12 months. This is consistent with our results which demonstrate that the blunting of the BMD response to strontium ranelate wears off at the spine after 6 months. As the iliac crest is a trabecular bone site, the study by Busse et al. does not provide any information on the changes in cortical bone where, in the present study, more prolonged blunting was observed.
This study investigates switching therapy from bisphosphonates to strontium ranelate. There are relatively few studies assessing the topic of switching therapy, although this is a frequent occurrence in clinical practice. This is an area which needs more research as the number of different treatments for osteoporosis increases. Switching between antiresorptives is unlikely to have major implications for the efficacy of the new therapy as the overall effects on bone are the same. In fact, studies demonstrate that switching from alendronate to denosumab [22] leads to further reductions in bone turnover, although it is still to be proven whether this confers any clinical benefits in terms of fracture reduction and there is even a possibility that prolonged over suppression of bone may be harmful [23]. For anabolic therapy, it has been demonstrated that prior oral bisphosphonate use inhibits the gains in BMD at the spine over the whole 2 years of treatment with teriparatide, while at total hip, BMD does not increase significantly during the first year of therapy [8, 9]. Finally, the present study has demonstrated that prior bisphosphonate use blunts the BMD response to strontium ranelate, although at the spine at least, this blunting appears to wear off as the antiresorptive efficacy of the bisphosphonate declines over time. Therefore, in clinical practice, careful consideration is needed when considering which initial treatment to use, with the choice of agent tailored for the individual patient, and when considering whether or not to switch class of treatment.
The most important issue regarding osteoporosis treatment is not the effect on BMD but the effect on fracture incidence. It is difficult to predict how the bisphosphonate-induced blunting of the BMD response to strontium will affect the ability of strontium ranelate to reduce fracture incidence. If strontium uptake into the skeleton is reduced, then it is plausible that this will reduce the effect of strontium on bone strength. However, it is also likely that prior bisphosphonate therapy will have a period of residual effect on fracture risk [4] which may provide protection during the transition period. This study demonstrated an increased incidence of vertebral fracture in those women with prior bisphosphonate exposure in both the first and second year. However, this was not a randomised study, and as such, there were differences between the groups at baseline in terms of age and spine BMD. Therefore, the prior bisphosphonate group was a higher risk population for fracture, and this may account for the differences in fracture incidence observed. This hypothesis is supported by the fact that the number of vertebral fractures in the prior bisphosphonate group was the same in the first year, during the period of most severe blunting, as in the second year, when spine BMD increased normally. Furthermore, women were entered into the prior bisphosphonate group if they had either an inadequate response to, or were intolerant of, bisphosphonates. Therefore, we cannot exclude the possibility that selection bias led to women in the prior bisphosphonate group being more resistant to treatment which may also account for the difference in observed fracture incidence.
The reduction in P1NP observed in the bisphosphonate-naïve group in the first year of this study persisted throughout the second year. This observation is in agreement with another study which reported a 14–19% reduction in P1NP in response to strontium ranelate [24]. This is an interesting observation as P1NP is derived from type 1 collagen synthesis [25]. A reduction in the synthesis of type 1 collagen in response to strontium ranelate would not be consistent with the theory that strontium ranelate alters the balance of bone turnover in favour of bone formation resulting in an overall increase in bone tissue [1, 7, 16]. An alternative explanation for the P1NP reduction could be the Adcal D3 tablets as vitamin D has been reported to reduce P1NP in patients with vitamin D levels of less than 30 nmol/l [26]. However, the average vitamin D level in the bisphosphonate-naive group was over 70 nmol/l, suggesting that it was not a vitamin D-deficient population, and 21 (37.5%) women were already taking calcium/vitamin D supplements prior to study entry. In contrast to our observed reduction in P1NP, Bruyere et al. report that strontium ranelate increases P1CP, the C-terminal propeptide of type 1 collagen, by 9.9% [15]. This is more in keeping with the change in bone-specific alkaline phosphatase, a marker of osteoblast activity [25], which is also reported to increase in response to strontium ranelate [1]. Given such mixed results from bone formation markers, it is difficult to draw any conclusions from bone markers regarding whether or not strontium ranelate does have an overall anabolic effect.
There are several limitations to this study. These include inability to randomise the subjects, different bisphosphonate usage prior to study entry and patient selection which have already been discussed with regard to the study [5]. The main limitation with the extension phase was that this was originally planned as a 1-year study, and therefore, subjects had to be enrolled and re-consented into the second year. A number of women chose not to continue into the second year, and this decision may have been influenced by their awareness of their BMD results after the first year. This does not appear to be the case as all but one of the women who decided not to continue into the second year had an increase in their spinal BMD after year 1, and the reasons given for not continuing were: preference for weekly therapy (five women); side effects (two women); and becoming eligible for teriparatide (one woman). Finally, a different statistic method had to be used in the analysis of the extension phase to account for the missing data due to the number of women who dropped out in the second year. This resulted in one statistical anomaly where the increase in hip BMD at 12 months (0.006 g/cm2) was just statistically significant (p = 0.048) in the current analysis, whereas in the original study, the same change was not statistically significant (p = 0.096). No other results were affected by the change in statistical method.
In summary, this study demonstrates that after bisphosphonate exposure, the BMD response to strontium ranelate is blunted for 6 months at the spine after which BMD increases normally. At the hip, a degree of blunting was observed throughout the 2 years of the study, although there was an overall increase in hip BMD in contrast to the heel where no increase in BMD was observed.
References
Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, Cannata J, Balogh A, Lemmel EM, Pors-Nielsen S, Rizzoli R, Genant HK, Reginster JY (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350:459–468
Reginster JY, Seeman E, De Vernejoul MC, Adami S, Compston J, Phenekos C, Devogelaer JP, Curiel MD, Sawicki A, Goemaere S, Sorensen OH, Felsenberg D, Meunier PJ (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90:2816–2822
National Institute for Health and Clinical Excellence (2008) Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. Available at: http://www.nice.org.uk/nicemedia/pdf/TA161quickrefguide.pdf
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Middleton ET, Steel SA, Aye M, Doherty SM (2010) The effect of prior bisphosphonate therapy on the subsequent BMD and bone turnover response to strontium ranelate. J Bone Miner Res 25(3):455–462
Li C, Paris O, Siegel S, Roschger P, Paschalis EP, Klaushofer K, Fratzl P (2010) Strontium is incorporated into mineral crystals only in newly formed bone during strontium ranelate treatment. J Bone Miner Res 25(5):968–975
Bruyère O, Collette J, Rizzoli R, Decock C, Ortolani S, Cormier C, Detilleux J, Reginster JY (2010) Relationship between 3-month changes in biochemical markers of bone remodelling and changes in bone mineral density and fracture incidence in patients treated with strontium ranelate for 3 years. Osteoporos Int 21(6):1031–1036
Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, Audran M, Barker C, Anastasilakis AD, Fraser WD, Nickelsen T (2008) Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 23(10):1591–1600
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19(5):745–751
Middleton ET, Steel SA (2008) Routine versus targeted vertebral fracture assessment for the detection of vertebral fractures. Osteoporos Int 19(8):1167–1173
Blake GM, Preston N, Patel R, Herd RJ, Fogelman I (2000) Monitoring skeletal response to treatment which site to measure in the femur? J Clin Densitom 3:149–155
Steel SA, Thorpe J (2002) The GE-lunar prodigy: in vivo precision and cross-calibration. Calcif Tissue Int 71:277–278
Boivin G, Farlay D, Khebbab MT, Jaurand X, Delmas PD, Meunier PJ (2010) In osteoporotic women treated with strontium ranelate, strontium is located in bone formed during treatment with a maintained degree of mineralization. Osteoporos Int 21(4):667–677
Nielsen SP, Slosman D, Sørensen OH, Basse-Cathalinat B, De Cassin P, Roux CR, Meunier PJ (1999) Influence of strontium on bone mineral density and bone mineral content measurements by dual X-ray absorptiometry. J Clin Densitom 2(4):371–379
Bruyere O, Collette J, Reginster J-Y (2010) The effects of strontium ranelate on biochemical markers of bone turnover and their relationship with bone mineral density: reply to Stepan et al. Osteoporos Int 21(6):1039–1041
Meunier PJ, Boivin G, Marie PJ (2009) About the comparison of two anabolic agents, teriparatide and strontium ranelate, in treated osteoporotic women. J Bone Miner Res 24(12):2066
Noble BS, Reeve J (2000) Osteocyte function, osteocyte death and bone fracture resistance. Mol Cell Endocrinol 159:7–13
Boivin G, Deloffre P, Perrat B (1996) Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S12911) administration. J Bone Miner Res 11:1302–1311
Liney GP, Bernard CP, Manton DJ, Turnbull LW, Langton CM (2007) Age, gender, and skeletal variation in bone marrow composition: a preliminary study at 3.0 Tesla. J Magn Reson Imaging 26:787–793
Rosen CJ, Bouxsein ML (2006) Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2:35–43
Busse B, Jobke B, Hahn M, Priemel M, Niecke M, Seitz S, Zustin J, Semler J, Amling M (2010) Effects of strontium ranelate administration on bisphosphonate-altered hydroxyapatite: matrix incorporation of strontium is accompanied by changes in mineralization and microstructure. Acta Biomater. doi:10.1016/j.actbio.2010.07.019
Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25(1):72–81
Visekruna M, Wilson D, McKiernan FE (2008) Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab 93(8):2948–2952
Recker RR, Marin F, Ish-Shalom S, Möricke R, Hawkins F, Kapetanos G, de la Peña MP, Kekow J, Farrerons J, Sanz B, Oertel H, Stepan J (2009) Comparative effects of teriparatide and strontium ranelate on bone biopsies and biochemical markers of bone turnover in postmenopausal women with osteoporosis. J Bone Miner Res 24(8):1358–1368
Seibel MJ (2005) Biochemical markers of bone turnover: I. Biochemistry and variability. Clin Biochem Rev 26:97–122
Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR (2009) High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int 20(8):1407–1415
Conflicts of interest
Servier Laboratories provided the strontium ranelate and a grant to fund the study. ProStrakan provided Adcal D3 as the calcium supplement but no financial support. Servier has paid speaker fees to Dr E Middleton (<£1,000) and Dr M Aye (<£2,000). All authors have no other conflicts of interest. The study design, the data collection, analysis and interpretation, and the manuscript were all carried out by the authors independent of Servier and ProStrakan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Middleton, E.T., Steel, S.A., Aye, M. et al. The effect of prior bisphosphonate therapy on the subsequent therapeutic effects of strontium ranelate over 2 years. Osteoporos Int 23, 295–303 (2012). https://doi.org/10.1007/s00198-011-1547-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1547-8