Abstract
Summary
A new Canadian WHO fracture risk assessment (FRAX®) tool to predict 10-year fracture probability was compared with observed 10-year fracture outcomes in a large Canadian population-based study (CaMos). The Canadian FRAX tool showed good calibration and discrimination for both hip and major osteoporotic fractures.
Introduction
The purpose of this study was to validate a new Canadian WHO fracture risk assessment (FRAX®) tool in a prospective, population-based cohort, the Canadian Multicentre Osteoporosis Study (CaMos).
Methods
A FRAX tool calibrated to the Canadian population was developed by the WHO Collaborating Centre for Metabolic Bone Diseases using national hip fracture and mortality data. Ten-year FRAX probabilities with and without bone mineral density (BMD) were derived for CaMos women (N = 4,778) and men (N = 1,919) and compared with observed fracture outcomes to 10 years (Kaplan–Meier method). Cox proportional hazard models were used to investigate the contribution of individual FRAX variables.
Results
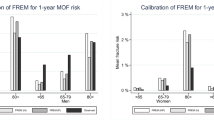
Mean overall 10-year FRAX probability with BMD for major osteoporotic fractures was not significantly different from the observed value in men [predicted 5.4% vs. observed 6.4% (95%CI 5.2–7.5%)] and only slightly lower in women [predicted 10.8% vs. observed 12.0% (95%CI 11.0–12.9%)]. FRAX was well calibrated for hip fracture assessment in women [predicted 2.7% vs. observed 2.7% (95%CI 2.2–3.2%)] but underestimated risk in men [predicted 1.3% vs. observed 2.4% (95%CI 1.7–3.1%)]. FRAX with BMD showed better fracture discrimination than FRAX without BMD or BMD alone. Age, body mass index, prior fragility fracture and femoral neck BMD were significant independent predictors of major osteoporotic fractures; sex, age, prior fragility fracture and femoral neck BMD were significant independent predictors of hip fractures.
Conclusion
The Canadian FRAX tool provides predictions consistent with observed fracture rates in Canadian women and men, thereby providing a valuable tool for Canadian clinicians assessing patients at risk of fracture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is common in Canada, affecting up to 21% of Canadian women and 5% of Canadian men over 50 years of age [1]. These numbers are expected to rise as the population ages [2]. It is projected that the burden on the Canadian health care system will be substantial, with increased rates of death in the acute care setting and increased lengths of fracture-related hospital stay [3, 4]. Furthermore, osteoporotic fractures have been found to be a source of significant morbidity and mortality [5, 6], and they contribute to long-term disability and decreased quality of life [7, 8]. Effective treatments for osteoporosis are now well established. Bisphosphonate therapy decreases vertebral and nonvertebral fracture risk by up to 50% and has been found to be cost-effective for the treatment of osteoporosis in Canada [9–11]. Therefore, identifying patients at risk for fracture in order to facilitate the introduction of appropriate medical therapy is of the utmost importance. Many clinicians use bone mineral density (BMD) alone to diagnose osteoporosis and predict fracture risk, with each standard deviation decrease in BMD being associated with at least a 50% increase in overall fracture risk and at least double the site concordant fracture risk [12]. However, using BMD alone will only identify the minority of patients who are destined to fracture and may result in the treatment of those who would not fracture [13, 14].
The World Health Organization (WHO) fracture risk assessment tool (FRAX®), introduced in 2008, uses country-specific fracture and mortality rates to predict an individual’s 10-year probability of major osteoporotic fracture (clinical spine, forearm, shoulder or hip) and hip fracture alone by combining multiple clinical risk factors (CRFs: age, sex, body mass index, parental history of hip fracture, current cigarette smoking, alcohol intake, rheumatoid arthritis, glucocorticoid use and previous fragility fracture) with or without femoral neck BMD measurement [15]. FRAX was found to have a high predictive ability for fractures when validated in independent cohorts from multiple countries [16]. The use of another country’s FRAX calculator is inappropriate for Canada given differences in Canadian fracture rates and mortality [17]. A Canadian FRAX model was recently developed using national hip fracture and mortality data (reference: “Construction of a FRAX® model for the assessment of fracture probability in Canada and implications for treatment”, Osteoporos Int). The Canadian Institute for Health Information provided the hip fracture and mortality data, and major osteoporotic fracture rates were imputed using major osteoporotic-to-hip fracture ratios from the U.S. FRAX tool (version 3.0) since there were no comprehensive national non-hip fracture data for Canada [18]. Thus, the calibration procedure was completely independent from the Canadian Multicentre Osteoporosis Study (CaMos). The risk factor weights in the generic FRAX model are based on nine prospective cohorts including 5-year data from the CaMos. Thus, the construction of the Canadian FRAX model is largely but not completely independent of the CaMos cohort. The purpose of this study was to validate the Canadian FRAX tool in a large, randomly selected, prospective population-based Canadian cohort using 10-year fracture data.
Methods
Study sample
The performance characteristics of the Canadian FRAX tool were studied in a sample selected from participants in an ongoing population-based longitudinal cohort study, the CaMos. We included all CaMos participants, with follow-up data, aged ≥50 years at study entry. The methodological details of CaMos have been described elsewhere [19]. Briefly, eligible participants were at least 25 years old at the start of the study, lived within a 50-km radius of one of nine Canadian cities (St John’s, Halifax, Quebec City, Toronto, Hamilton, Kingston, Saskatoon, Calgary and Vancouver) and were able to converse in English, French or Chinese (Toronto and Vancouver). Households were randomly selected from a list of residential phone numbers and participants were randomly selected from eligible household members using a standard protocol. Of those selected, 43% agreed to participate and had a baseline interview. All study participants gave written informed consent in accordance with the Helsinki declaration. Ethics approval was granted through McGill University and the appropriate ethics review boards for each participating centre.
Data collection
Participants completed a standardized interviewer-administered questionnaire (CaMos questionnaire© 1995) at baseline, with assessment of demographics, general health, nutrition, medication use and medical history. The questionnaire was designed to capture detailed information about risk factors for fractures, including information about prior fractures, and as such assessed: all previous fractures (fracture site, date and circumstances), family history of osteoporosis/fracture and falls in the past month. Participants had a baseline clinical assessment that included measurement of height, weight and BMD.
Bone mineral density measurements
BMD was measured at the lumbar spine (L1–L4) and proximal femur. Seven centres used Hologic densitometers and two used GE Lunar densitometers. All Lunar measurements were converted to equivalent Hologic values using standard reference formulae [20, 21].
All densitometers were cross-calibrated using a European spine phantom circulated between study centres. A more detailed description of BMD quality control appears elsewhere [22]. Femoral neck T-scores were calculated in both men and women using the NHANES III white female reference values as recommended by the WHO Collaborating Centre. Lumbar spine T-scores were calculated using the manufacturer white female reference values.
Other FRAX variables
Self-reports of rheumatoid arthritis may not be reliable due to confusion with osteoarthritis; therefore, we derived a variable for rheumatoid arthritis based on self-report diagnosis plus treatment using the drug codes for methotrexate, hydroxychloroquine or corticosteroids. Corticosteroid use was based on the drug codes for oral or intravenous glucocorticoids. History of osteoporotic fracture after the age of 50 was assessed using the baseline questionnaire and excluded fractures of the head, hands, ankle, or feet and those due to high trauma. All FRAX estimates were based on baseline CRFs with the exception of parental hip fracture which was a composite; history of parental hip fracture was used for everyone with year 5 data, whereas history of any parental osteoporotic fracture was used from the baseline questionnaire for those without year 5 data.
Fracture probability
The WHO Coordinating Centre used the Canadian FRAX tool calibrated using national hip fracture and mortality data along with the FRAX predictor variables from CaMos to calculate 10-year fracture probability. Fracture probabilities for hip and major osteoporotic sites were calculated using femoral neck BMD combined with CRFs and using CRFs alone. The WHO Coordinating Centre was blinded to fracture outcomes in CaMos.
Fracture assessment
Self-reported incident clinical fractures were identified by yearly postal questionnaire or at the scheduled interval for in-person reassessment (third, fifth and tenth year after study entry). When fractures were identified by yearly postal questionnaire, confirmation and further information concerning the fracture was gathered using a structured interview that included date, fracture site, circumstances leading to fracture, and medical treatment. Participants who reported fractures were asked for consent to contact the treating physician or hospital for verification and for acquisition of further details. For the current analysis, we included all-cause incident fractures and used the FRAX definition of major osteoporotic fractures (hip, clinical spine, humerus, forearm/wrist} occurring before the year 10 annual follow-up.
Statistical methods
Descriptive statistics for demographic and baseline characteristics are presented as mean ± SD for continuous variables or percentages for categorical variables. Univariate comparisons of women and men were performed with the chi-square test (for categorical data) and the t test (for continuous data). To control for incomplete follow-up (18.7% of the study population), survival methods were used. Ten-year estimates of observed major osteoporotic and hip fracture probability were derived using the Kaplan–Meier method with incomplete observations censored and death treated as a competing risk. Separate analyses were performed for women and men. For calibration of the Canadian FRAX tool, overall predicted vs. observed fracture rates were calculated. Individuals were also grouped into quintiles based on their hip and major osteoporotic FRAX probabilities (using BMD), with men and women categorized separately. Within each subgroup, the average predicted 10-year probability was then compared with the observed 10-year fracture probability using graphical methods. In a well-calibrated risk prediction system, predicted and observed rates should track the line of identity. Areas under the receiver operator curve (ROC) were calculated to explore the incremental gain in fracture risk stratification using FRAX (with and without BMD) vs. BMD alone (femoral neck T-score or minimum site). Cox proportional hazards models were constructed for major osteoporotic and hip fracture prediction to determine the contributions of the individual FRAX variables. Statistical analyses were performed with Statistica (version 6.1, StatSoft Inc, Tulsa, OK, USA) and SPSS for Windows (version 16.0, SPSS Inc., Chicago, IL, USA).
Results
Population 10-year probability of fracture
The final study sample consisted of 4,778 women and 1,919 men (combined 6,697). Baseline characteristics are presented in Table 1. In the study sample, Canadian FRAX probability estimates for major osteoporotic fracture and hip fracture were greater for women than men (FRAX with BMD or CRF only, all p < 0.001; Table 2). As shown in Fig. 1, the 10-year observed probability of major osteoporotic fracture in women was 12.0% (95%CI 11.0–12.9%), which was slightly higher than the mean predicted probability of 10.8% by FRAX with BMD or 10.6% by FRAX with CRF only. The 10-year observed probability of major osteoporotic fracture in men (estimated by the Kaplan–Meier method with censoring for incomplete follow-up except in the case of death) was 6.4% (95%CI 5.2–7.5%), which was close to the mean predicted probability of 5.4% by FRAX with BMD. The 10-year observed probability of hip fracture in men was 2.4% (95%CI 1.7–3.1%), whilst the mean predicted probability was 1.3% by FRAX with BMD. Finally, the 10-year observed probability of hip fractures for all women was 2.7% (95%CI 2.2–3.2%), with point estimate equal to the mean predicted probability of 2.7% by FRAX with BMD.
Fracture probability by quintile
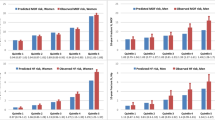
Figure 2 shows the predicted vs. observed fracture probability when women and men were stratified by major osteoporotic fracture and hip fracture probability quintile. For major osteoporotic fracture probability in women, the Canadian FRAX tool gave probability predictions that were within the 95%CI for the observed probability in all quintiles, except for the middle probability quintile in which the observed probability [10.92% (95%CI 8.85–12.99%)] was slightly higher than the predicted probability (8.48%). The regression slope for women was 1.07. In men, the Canadian FRAX tool gave probability predictions for major osteoporotic fracture probability that were within the 95%CI for the observed probability in all quintiles, except the second lowest quintile where the observed probability 1.94% (95%CI 0.51–3.37) was slightly lower than the predicted probability (3.72%). The regression slope for men was 1.26.
When women were stratified by hip fracture probability quintile, the Canadian FRAX tool gave probability predictions that were within the 95%CI for the observed probability in all quintiles. The regression slope for women was 0.93. An unexpectedly high number of men with hip fractures was observed in the highest two quintiles. This is evident in Fig. 2(b) and is reflected by a regression slope for men of 1.83.
ROC analyses
As seen in Table 3, the FRAX estimates using BMD and CRFs were superior to BMD alone or CRFs alone for both major osteoporotic fractures and hip fractures. For major osteoporotic fractures, FRAX with BMD gave area under the curve of 0.69 (95%CI 0.67–0.71) vs. FRAX without BMD 0.66 (95%CI 0.63–0.68) and femoral neck T-score alone 0.66 (95%CI 0.64–69). For hip fractures, FRAX with BMD gave area under the curve of 0.80 (95%CI 0.77–0.83) vs. FRAX without BMD 0.77 (95%CI 0.73–0.80) and femoral neck T-score alone 0.76 (95%CI 0.72–0.79).
Cox proportional hazards models
Hazard ratios (HR) for the contribution of individual CRFs and BMD to observed fracture outcomes are shown in Table 4. For major osteoporotic fractures, age, BMI, prior fragility fracture and femoral neck BMD were significant independent predictors. Male sex, age, prior fragility fracture and femoral neck BMD were significant independent predictors of hip fracture. Despite the important weight given to parental hip fracture in the FRAX calculations, this variable was not a significant independent risk factor for major osteoporotic fracture [HR 0.92 (95%CI 0.69–1.20)] or hip fracture [HR 1.14 (95%CI 0.67–1.96)].
Discussion
In this analysis, we tested a Canadian version of the WHO FRAX tool in a prospective population-based Canadian cohort derived from CaMos. When the 10-year estimates of major osteoporotic fracture and hip fracture probability were calculated by FRAX and compared with the observed 10-year fracture outcomes from CaMos, FRAX was found to be an accurate predictor of fracture risk in both men and women. FRAX probabilities based on both CRFs and BMD were more accurate predictors of 10-year fracture outcomes than BMD alone or FRAX estimates based on CRFs alone. Age, femoral neck BMD and several CRFs were found to independently predict major osteoporotic fractures and hip fractures and thereby contribute to the predictive ability of the Canadian FRAX tool.
Published in 2008, FRAX was created as a quantitative fracture risk tool and is available free of charge on the Internet at www.shef.ac.uk/FRAX [15]. FRAX was derived from nine prospective cohorts (which included 5-year data from CaMos) and was validated in 11 different population-based prospective cohorts from Europe, the USA, Australia and Japan [16]. More recently, the Canadian FRAX tool was validated in Manitoba, a Canadian province that did not participate in CaMos or the initial development of FRAX [23]. These two separate and independent validations are complementary in that they cover different geographic regions within Canada and show good concordance despite differences in study design. Fracture rates vary significantly between countries; therefore, FRAX can be calibrated to the fracture rates and mortality of a country and used to calculate country-specific fracture probability for individuals [24, 25]. Such models have been developed for multiple countries including the UK, Japan, Switzerland and the USA [15, 26–28]. In the absence of a Canadian-specific FRAX tool, Swedish fracture data have been used by Canadians when estimating fracture risk [29]. This is not optimal because of possible miscalibration due to known variations in fracture risk between countries [17, 24]. Our study further validates calibration and discrimination of the Canadian FRAX tool in a population-based Canadian cohort and supports its clinical role as a useful fracture prediction tool across Canada.
FRAX considers the interaction of multiple CRFs, either alone or together with BMD, to give a more accurate prediction of fracture risk than would be obtained by any of these variables alone [15]. The FRAX model has not been previously validated in a population-based Canadian cohort; however, previous Canadian studies have shown incremental benefit in osteoporosis and fracture prediction when certain CRFs are combined with BMD measurements [30, 31]. A further study found that vertebral fracture status also enhances the estimate of fracture risk [32]. Our study findings are consistent with these and other studies showing that FRAX (using CRFs and BMD) is more predictive of fractures than using CRFs or BMD alone [16, 33].
Although BMD is an important risk factor for fracture [12, 34], all fractures cannot be explained by low BMD [35]. In a study examining a large cohort of Canadian postmenopausal women and in a previous CaMos study, most factures occurred in subjects who had BMD values above the osteoporotic range [13, 14]. This finding has been seen in other studies in both women and men [36–38]. The CRFs included in FRAX have all been found to be related to fracture risk independent of BMD [39–44]. Furthermore, a model including these CRFs has been shown to increase sensitivity when identifying individuals at risk of fracture [16]. CaMos was one of the nine prospective population-based cohort studies used to identify CRFs for the original FRAX tool development; therefore, it is not surprising that many of the CRFs used in FRAX were found to be independent predictors of fracture in the present study. We found age, BMI and prior fragility fracture to be independently predictive of major osteoporotic fracture, and sex, age and prior fragility fracture to be independently predictive of hip fracture. In the original FRAX tool development studies, each CRF contributed independently to fracture probability [15]. Possible reasons why some risk factors were not independently predictive of fracture in CaMos could be their low prevalence (resulting in wide CIs), potential misclassification in CRFs that are difficult to assess in epidemiologic studies (such as parental hip fracture, rheumatoid arthritis and alcohol intake which might dilute any effect), or other population characteristics. The question of whether these CRFs should be included in the Canadian FRAX model is not answered by this study.
The FRAX tool is able to capture the independent contribution of multiple different CRFs and combine these with BMD. The ability of FRAX to estimate risk with and without BMD is a major advantage of the tool as BMD measurements are not always readily available to clinicians when assessing patients, especially for men. FRAX gives these clinicians the ability to assess risk and consider treatment options without delay or additional clinical assessment. We found the combination of CRFs and BMD to be the most predictive of fracture; however, using FRAX with CRFs alone still provided accurate 10-year fracture estimates (and, in the case of hip fracture, better than BMD alone).
This study is limited by the relatively small number of fractures, especially in men. However, the men in CaMos had an unexpectedly high hip fracture rate (Fig. 2). Possible reasons include: sample size issues (too few hip fractures for robust estimates), selection bias (men at higher fracture risk more likely to stay in the CaMos study) or poor identification of high-risk men by FRAX CRFs. Based on the population studied, findings cannot be extrapolated to individuals aged <50, though the FRAX model was developed to be applicable from ages 40 to 90. Additionally, CaMos consists of community-dwelling ambulatory subjects; therefore, results cannot be extrapolated to individuals living in long-term care facilities. There are no reports to date of FRAX validation in any long-term care population. We also note that there was both loss to follow-up and death within the cohort, and those who remained in the cohort were healthier than those with incomplete follow-up. We used survival analysis with death treated as a competing risk, but it is still possible that those lost for reasons other than death may have differed from the cohort in risk factors not included in the FRAX model. There were too few participants of non-European ancestry for subgroup analysis, and this may limit the generalizability of our study findings to these ethnic groups. Finally, the study does not examine or make recommendations for intervention thresholds. Further cost effectiveness analyses need to be performed in the Canadian population to determine appropriate treatment thresholds [45].
In summary, by comparing 10-year fracture probability as predicted by the Canadian FRAX tool with observed fracture outcomes in CaMos, we found that the Canadian FRAX tool was a good predictor of fractures for Canadian men and women. This validates the Canadian FRAX model in a cohort representative of the Canadian population. The Canadian FRAX model is a useful clinical tool to assess fracture risk in Canadian women and men.
References
Berger C, Goltzman D, Langsetmo L, Joseph L, Kreiger N, Tenenhouse A, Davison KS, Josse R, Prior J, Hanley D, CaMos Research Group (2010) Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res 25:1948–1957
Hanley DA, Josse RG (1996) Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. 1. Introduction. CMAJ 155(7):921–923
Jaglal SB, Sherry PG, Schatzker J (1996) The impact and consequences of hip fracture in Ontario. Can J Surg 39(2):105–111
Melton LJ III (2003) Epidemiology worldwide. Endocrinol Metab Clin North Am 32(1):1–13, v
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733
Ioannidis G, Papaioannou A, Hopman WM, khtar-Danesh N, Anastassiades T, Pickard L, Kennedy CC, Prior JC, Olszynski WP, Davison KS, Goltzman D, Thabane L, Gafni A, Papadimitropoulos EA, Brown JP, Josse RG, Hanley DA, Adachi JD (2009) Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ 181(5):265–271
Adachi JD, Ioannidis G, Berger C, Joseph L, Papaioannou A, Pickard L, Papadimitropoulos EA, Hopman W, Poliquin S, Prior JC, Hanley DA, Olszynski WP, Anastassiades T, Brown JP, Murray T, Jackson SA, Tenenhouse A (2001) The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int 12(11):903–908
Hallberg I, Rosenqvist AM, Kartous L, Lofman O, Wahlstrom O, Toss G (2004) Health-related quality of life after osteoporotic fractures. Osteoporos Int 15(10):834–841
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH III, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282(14):1344–1352
Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C (2002) Meta-analyses of therapies for postmenopausal osteoporosis. IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev 23(4):570–578
Goeree R, Blackhouse G, Adachi J (2006) Cost-effectiveness of alternative treatments for women with osteoporosis in Canada. Curr Med Res Opin 22(7):1425–1436
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312(7041):1254–1259
Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD (2007) Low bone mineral density and fracture burden in postmenopausal women. CMAJ 177(6):575–580
Langsetmo L, Goltzman D, Kovacs CS, Adachi JD, Hanley DA, Kreiger N, Josse R, Papaioannou A, Olszynski WP, Jamal SA (2009) Repeat low-trauma fractures occur frequently among men and women who have osteopenic bone mineral density. J Bone Miner Res 24(9):1515–1522
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19(4):385–397
Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman JA, Fujiwara S, Gluer C, Goltzman D, Hans D, Krieg MA, La CA, McCloskey E, Mellstrom D, Melton LJ III, Pols H, Reeve J, Sanders K, Schott AM, Silman A, Torgerson D, van ST W, NB YN (2007) The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int 18(8):1033–1046
Leslie WD, O'Donnell S, Lagace C, Walsh P, Bancej C, Jean S, Siminoski K, Kaiser S, Kendler DL, Jaglal S (2009) Population-based Canadian hip fracture rates with international comparisons. Osteoporos Int 21:1317–1322
Ettinger B, Black DM, wson-Hughes B, Pressman AR, Melton LJ III (2010) Updated fracture incidence rates for the US version of FRAX. Osteoporos Int 21(1):25–33
Kreiger N, Tenenhouse A, Joseph L, MacKenzie T, Poliquin S, Brown JP, Prior JC, Rittmaster (1999) Research notes: the Canadian Multicentre Osteoporosis Study (CaMos)—background, rationale, methods. Can J Aging 18:376–387
Lu Y, Fuerst T, Hui S, Genant HK (2001) Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int 12(6):438–444
Genant HK (1995) Universal standardization for dual X-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res 10(6):997–998
Berger C, Langsetmo L, Joseph L, Hanley D, Davison KS, Josse RG, Kreiger N, Tenenhouse A, Goltzman D (2008) Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. Can Med Assoc J 178(13):1660–1668
Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA (2010) Independent clinical validation of a Canadian FRAX® tool: fracture prediction and model calibration. J Bone Miner Res. doi:10.1002/jbmr.123
Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A, Ogelsby AK (2002) International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res 17(7):1237–1244
Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E (2009) FRAX and its applications to clinical practice. Bone 44(5):734–743
Fujiwara S, Nakamura T, Orimo H, Hosoi T, Gorai I, Oden A, Johansson H, Kanis JA (2008) Development and application of a Japanese model of the WHO fracture risk assessment tool (FRAX). Osteoporos Int 19(4):429–435
Dawson-Hughes B, Tosteson AN, Melton LJ III, Baim S, Favus MJ, Khosla S, Lindsay RL (2008) Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int 19(4):449–458
Lippuner K, Johansson H, Kanis JA, Rizzoli R (2010) FRAX assessment of osteoporotic fracture probability in Switzerland. Osteoporos Int 21(3):381–389
Siminoski K, Leslie WD, Frame H, Hodsman A, Josse RG, Khan A, Lentle BC, Levesque J, Lyons DJ, Tarulli G, Brown JP (2005) Recommendations for bone mineral density reporting in Canada. Can Assoc Radiol J 56(3):178–188
Richards JB, Leslie WD, Joseph L, Siminoski K, Hanley DA, Adachi JD, Brown JP, Morin S, Papaioannou A, Josse RG, Prior JC, Davison KS, Tenenhouse A, Goltzman D (2007) Changes to osteoporosis prevalence according to method of risk assessment. J Bone Miner Res 22(2):228–234
Leslie WD, Tsang JF, Lix LM (2009) Simplified system for absolute fracture risk assessment: clinical validation in Canadian women. J Bone Miner Res 24(2):353–360
Chen P, Krege JH, Adachi JD, Prior JC, Tenenhouse A, Brown JP, Papadimitropoulos E, Kreiger N, Olszynski WP, Josse RG, Goltzman D (2009) Vertebral fracture status and the World Health Organization risk factors for predicting osteoporotic fracture risk. J Bone Miner Res 24(3):495–502
Johansson H, Kanis JA, Oden A, Johnell O, McCloskey E (2009) BMD, clinical risk factors and their combination for hip fracture prevention. Osteoporos Int 20(10):1675–1682
Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ III, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20(7):1185–1194
Leslie WD, Metge C, Ward L (2003) Contribution of clinical risk factors to bone density-based absolute fracture risk assessment in postmenopausal women. Osteoporos Int 14(4):334–338
Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34(1):195–202
Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, Berger ML (2004) Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 164(10):1108–1112
Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR (2003) BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 18(11):1947–1954
De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ III, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16(11):1330–1338
Kanis JA, Johansson H, Oden A, Johnell O, De Laet C, Eisman JA, McCloskey EV, Mellstrom D, Melton LJ III, Pols HA, Reeve J, Silman AJ, Tenenhouse A (2004) A family history of fracture and fracture risk: a meta-analysis. Bone 35(5):1029–1037
Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35(2):375–382
Kanis JA, Johansson H, Oden A, Johnell O, de LC M, LJ III, Tenenhouse A, Reeve J, Silman AJ, Pols HA, Eisman JA, McCloskey EV, Mellstrom D (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19(6):893–899
Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, Pols H, Tenenhouse A (2005) Alcohol intake as a risk factor for fracture. Osteoporos Int 16(7):737–742
Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, Fujiwara S, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2005) Smoking and fracture risk: a meta-analysis. Osteoporos Int 16(2):155–162
Borgstrom F, Johnell O, Kanis JA, Jonsson B, Rehnberg C (2006) At what hip fracture risk is it cost-effective to treat? International intervention thresholds for the treatment of osteoporosis. Osteoporos Int 17(10):1459–1471
Acknowledgements
We thank all those participants in CaMos whose careful responses and attendance made this analysis possible. Dr. Fraser is supported by the University of Western Ontario Resident Research Career Development Program. The Canadian Multicentre Osteoporosis Study was funded by the Canadian Institutes of Health Research (CIHR); Merck Frosst Canada Ltd.; Eli Lilly Canada Inc.; Novartis Pharmaceuticals Inc.; The Alliance: sanofi-aventis & Procter and Gamble Pharmaceuticals Canada Inc.; Servier Canada Inc.; Amgen Canada Inc.; The Dairy Farmers of Canada; and The Arthritis Society.
CaMos Research Group
David Goltzman (co-principal investigator, McGill University), Nancy Kreiger (co-principal investigator, Toronto), Alan Tenenhouse (principal investigator emeritus, Toronto)
CaMos Coordinating Centre, McGill University, Montreal, Quebec: Suzette Poliquin (national coordinator), Suzanne Godmaire (research assistant), Claudie Berger (study statistician)
Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher Kovacs (co-director), Emma Sheppard (coordinator)
Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (co-directors), Barbara Stanfield (coordinator)
Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), Marc Gendreau (coordinator)
Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (co-director), Barbara Matthews (coordinator)
University of Toronto, Toronto, Ontario: Bob Josse (director), Sophie A Jamal (co-director), Tim Murray (past director), Barbara Gardner-Bray (coordinator)
McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director), Laura Pickard (coordinator)
University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison(co-director), Jola Thingvold (coordinator)
University of Calgary, Calgary, Alberta: David A. Hanley (director), Jane Allan (coordinator)
University British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Milan Patel (co-director),Yvette Vigna (coordinator); Brian C. Lentle (radiologist)
Conflicts of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Fraser, LA., Langsetmo, L., Berger, C. et al. Fracture prediction and calibration of a Canadian FRAX® tool: a population-based report from CaMos. Osteoporos Int 22, 829–837 (2011). https://doi.org/10.1007/s00198-010-1465-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1465-1