Abstract
Summary
We describe the creation of a FRAX® model for the assessment of fracture probability in Canadian men and women, calibrated from national hip fracture and mortality data. This FRAX tool was used to examine possible thresholds for therapeutic intervention in Canada in two large complementary cohorts of women and men.
Objective
To evaluate a Canadian World Health Organization (WHO) fracture risk assessment (FRAX®) tool for computing 10-year probabilities of osteoporotic fracture.
Methods
Fracture probabilities were computed from national hip fracture data (2005) and death hazards (2004) for Canada. Probabilities took account of age, sex, clinical risk factors (CRFs), and femoral neck bone mineral density (BMD). Treatment implications were studied in two large cohorts of individuals age 50 years and older: the population-based Canadian Multicentre Osteoporosis Study (4,778 women and 1,919 men) and the clinically referred Manitoba BMD Cohort (36,730 women and 2,873 men).
Results
Fracture probabilities increased with age, decreasing femoral neck T-score, and number of CRFs. Among women, 10.1–11.3% would be designated high risk based upon 10-year major osteoporotic fracture probability exceeding 20%. A much larger proportion would be designated high risk based upon 10-year hip fracture probability exceeding 3% (25.7–28.0%) or osteoporotic BMD (27.1–30.9%), and relatively few from prior hip or clinical spine fracture (1.6–4.2%). One or more criteria for intervention were met by 29.2–34.0% of women excluding hip fracture probability (35.3–41.0% including hip fracture probability). Lower intervention rates were seen among CaMos (Canadian Multicentre Osteoporosis Study) men (6.8–12.9%), but in clinically referred men from the Manitoba BMD Cohort, one or more criteria for high risk were seen for 26.4% excluding hip fracture probability (42.4% including hip fracture probability).
Conclusions
The FRAX tool can be used to identify intervention thresholds in Canada. The FRAX model supports a shift from a dual X-ray absorptiometry (DXA)-based intervention strategy, towards a strategy based on fracture probability for a major osteoporotic fracture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a common condition, affecting a large number of women and men over age 50 in Canada [1]. More than 30,000 new hip fractures occur in Canada each year, with many more fractures affecting other skeletal sites [2]. The consequences of fracture include increased mortality, morbidity, institutionalization and economic costs [3, 4]. An individual with a hip or vertebral fracture has an excess risk of death that continues beyond the first year [5–7], and has been attributed to underlying co-morbidity [8, 9]. Moreover, all osteoporosis related fractures can lead to significant long-term disability and decreased quality of life [10, 11].
In the absence of a defining fracture, the diagnosis of osteoporosis is based on the measurement of bone mineral density (BMD) by dual X-ray absorptiometry (DXA). The World Health Organization has provided an operational definition of osteoporosis given as a BMD that lies 2.5 standard deviations (SD) or more below the average mean value for young healthy women (T-score ≤ −2.5 SD) based upon a standardised reference site (the femoral neck) and a standard reference range for both men and women (the NHANES III data for women aged 20–29 years) [12–14]. Although reduced bone mass is an important and easily quantifiable measurement, studies have shown that most fractures occur in individuals with a BMD T-score above the operational threshold [15]. Recently, the use of clinical risk factors (CRFs) has been shown to enhance the performance of BMD in the prediction of hip and major osteoporotic fractures in men and women [16]. In order to identify the CRFs for osteoporotic fracture, data were analyzed from nine prospective primary cohorts and 11 prospective validation cohorts, including more than 275,000 persons corresponding to 1.4 million person-years with more than 22,711 reported fractures [16]. In addition to a prior fragility fracture, age, sex, body mass index and additional risk factors for fractures were identified including the prior use of glucocorticoids, secondary osteoporosis, rheumatoid arthritis, a parental history of hip fracture, current cigarette smoking, and alcohol intake of 3 or more units/day. The fracture risk assessment tool (FRAX®) allows for estimation of individual 10-year osteoporotic and hip fracture probability [17]. Analyses have confirmed that there is an improvement in fracture prediction in the use of BMD and CRFs together when compared with BMD alone or CRFs alone [15, 16]. This has led to endorsement of FRAX and integration into clinical practice guidelines by an increasing number of national bodies.
Fracture and mortality rates are known to vary widely between countries [18]. Therefore, population-specific FRAX tools can be customized based upon fracture and mortality epidemiology in that specific region. [17] At present FRAX® models are available for Argentina, Austria, Belgium, China, Finland, France, Germany, Hong Kong, Italy, Japan, Lebanon, New Zealand, Spain, Sweden, Switzerland, Turkey, the UK and the US [19–22], and several others are being developed. Recent analyses have shown that mortality and hip fracture epidemiology in Canada differs from that in the United States, and this justifies creation of a Canadian FRAX tool calibrated with Canadian fracture data [2].
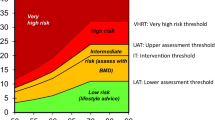
Previous guidelines from Osteoporosis Canada have endorsed absolute 10-year fracture probability for risk assessment [23]. Specifically, a 10-year major osteoporotic fracture probability of greater than 20% is considered high risk, less than 10% is considered low risk, with intermediate values considered moderate risk. The high risk threshold of 20% is consistent with the recommendation from the National Osteoporosis Foundation (NOF) based upon cost-effectiveness analyses for the United States [20, 24]. The Canadian risk assessment system currently does not have a recommendation for the use of hip fracture probability in management, though the NOF recommends that a 10-year hip fracture probability of 3% or greater be considered for intervention. The NOF also recommends intervention in individuals with prior fragility fractures of the hip or spine, and individuals with osteoporotic BMD (T-score –2.5 or lower), independent of the FRAX score.
The current report details the construction of a Canadian FRAX tool. Potential treatment implications based upon this risk assessment system were evaluated in two complementary populations, namely the Canadian Multicentre Osteoporosis Study (CaMos) and the Manitoba Bone Density Program cohort. CaMos is a population-based study and therefore provides information on potential intervention rates for the general population. The Manitoba Bone Density Program cohort is a large clinical referral population and therefore provides information on implications for routine clinical practice.
Methods
Canadian fracture data
National hip fracture data for 2005 and national mortality data for 2004 (the most recent available) were used to construct the Canadian FRAX tool using previously described data sources [2]. Ideally, detailed fracture epidemiology for major non-hip fracture sites (clinical spine, forearm, humerus) should be used in FRAX tool construction but these were not available at a national level. Therefore, non-hip fracture rates were imputed based upon the assumption that the ratios would be similar to those for the United States, as recently described in the construction of the updated United States FRAX tool [25–27].
Briefly, the Canadian Institute for Health Information (CIHI) collects and analyzes information on health and health care in Canada and makes this publicly available. The Hospital Morbidity Database (HMDB), a database housed at CIHI, includes administrative, clinical and demographic information on hospital inpatient events and provides national discharge statistics from Canadian health care facilities by diagnoses and procedures. The HMDB is comprised of a subset of the Discharge Abstract Database (DAD) data and it appends data from provinces/territories that are not participating in DAD in order to provide a national database. Hospital discharges in the HMDB for 2005 were coded using the Tenth International Classification of Diseases, Canadian Enhancement (ICD-10-CA) system following standardized and mandatory coding methods [28, 29]. CIHI ensures a high quality of information in the HMDB through a data quality enhancement program [28, 29]. We identified all 2005 hospitalizations from the HMDB in which the most responsible diagnosis was a hip (proximal femoral) fracture using the following diagnosis codes: ICD-10-CA S72.0–S72.2. The annual number of hip fractures was tabulated by sex and age (5-year intervals). The denominator was stratified in the same fashion using national census data.
Construction of the FRAX tool
Models were constructed to compute the 10-year probability of hip fracture and a major osteoporosis related fracture in Canada using the methodology previously described for the development of FRAX in the UK [19]. A major osteoporosis-related fracture was defined as a clinical spine, hip, proximal humeral or forearm fracture. Poisson models were used to calculate the hazard functions of fracture and death. The relationship between the hazard functions was used to calculate the 10-year probability or fracture for a combination of given risk factors. Since the incidence of other fractures was not known in Canada, we assumed that the age- and sex-specific ratio of index fracture to hip fracture in Canada was the same as found in the United States [27]. The relationship of CRFs to fracture outcomes and death was assumed to be the same as that determined in a large meta-analysis of risk factors derived from prospectively studied population-based cohorts from Europe, Australia, North America and Asia. The independent contribution of each risk factor was used to compute probabilities of fracture in the absence of CRFs or in the presence of any combination [16].
Canadian multicentre osteoporosis study
The CaMos is an ongoing population-based longitudinal cohort study that began in 1996. The methodological details have been described elsewhere [30]. For the present report, we included all CaMos participants aged 50 years and over at study entry for whom follow-up data and BMD measurements were available. Briefly, eligible participants were at least aged 25 years at the start of the study, lived within a 50-km radius of one of nine recruitment cities. Households were randomly selected from a list of residential phone numbers and participants were randomly selected from eligible household members using a standard protocol. Ethics approval was granted through McGill University and the appropriate ethics review boards for each participating center.
Participants completed a standardized interviewer-administered questionnaire (CaMos questionnaire ©1995) at baseline, which assessed demographics, general health, nutrition, medication use, and medical history. The questionnaire was designed to capture detailed information about risk factors for fractures, including information about prior fractures (fracture site, date and circumstances) and other CRFs required for FRAX. Participants had a baseline clinical assessment that included measurement of height, weight, and BMD. BMD was measured at the lumbar spine (L1–L4) and proximal femur (femoral neck, trochanter and total hip). Seven centers used Hologic densitometers and two used GE Lunar densitometers. All Lunar measurements were converted to equivalent Hologic values using standard reference formulas [31]. All densitometers were cross-calibrated using a European spine phantom circulated among study centers. A more detailed description of BMD quality control appears elsewhere [32].
Manitoba BMD cohort
Bone density testing with DXA has been available in Manitoba since 1990 and managed as an integrated program since 1997 using targeted case-finding and standard criteria as previously published [33, 34]. The program maintains a database of all DXA results which can be linked with other population-based computerized health databases through an anonymous personal identifier as previously described [35]. The clinical study population consisted of all women and men in the Province of Manitoba, Canada, 50 years or older at the time of baseline DXA testing at the lumbar spine (L1–L4) and proximal femur (femoral neck, trochanter and total hip) between January 1990 and March 2007. Subjects were required to have medical coverage from Manitoba Health during the observation period ending March 2008 without other exclusions. For those with more than one eligible set of measurements, only the first record was included. The study was approved by the Research Ethics Board for the University of Manitoba.
Prior to 2000, DXA measurements were performed with a pencil-beam instrument (Lunar DPX; GE Lunar, Madison, WI) and after this date a fan-beam instrument was used (Lunar Prodigy, GE Lunar). Instruments were cross-calibrated using anthropomorphic phantoms and 59 volunteers. Densitometers showed stable long-term performance (coefficient of variation [CV] <0.5%) and satisfactory in vivo precision [36]. Fractures and other CRFs required for FRAX were assessed through a combination of hospital discharge abstracts (diagnoses and procedures coded using the ICD-9-CM prior to 2004 and ICD-10-CA thereafter) and physician billing claims (coded using ICD-9-CM) [37]. Use of systemic glucocorticoids (over 90 days dispensed in the year prior to DXA testing at a mean prednisone-equivalent dose of 7.5 mg/day or greater) was obtained from the provincial pharmacy database. For purposes of the FRAX calculation, prior fragility fracture was taken to be a major fracture (hip, clinical vertebral, forearm, and humerus fracture) prior to BMD testing [38]. A diagnosis of rheumatoid arthritis testing was taken from physician office visits and/or hospitalizations with a compatible ICD-9-CM/ICD-10-CA code in a 3-year period prior to BMD testing. Proxies were used for smoking (COPD diagnosis) and high alcohol intake (alcohol or substance abuse diagnosis). Parental hip fracture information was only collected in the last 2 years (2005 and onwards) and was therefore missing for earlier cases. To adjust for the effect of missing parental hip fracture information on FRAX estimates prior to 2005, age- and sex-specific estimates of the effect of a positive response was determined using the later years of data (2005–2007) as previously described [39]. This averaged effect incorporates both the prevalence of a positive response as well as the relative change in risk. Weight and height were recorded at the time of the DXA examination. BMI (in kg/m2) was calculated as weight (in kilograms) divided by height squared (in meters).
Statistical methods
Ten-year osteoporotic and hip fracture probabilities were estimated for each individual in the study cohorts by the WHO Collaborating Centre using the Canadian FRAX tool. For comparative purposes, estimates from the Canadian FRAX tool were compared for a hypothetical woman or man without additional CRFs in which age was varied from 50 to 90 years (femoral neck T-score fixed at −2.5 SD) and in which the femoral neck T-score is varied from +1 to −4 SD (age fixed at 65 years). The numbers of individuals from the CaMos and Manitoba BMD Program cohorts satisfying different high risk criteria, individually and in combination, were tabulated according to sex and age stratum (5-year increments). The criteria that were considered were FRAX major osteoporotic fracture probability greater than 20%, FRAX hip fracture probability greater than 3%, prior hip or clinical spine fracture, and osteoporotic BMD (minimum T-score −2.5 or lower using NHANES III white female reference data for hip measurements and manufacturer white female reference data for spine measurements).
Results
Age-specific mortality and hip fracture data are summarized in Table 1. Mortality rates showed the expected steep increase with older age, and men had higher age-specific mortality than women across the age spectrum. Hip fracture rates in men and women showed a similar steep age-dependent increase. Hip fractures were rare prior to age 65 years but then increased sharply in both sexes. Men had higher hip fracture rates than women prior to age 55 years, after which women had substantially higher hip fracture risk.
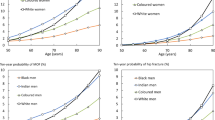
The effects of age and femoral neck BMD on the FRAX estimates for major osteoporotic and hip fractures were assessed for a hypothetical individual without additional CRFs (Figs. 1 and 2). The strong effect of declining femoral neck T-score on major osteoporotic fracture risk was clearly evident and consistent with an exponential risk relationship in both men and women (Fig. 1a). At any given T-score, men had lower 10-year major osteoporotic fracture probability than women (9.2% vs. 11.0% respectively at age 65 years for a T-score of −2.5 SD) (Fig. 2). For a man at the osteoporosis threshold (femoral neck T-score −2.5 SD), the 10-year probability of a major osteoporotic fracture increased from 5.4% at age 50 years to a maximum of 11% at age 80 years, and then declined reflecting the effect of competing mortality. For a woman the corresponding values were 5.3% at age 50 and 21% at age 85 years (Fig. 1b).
Ten-year hip fracture probability estimated by FRAX showed a slightly different pattern (Fig. 2). For a given femoral neck BMD, a 65-year-old man had equal or slightly greater fracture probability than a 65-year-old woman without additional CRFs (3.1% vs. 2.5% respectively at a T-score of −2.5 SD). Ten-year hip risk (for femoral neck T-score −2.5 SD) increased from 1.8% at age 50 years to 5.1% at age 80 years for a man, and from 1.8% at age 50 years to 7.2% at age 85 years for a woman. There was a transition point around age 70–75 years below which hip fracture probability was greater for a man at the osteoporotic threshold, and above which hip fracture probability was greater for a woman.
The presence of additional CRFs increased fracture probabilities in a dose-dependent fashion. For the CaMos cohort, average probability of major osteoporotic fracture calculated with BMD was 7.6% for those with no additional risk factors, 11.8% for one additional risk factor, 16.4% for two additional risk factors, and 34.1% for three or more additional risk factor (1.6%, 3.4%, 5.5% and 17.9% respectively for hip fracture probability calculated with BMD). There was a similar monotonic increase in the Manitoba Bone Density Program cohort for average probability of major osteoporotic fracture calculated with BMD (8.8%, 14.8%, 19.2% and 27.0%) and hip fracture calculated with BMD (1.9%, 4.3%, 7.6%, 13.0%). Multiple regression confirmed a significant positive correlation between FRAX probabilities and number of CRFs adjusted for sex, age, BMD and BMI (all P for trend <0.001). Similar results were seen using FRAX without BMD (data not shown).
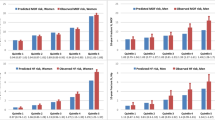
Risk categorization with the Canadian FRAX tool was assessed in 4,766 women and 1,916 men from the CaMos cohort. Prior to the age of 60 years, the 10-year probability of a major osteoporotic fracture rarely exceeded 20% (less than 1%). After age 85 years, 63.6% of the women exceeded the high risk threshold. A higher prevalence of hip fracture probability greater than the 3% threshold was seen across the age spectrum and peaked at 96.6% for women age 85 years and older. Overall, 10.1% of women would be considered high risk based upon major osteoporotic FRAX probability and 25.7% would be considered high risk based upon the hip FRAX probability (Table 2). Osteoporotic BMD (minimum T-score −2.5 SD or lower) was seen in 8.1% of women age 50–54 years and this showed a progressive increase to 59.1% after age 85 years (overall 27.1%). The prevalence of prior spine or hip fracture was only 0.2% in women age 50–54 years and increased to 8.0% in those age 85 years and older (overall 1.6%). The effect of combining these criteria was evaluated. Based upon major osteoporotic fracture probability (but not hip fracture probability) in addition to osteoporotic BMD or prior fragility fracture (hip or clinical spine), one or more of these criteria was satisfied by 8.1% of women age 50–54 years and 71.6% of women age 85 years and older (overall 29.2%). Inclusion of the hip fracture probability criterion increased the overall high risk designation to 8.4% in women age 50–54 years and 96.6% in women age 85 years and older (overall 35.3%). Intervention rates weighted for the 2006 Canadian population structure were also estimated and were virtually identical.
All of the high risk criteria for therapeutic intervention showed lower prevalence among CaMos men than CaMos women, but the same age-related increase was evident. The criterion with the greatest prevalence was 10-year hip fracture probability greater than 3% (overall 10.2%), which contrasted sharply with 10-year probability of a major osteoporotic fracture greater than 20% (overall 0.8%). Prevalence of osteoporotic BMD was intermediate (overall 6.4%) and prior fragility fracture was infrequent (overall 0.4%). If the hip fracture criterion was excluded then 6.8% of all men satisfied one or more of the high risk criteria; this increased to 12.9% if hip fracture probability was also included.
Risk categorization was also assessed in 36,730 women and 2,873 men from the Manitoba Bone Density Program cohort (Table 2 and Fig. 3). The most common single criterion leading to a designation of high risk was osteoporotic BMD (overall 30.9%), followed by hip fracture probability greater than 3% (28.0%), major osteoporotic fracture probability greater than 20% (overall 11.3%) and finally prior hip or clinical spine fracture (overall 4.2%). When the hip fracture probability criterion was not included, 34.0% of the women satisfied one or more high risk criteria; with inclusion of hip fracture probability this increased to 41.0%. Rates were slightly but not markedly higher among the clinically referred Manitoba women when compared with the randomly selected CaMos women. On the other hand, men from the Manitoba cohort showed a higher prevalence of high risk criteria (individually and in combination) than in the CaMos cohort, consistent with greater referral bias among men. A marked discordance was again seen in designation of high risk in men based upon hip fracture probability (overall 33.3%) compared with major osteoporotic fracture probability (overall 2.0%). Osteoporotic BMD (overall 19.3%) and prior hip or clinical spine fracture (10.0%) showed intermediate prevalence. One or more criterion for high risk were seen for 26.4% of the men if hip fracture probability was not included, and 42.4% if hip fracture probability was included.
To directly compare the relative contributions of major osteoporotic fracture probability greater than 20% versus hip fracture probability greater than 3%, we assessed these criteria among all individuals (women and men combined) designated as high risk by either of these FRAX criteria. Overall, 64.0% of the CaMos cohort with a high risk FRAX probability were identified from only the hip fracture criterion (with major osteoporotic fracture probability less than 20%) versus 2.7% identified solely from major osteoporotic fracture criterion (with hip fracture probability less than 3%). Similar results were seen in the Manitoba cohort among individuals with a high risk FRAX probability, with 61.6% identified solely on the basis of hip fracture probability greater than 3% (with major osteoporotic fracture probability less than 20%) and only 1.0% identified solely on the basis of major osteoporotic fracture probability greater than 20% (with hip fracture probability less than 3%). After age 70 years, no individual met the major osteoporotic fracture criterion who did not also have hip fracture criterion.
Discussion
The present study describes the FRAX® model for the assessment of fracture probability in Canadian men and women. The model has been calibrated to the epidemiology of hip fractures which has recently been characterised for Canada [2]. The Canadian FRAX tool was calibrated based upon national hip fracture data (for 2005) and national mortality data (for 2004) according to the procedure established by the WHO Collaborating Centre. National data on non-hip fracture epidemiology is not available, and was therefore assumed to show the same relationship with hip fractures as in construction of the US white FRAX tool [27]. The strengths and limitations of FRAX have been extensively reported [17, 40]. The technology permits an estimate of fracture probability that integrates in a quantitative manner the information from multiple risk factors, with or without information on BMD. The inclusion of the risk factors improves the performance of assessment by increasing sensitivity (detection rate of who will fracture) without sacrificing specificity [16, 41]. The model described in this study has been validated in 11 independent prospectively studied cohorts with in excess of 1 million patient years [16].
The contribution of FRAX for identifying high-risk women and men was assessed in two complementary cohorts, one based upon the general population (CaMos) and the other from a clinical referral population (Manitoba). High risk thresholds were taken from Osteoporosis Canada and the National Osteoporosis Foundation as 10-year major osteoporotic fractures greater than 20% and 10-year hip fracture probability greater than 3%. Based upon these thresholds, hip fracture probability was responsible for virtually all of the high risk designation with minimal contribution of major osteoporotic fracture probability. Of course, this relationship could be very different if other thresholds were used. It is notable that virtually all women (greater than 90%) in the oldest subgroup age 85 years and older have a high hip fracture probability designation, as do the majority (greater than 70%) of clinically referred men. The intervention criterion based upon hip fracture probability may be overly sensitive given the near universal categorization of high risk in older individuals, and suggests that the primary designation of risk should be based upon the global assessment of major osteoporotic fracture probability as has been suggested for the UK [42].
Importance of age in the risk assessment process has previously been highlighted for women. It was found that prior to age 65 years, osteoporotic BMD categorized more women at high risk than did the 10-year fracture probability with a reversal in this relationship after age 65 years [43, 44]. These previous analyses were based upon estimates of 10-year probability of major osteoporotic fracture for Swedish women and did not use the finalized FRAX model. The current analysis updates these studies by examining the newly created Canadian FRAX tool in men as well as women. Under the current Canadian FRAX tool, osteoporotic BMD results in the highest yield of women identified in need of treatment. Before the age of 65 years, very few women (less than 5%) would be designated high risk based upon major osteoporotic fracture probability greater than 20%. Even after age 65, using major osteoporotic fracture probability greater than 20% as a treatment criterion identified very few additional women who were not already identified from an osteoporotic BMD or prior hip/spine fracture. These findings were even more evident among men, in whom very few met the criterion of high major osteoporotic fracture probability (0.8% of the CaMos cohort and 2.9% of the Manitoba cohort).
The major challenge is to integrate these findings into a management paradigm. Treatment would be relatively easy if all risk assessment criteria were concordant, but this is clearly not the case. Where clinical trial evidence exists, such as for the treatment of individuals following hip fracture or vertebral fracture [45, 46], then this can guide treatment initiation. There is evidence from two clinical trials of greater fracture reduction at higher fracture probabilities [47, 48]. Where there is discordance, such as the younger patient with osteoporotic BMD but relatively low 10-year fracture probability, or the non-osteoporotic elderly with high fracture probability, more research is required to better define the magnitude of the absolute risk reduction (the frequently overlooked counterpoint to absolute fracture risk). In the absence of definitive data, patients and their health care providers must use best judgment and attempt to integrate the information provided from these various sources. Individual preferences need to be given particular weight where firm evidence-based recommendations cannot be made.
The NOF for the US and the National Osteoporosis Guideline Group (NOGG) for the UK describe treatment intervention thresholds using fracture probability estimates from FRAX. Both are supported by health economic analyses, though the approaches differ. In the US, the thresholds are set by cost-effectiveness analyses. In the UK, practice guidelines are translated to probabilities which are shown to be cost-effective. The NOF Clinician’s Guide states that postmenopausal women or men older than 50 years with a T-score of ≤ –2.5 SD at the hip or spine, should be treated, regardless of prior fracture status. [49, 50] Similarly, patients with a prior hip or spine fracture should be treated regardless of BMD. In addition, based on risk calculations from the US FRAX tool, patients with low bone mass (T-score between −1.0 and −2.5 SD at the femoral neck, total hip or spine) should be treated when there is a 10-year probability of hip fracture that is ≥ 3% or a 10-year probability of a major osteoporosis related fracture that is ≥ 20%.
NOGG suggests an age-dependent intervention threshold (equivalent to the probability of a woman with no risk factors other than a prior fragility fracture) which varies from a 10-year probability of a major osteoporotic fracture of 7.5% at age 50 years to 30% at the age of 80 years [42]. Assessment thresholds for testing individuals with BMD are also proposed by NOGG and would apply to 6–9% of the population at the age of 50 years, rising to 18–36% at the age of 80 years. The overall use of the NOGG thresholds in a case-finding strategy was projected to identify 6–20% women as eligible for BMD testing and 23–46% as eligible for treatment, depending on age [42].
The NOF approach would treat a much larger proportion of the population than under the NOGG guidelines, even after the recent downwards re-calibration in the US FRAX tool risk estimates; thus NOF guidelines are estimated to treat 40.5% of white women over age 50, rising to 67.9–90.8% after age 70 [25, 51]. For comparison, a women age 68 years or older with femoral neck T-score −2.4 SD and no other risk factors would be recommended for treatment under the NOF guidelines but not under the NOGG guidelines. It is unclear which approach would be better suited to the Canadian context. Cost-effectiveness studies using Canadian cost data and the currently Canadian FRAX tool are required. The choice of an intervention threshold of 20% risk of major osteoporotic fractures is therefore an evolving target that may change as additional information emerges. National cohorts, such as CaMos, and the rich administrative data collection systems that exist across Canada offer the opportunity to assess the impact of FRAX on clinically relevant outcomes, and thereby inform future iterations of the Canadian FRAX tool.
Strengths of this analysis include the use of two large, independent and complementary cohorts. Similar findings in both population-based and clinical referral populations increased confidence in the robustness of the findings. Limitations are acknowledged. This report does not examine fracture outcomes in relation to FRAX. Confirmation that there is agreement between the predicted and observed fracture rates is a critically important step before widespread clinical application and these analyses are reported elsewhere [52, 53]. The relatively small number of men in both cohorts, particularly among the oldest age categories, limits the analysis. There were also differences in the way that CRFs were defined for purposes of the FRAX calculations reflecting the nature of the data sources. The definition of prior fragility fracture was more inclusive with CaMos, whereas the Manitoba cohort restricted this to the fracture types that the NOF considers for empirical treatment initiation. Conversely, the administrative data sources for the Manitoba cohort used proxy measurements for smoking and alcohol intake, and had incomplete parental hip fracture information. Despite these limitations, the overall consistency in the observed for the two cohorts suggests that conclusions are robust and generalizable.
In conclusion, the Canadian FRAX tool provides an important assessment of fracture risk that complements other indicators such as prior fragility fracture and osteoporotic BMD. Integrating the Canadian FRAX tool into a management paradigm will require careful consideration since discordance between the various indicators is common.
References
Tenenhouse A, Joseph L, Kreiger N et al (2000) Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int 11:897–904
Leslie WD, O’Donnell S, Lagace C et al (2009) Population-based Canadian hip fracture rates with international comparisons. Osteoporos Int 21:1317–1322
Wiktorowicz ME, Goeree R, Papaioannou A et al (2001) Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteoporos Int 12:271–278
Papaioannou A, Adachi JD, Parkinson W et al (2001) Lengthy hospitalization associated with vertebral fractures despite control for comorbid conditions. Osteoporos Int 12:870–874
Center JR, Nguyen TV, Schneider D et al (1999) Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 353:878–882
Johnell O, Kanis JA, Oden A et al (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42
Ioannidis G, Papaioannou A, Hopman WM et al (2009) Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ 181:265–271
Kanis JA, Oden A, Johnell O et al (2003) The components of excess mortality after hip fracture. Bone 32:468–473
Kanis JA, Oden A, Johnell O et al (2004) Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 15:108–112
Adachi JD, Ioannidis G, Berger C et al (2001) The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int 12:903–908
Hallberg I, Rosenqvist AM, Kartous L et al (2004) Health-related quality of life after osteoporotic fractures. Osteoporos Int 15:834–841
Kanis JA, Melton LJ III, Christiansen C et al (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Looker AC, Wahner HW, Dunn WL et al (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489
Kanis JA, McCloskey EV, Johansson H et al (2008) A reference standard for the description of osteoporosis. Bone 42:467–475
Cranney A, Jamal SA, Tsang JF et al (2007) Low bone mineral density and fracture burden in postmenopausal women. CMAJ 177:575–580
Kanis JA, Oden A, Johnell O et al (2007) The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int 18:1033–1046
Kanis JA, Oden A, Johansson H et al (2009) FRAX and its applications to clinical practice. Bone 44:734–743
Kanis JA, Johnell O, De Laet C et al (2002) International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res 17:1237–1244
Kanis JA, Johnell O, Oden A et al (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
Dawson-Hughes B, Tosteson AN, Melton LJ III et al (2008) Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int 19:449–458
Fujiwara S, Nakamura T, Orimo H et al (2008) Development and application of a Japanese model of the WHO fracture risk assessment tool (FRAX). Osteoporos Int 19:429–435
Lippuner K, Johansson H, Kanis JA et al (2009) Remaining lifetime and absolute 10-year probabilities of osteoporotic fracture in Swiss men and women. Osteoporos Int 20:1131–1140
Siminoski K, Leslie WD, Frame H et al (2005) Recommendations for bone mineral density reporting in Canada. Can Assoc Radiol J 56:178–188
Dawson-Hughes B (2008) A revised clinician’s guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab 93:2463–2465
Dawson-Hughes B, Looker AC, Tosteson AN et al (2010) The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporos Int 21:41–52
Kanis JA, Johansson H, Oden A et al (2010) The effects of a FRAX® revision for the USA. Osteoporos Int 21:35–40
Ettinger B, Black DM, Dawson-Hughes B et al (2010) Updated fracture incidence rates for the US version of FRAX. Osteoporos Int 21:25–33
Richards J, Brown A, Homan C (2001) The data quality study of the Canadian discharge abstract database: a methodological perspective. Proceedings of Statistics Canada Symposium: Achieving data quality in a statistical agency. Statistics Canada, Ottawa Last accessed: June 6, 2009. URL: http://secure.cihi.ca/cihiweb/en/downloads/quality_dadconfpaper_e.pdf
Canadian Institute for Health Information (2008) Quality Assurance Processes Applied to the Discharge Abstract and Hospital Morbidity Databases. CIHI, Ottawa Last accessed: Aug. 3, 2009. URL: http://secure.cihi.ca/cihiweb/en/downloads/quality_assurance_proc_apr08_e.pdf
Kreiger N, Tenenhouse A, Joseph L et al (1999) Research notes: the Canadian Multicentre Osteoporosis Study (CaMos)—background, rationale, methods. Can J Aging 18:376–387
Genant HK (1995) Universal standardization for dual X-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res 10:997–998
Berger C, Langsetmo L, Joseph L et al (2008) Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. Can Med Assoc J 178:1660–1668
Leslie WD, Metge C (2003) Establishing a regional bone density program: lessons from the Manitoba experience. J Clin Densitom 6:275–282
Leslie WD, MacWilliam L, Lix L et al (2005) A population-based study of osteoporosis testing and treatment following introduction of a new bone densitometry service. Osteoporos Int 16:773–782
Leslie WD, Caetano PA, MacWilliam LR et al (2005) Construction and validation of a population-based bone densitometry database. J Clin Densitom 8:25–30
Leslie WD (2006) The importance of spectrum bias on bone density monitoring in clinical practice. Bone 39:361–368
Roos NP, Shapiro E (1999) Revisiting the Manitoba Centre for Health Policy and Evaluation and its population-based health information system. Med Care 37:JS10–JS14
Giangregorio L, Leslie WD (2010) Time since prior fracture is a risk modifier for ten year osteoporotic fractures. J Bone Miner Res (in press)
Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA (2010) Independent clinical validation of a Canadian FRAX® Tool: fracture prediction and model calibration. J Bone Miner Res
Kanis JA (2007) WHO Scientific group on the assessment of osteoporosis at primary health care level. Brussels, Belgium
Johansson H, Kanis JA, Oden A et al (2009) BMD, clinical risk factors and their combination for hip fracture prevention. Osteoporos Int 20:1675–1682
Kanis JA, McCloskey EV, Johansson H et al (2008) Case finding for the management of osteoporosis with FRAX((R))—assessment and intervention thresholds for the UK. Osteoporos Int 19:1395–1408
Leslie WD, Siminoski K, Brown JP (2007) Comparative effects of densitometric and absolute fracture risk classification systems on projected intervention rates in postmenopausal women. J Clin Densitom 10:124–131
Richards JB, Leslie WD, Joseph L et al (2007) Changes to osteoporosis prevalence according to method of risk assessment. J Bone Miner Res 22:228–234
Lyles KW, Colon-Emeric CS, Magaziner JS et al (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809
Kanis JA, Barton IP, Johnell O (2005) Risedronate decreases fracture risk in patients selected solely on the basis of prior vertebral fracture. Osteoporos Int 16:475–482
Kanis JA, Johansson H, Oden A et al (2009) Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone 44:1049–1054
McCloskey EV, Johansson H, Oden A et al (2009) Ten-year fracture probability identifies women who will benefit from clodronate therapy—additional results from a double-blind, placebo-controlled randomised study. Osteoporos Int 20:811–817
Tosteson AN, Melton LJ III, Dawson-Hughes B et al (2008) Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int 19:437–447
Tosteson AN, Burge RT, Marshall DA et al (2008) Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care 14:605–615
Donaldson MG, Cawthon PM, Lui LY et al (2009) Estimates of the proportion of older white women who would be recommended for pharmacologic treatment by the new U.S. National Osteoporosis Foundation Guidelines. J Bone Miner Res 24:675–680
Fraser LA, Langsetmo L, Berger C et al (2010) Fracture prediction and calibration of a Canadian FRAX® tool: a population-based report from CaMos. Osteoporos Int (in press)
Leslie WD, Lix LM, Johansson H et al (2010) Independent clinical validation of a Canadian FRAX((R)) tool: Fracture prediction and model calibration. J Bone Miner Res [epub ahead of print]
Acknowledgements
The development of FRAX® was in part supported by a non restricted grant from the International Osteoporosis Foundation and the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis. The hip fracture and mortality statistics were generously provided by the Public Health Agency of Canada using manipulated Canadian Institutes of Health Information data. We thank all those participants in CaMos whose careful responses and attendance made this analysis possible. The authors are indebted to Manitoba Health for the provision of data (HIPC File No. 2007/2008-49). The results and conclusions are those of the authors, and no official endorsement by Manitoba Health is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee. The analyses and conclusions in this report reflect the opinions of individual experts and not their affiliated organizations.
Conflicts of interest
William D. Leslie
Speaker fees and unrestricted research grants from Merck Frosst Canada Ltd; unrestricted research grants from Sanofi-Aventis, Procter & Gamble Pharmaceuticals Canada, Inc., Novartis, Amgen Pharmaceuticals Canada, Inc., Innovus 3M, Genzyme Canada; advisory boards for Genzyme Canada, Novartis, and Amgen Pharmaceuticals Canada, Inc.
Lisa M. Lix
Unrestricted research grants from Amgen Pharmaceuticals Canada, Inc. and innovus 3M.
David Goltzman
Consultant for Eli Lily, Novartis, Merck, Proctor & Gamble, and Amgen.
David A. Hanley
Consultant and grants from Amgen, Eli Lilly, Merck, Novartis, Proctor & Gamble, Warner-Chilcott, Sanofi-Aventis, Servier, Wyeth-Ayerst, Nycomed.
Jonathan D. Adachi
Consultant/Speaker or research grants from: Amgen, Astra Zeneca, Eli Lilly, GSK, Merck, Novartis, Nycomed, Pfizer, Procter & Gamble, Roche, Sanofi Aventis, Servier, Wyeth, Bristol-Myers Squibb.
Eugene McCloskey
Speaker fees and/or unrestricted research grants from Novartis, Amgen, AstraZeneca, Pfizer, Bayer, Procter & Gamble, Lilly, Roche, Servier and Hologic.
John A Kanis
Nothing to declare for FRAX and the context of this paper.
Others: None
Sources of support
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
CaMos Research Group
David Goltzman (co-principal investigator, McGill University), Nancy Kreiger (co-principal investigator, Toronto), Alan Tenenhouse (principal investigator emeritus, Toronto). CaMos Coordinating Centre, McGill University, Montreal, Quebec: Suzette Poliquin (national coordinator), Suzanne Godmaire (research assistant), Claudie Berger (study statistician). Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher Kovacs (co-director), Emma Sheppard (coordinator). Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (co-directors), Barbara Stanfield (coordinator). Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), Marc Gendreau (coordinator). Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (co-director), Barbara Matthews (coordinator). University of Toronto, Toronto, Ontario: Bob Josse (director), Sophie A Jamal (co-director), Tim Murray (past director), Barbara Gardner-Bray (coordinator). McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director), Laura Pickard (coordinator). University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison(co-director), Jola Thingvold (coordinator). University of Calgary, Calgary, Alberta: David A. Hanley (director), Jane Allan (coordinator). University British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Milan Patel (co-director),Yvette Vigna (coordinator); Brian C. Lentle (radiologist).
Rights and permissions
About this article
Cite this article
Leslie, W.D., Lix, L.M., Langsetmo, L. et al. Construction of a FRAX® model for the assessment of fracture probability in Canada and implications for treatment. Osteoporos Int 22, 817–827 (2011). https://doi.org/10.1007/s00198-010-1464-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1464-2