Abstract
Summary
Postmenopausal hemodialysis patients are at risk of complications related to renal mineral and bone disorder, and postmenopausal osteoporosis. In 112 postmenopausal hemodialysis patients, free estrogen index was positively correlated with bone mineral density (BMD) Z-score and the annual percent change of BMD in multiple regression analysis. Endogenous estrogen may prevent bone loss in postmenopausal hemodialysis patients throughout life.
Introduction
Women on dialysis are not only at risk of developing mineral and bone disorder, but also suffer from postmenopausal osteoporosis. We assessed the effect of sex hormones on bone metabolism in postmenopausal hemodialysis patients.
Methods
We enrolled 112 postmenopausal hemodialysis patients with a mean age of 68.4 ± 10.4 years. We measured the serum levels of estradiol, testosterone, sex hormone-binding globulin (SHBG), and intact parathyroid hormone (intact-PTH), as well as bone metabolism parameters and radial bone mineral density (BMD). The free estrogen index (FEI) was calculated from the estradiol and SHBG values. After conventional dialysis was performed for 12 months, BMD was measured again and the annual percent change was calculated. Estradiol and SHBG were also measured in 25 postmenopausal women without chronic kidney disease.
Results
Estradiol levels were higher in the hemodialysis patients than in the postmenopausal women without chronic kidney disease. In patients with relatively normal bone turnover (intact-PTH: from 150 to 300 pg/ml), the FEI showed a positive correlation with the BMD Z-score. The annual percent change of BMD showed a positive correlation with the FEI according to multiple regression analysis.
Conclusions
Endogenous estrogen may prevent bone loss in postmenopausal hemodialysis patients throughout life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A significant decrease of the bone mineral density (BMD) has been reported in hemodialysis patients compared with the general population and this decline of BMD becomes more marked as the duration of dialysis lengthens [1]. Hemodialysis patients often have secondary hyperparathyroidism due to hyperphosphatemia, impaired vitamin D activation, and hypocalcemia. Hyperparathyroidism not only leads to a low BMD and high fracture rate, but also impairs the health-related quality of life [2].
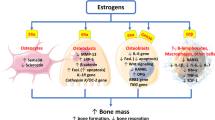
Estrogen is a sex hormone that is known to inhibit bone resorption [3]. Estrogen deficiency stimulates the proliferation and differentiation of osteoclast precursors, and activates mature osteoclasts [3, 4]. In addition, estrogen activates osteoblasts both directly and indirectly via growth factors such as insulin-like growth factor (IGF) I and II [5]. For these reasons, estrogen deficiency after menopause leads to a decrease of BMD. Postmenopausal women with an undetectable serum estradiol level have a higher risk of fracture than women with a serum estradiol level ≥5 pg/ml [6]. Thus, endogenous estrogen production still has an important influence on bone strength after menopause.
Despite frequent contact with medical care providers, women’s health issues may receive less attention in patients on dialysis compared with women in the general population [7]. Women on dialysis are at risk of developing complications related to both mineral and bone disorder and postmenopausal osteoporosis, but there has been little investigation of the relationship between endogenous hormones and bone metabolism. Therefore, we studied the influence of endogenous sex hormones on bone metabolism in postmenopausal Japanese women receiving hemodialysis.
Subjects and methods
Subjects
We enrolled postmenopausal women (all ethnic Japanese) who had been on hemodialysis for over 1 year at Hakuai Clinic (Kure, Japan), Clear Yakeyama Clinic (Kure, Japan), and Chuonaika Clinic (Kure, Japan). We excluded patients who had received hormone replacement therapy, parathyroidectomy, or kidney transplantation. Patients who had undergone limb amputation were also excluded because of difficulty in calculating the body mass index (BMI). Furthermore, we excluded patients who were on steroid therapy. We also enrolled 25 postmenopausal women without chronic kidney disease (serum creatinine: <1.0 mg/dl). The definition of menopause was the same as for the hemodialysis group. None of the subjects had been on estrogen replacement therapy or had undergone oophorectomy.
The definition of menopause according to the World Health Organization is “The permanent cessation of menstruation resulting from loss of ovarian follicular activity.” In our study, menopausal status was defined as a history of bilateral oophorectomy or an age ≥55 years without menstruation for over 1 year, because more than 80% of women in the general population are postmenopausal by the age of 55 years [8]. Women younger than 55 years who had been without menstruation for over 1 year or who had received hysterectomy or oophorectomy were considered to be menopausal if they had a follicle-stimulating hormone (FSH) level ≥ 30 mIU/mL. We measured the serum FSH of the 12 patients who were under 55 years old, and excluded one patient with an FSH level <30 mIU/ml. Accordingly, we enrolled a total of 112 patients in this study and they continued conventional hemodialysis for 12 months. During the study period, ten patients were lost to follow-up because of transfer to another hospital or death, so a total of 102 patients could be followed for 12 months.
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the hospital ethics committees of the participating hospitals. All of the subjects gave informed consent to participation.
Measurement of the radial BMD
We measured the BMD at the distal one-third of the radius by dual-energy X-ray absorptiometry (DOS-600, Aloka, Tokyo). The BMD Z-score was calculated by the following equation: (actual BMD – average BMD for the same age and gender)/standard deviation of the BMD for the same age and gender. After 12 months, we measured the BMD again and calculated the annual percent change as follows: 100 × (follow-up BMD – baseline BMD)/baseline BMD.
Biochemical parameters
At the time of measuring the baseline BMD, venous blood samples were collected after an overnight fast for measurement of the serum concentrations of intact parathyroid hormone (intact-PTH), calcium, phosphate, bone-specific alkaline phosphatase (B-ALP), cross-linked N-terminal telopeptide of type I collagen (NTx), tartrate-resistant acid phosphatase (TRAP), estradiol, testosterone, and sex hormone-binding globulin (SHBG). For calcium and phosphate levels, the mean values were determined over a period of 3 months. The adjusted calcium level was calculated by Payne’s formula [9].
Estradiol was measured with a DPC estradiol double-antibody kit (Mitsubishikagaku Yatoron, Tokyo, Japan) and all samples were evaluated in duplicate. The DPC estradiol double-antibody kit is a highly sensitive assay with a detection limit of 2.5 pg/ml. Intact-PTH was measured with an intact-PTH kit (Roche Diagnostics, Tokyo, Japan). B-ALP was measured by an immunoassay using microtiter strips coated with a monoclonal anti-B-ALP antibody (Metra Biosystems, Mountain View, CA, USA), NTx was measured with an Osteomark-NTx serum kit (Ostex International, Seattle, WA, USA), and TRAP was measured with an N-Assay ACP Nittobo kit (Nitto Boseki, Tokyo, Japan). Testosterone was measured with an Immulite-1000 Testosterone kit (Mitsubishikagaku Yatoron), and SHBG was measured with an Immulite-2000 SHBG kit (Mayo Medical Laboratories, Rochester, MN, USA). The free estrogen index (FEI) was calculated from total estradiol and SHBG by the following equation: \( {\hbox{FEI}} = {\hbox{estradiol }}\left( {{\hbox{pg}}/{\hbox{ml}}} \right) \times 0.{367 }/{\hbox{ SHBG }}\left( {{\hbox{nmol}}/{\hbox{l}}} \right) \). The free androgen index (FAI) was calculated from total testosterone and SHBG by the following equation: \( {\hbox{FAI}} = {\hbox{testosterone }}\left( {{\hbox{ng}}/{\hbox{ml}}} \right) \times {3}.{47} \times {1}00{ }/{\hbox{ SHBG }}\left( {{\hbox{nmol}}/{\hbox{l}}} \right) \) [10, 11].
Statistical analysis
All variables were expressed as the mean ± SD or median and interquartile range (25th to 75th percentiles), unless otherwise indicated. The patients were divided into two groups according to whether the serum estradiol level was <2.5 pg/ml or ≥2.5 pg/ml. Statistical analysis was performed by the Mann-Whitney U test, or the χ 2 test was used for categorical data.
The following variables were included in univariate and multivariate models: age (1-year intervals), duration of hemodialysis (1-month intervals), diabetes (present/absent), vitamin D therapy (present/absent). In multiple regression analysis, we used log-transformed (log10) values for the following parameters: duration of hemodialysis, intact-PTH, B-ALP, NTx, and TRAP.
Multiple regression analysis with forward elimination was used to evaluate possible independent predictors of the FEI by testing a total of 13 variables (age, duration of hemodialysis, diabetes, BMI, vitamin D therapy, intact-PTH, adjusted calcium, phosphate, B-ALP, NTx, TRAP, FAI, and SHBG).
We examined the relationship between the BMD Z-score and the following factors using Spearman’s rank correlation analysis: duration of hemodialysis, diabetes, BMI, vitamin D therapy, dose of oral calcium, dose of sevelamer hydrochloride, intact PTH, adjusted calcium, phosphate, B-ALP, NTx, TRAP, FEI, FAI, and SHBG. Furthermore, multiple regression analysis with forward elimination was used to evaluate possible predictors of the BMD Z-score by testing a total of 15 variables (duration of hemodialysis, diabetes, BMI, vitamin D therapy, dose of oral calcium, dose of sevelamer hydrochloride, intact-PTH, adjusted calcium, phosphate, B-ALP, NTx, TRAP, FEI, FAI, and SHBG). Then we selected a subgroup of patients with relatively normal bone turnover who had intact-PTH levels ranging from 150 to 300 pg/ml. In all of the patients and in this subgroup, Spearman’s rank correlation analysis was used to assess the relation between the BMD Z-score and the FEI. The BMD Z-score was used instead of raw BMD data because employing a BMD Z-score adjusted for age and sex made the model much closer to ideal. Finally, we examined the annual percent change of BMD by Spearman’s rank correlation analysis and multiple regression analysis with forward elimination. The variables employed were the same as for the cross-sectional analysis of BMD Z-score.
Results
Although age and SHBG did not differ between the postmenopausal hemodialysis patients and postmenopausal women without chronic kidney disease (age: 68.4 ± 10.4 vs. 69.7 ± 8.6, P = 0.393; SHBG: 67.4 ± 25.4 vs. 67.7 ± 25.6, P = 0.850, respectively), estrogen levels were higher in the hemodialysis patients. Despite using a highly sensitive estradiol kit, 43 out of 112 postmenopausal hemodialysis patients had undetectable estradiol levels versus 22 out of 25 women without chronic kidney disease (38.4% vs. 88.0%, P < 0.0001).
We stratified the subjects into two groups based on a serum estradiol level <2.5 pg/ml or ≥2.5 pg/ml. Table 1 shows the clinical and laboratory parameters of these two groups. In the group with a serum estradiol level <2.5 pg/ml, the levels of intact-PTH, B-ALP, and NTx, as well as the BMD, BMD Z-score, and BMD T-score, were all smaller than in the group with a serum estradiol level >2.5 pg/ml, but these differences did not reach significance.
Stepwise multiple regression analysis was performed for all 112 patients to find independent predictors of the FEI (Table 2). As a result, the FEI showed a positive correlation with the FAI, diabetes, and intact-PTH, as well as a negative correlation with SHBG.
Next, we examined the factors that influenced the BMD Z-score by Spearman’s rank correlation analysis. There was a negative correlation with the duration of hemodialysis, vitamin D therapy, B-ALP, and NTx. When stepwise multiple regression analysis was performed in all 112 patients to find independent predictors of the BMD Z-score, there was a negative correlation with duration of dialysis and B-ALP, as well as a positive correlation with the FEI (Table 3).
Secondary hyperparathyroidism is strongly associated with a decrease of the BMD [12], so we also investigated correlations in our patients with relatively normal bone turnover who had intact-PTH levels in the range from 150 to 300 pg/ml. In this subgroup (n = 32), the FEI showed a positive correlation with the BMD Z-score (r = 0.658, P < 0.001), despite showing no correlation with the Z-score (r = 0.136, P = 0.155) in all patients (n = 112). When we examined factors that influenced the annual percent change of BMD by Spearman’s rank correlation analysis, there was a positive correlation with the FEI, while there was a negative correlation with intact-PTH, NTx, and TRAP. Stepwise multiple regression analysis was performed to find independent predictors of the annual percent change of BMD, showing a positive correlation with the FEI, as well as a negative correlation with intact-PTH and NTx (Table 4).
Discussion
Women on dialysis are at risk of suffering from renal mineral and bone disorders as well as postmenopausal osteoporosis. In this study, we investigated the effect of sex hormones on bone metabolism in postmenopausal hemodialysis patients, and we found that estradiol levels were higher in these patients than in women without chronic kidney disease. In patients with relatively normal bone turnover (intact-PTH: 150-300 pg/ml), the FEI had a positive correlation with the BMD Z-score. The annual percent change of BMD showed a positive correlation with the FEI according to multiple regression analysis. These findings suggest that endogenous estrogen prevents bone loss in postmenopausal hemodialysis patients throughout life.
In the present study, the FEI demonstrated a positive correlation with intact-PTH (Table 2). Estrogen has been reported to promote PTH secretion [13], while expression of estrogen receptor mRNA has been demonstrated in rat parathyroid tissue and binding of estrogen to the parathyroid glands has been shown by immunohistochemistry [14]. These findings suggest that estrogen might promote PTH secretion in postmenopausal hemodialysis patients as it does in healthy postmenopausal women. During early menopause, a sudden decrease of estrogen leads to high bone turnover, increased bone resorption, and a reduction of PTH. In late menopause, however, low intestinal calcium absorption [15] and low renal calcium handling [16] are primarily responsible for a higher serum PTH level [17]. Therefore, healthy postmenopausal women first show a decrease of the PTH level and then it gradually rises with increasing age. This suggests that there might be no correlation between estradiol and PTH in healthy postmenopausal women.
BMD has been reported to show a strong correlation with intact-PTH [12]. Therefore, we investigated the relation between the FEI and BMD Z-score in our patients with relatively normal bone turnover (an intact-PTH level from 150 to 300 pg/ml). We considered that the intact-PTH level would have little influence on the BMD of this subgroup. As a result, we found that the FEI had a positive correlation with the BMD Z-score in this subgroup. Estrogen deficiency was reported to increase the secretion of interleukin-1, interleukin-6, interleukin-11, and tumor necrosis factor-α and -β [18–20], which activate mature osteoclasts indirectly via a primary effect on osteoblasts and by stimulating the proliferation and differentiation of osteoclast precursors [3, 4]. These findings suggest that estrogen could inhibit bone loss in postmenopausal hemodialysis patients.
In the present study, the BMD Z-score showed a positive correlation with the FEI and a negative correlation with B-ALP according to multiple regression analysis (Table 3). In addition, the annual percent change of BMD was positively correlated with the FEI according to multiple regression analysis, as well as being negatively correlated with intact-PTH and NTx. The negative correlation between BMD and B-ALP indicates that BMD was lower in patients with a high bone turnover. B-ALP showed a strong correlation with intact-PTH. On the other hand, PTH secretion was promoted by estrogen, even though the FEI showed a positive correlation with both the BMD Z-score and the annual percent change of BMD. Thus, estrogen may have two opposing effects on bone metabolism in postmenopausal hemodialysis patients. Estrogen is well known to directly inhibit bone resorption [3]. In addition, estrogen activates osteoblasts both directly and indirectly via the action of growth factors such as IGF-I and -II [5]. Thus, estrogen is thought to decrease bone resorption and increase bone formation in postmenopausal hemodialysis patients. On the other hand, estrogen has previously been reported to promote PTH secretion [13], and we also showed a positive correlation between the FEI and intact-PTH. An increase of PTH increases bone remodeling. In hemodialysis patients, however, a high PTH level is strongly associated with a decrease of BMD. If estrogen only acted to promote PTH secretion, BMD would decrease. However, our study showed that the FEI was positively correlated with the BMD Z-score and the annual percent change of BMD, so the direct effect of estrogen on bone appears to outweigh its indirect effect via PTH.
In the present study, the FEI showed a positive correlation with the presence of diabetes according to multiple regression analysis (Table 2). Many studies have assessed the relation between estrogen and diabetes, and it has been reported that the plasma estradiol level is positively associated with insulin resistance in postmenopausal women [21]. In addition, higher plasma estradiol levels are prospectively related to an increased risk of type 2 diabetes in postmenopausal women [22]. Furthermore, exposure to estradiol induces an increase of pancreatic β-cell insulin in mice and leads to chronic hyperinsulinemia, while longer exposure to estradiol enhances the risk of type 2 diabetes [23].
Weisinger et al. [24] reported on the correlation between serum estradiol and BMD in women under 50 years old. They showed that persistently amenorrheic younger women on dialysis had a lower trabecular BMD compared with normally menstruating women on dialysis, and they found that lumbar spine BMD was significantly correlated with the total estradiol level in the amenorrheic group. We studied postmenopausal women under 85 years old on hemodialysis and showed that the serum estradiol level had a positive correlation with the BMD-Z score. Accordingly, estradiol seems to influence bone metabolism in postmenopausal women on hemodialysis throughout life. Cummings et al. [6] reported that an undetectable serum estradiol level was a risk factor for fracture in postmenopausal women. Our results indicate that patients with a low FEI have a low BMD and might have a higher risk of fracture. Hemodialysis patients already show an increased risk of fracture compared with healthy persons, so we have to pay close attention to the estradiol level in postmenopausal women on hemodialysis.
There have been conflicting reports about serum estradiol levels in postmenopausal women with end-stage renal disease [25–27]. Tanaka et al. [27] reported that estradiol levels were higher in hemodialysis patients and our findings support their results.
One of the limitations of this study is that we only measured the BMD at the radius. However, Ettinger et al. [28] reported that women with estradiol levels from 10 to 25 pg/ml had a 4.9%, 9.6%, 7.3%, and 6.8% higher BMD of the total hip, calcaneus, proximal radius, and spine, respectively, than women with estradiol levels below 5 pg/ml. According to their report, estradiol prevents both cortical and trabecular bone loss in healthy postmenopausal women.
In healthy postmenopausal women, the risk of breast cancer, pulmonary embolism, coronary artery disease, and cerebrovascular disease is increased by long-term combined estrogen and progesterone therapy [29]. However, treatment with raloxifene (a selective estrogen receptor modulator) seems to be less harmful in women with osteoporosis, and 3 years of raloxifene therapy increases the lumbar spine BMD along with a marked decrease of vertebral fractures [30]. Thus, newer therapeutic regimes for postmenopausal hemodialysis patients are expected to include raloxifene.
In conclusion, this study revealed that the FEI was positively correlated with the BMD Z-score and the annual percent change of BMD in postmenopausal women on hemodialysis. Endogenous estrogen may prevent bone loss in postmenopausal hemodialysis patients throughout life.
References
Fontain MA, Albert A, Dubois B et al (2000) Fracture and bone mineral density in hemodialysis patients. Clin Nephrol 54:218–226
Cunningham J, Danese M, Olson K et al (2005) Effects of the calcimimetic cinacalcet HCI on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int 68:1793–1800
Horowitz MC (1993) Cytokines and estrogen in bone: anti-osteoporotic effects. Science 260:626–627
Kitazawa R, Kimble RB, Jl V et al (1994) Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J Clin Invest 94:2397–2406
Mendez-Davila C, Garcia-Moreno C, Turbi C et al (2004) Effects of 17 beta-estradiol, tamoxifen and raloxifene on the protein and mRNA expression of interleukin-6, transforming growth factor-beta1 and insulin-like growth factor-1 in primary human osteoblast cultures. J Endocrinol Investig 27:904–912
Cummings SR, Browner WS, Bauer D et al (1998) Endogenous hormones and the risk of hip and vertebral fractures among older women. N Engl J Med 339:733–738
Rush H, Neugarten J, Coco M (2000) Women’s health issues in a dialysis population. Clin Nephrol 54:455–462
McKinlay SM, Brambilla DJ, Posner JG (1992) The normal menopause transition. Maturitas 14:103–115
Payne RB, Little AJ, Williams RB et al (1973) Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J 4:643–646
Lambrinoudaki I, Christodoulakos G, Aravantinous L et al (2006) Endogenous sex steroids and bone mineral density in healthy Greek postmenopausal women. J Bone Miner Metab 24:65–71
Selby C (1990) Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem 27:532–541
Nakashima A, Yorioka N, Tanji C et al (2003) Bone mineral density may be related to atherosclerosis in hemodialysis patients. Osteoporos Int 14:369–373
Duarte B, Hargis GK, Kukreja SC (1988) Effects of estradiol and progesterone on parathyroid hormone secretion from human parathyroid tissue. J Clin Endocrinol Metab 66:584–587
Naveh-Many T, Almogi G, Livni N et al (1992) Estrogen receptors and biologic response in rat parathyroid tissue and C-cells. J Clin Invest 90:2434–2438
Gennari C, Agnusdei D, Nardi P et al (1990) Estrogen preserves a normal intestinal responsiveness to 1, 25-dihydroxyvitamin D3 in oophorectomized women. J Clin Endocrinol Metab 71:1288–1293
McKane WR, Khosla S, Burritt MF et al (1995) Mechanism of renal calcium conservation with estrogen replacement therapy in women in early postmenopause—a clinical research center study. J Clin Endocrinol Metab 80:3458–3464
Khosla S, Atkinson EJ, Melton LJ 3rd et al (1997) Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population-based study. J Clin Endocrinol Metab 82:1522–1527
Pacifici R, Rifas L, McCracken R et al (1989) Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci USA 86:2398–2402
Jilka RL, Hangoc G, Girasole G et al (1992) Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257:88–91
Roggia C, Gao Y, Cenci S et al (2001) Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 98:13960–13965
Golden SH, Dobs AS, Vaidya D et al (2007) Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab 92:1289–1295
Ding EL, Song Y, Manson JE et al (2007) Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia 50:2076–2084
Alonso-Magdalena P, Morimoto S, Ripoll C et al (2006) The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ Health Perspect 114:106–112
Weisinger JR, Gonzalez L, Alvarez H et al (2000) Role of persistent amenorrhea in bone mineral metabolism of young hemodialyzed women. Kidney Int 58:331–335
Holley JL, Schmidt RJ, Bender FH et al (1997) Gynecologic and reproductive issues in women on dialysis. Am J Kidney Dis 29:685–690
Kramer HM, Curhan G, Singh A, HELP Study Group (2003) Hemodialysis and estrogen levels in postmenopausal (HELP) patients: the multicenter HELP study. Am J Kidney Dis 41:1240–1246
Tanaka M, Itoh K, Matsushita K et al (2005) High serum estradiol concentrations in postmenopausal women with end-stage renal disease. Clin Nephrol 64:394–396
Ettinger B, Pressman A, Sklarin P et al (2006) Associations between low levels of serum estradiol, bone density, and fractures among elderly women: The study of osteoporotic fractures. J Clin Endocrinol Metab 91:3791–3797
Writing Group for the Women’s Health Initiative Investigators (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 288:321–333
Ettinger B, Black DM, Mitlak BH et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 282:637–645
Acknowledgments
This study was supported by Grants-in-Aid for kidney failure and hemodialysis research from the Japanese Association of Dialysis Physicians.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugiya, N., Nakashima, A., Takasugi, N. et al. Endogenous estrogen may prevent bone loss in postmenopausal hemodialysis patients throughout life. Osteoporos Int 22, 1573–1579 (2011). https://doi.org/10.1007/s00198-010-1350-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1350-y